ABSTRACT
Idiopathic granulomatous mastitis (IGM) is a debilitating, chronic, inflammatory condition of the breast. Several studies have emerged evaluating intralesional steroid (ILS) injection and topical steroid administration as a treatment for IGM. However, there is a dearth of international consensuses with regards to the management of IGM. Therefore, we have systematically reviewed the effectiveness of ILS in the management of IGM. A systematic search was conducted in PubMed and Cochrane Library databases, the Google Scholar website and by citation searching up to June 15th, 2023. Eight articles were selected and analyzed. A total of 397 IGM patients were included in the review. The mean patient age was 35.7 years, ranging from 23–62 years. The mean pre-treatment diameter of lesions was 27.5 mm. A total of 184 patients were treated with ILS. The mean complete clinical response time was 2.6 months. The overall complete response rate was 92.8%. Complications following ILS were minor, with hematoma, skin atrophy and hyperemia being commonly described, while avoiding the systemic side effects of oral steroid use, such as weight gain and hirsutism, which were the most commonly reported side effects with oral steroids. The recurrence rates in the ILS group (6.6%) appear to be lower than in the oral steroid group (25.8%) and surgery group (26.3%). ILS seem to show a favorable outcome in terms of complete response rate, complete clinical response time and has a lower recurrence rate and complication rate when compared to other intervention strategies. However, more comparative studies with standardized protocols are necessary to ascertain the optimum type, dosage and frequency of ILS regimens.
Keywords: Idiopathic granulomatous mastitis, corticosteroids, intralesional steroid
Key Points
• Idiopathic granulomatous mastitis (IGM) is a debilitating chronic inflammatory condition of the breast.
• Intralesional steroid injection has become a promising treatment option for IGM.
• However, there is a dearth of international consensuses with regards to the management of IGM.
• This study is a systematic review of the effectiveness of intralesional steroids in the management of IGM to help understand the usage and efficacy of intralesional steroids.
Introduction
Idiopathic granulomatous mastitis (IGM) is a rare, chronic, benign inflammatory condition of the breast which commonly affects women of childbearing age with a history of breastfeeding (1). Infrequently, IGM has been reported in nulliparous women (2) and in men (3). The condition was first described in 1972 (4). Women from Southeast Asia and the Middle East may have a higher incidence of IGM than those of European descent (5). It has also been shown that IGM is commoner in those of Hispanic ethnicity (6).
Despite being described in the literature for over 50 years, the possible etiology for IGM remains elusive. Pregnancy, hyperprolactinemia (7), Corynebacterium infections (8), reactions caused by oral contraceptives and autoimmune reactions (9) seem to be associated with IGM. The strong link between IGM and lactation may be due to micro-trauma caused by milk stasis and breastfeeding (1).
Patients with IGM commonly present with a breast mass, pain, redness, peau d’orange appearance and axillary lymph node enlargement (10, 11). Radiologically, ultrasound features include circumscribed heterogeneous hypoechoic masses with tubular formations, while the commonest mammography findings are focal or diffuse asymmetrical density (12, 13). Magnetic resonance imaging is not routinely used in the workup of IGM (12, 13). Importantly, IGM is indistinguishable from malignancy both clinically and radiologically (14) and can only be reliably diagnosed by histopathological examination of a biopsy (1).
There is a dearth of international consensuses with regards to the management of IGM. Although it may be self-limiting, with observation alone leading to complete resolution within 5–20 months (15), the morbidity, persistence and progression of the condition in some, especially those with large (>5 cm), bilateral lesions or lesions complicated by abscesses and fistulae may necessitate intervention (16). Etiology-specific treatment, such as bromocriptine for hyperprolactinemia and antibiotics for Corynebacterium infection, have been described (17). Surgical measures, though effective, are plagued with adverse outcomes, such as scarring, poor wound healing, recurrence, fistula formation and mastectomy (18) and is generally limited to those with refractory or recurrent disease (17).
Oral steroid (OS) use in the management of IGM was first described in 1980 and acts by mitigating inflammation and autoimmune reactions that may be a causative factor in IGM (19). Oral steroids have been shown to reduce the extent of surgery, or even alleviating the need for surgery in selected cases (17). Therefore, OS is generally considered a first line treatment option. However, its use is associated with side effects such as Cushing syndrome, weight gain, hyperglycemia and opportunistic infections (1).
Methotrexate (MTX) has also been described as a steroid sparing agent in the treatment of IGM, but its efficacy is controversial and its adverse effect profile, especially among women of reproductive age, amongst whom this disease is commonest, has resulted in limited use of this treatment modality (17).
Intralesional steroid (ILS) use was first described for the management of IGM in 2012 by Munot et al. (19) amongst a cohort of four subjects, all of whom showed a complete response, with no local or systemic side effects and no recurrence within a year of treatment. This initial success sparked an interest in the use of this novel method, and several studies have emerged evaluating ILS injection and topical steroid administration as a treatment for IGM. The results seem promising but there is heterogeneity within the published studies.
