Abstract
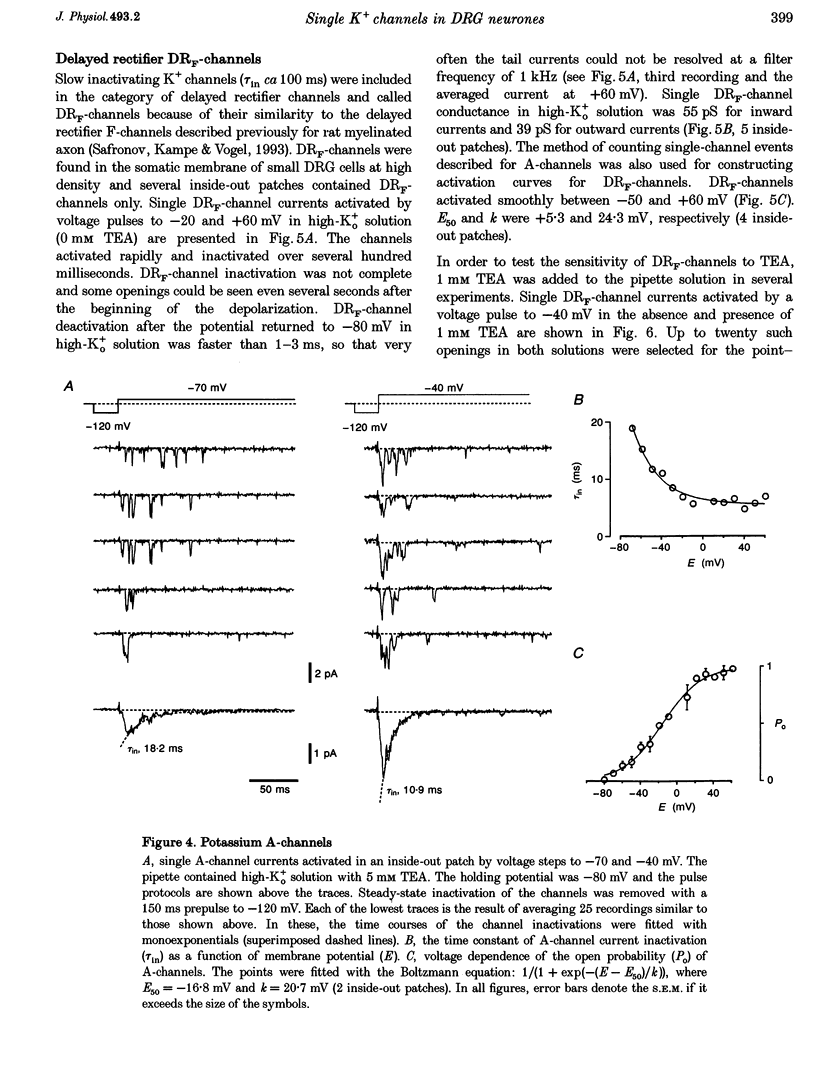
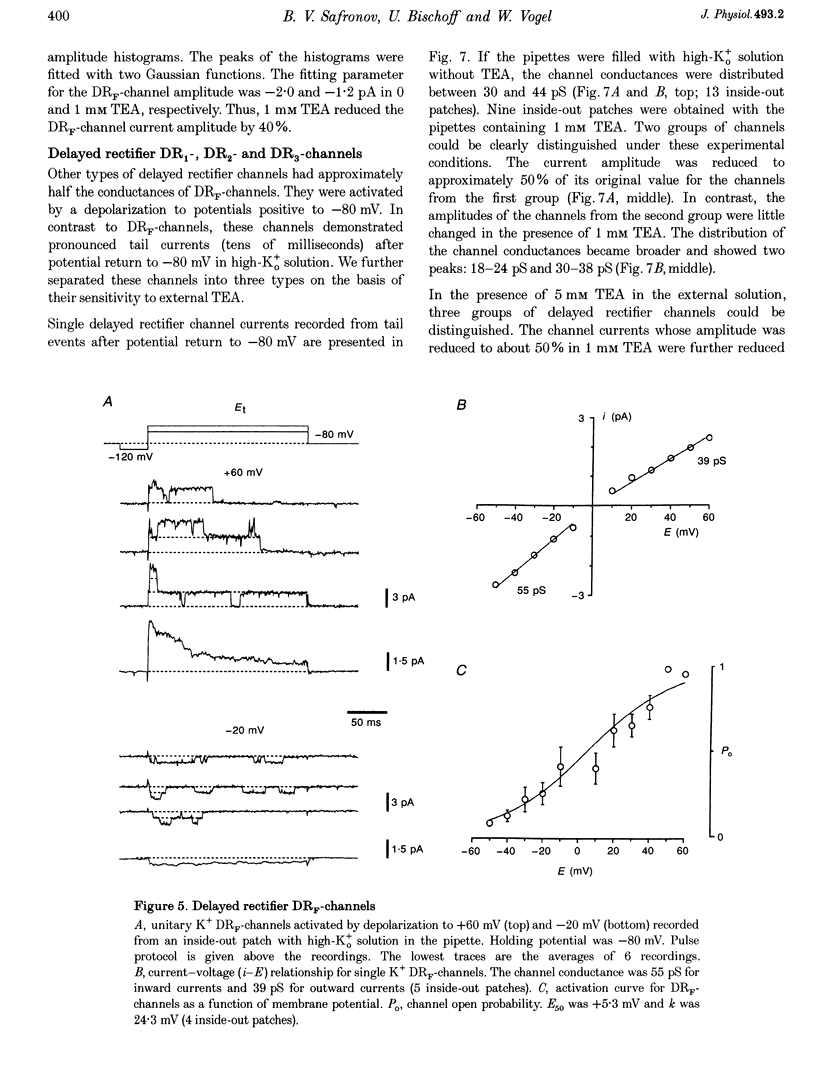
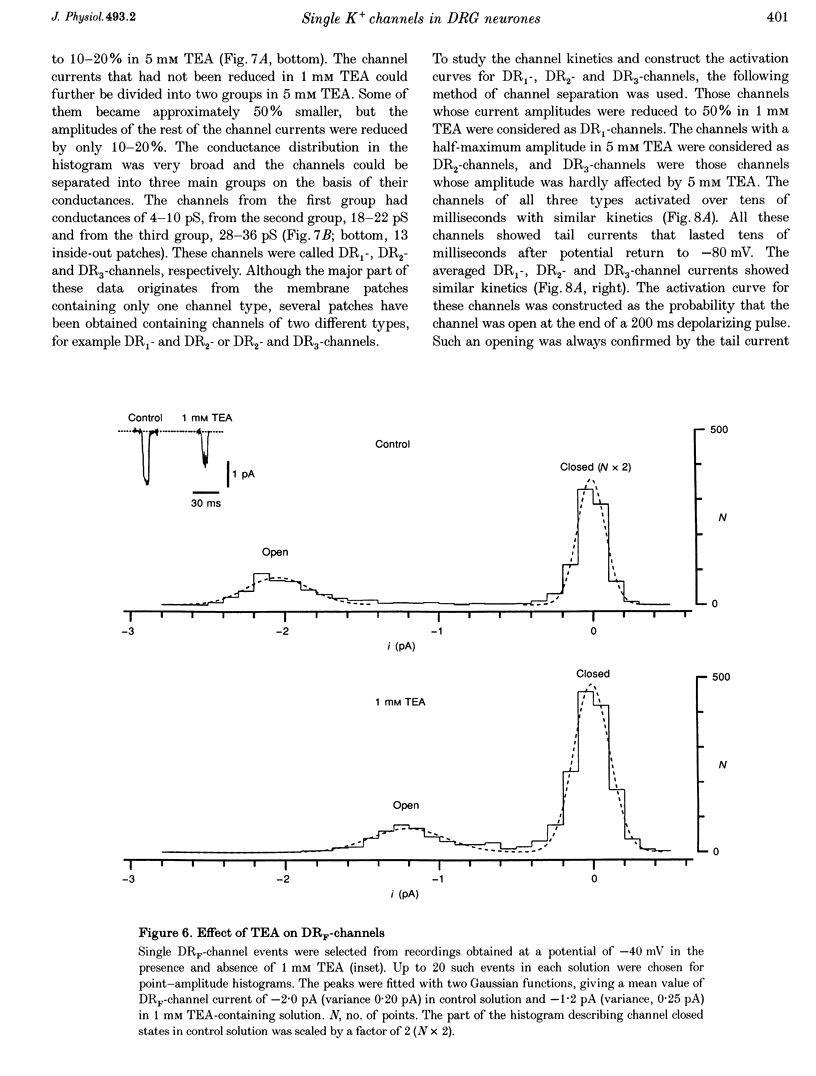
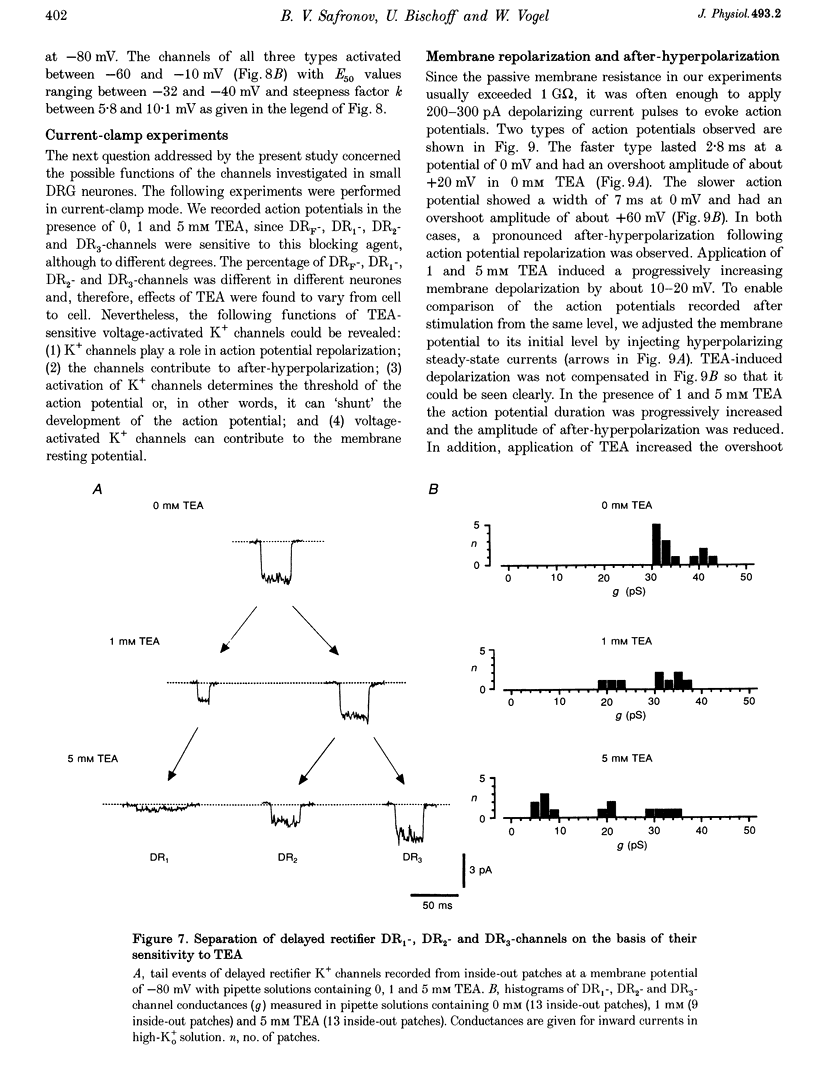
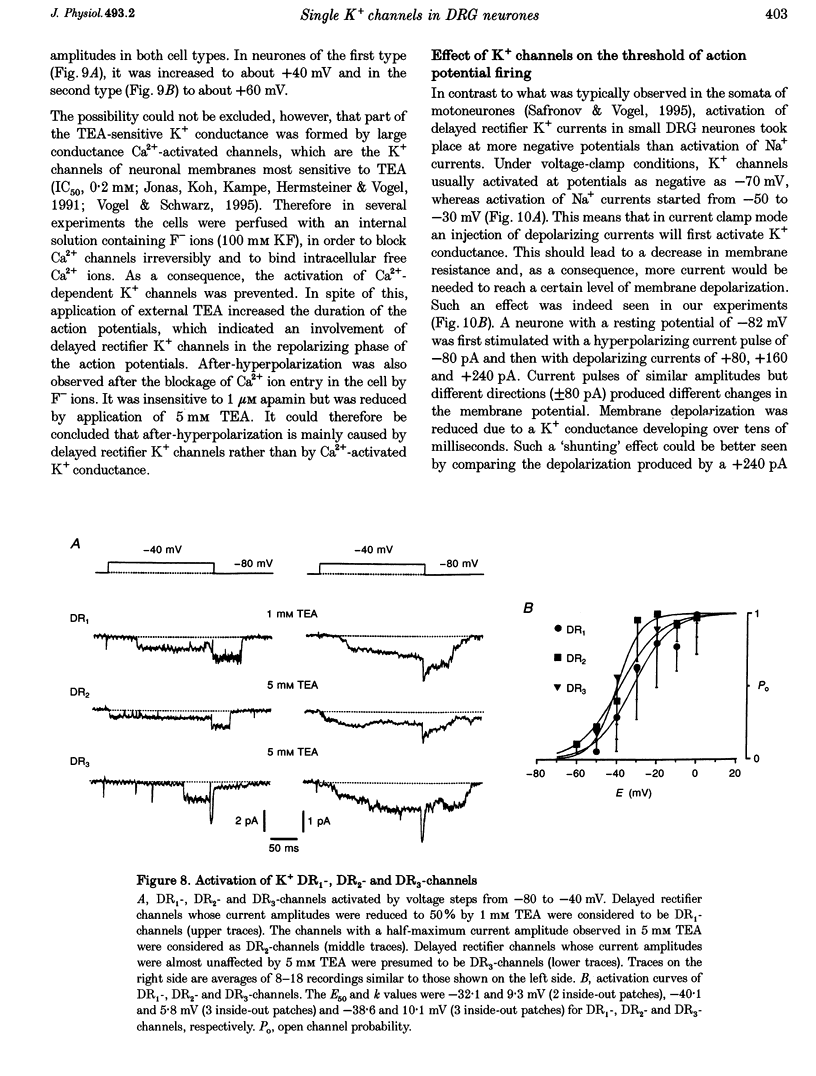
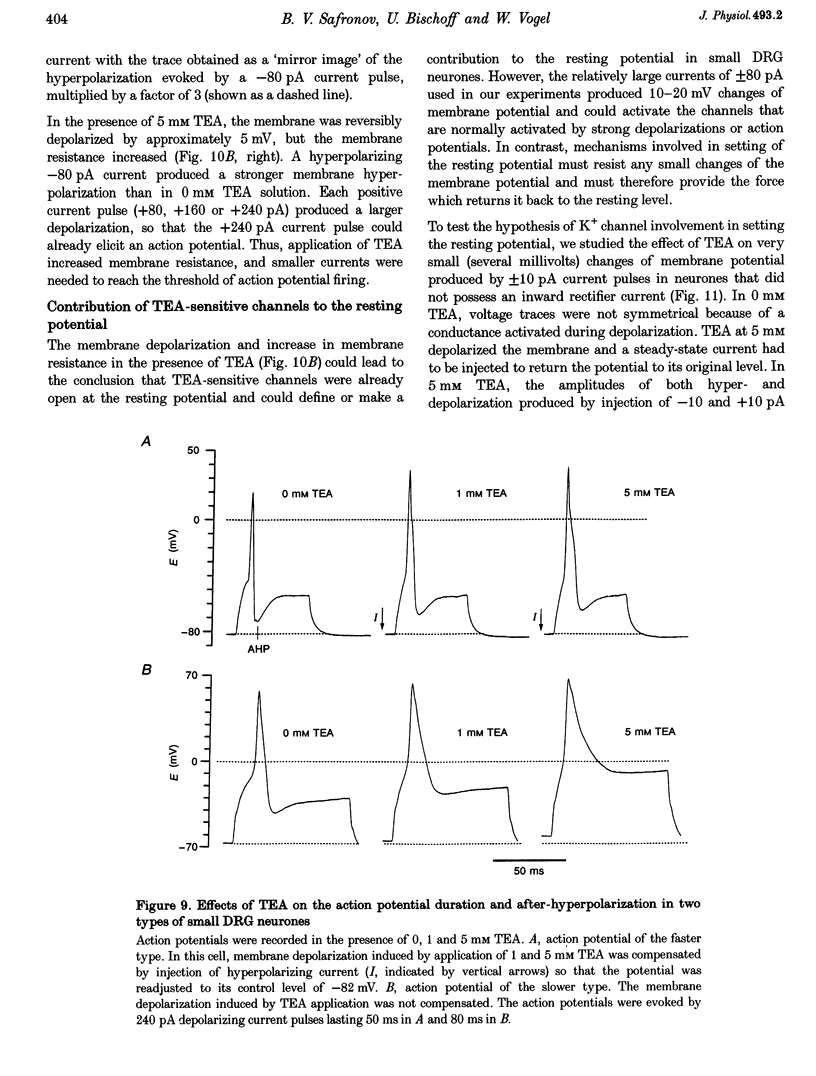
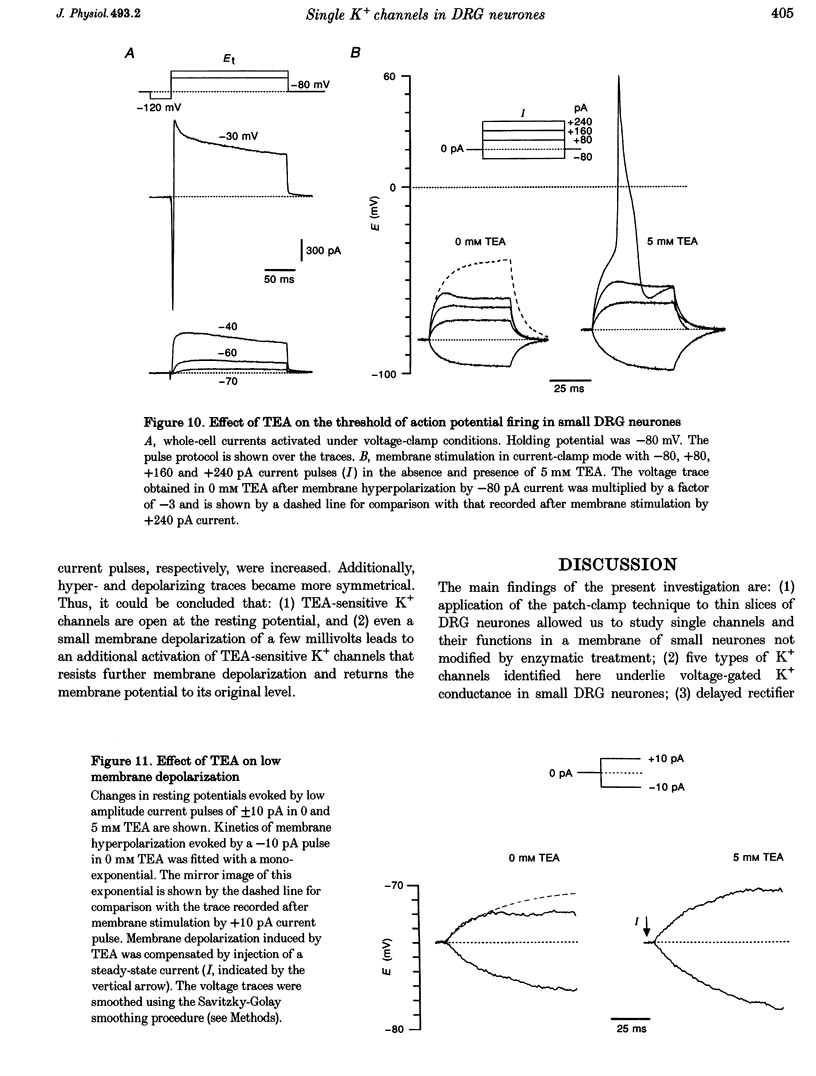
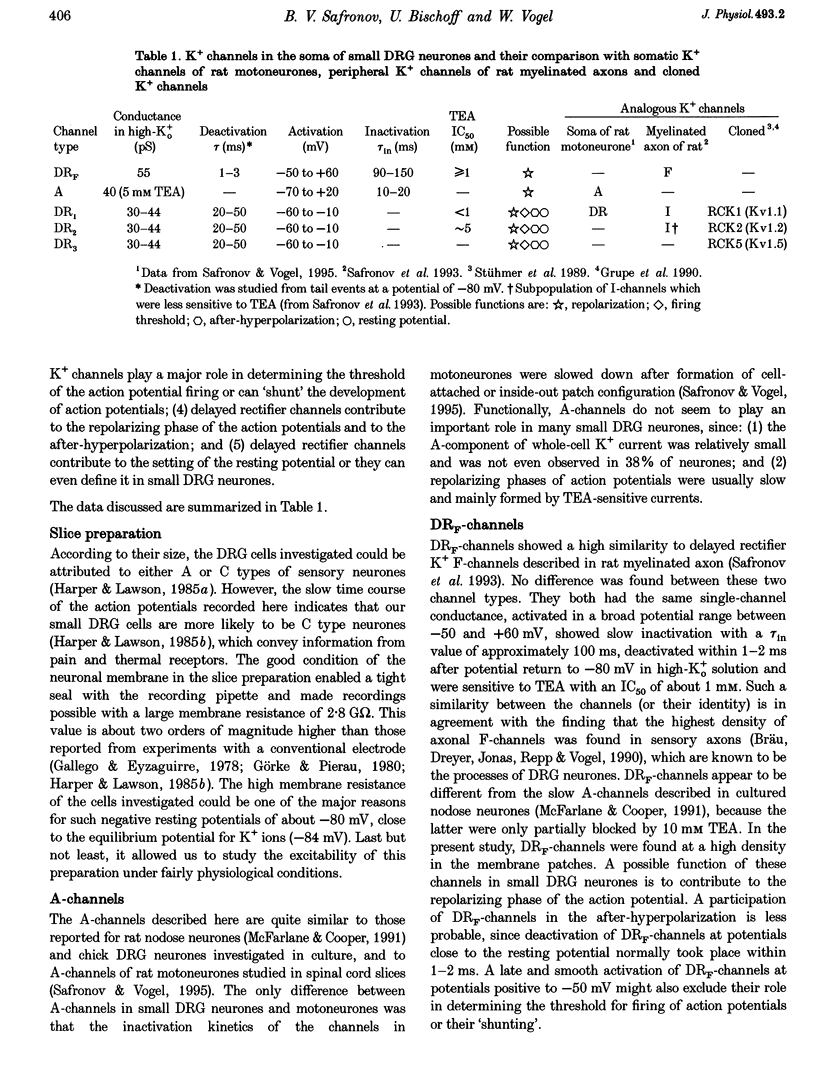
1. Single voltage-activated K+ channels were investigated by means of the patch-clamp technique in small dorsal root ganglion (DRG) neurones in 150 microns thin slices of new-born rat DRG. It was found that K+ conductance in small DRG neurones is formed by one type of fast inactivating A-channel and four types of delayed rectifier K+ channels, which could be separated on the basis of their single-channel conductance, kinetics and sensitivity to external tetraethylammonium (TEA). 