Abstract
Human MxA is an alpha/beta interferon-inducible intracytoplasmic protein that mediates antiviral activity against several RNA viruses. We had previously shown that overexpression of the hepatitis B virus (HBV) capsid led to selective downregulation of MxA gene expression, suggesting a mechanism by which the virus escapes from the host defense system (O. Rosmorduc, H. Sirma, P. Soussan, E. Gordien, P. Lebon, M. Horisberger, C. Brechot and D. Kremsdorf, J. Gen. Virol. 80:1253–1262, 1999). In the present study, we investigated the antiviral activity of MxA protein against HBV. MxA-expressing HuH7 clones were established and transiently transfected with HBV, and viral replication was then studied. Viral protein secretion was profoundly reduced in MxA-expressing clones by 80% for HBV surface antigen (HBsAg) and 70% for HBV e antigen (HBeAg). The levels of intracytoplasmic HBsAg and HBeAg were reduced by about 80 and 50% in the two MxA-positive clones tested. A nearly complete disappearance of HBV DNA replicative intermediates was observed in MxA-expressing clones. Although the expression of total viral RNAs was not modified, two- to fourfold reductions in HBV cytoplasmic RNAs were found in MxA-expressing clones. This suggests the inhibition of HBV replication at a posttranscriptional level. Indeed, using the well-characterized posttranscriptional regulation element (PRE) reporter system, we were able to demonstrate a marked reduction (three- to eightfold) in the nucleocytoplasmic export of unspliced RNA in MxA-expressing clones. In addition, MxA protein did not interact with HBV nucleocapsid or interfere with HBV nucleocapsid formation. Our results show an antiviral effect of MxA protein on a DNA virus for the first time. MxA protein acts, at least in part, by inhibiting the nucleocytoplasmic export of viral mRNA via the PRE sequence.
The hepatitis B virus (HBV) is a major human pathogen, belonging to the family of hepadnaviruses, a group of small enveloped viruses with major liver tropism (47). The HBV genome consists of a relaxed, circular, partially double-stranded 3.2-kb DNA molecule. One of the striking features of HBV is that its replication involves reverse transcription of a greater-than-genome-length pregenomic RNA (3.5 kb) (22, 29). This reverse transcription process occurs exclusively in the core particle, which is assembled through complex interactions between pregenomic RNA, core protein, polymerase, and several cellular proteins (22, 29). The core particles containing the replicative intermediates are then transported back to the nucleus, for the establishment of a pool of covalently closed circular DNA, or to the endoplasmic reticulum to be released after association with the viral envelope, as infectious mature virions (for a review, see reference 22).
HBV infection may lead to acute liver disease, chronic active hepatitis, liver cirrhosis, and hepatocellular carcinoma. Over 300 million people worldwide are estimated to be infected chronically by HBV and are therefore at risk of liver failure, cirrhosis, or hepatocellular carcinoma. The principal treatment for chronic hepatitis B involves the use of alpha interferon (IFN-α) or nucleoside analogs (9, 42). IFN-α belongs to the IFN-α/β system, which mediates antiviral, antiproliferative, immune, and other cellular effects (8). In humans, IFN-α antiviral action is mediated by the induction of at least three major proteins, 2′,5′-oligoadenylate synthetase, protein kinase R, and MxA. IFN-α likely acts by combining stimulation of the immune response and a direct viral effect. However, the specific mechanisms responsible for an improvement in HBV-related hepatitis following IFN treatment are not clearly understood. To date, IFN-α antiviral mechanisms against HBV have mainly been examined in vitro using hepatoma cell lines. These experiments showed that IFN-α brought about changes to the expression of viral antigens and/or steady-state levels of viral RNAs or replicative intermediates, depending on the experimental model employed (2, 4, 7, 17, 21, 26, 35, 36, 51, 54).
Although the use of IFN-α has improved the treatment of chronically infected HBV patients, an effective reduction in virus load is only observed in 30% of treated patients. The molecular basis for resistance to IFN-α therapy is not clearly defined. However, studies have suggested that HBV may play a direct role in the development of resistance to endogenous or exogenous IFN. In vitro, HBV genome expression has been shown to reduce sensitivity to IFN, as measured by inhibition of the cytopathic effect of Sindbis virus challenge (30). HBV capsid and polymerase terminal proteins have been shown to reduce expression of the IFN-β and IFN-induced 6-16 genes, respectively (11, 52, 53). Furthermore, several in vivo studies have demonstrated a lack of IFN system activation in patients with acute or chronic hepatitis B. In particular, impaired induction of the IFN-inducible MxA protein was evidenced in acute and chronic HBV infection (10, 20).