Therefore, this systematic review was conducted to assess the efficacy of this treatment, as it potentially mitigates the adverse effects of surgery and OS use. We have systematically reviewed the effectiveness of intralesional corticosteroids in the management of IGM.
This study has been registered in PROSPERO on 10.08.2023. ID: CRD42023449788.
Materials and Methods
Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline was used for the study design, search strategy, screening, and reporting. A systematic search was conducted using MeSH keywords as follows: (All available MeSH terms for “steroids”) AND “idiopathic granulomatous mastitis” AND “intralesional” in the PubMed and Cochrane Library databases, the Google Scholar website and by citation searching up to June 15th, 2023. Only publications in English and human interventional studies were included.
Study Selection Criteria
Studies were independently selected by two members of the research group. In case of disagreement, a discussion was held between the two and the third member until the matter was resolved. The following criteria were used to include studies in this systematic review: (1) human studies which used intra-lesional corticosteroids to treat IGM, (2) studies confirming IGM by histopathological diagnosis, and (3) studies reporting complete clinical response rates. Studies were excluded if they were case reports or case series without individual outcome data, review articles, conference abstracts, letters, animal studies, or in vitro studies; duplicate publications; or if the desired parameters such as complete clinical response rate were not reported.
The literature search protocol is summarized in Figure 1.
Figure 1.
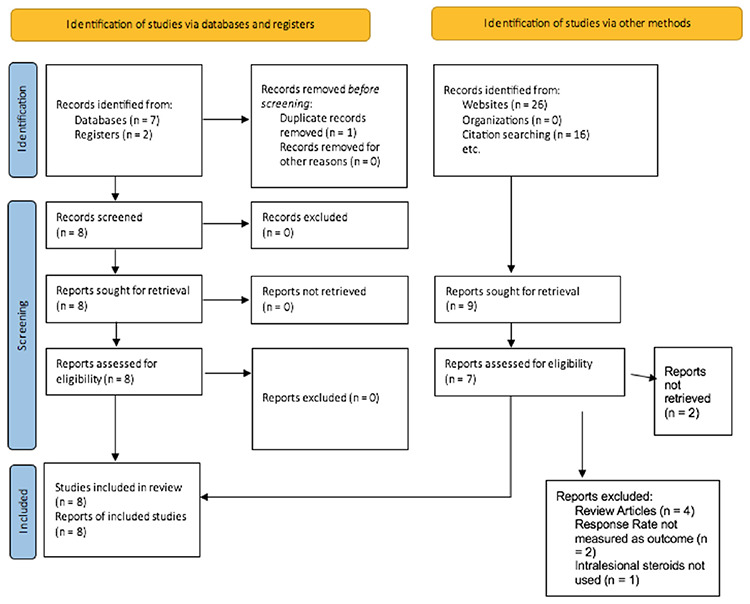
PRISMA flowchart
Data Extraction and Quality Assessment
Two members of the group independently assessed the quality of each selected study and extracted data from the papers and results were compared. Any conflicts were discussed and resolved with a third investigator. The data extraction checklist included the name of the first author, period of data collection, year of publication, country where the study was performed, type of study, number of patients in each intervention, mean age, location of lesion (s), clinical presentation, the type, dose, frequency and duration of intralesional and/or OS use, evaluation frequency and mean follow-up time, complete clinical response rate, mean complete response time period, the number and types of adverse effects and the complication rate of each intervention.
Quality Assessment
The modified downs and black scale (20) was used to assess the quality of the included studies. A 27-point scale was used and was categorized as follows; Excellent (26–27), Good (20–25), Fair (15–19) and Poor (⩽14). All studies achieved a “Fair” or greater score and were included in the systematic review (Table 1).
Table 1. Characteristics of studies included in systematic review.
|
Study |
Country |
Type of study |
Number of patients |
Number treated with ILS |
Number treated with OS |
Number treated with surgery |
Number treated with combination/observation |
Quality assessment* |
|
Alper et al. (22) 2020 |
Turkey |
Prospective cohort |
28 |
28 |
0 |
0 |
0 |
Fair (15/27) |
|
Ertürk et al. (23) 2022 |
Turkey |
Retrospective descriptive |
86 |
38 |
0 |
48 |
0 |
Fair (19/27) |
|
Karami et al. (21) 2022 |
Iran |
Randomized clinical trial |
99 |
31 |
30 |
0 |
38 (Combination) |
Good (23/27) |
|
Kim et al. (24) 2016 |
South Korea |
Retrospective descriptive |
15 |
15 |
0 |
0 |
0 |
Fair (16/27) |
|
Tang et al. (25) 2020 |
USA |
Retrospective descriptive |
49 |
12 |
0 |
9 |
28 (Observation) |
Fair (17/27) |
|
Toktas et al. (26) 2021 |
Turkey |
Retrospective descriptive |
78 |
46 |
32 |
0 |
0 |
Good (20/27) |
|
Toktas and Toprak (27) 2021 |
Turkey |
Retrospective descriptive |
6 |
6 |
0 |
0 |
0 |
Fair (15/27) |
|
Yildirim et al. (28) 2021 |
Turkey |
Randomized clinical trial |
36 |
17 |
19 |
0 |
0 |
Good (23/27) |
*Downs and black scale was used for quality assessment; ILS: Intralesional steroid; OS: Oral steroid
Data Analysis
Data were analyzed based on subgroups of patients classified according to treatment modalities used and were classified as the ILS group (Group 1), OS group (Group 2), Surgery Group (Group 3) and Combined Therapy Group (Group 4). In addition, patients who were given OS in addition to ILS for only a short duration and for whom individual outcome data was not published were included in the ILS group.