2. Potassium A-channels were observed at relatively moderate density. They were weakly sensitive to TEA and activated between -70 and +20 mV. The conductance of A-channels was about 40 pS for inward currents in symmetrical high-K+ solutions with external 5 mM TEA added to suppress other types of K+ channels. The time constant of channel inactivation (tau in) was 18.8 ms at -70 mV and 6 ms at potentials positive to -20 mV. 3. A fast delayed rectifier (DRF) channel with a conductance of 55 pS in symmetrical high-K+ solutions was the most frequent type of K+ channel. The channel activated in a broad potential range between -50 and +60 mV and demonstrated a fast deactivation within 1-3 ms after potential return to -80 mV in high-Ko+ solution. The tau in value was 90-150 ms at positive membrane potentials. The single-channel current amplitudes were blocked to 55% by 1 mM TEA. 4. Three further types of delayed rectifier K+ channels were called DR1-, DR2- and DR3- channels. Their single-channel conductances for inward currents in symmetrical high-K+ solutions were distributed between 30 and 44 pS. The channels activated in almost the same voltage range between -60 and -10 mV. Deactivation of the channels at -80 mV lasted tens of milliseconds. The channels were separated on the basis of their sensitivities to TEA. DR1-channel currents were reduced to 50% in the presence of 1 mM TEA, DR2-channel currents were reduced to about 50% by 5 mM TEA, whereas the amplitudes of currents through DR3-channels were almost unaffected by 5 mM TEA. 5. Addition of external 1 and 5 mM TEA to whole cells under current-clamp condition depolarized the cell membrane, lowered the threshold for action potential firing, prolonged action potential duration and reduced the amplitude of after-hyperpolarization. 