MxA is a 76-kDa GTPase protein belonging to the superfamily of large GTPases, which accumulate in the cytoplasm in response to IFN-α/β (16). In vitro, in different cellular models, or in vivo, in MxA transgenic mice, MxA protein is able to inhibit a broad spectrum of negative-stranded RNA viruses, including influenza virus, Thogoto virus, vesicular stomatitis virus, measles virus, and bunyavirus (12, 13, 33, 46, 57). Recently, antiviral activity has been demonstrated against a positive-stranded RNA virus, Semliki Forest virus (28). The mechanisms through which MxA is able to inhibit such a variety of viruses are yet to be precisely defined. Several studies have shown that MxA may act at different levels of the virus replication cycle, depending on the virus species and the cellular models used. Indeed, MxA is capable of blocking viral replication at primary transcriptional steps (Semliki Forest and vesicular stomatitis viruses) (28, 46) or following primary transcription (influenza virus) (32). In the case of measles virus, MxA seems to have an inhibitory effect on either viral RNA or glycoprotein synthesis, depending on the cellular model (43, 44).
In a previous study, we showed evidence for HBV defective particles, characterized by a singly spliced HBV RNA, which had been encapsidated and retrotranscribed, giving rise to a defective HBV genome (49). We also demonstrated an association between those defective particles and the establishment of a chronic carrier state (37). In vitro, we showed that expression of this defective genome led to a reduction in the antiviral activity of IFN, as determined using the virus yield reduction assay, and that this modulation involved a selective inhibition of MxA protein induction via overexpression of the HBV capsid protein (38). This led us to suggest that MxA might play a major role in antiviral activity against HBV.
The aim of the present study was to determine whether the antiviral spectrum of the MxA protein extended to cover HBV. We therefore established HuH7 cell lines stably expressing the MxA protein and performed transient-transfection experiments using an HBV-expressing plasmid. We found that MxA inhibited HBV replication through a significant reduction in the synthesis of viral proteins, cytoplasmic RNAs, and DNA replicative intermediates. We demonstrate that MxA antiviral action against HBV occurs, partly at least, at a posttranscriptional level, by inhibiting the nuclear export of viral RNAs.
MATERIALS AND METHODS
Plasmids.
The MxA gene, derived from the cDNA clone p78-8b (gift from M. A. Horisberger), was flanked by two HindIII sites, one located 24 bp upstream of the ATG initiation codon and the other introduced by adding a linker at the SmaI site 51 bp downstream of the TAA termination codon. The resulting 2,070-bp HindIII fragment, comprising the full MxA coding sequence, was subcloned at the HindIII site into the pHβApr-3-neo expression vector (15). The pTHBV1.1 plasmid (gift from H. Schaller), corresponding to the ayw HBV complete sequence under the control of the C gene promoter, has been described previously (14). The PCMVHBc plasmid, expressing the capsid under a cytomegalovirus (CMV) promoter, was a kind gift from P. Soussan. The pRSV138PDM-CAT, pRSV138PRE-CAT, and pRSV-CAT plasmid constructs (kindly provided by M. Dobbelstein) have been described previously (39). β-Galactosidase expression plasmids, pCH110 or pRSV (in which β-galactosidase is driven by the Rous sarcoma virus promoter) (Pharmacia Biotech) were cotransfected in each experiment to monitor the efficiency of transfection.
Cell culture and transfection.