Complete response was defined >90% clinical resolution, based on a previous study (21).
Recurrence was defined as clinical re-emergence of lesions following complete or partial response.
Continuous variables are presented as mean with minimum and maximum values, and categorical variables as numbers and percentages. All missing information, including outcome data in patients lost to follow-up was considered as such, and no assumptions were made. Patients with missing data for a specific variable were not included in the statistical analysis.
Results
Description of Studies
Eight studies were selected that included 397 IGM patients and were analyzed for this review. The mean (range) patient age was 35.7 (23–62) years. The mean pre-treatment diameter of lesions was 27.5 (22.2–37.2) mm. Bilateral or multifocal disease was noted in only a minority (11.9%). The mean duration of symptoms upon presentation was 7.8 months. The majority presented with a painful mass, with or without features of inflammation. Other notable presentations included firmness of skin and soft tissue changes, such as purulence, abscesses, ulceration, and fistulation. The mean follow-up frequency was 4.7 weeks while the mean follow-up time was 12.4 months. The characteristics of studies included in this review are given in Table 1.
Intra-Lesional Steroid Group (Group 1)
All studies (21–28) contained an ILS subgroup. A total of 193 (48.6%) patients were treated with ILS of which 9 were lost to follow-up in one study (26), hence the outcome data was not available, and was thus calculated for 184 patients with outcome data. The number of ILS dosages ranged from 1–7 injections. The frequency of dosing was 1-weekly in two studies, 2-weekly in two studies, 4-weekly in three studies, while one study had a single dosage regimen only. Most studies (5/8; 62.5%) used triamcinolone as the ILS, while two studies used methylprednisolone, and a single study used betamethasone disodium phosphate. In one study (24), oral prednisolone (10 mg daily) was combined in 5 patients with multiple, large, or painful abscesses in the early period, before ILS was an established treatment modality. This heterogeneity of ILS regimens was based on common denominators such as the severity, number, and size of the lesions. Additionally, three studies used topical steroids for one month, of which two used triamcinolone and one used prednisolone. A summary of ILS treatment regimens used in the studies included in this review is given in Table 2.
Table 2. Intra-lesional steroid regimes used in studies included in the systematic review.
|
Study |
ILS type |
ILS single dose (mg) |
Dosage range |
Total dosage range (mg) |
Frequency of dosage |
Topical steroid use |
|
Alper et al. (22) 2020 |
Methylprednisolone acetate |
40 |
2–7 |
80–280 |
3–4 weekly |
No |
|
Toktas and Toprak (27) 2021 |
Methylprednisolone acetate |
40 |
1–2 |
40–80 |
2-weekly |
0.125% prednisolone twice a day, EOD for 1 month |
|
Ertürk et al. (23) 2022 |
Triamcinolone acetonide |
40–80 |
1–5 |
40–400 |
4-weekly |
Triamcinolone Daily - 1 month (after ILS) |
|
Kim et al. (24) 2016 |
Triamcinolone acetonide |
40 |
2–6 |
80–240 |
1–2 weekly |
No |
|
Tang et al. (25) 2020 |
Triamcinolone acetonide |
80–160 |
1 |
80–160 |
Single dose |
No |
|
Toktas et al. (26) 2021 |
Triamcinolone acetonide |
20 mg |
1–3 |
20 mg up to 3 times |
4-weekly |
Triamcinolone acetonide 0.1%, twice a day, EOD for 1 month |
|
Yildirim et al. (28) 2021 |
Triamcinolone acetonide |
40 |
1–5 |
40–200 |
1 weekly |
No |
|
Karami et al. (21) 2022 |
Betamethasone disodium phosphate + betamethasone acetate |
6 |
1–4 |
6–24 |
1 weekly |
No |
ILS: Intralesional steroid
The mean complete clinical response time was 2.6 months. The overall complete response rate was 92.8% (n = 171), while the partial response rate was 6.0% (n = 11). There were only 2 non-responders in this group. The recurrence rate during the respective periods of follow-up was 6.6% (n = 11) (21, 22, 23, 24, 25, 26, 27). In one study (23), the two partial responders were followed up without active intervention and the lesions remained stable throughout the follow-up period. In another study (26), one non-responder underwent total mastectomy due to diffuse multifocal disease. The outcomes of the remaining partial responders, non-responders and recurrences were not reported. Seven patients (3.8%) reported minor complications following local steroid therapy. Three patients (0.8%) reported skin atrophy, 2 patients (0.5%) reported hematoma and two patients (0.5%) reported skin hyperemia as adverse effects. These side effects were observed in study groups prescribing topical and ILS as well as ILS-only group and were only observed in groups using Triamcinolone as the intra-lesional steroid.