6. It is concluded that potassium A-, DRF-, DR1-, DR2- and DR3-channels play multiple roles in the excitability of DRG neurones. Possible influences of these channels on the shape of the action potential, its firing threshold and the resting membrane potential of small DRG neurones are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräu M. E., Dreyer F., Jonas P., Repp H., Vogel W. A K+ channel in Xenopus nerve fibres selectively blocked by bee and snake toxins: binding and voltage-clamp experiments. J Physiol. 1990 Jan;420:365–385. doi: 10.1113/jphysiol.1990.sp017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Eng D. L., Black J. A., Waxman S. G., Kocsis J. D. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992 Oct 2;592(1-2):283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Campbell D. T. Single-channel current/voltage relationships of two kinds of Na+ channel in vertebrate sensory neurons. Pflugers Arch. 1993 Jun;423(5-6):492–496. doi: 10.1007/BF00374946. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Elliott A. A., Elliott J. R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993 Apr;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio S. K., Westbrook C. D., Vasko M. R., Bauer R. J., Kenyon J. L. Transient potassium currents in avian sensory neurons. J Neurophysiol. 1990 Apr;63(4):725–737. doi: 10.1152/jn.1990.63.4.725. [DOI] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C. Membrane and action potential characteristics of A and C nodose ganglion cells studied in whole ganglia and in tissue slices. J Neurophysiol. 1978 Sep;41(5):1217–1232. doi: 10.1152/jn.1978.41.5.1217. [DOI] [PubMed] [Google Scholar]