The human hepatoma cell line HuH7 was maintained in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with 10% fetal calf serum plus 100 IU of penicillin and 100 μg of streptomycin (Gibco-BRL) per ml at 37°C in a 5% CO2–95% air humidified atmosphere. To obtain cells which stably expressed MxA, 2 × 106 HuH7 cells were transfected with 20 μg of either pHβApr-3-neo-MxA or pHβApr-3-neo plasmid, using the calcium phosphate precipitation method. Sixteen hours later, the cells were washed and grown for a further 24 h. The cells were then trypsinized and seeded into new dishes at a splitting ratio of 1:4, and clones were selected in medium containing 400 μg of geneticin (G418 sulfate; Gibco-BRL) per ml. The neomycin-resistant clones were analyzed by Western immunoblot for MxA expression. One clone expressing the neomycin resistance gene (neo clone) and two clones expressing MxA (MxA6 and MxA10) were selected for the study. The growth curve of these different clones was studied as follows. The cells were seeded at a confluence of 20,000 cells per well of six-well plates and counted daily in duplicate. The experiment was done twice. The MxA-positive clones (MxA6 and MxA10) and the neo clone left untreated or treated with IFN-α (500 IU of recombinant human IFN-α-2b per ml [Schering-Plough] applied for 20 h prior to transfection) were transiently transfected with the plasmids specified (10 to 20 μg per 10-cm plates, 0.5 to 3 μg per six-well plate). Two micrograms per 10-cm plate or 0.3 μg per six-well plate of β-galactosidase expression plasmid was added to the DNA transfection mixtures to monitor the efficiency of transfection. An aliquot of the transfected cells was lysed in NP-40 buffer (10 mM Tris [pH 8], 100 mM NaCl, 1 mM EDTA, and 1% Nonidet P-40) containing a cocktail of protease inhibitors (Complete Boehringer) and then subjected to a β-galactosidase assay. The values obtained were used to normalize the efficiency of transfection.
Protein analysis.
For cellular MxA protein analysis, cells were lysed from a confluent 10-cm-diameter dish by the addition of 500 μl of NP-40 buffer. The protein content was measured by the Bradford technique (Bio-Rad). From 20 to 40 μg of protein was boiled in 4× Laemmli buffer and resolved by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE). After gel transfer onto a polyvinylidene difluoride membrane (Immobilon P; Millipore), the blots were incubated with a monoclonal anti-MxA (1:3,000) antibody (kindly provided by M. A. Horisberger) or with a monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:1,000) (Interchim). Detection was carried out using a horseradish peroxidase-conjugated secondary antibody (1:2,000) (Amersham) and subsequent chemilumiscent revelation (ECL; Amersham).
Viral envelope (HBsAg), capsid (HBcAg), and HBeAg protein expression was determined-using standard enzyme-linked immunosorbent assay (ELISA) (Abbott) in supernatants and cell extracts from HBV-transfected HuH7 neo and MxA clones harvested 2 or 3 days posttransfection. All experiments were performed at least three times.
The in vitro interaction between MxA and HBV capsid was evaluated using a cosedimentation assay in a discontinuous glycerol gradient (70 and 60%) in the presence of GTP-γS, as described elsewhere by Kocks et al. (24). For this experiment, the MxA lysate was prepared from the 3T3 MxA-expressing cell clone and mixed with either Thogoto virus (THOV)-infected cell lysates (kindly provided by G. Kochs) or purified capsid protein (kind gift from M.-A. Petit). Seven fractions (80 μl each) were collected from the bottom of the gradient and analyzed as described above by Western blotting, using polyclonal rabbit anti-MxA and anti-THOV antibodies (gift from G. Kochs) and an anti-HBe/c antibody (gift from M.-A. Petit).
We analyzed the ability of MxA protein to interfere with capsid self-assembly into particles using the sucrose gradient sedimentation analysis described by Koschel et al. (27). Briefly, HuH7 and positive MxA (MxA10) cells were transfected with 20 μg of the PCMVHBc plasmid and lysed 4 days posttransfection in 1 ml of lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 0.2% Nonidet P-40). The lysates were applied to a 15 to 60% discontinuous sucrose gradient. Eleven fractions (380 μl each) were collected from the bottom, and 30 μl of each fraction was analyzed by Western blot (as described above) using polyclonal anti-HBe/c antibody.
Analysis of viral nucleic acids.