Oral Steroid Comparative Group (Group 2)
Three studies contained a comparative OS subgroup, which provided outcome data (21, 26, 28). Accordingly, 81 (20.4%) patients who were treated solely with OS were included in this subgroup.
Two studies used oral methylprednisolone (26, 28), while the third study used prednisolone (21). The third study (21) also used oral MTX 10 mg per week for 1 month then 15 mg weekly until prednisolone was discontinued. In addition, daily Calcium-D and folic acid supplements were given to all patients in the third study.
The dosage of OS was heterogenous, with one study giving a fixed dose of 32 mg, the second study dosing based on the size, number of lesions and bodyweight (Unilateral, single lesions less than 5 cm: 0.5 mg/kg/day; bilateral, multiple or lesions exceeding 5 cm or with ulceration: 1 mg/kg/day), while the third study gave a tapering OS dose (50 mg/day for two weeks followed by 25 mg/day for 1 month, then 12.5 mg/day 1 month, then 10 mg/day for 1 month and 5 mg/day for 1 month for a total of 4 months, 2 weeks). All three studies had daily dosing regimens.
The total duration of dosage was 1 month in the first and second studies (with an additional 1 month of dosage in 5 patients with no response in the second study), and 4 months and 2 weeks in the third study.
A summary of OS treatment regimens used in the studies included in this review is given in Table 3. The mean complete response time was reported as 6.36 months (range; 6–9) in one study (21). The overall complete response rate was 86.4% (n = 70), with 4 patients (4.9%) showing a partial clinical response. The non-response rate was 8.6% (n = 7). Recurrence data was available in two studies (21, 26), with the overall recurrence rate in complete and partial responders in the two studies being 25.8% (n = 16). Notably, 93.8% (n = 15) of recurrences occurred in the study not using MTX (26). In one study (26), 5 patients with complete response who then developed recurrence were treated with successive doses of oral steroids, while surgery was performed on 4 patients with no response or recurrent disease including lumpectomy (n = 3) and mastectomy (n = 1) for diffuse disease. The final outcome of partial responders, non-responders and recurrences were not reported in the other two studies. The overall complication rate was 9.9% (n = 8) following OS therapy, with systemic side effects such as weight gain (n = 3) and hirsutism (n = 2).
Table 3. Oral steroid regimes used in studies included in systematic review.
|
Study |
Number of patients treated with Oral steroid |
Oral steroid type |
Oral steroid dose |
Frequency of dosage |
Duration of treatment (months) |
|
Karami et al. (21) 2022 |
30 |
Prednisolone |
Tapering dose of 50 to 5 mg |
Daily |
4.5 |
|
Toktas et al. (26) 2021 |
32 |
Methylprednisolone |
32 mg |
Daily |
1 |
|
Yildirim et al. (28) 2021 |
19 |
Methylprednisolone |
0.5–1 mg/kg/day based on lesion characteristics |
Daily |
1–2 |
Surgery Group (Group 3)
Two studies had cohorts that were treated exclusively with surgery (23, 25). A total of 57 (14.4%) patients were treated with surgery only. The majority underwent local excision (91.2%, n = 52) and only 5 (8.8%) patients required mastectomy. Only one study (23) reported a recurrence rate after surgery, which was 31.2% (n = 15/48), and this was reported at a 12-month follow-up after surgery. The same study (23) reported a complication rate of 8.3% (n = 4), of which three were surgical site infections and one was a hematoma. This study also noted that post treatment median pain score was significantly higher in patients who underwent surgery compared to those who underwent ILS therapy (p<0.001). Notably, the aesthetic outcome of surgery was not assessed in either study.
Combined Group (Group 4)
A single study described a cohort with a combination of oral and ILS with outcome data (21). In this study, patients received intralesional betamethasone acetate (3 mg) and betamethasone disodium phosphate (3 mg/mL) in a weekly dosage between 1–4 times, combined with a tapering dose of oral prednisolone (50 mg/day for two weeks, followed by a taper to 5 mg/day in 4 months: 25 mg/day for 1 month followed by 12.5 mg/day 1 month, then 10 mg/day for 1 month and 5 mg/day for 1 month) and weekly doses of oral MTX (10 mg per week for 1 month then 15 mg per week until prednisolone was discontinued).
A total of 38 (9.6%) of patients were treated with combined therapy. The mean complete response time was 4.33 months (range: 1–6). The complete clinical response rate was 89.5% (n = 34). Two patients (5.3%) had a partial clinical response, while 2 more patients were non-responders. Five patients (13.2%) were documented to have recurrence in the combined subgroup. Four patients (10.5%) had systemic complications following combined therapy.