- Grupe A., Schröter K. H., Ruppersberg J. P., Stocker M., Drewes T., Beckh S., Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990 Jun;9(6):1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görke K., Pierau F. K. Spike potentials and membrane properties of dorsal root ganglion cells in pigeons. Pflugers Arch. 1980 Jul;386(1):21–28. doi: 10.1007/BF00584182. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985 Feb;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Koh D. S., Kampe K., Hermsteiner M., Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991 Mar;418(1-2):68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Kasai H., Kameyama M., Yamaguchi K., Fukuda J. Single transient K channels in mammalian sensory neurons. Biophys J. 1986 Jun;49(6):1243–1247. doi: 10.1016/S0006-3495(86)83754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-III. Potassium currents. Neuroscience. 1981;6(12):2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Streit J., Quadroni R., Lüscher H. R. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994 Aug;72(2):622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- McFarlane S., Cooper E. Kinetics and voltage dependence of A-type currents on neonatal rat sensory neurons. J Neurophysiol. 1991 Oct;66(4):1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- Safronov B. V., Kampe K., Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol. 1993 Jan;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov B. V., Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. J Physiol. 1995 Aug 15;487(1):91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld C., Feltz A. Dendrotoxin-sensitive K+ channels in dorsal root ganglion cells. Neurosci Lett. 1988 Oct 31;93(1):49–55. doi: 10.1016/0304-3940(88)90011-0. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]