HBV DNA replication following the transient transfection of MxA clones was assessed using Southern blot analysis of viral DNA extracted from immunoprecipitated intracellular core particles. Encapsidated viral DNA was prepared as follows, after culture for 3 days of HBV-transfected HuH7 clones. The cytoplasmic lysates, prepared as described above, were first cleared by incubation for 3 h at 4°C in protein A-Sepharose CL-4B bead (Pharmacia Biotech) solution (50% protein A-Sepharose beads in NP-40 buffer). In order to eliminate any residual transfected DNA, the lysates were digested with DNase I (100 μg/ml) in NP-40 buffer containing magnesium acetate (10 mM) for 30 min at 37°C. Viral capsids were immunoprecipitated overnight at 4°C, using a rabbit polyclonal anti-HBe/c antibody. Protein A-Sepharose solution was then added for 3 h at 4°C. Beads were collected by centrifugation, and HBV DNA was extracted by proteinase K (1 mg/ml) digestion in lysis buffer (50 mM Tris [pH 8], 1 mM EDTA, 100 mM NaCl, 1% SDS). After incubation at 55°C for 4 h, viral DNA was purified from the lysate by phenol-chloroform extraction and then precipitated using ethanol. Viral DNA replicative forms were loaded onto a 0.8% agarose gel and transferred onto Hybond N+ (Amersham) filters by the Southern blot method. Membranes were hybridized overnight at 42°C in a buffer containing 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1× SDS, 40 mM phosphate buffer [pH 6.5], 5× Denhardt's solution, and a 32P-labeled HBV DNA probe (Megaprime; Stratagene). In order to ensure the homogeneity of the results, all immunoprecipitation experiments were performed using the same amount of protein lysate, and the core-extracted DNA was loaded onto the agarose gel according to the transfection efficiency.
Northern blot analysis of total or cytoplasmic viral RNAs was performed 48 h posttransfection. Total or cytoplasmic RNAs were extracted using Trizol reagent (Gibco-BRL) or the Qiagen RNeasy kit, as recommended by the manufacturer. Ten micrograms of total or cytoplasmic RNA was subjected to electrophoresis on a 1% formaldehyde–agarose gel and then transferred onto Hybond N+ (Amersham). Total and cytoplasmic HBV RNAs were detected with a 32P-labeled HBV DNA probe (Megaprime; Stratagene) in Church hybridization buffer (6). The blots were then stripped and rehybridized with a 32P-labeled GAPDH probe for normalization. The autoradiograms were scanned using NIH image 1.62/ppc software. The values of HBV bands were normalized to the β-galactosidase activity (transfection efficiency) and the corresponding GAPDH scanned band value. All experiments were performed at least twice.
CAT assays.
Two days after transfection of the different clones with pRSV138PDM-CAT, pRSV138PRE-CAT, and pRSV-CAT plasmids, cells were harvested and lysed in NP-40 buffer for the quantification of cytoplasmic chloramphenicol acetyltransferase (CAT) activity using the Quant-T-CAT assay kit (Amersham), as recommended by the manufacturer. An aliquot of each lysate was assayed for β-galactosidase activity to monitor transfection efficiency. Two independent transfection experiments were performed, in duplicate.
RESULTS
Stably transfected HuH7 cells expressing MxA protein.
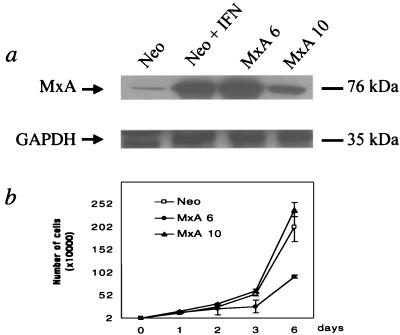
In order to investigate the specific influence of MxA protein on HBV replication, we established cell lines which constitutively express MxA by stably transfecting well-differentiated human hepatoma HuH7 cells with an MxA plasmid, as described in Materials and Methods. HuH7 cells were chosen for their capacity to support efficient HBV replication (5). Clones were tested for their MxA expression by Western blot using a mouse monoclonal MxA antibody. We selected two independent clones (MxA6 and MxA10) which expressed the MxA protein at a level comparable to that found in IFN-induced parental HuH7 cells (data not shown) or neo-expressing clone (Fig. 1a). A clone expressing the neomycin resistance gene only was also selected as a negative control (Fig. 1a). A normal cytoplasmic localization of the MxA protein was found (data not shown). The influence of MxA protein on cell growth was assessed (Fig. 1b). No significant effect on the growth rate of the MxA10 clone was seen in comparison with the neo clone. A reduction in cell growth was observed in the MxA6 clone after 2 days of culture; this probably reflected a cell-cloning event independent of MxA expression, since no modification was seen in the MxA10 clone. In addition, identical expression of GAPDH protein was observed in the different cell clones (Fig. 1a).