Comparison of Outcomes in the ILS Group
Due to the heterogeneity of the studies, as discussed below, a comprehensive meta-analysis of the efficacy of the ILS regimens is not feasible. However, preliminary comparisons were carried out in this study.
The complete response rates of studies using Methylprednisolone (91.2%), Triamcinolone (94.1%) and Betamethasone (90.3%) appear to be similar. The recurrence rate of the single study (21) that used Betamethasone (19.4%) appears to be higher than that of studies that used Methylprednisolone (2.9%) and Triamcinolone (3.4%). Also, studies that used Triamcinolone were the only studies that reported local complications (n = 7). Three patients (42.9%) reported skin atrophy, 2 patients (28.6%) reported hematoma and 2 patients (28.6%) reported skin hyperemia as adverse effects. The comparison of each type of ILS is summarized in Table 4.
Table 4. Comparison of outcomes of intra lesional steroid group.
|
Steroid type |
Total treated |
Complete response no |
Complete response rate (%) |
Recurrence no |
Recurrence rate (%) |
No of complications |
Complication rate (%) |
|
Methylprednisolone |
34 |
31 |
91.2 |
1 |
2.9 |
0 |
0 |
|
Triamcinolone |
119 |
112 |
94.1 |
4 |
3.4 |
7 |
5.9 |
|
Betamethasone |
31 |
28 |
90.3 |
6 |
19.4 |
0 |
0 |
|
Total |
184 |
171 |
92.9 |
11 |
6.0 |
7 |
3.8 |
The comparison of the outcomes of each group are detailed in Table 5.
Table 5. Comparison of outcomes of each group.
|
Study |
Steroid used |
Complete response rate |
Mean complete response time (months) |
Recurrence rate |
|
Group 1 (ILS) | ||||
|
Alper et al. (22) 2020 |
Methylprednisolone |
25 (89.3%) |
NAD |
0 (0%) |
|
Toktas and Toprak (27) 2021 |
Methylprednisolone |
6 (100%) |
1.2 |
1 (16.7%) |
|
Ertürk et al. (23) 2022 |
Triamcinolone acetonide |
36 (94.5%) |
Large lesions-3 Small lesions-2 (Median) Range: 1-5 |
0 (0%) |
|
Kim et al. (24) 2016 |
Triamcinolone |
15 (100%) |
3.8 |
0 (0%) |
|
Tang et al. (25) 2020 |
Triamcinolone |
12 (100%) |
2 (Median) |
0 (0%) |
|
Toktas et al. (26) 2021 |
Triamcinolone acetonide |
34 (91.2%) |
NAD |
4 (10.8%) |
|
Yildirim et al. (28) 2021 |
Triamcinolone acetonide |
15 (88.2%) |
NAD |
NAD |
|
Karami et al. (21) 2022 |
Betamethasone disodium |
28 (90.3%) |
3.17 Range: 1–6 |
6 (16.3%) |
|
Group 2 (OS) | ||||
|
Karami et al. (21) 2022 |
Prednisolone |
24 (80%) |
6.37 Range: 6–9 |
1 (3.3%) |
|
Toktas et al. (26) 2021 |
Methylprednisolone |
29 (90.6%) |
2.1 Range: 1–3 |
15 (48.4%) |
|
Yildirim et al. (28) 2021 |
Methylprednisolone |
17 (89.5%) |
1.82 Range: 1–3 |
NAD |
|
Group 3 (Surgery) | ||||
|
Ertürk et al. (23) 2022 |
N/A |
48 (100%) |
N/A |
15 (31.2%) |
|
Tang et al. (25) 2020 |
N/A |
9 (100%) |
N/A |
0 (0%) |
|
Group 4 (Combined) | ||||
|
Karami et al. (21) 2022 |
IL betamethasone + OS prednisolone |
34 (89.5%) |
4.33 Range: 1–6 |
5 (13.2%) |
NAD: No available data; N/A: Not applicable; OS: Oral steroid; ILS: Intralesional steroid
Discussion and Conclusion
In this systematic review, we analyzed eight studies that used ILS. Methylprednisolone, Triamcinolone, Betamethasone and Prednisolone were the steroids used. ILS use is defined as the administration of steroids directly into a lesion, thereby bypassing the metabolic first pass effects and reducing the well-known systemic adverse effects of steroids, such as hypertension, osteoporosis, gastrointestinal disturbances, weight gain and diabetes mellitus (29), and allowing higher doses to be used (30). This technique creates a subepidermal depot which bypasses the superficial barrier zone (31). The use of ILS was first described in the management of dermatoses in 1961 (32). Since then, a variety of dermatological, rheumatological and surgical uses have been described.
ILS has a wide range of applications in dermatology and the dose per session generally depends on the size of the skin lesions, while the number of treatments depends on many clinical factors, including the disease, site of lesions, age of the patient and response to previous injections. The duration between treatment sessions is around 3–6 weeks (33). A similar rationale to that used in dermatological conditions was observed in the dosing regimens of the studies that used ILS in the management of IGM.
Comparison of Efficacy
In all eight studies, we noted a heterogeneity in the prescription of steroids with varying potencies, dosage, and frequencies. The basis for steroid regimes differed, with some studies (24, 28) citing regimes used in other inflammatory conditions in which ILS use is established, such as acute and chronic skin lesions and capsulitis (34), while others based on the number, size and distance of lesions (23), and on the clinical experience of the treating clinician (25, 26). In the ILS group the complete response rates of studies were 91.7% for Methylprednisolone, Triamcinolone (94.1%) and Betamethasone (90.3%). This shows that all three types of steroids have similar efficacy when used intralesionally. In comparison, the studies that used OS regimens, showed an 80% complete response rate in the prednisolone group and 90.1% in the methylprednisolone group (26, 28). The single study that used a combined treatment with both oral and ILS also showed a complete response in 89.5%.
The dosage or the frequency of injection did not show a correlation with the complete response rate. The main determinants of these factors were the severity of the disease.
Similar observations were noted in the complete response time. In the ILS group this ranged from one to six months with a mean of 2.6 months whereas, in the OS group it ranged from one to nine months with a mean of 6.4 months. The oral Prednisolone group also appears to have had a longer mean clinical response time of 6.4 months (21) compared to the studies using Methylprednisolone with response times of 1.8 (28) and 2.1 months (26). The combined group showed a mean complete response time of 4.3 months.
Therefore, the efficacy of ILS use in IGM was comparable to the oral and combined steroid groups.
Comparison of Complications Related to Treatment
The overall complication rate also appears to be lower in the ILS Group (3.8%) compared to the OS (9.9%), surgery (8.3%) and combined treatment (10.5%) groups. Most importantly, complications following ILS were minor, with hematomas, skin atrophy and hyperemia being commonly described. Three patients treated with ILS had skin atrophy, of which two were from groups that did not concurrently use topical steroids. The ILS group avoided systemic side effects of OS use such as weight gain and hirsutism, which were the most widely reported side effects in the OS and combined group. These systemic side effects have significant medical and psychological impacts in the demographic that is affected by IGM.
Post-operative pain is a significant complication of surgical excision, with the study done by Ertürk et al. (23) demonstrating significantly higher pain scores in the surgical group as compared to the ILS group. In addition, the inherent poorer cosmetic outcomes of surgery add to the unfavorable outcomes of that intervention. However, aesthetic outcome has not been described in any of the selected studies. Combined therapies, such as those with MTX have the highest complication rate, with other factors such as problems with compliance making this modality questionable, more so considering the non-inferiority of ILS monotherapy in terms of complete response rates, response times and minimal complications
Comparison of Recurrence
Within the ILS group, the recurrence rate of the single study (21) that used Betamethasone (19.35%) appears to be higher than that of studies that used Methylprednisolone (2.94%) and Triamcinolone (3.36%). Possible causes for this discrepancy could be due to the heterogeneity of dosage and frequency, and further comparative studies would be useful to establish a significant difference.
In comparison the recurrence rates in the ILS Group (6.6%) and Combined Group (13.2%) appear to be lower than in the OS Group (25.8%) and Surgery Group (26.3%). The recurrence rate of oral steroids appears to be similar in other studies focusing on recurrence with OS use, which highlighted patient age, radiological residual disease, and non-compliance as independent risk factors (35, 36). One possible explanation for this discrepancy could be because intralesional steroids achieve persistently high therapeutic levels of steroid concentration at the target site compared to oral steroids alone, resulting in prolonged resolution. The high recurrence rates of surgical intervention are also comparable to other reported studies (37, 38). The higher recurrence rates in surgery have mainly been attributed to residual disease post excision, which can be mitigated with repeated ILS use, which is less invasive.
Study Limitations
A major limitation of the studies included was that the distributions of principal confounders in each group of subjects to be compared were not clearly described. Furthermore, there was also a lack of adequate adjustment for confounding factors in the analyses from which the main findings were drawn. Factors, such as severity of the disease condition, the presence of complications such as abscesses and fistulae (and the additional management of such complications), the use of ultrasound to guide intralesional injections, the exact formulation of intralesional injections (diluents, etc.), the use of other treatment modalities such as MTX and antibiotics, as well as the variability of patients’ perception of the efficacy of each modality of treatment and clinical reasoning which led to selection of treatment modalities were not clearly defined. The statistical power of individual studies was also limited as the sample sizes were limited, and the required sample size to detect a significant difference was not calculated in most studies. Other limitations included the lack of randomization and blinding of patients and evaluators.
In conclusion, ILS seem to show a favorable outcome in terms of complete response rate, complete clinical response time and have a lower recurrence rate and complication rate as compared to other intervention strategies and may be considered as first-line therapy in the management of IGM. However, more comparative studies with standardized protocols are necessary to ascertain the optimum type, dosage, and frequency of ILS regimens.
Footnotes
Authorship Contributions: Surgical and Medical Practices: A.W., J.S., B.W., J.F., U.J., A.D.S., K.W.; Concept: A.W., A.D.S., K.W.; Design: A.W., K.L., J.S., B.W., J.F., U.J., A.D.S., K.W.; Data Collection or Processing: A.W., K.L., J.S., B.W., J.F., U.J., A.D.S., K.W.; Analysis or Interpretation: A.W., K.L., B.W., J.F., U.J., A.D.S., K.W.; Literature Search: A.W., J.S., B.W., J.F., U.J., A.D.S., K.W.; Writing: A.W., J.S., U.J., A.D.S., K.W.
Conflict of Interest: No conflict of interest declared by the author.
Financial Disclosure: The author declare that this study received no financial disclosure.
References
- 1.Benson JR, Dumitru D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol . 2016;12(11):1381–1394. doi: 10.2217/fon-2015-0038. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, Pabolu S. A Rare Case of Idiopathic Granulomatous Mastitis in a Nulliparous Woman with Hyperprolactinemia. Cureus . 2019;11:e4680. doi: 10.7759/cureus.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Manasra AR, Al-Hurani MF. Granulomatous Mastitis: A Rare Cause of Male Breast Lump. Case Rep Oncol . 2016;9(2):516–519. doi: 10.1159/000448990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol . 1972;58(6):642–646. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MH, Molland JG, Kennedy S, Gray TJ, Limaye S. Idiopathic granulomatous mastitis: case series and clinical review. Intern Med J . 2021;51(11):1791–1797. doi: 10.1111/imj.15112. [DOI] [PubMed] [Google Scholar]
- 6.Barreto DS, Sedgwick EL, Nagi CS, Benveniste AP. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat . 2018;171(3):527–534. doi: 10.1007/s10549-018-4870-3. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaev A, Blake CN, Carlson DL. Association between Hyperprolactinemia and Granulomatous Mastitis. Breast J . 2016;22(2):224–231. doi: 10.1111/tbj.12552. [DOI] [PubMed] [Google Scholar]
- 8.Troxell ML, Gordon NT, Doggett JS, Ballard M, Vetto JT, Pommier RF. Cystic Neutrophilic Granulomatous Mastitis: Association With Gram-Positive Bacilli and Corynebacterium. Am J Clin Pathol . 2016;145:635–645. doi: 10.1093/ajcp/aqw046. [DOI] [PubMed] [Google Scholar]
- 9.Ramadan R, Koryem IM, Fayed H. Idiopathic granulomatous mastitis: Risk factors and management. Breast Dis . 2022;41(1):413–420. doi: 10.3233/BD-220047. [DOI] [PubMed] [Google Scholar]
- 10.Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg . 2007;31(8):1677–1681. doi: 10.1007/s00268-007-9116-1. [DOI] [PubMed] [Google Scholar]
- 11.Freeman CM, Xia BT, Wilson GC, Lewis JD, Khan S, Lee SJ. Idiopathic granulomatous mastitis: A diagnostic and therapeutic challenge. Am J Surg . 2017;214:701–706. doi: 10.1016/j.amjsurg.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Dursun M, Yilmaz S, Yahyayev A, Salmaslioglu A, Yavuz E, Igci A. Multimodality imaging features of idiopathic granulomatous mastitis: outcome of 12 years of experience. Radiol Med . 2012;117:529–538. doi: 10.1007/s11547-011-0733-2. [DOI] [PubMed] [Google Scholar]
- 13.Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol . 2009;193:574–581. doi: 10.2214/AJR.08.1528. [DOI] [PubMed] [Google Scholar]
- 14.Alper F, Abbasguliyev H, Özmen S, Yalçin A, Yılmaz Çankaya B, Akçay MN. Clinical, Histopathological, Imaging, and Treatment Perspectives of Inflammatory Granulomatous Mastitis: Review of the Literature. Eurasian J Med . 2022;54(Suppl 1):172–178. doi: 10.5152/eurasianjmed.2022.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J, Cocco D, Matz S, Hsu CH, Brown MJ, Lee J. Re-evaluating if observation continues to be the best management of idiopathic granulomatous mastitis. Surgery . 2019;166:1176–1180. doi: 10.1016/j.surg.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Basim P, Argun D, Argun F. Risk Factors for Idiopathic Granulomatous Mastitis Recurrence after Patient-Tailored Treatment: Do We Need an Escalating Treatment Algorithm? Breast Care (Basel) . 2022;17:172–179. doi: 10.1159/000517399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan QQ, Xiao SY, Farouk O, Du YT, Sheybani F, Tan QT. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition). Mil Med Res . 2022;9:20. doi: 10.1186/s40779-022-00380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bede K, Valente SA. Idiopathic granulomatous mastitis. Ann Breast Surg . 2020;4:24. [Google Scholar]
- 19.Munot K, Nicholson S, Birkett V. Granulomatous mastitis - A novel method of treatment. EJSO . 2012;38:461–462. [Google Scholar]
- 20.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health . 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karami MY, Zangouri V, Habibagahi Z, Tahmasebi S, Ranjbar A, Seyyedy MS, et al. The effectiveness of local steroid injection for the treatment of breast-limited idiopathic granulomatous mastitis: A randomized controlled clinical trial study. 2022. [Google Scholar]
- 22.Alper F, Karadeniz E, Güven F, Yılmaz Çankaya B, Özden K, Akçay MN. The evaluation of the efficacy of local steroid administration in idiopathic granulomatous mastitis: The preliminary results. Breast J . 2020;26(2):309–311. doi: 10.1111/tbj.13588. [DOI] [PubMed] [Google Scholar]
- 23.Ertürk TF, Çakır Ö, Yaprak Bayrak B, Güneş A, Aydemir S, Utkan NZ. Local steroid treatment: An effective procedure for idiopathic granulomatous mastitis, including complicated cases. J Invest Surg . 2022;35:745–751. doi: 10.1080/08941939.2021.1933272. [DOI] [PubMed] [Google Scholar]
- 24.Kim BS, Koo BY, Eom TI. Usefulness of ultrasound-guided intralesional steroid injection in management of idiopathic granulomatous mastitis. J Surg Ultrasound . 2016;3:40–45. [Google Scholar]
- 25.Tang A, Dominguez DA, Edquilang JK, Green AJ, Khoury AL, Godfrey RS. Granulomatous Mastitis: Comparison of Novel Treatment of Steroid Injection and Current Management. J Surg Res . 2020;254:300–305. doi: 10.1016/j.jss.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Toktas O, Konca C, Trabulus Didem C, Soyder A, Koksal H, Karanlik H. A Novel First-Line Treatment Alternative for Noncomplicated Idiopathic Granulomatous Mastitis: Combined İntralesional Steroid İnjection with Topical Steroid Administration. Breast Care (Basel) . 2021;16:181–187. doi: 10.1159/000507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toktas O, Toprak N. Treatment Results of Intralesional Steroid Injection and Topical Steroid Administration in Pregnant Women with Idiopathic Granulomatous Mastitis. Eur J Breast Health . 2021;17:283–287. doi: 10.4274/ejbh.galenos.2021.2021-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildirim E, Kayadibi Y, Bektas S, Ucar N, Oymak A, Er AM. Comparison of the efficiency of systemic therapy and intralesional steroid administration in the treatment of idiopathic granulomatous Mastitis. The novel treatment for Granulomatous Mastitis. Ann Ital Chir . 2021;92:234–241. [PubMed] [Google Scholar]
- 29.Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin Ther . 2017;39:2216–2229. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Deshmukh NS, Belgaumkar VA, Mhaske CB, Doshi BR. Intralesional drug therapy in dermatology. Indian J Dermatol Venereol Leprol . 2017;83:127–132. doi: 10.4103/0378-6323.190870. [DOI] [PubMed] [Google Scholar]
- 31.Savant S. Textbook of dermatosurgery and cosmetology. Indian Journal of Dermatology, Venereology, and Leprology 2005; 71: 304. doi: 10.4103/0378-6323.16778. [DOI] [PubMed] [Google Scholar]
- 32.Hollander A. Intralesional injections of triamcinolone acetonide; a therapy for dermatoses. Antibiotic Med Clin Ther (New York) . 1961;8:78–83. [PubMed] [Google Scholar]
- 33.Verbov J. The place of intralesional steroid therapy in dermatology. Br J Dermatol . 1976;94(Suppl 12):51–58. doi: 10.1111/j.1365-2133.1976.tb02269.x. [DOI] [PubMed] [Google Scholar]
- 34.de Jong BA, Dahmen R, Hogeweg JA, Marti RK. Intra-articular triamcinolone acetonide injection in patients with capsulitis of the shoulder: a comparative study of two dose regimens. Clin Rehabil . 1998;12:211–215. doi: 10.1191/026921598673772420. [DOI] [PubMed] [Google Scholar]
- 35.Çetin K, Sıkar HE, Feratoğlu F, Taşdoğan B, Güllüoğlu BM. Treatment of Granulomatous Mastitis With Steroids: Should the Decision to End the Treatment be Made Radiologically? Eur J Breast Health . 2024;20:25–30. doi: 10.4274/ejbh.galenos.2023.2023-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan QW, Zhang YN, Jia YP, Gou J, Lv Q, Yang XQ. Methylprednisolone for idiopathic granulomatous mastitis: a prospective observational cohort study. Gland Surg . 2022;11(9):1538–1545. doi: 10.21037/gs-22-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin YD, Park SS, Song YJ, Son SM, Choi YJ. Is surgical excision necessary for the treatment of Granulomatous lobular mastitis? BMC Womens Health . 2017;17:49. doi: 10.1186/s12905-017-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kok KY, Telisinghe PU. Granulomatous mastitis: presentation, treatment and outcome in 43 patients. Surgeon . 2010;8(4):197–201. doi: 10.1016/j.surge.2010.02.002. [DOI] [PubMed] [Google Scholar]