FIG. 1.
Analysis of MxA clones. (a) Expression of MxA protein in stably transfected HuH7 clones. Western blot analysis of cytoplasmic extracts from HuH7 clones expressing the neomycin resistance gene, untreated (Neo), or treated (Neo + IFN) with IFN-α for 20 h, and from two MxA-expressing clones (MxA6 and MxA10) using a monoclonal mouse anti-MxA antibody. Level of protein expression was monitored by using a mouse monoclonal anti-GAPDH antibody. (b) Typical curve of cell growth obtained for neo (Neo) and MxA-positive (MxA6 and MxA10) clones. Cells were seeded at a confluence of 20,000 cells per well of six-well plates, trypsinized, and counted daily. Experiments were done in duplicate at least twice, and bars show standard errors.
MxA protein reduces the synthesis of HBV proteins.
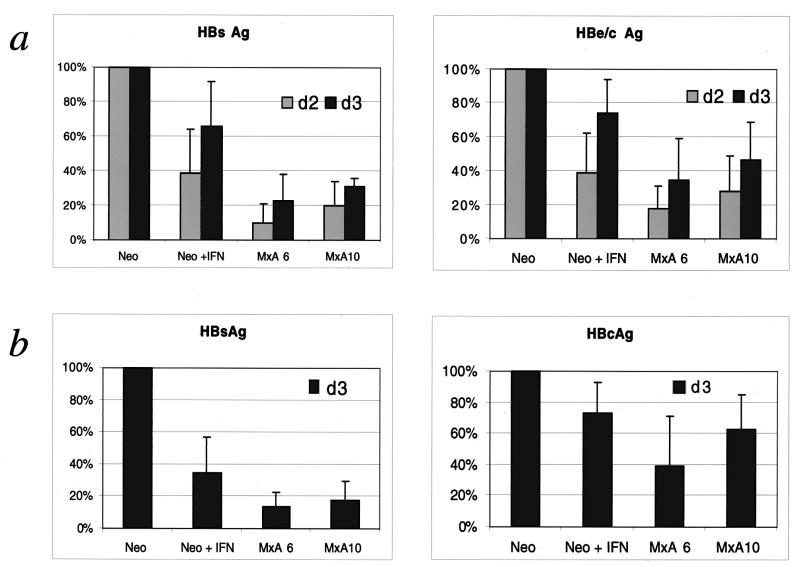
In order to demonstrate the inhibitory effect of MxA protein on HBV protein synthesis, the different clones were transiently transfected with the pTHBV1.1 plasmid. We first investigated HBsAg and HBeAg secretion, 2 and 3 days posttransfection, using standard immunoassays. The secretion of HBs and HBe Ags was profoundly reduced in MxA-expressing clones (MxA6 and MxA10), by about 80% for HBsAg and 70% for HBeAg, compared to the neo clone (Fig. 2a). In the IFN-treated neo clone, the reductions were about 40 to 60% for HBs and HBe. This difference could be linked to the lower level of MxA expression. In order to establish whether this effect was due to intracellular accumulation of synthesized viral proteins, we analyzed the amounts of cytosolic HBsAg and HBe/cAg present 3 days posttransfection, using the same ELISA tests. The level of intracytoplasmic HBsAg was reduced by about 80% in MxA-positive clones and about 60% in the IFN-induced neo clone (Fig. 2b). In the case of HBe/cAg, we observed a reduction of about 40 to 60% in MxA-positive clones and 30% in the IFN-induced neo clone (Fig. 2b). Taken together, our results are consistent with a marked reduction in HBV protein expression in MxA-positive clones.
FIG. 2.
Inhibition of HBV protein synthesis in MxA-expressing clones. MxA clones (MxA6 and MxA10) and untreated (Neo) or IFN-treated (Neo + IFN) neo clones were transfected with pTHBV1.1 plasmid as described in Materials and Methods. Two (d2) or 3 days (d3) after transfection, culture supernatants (a) and cell lysates (b) were collected and assessed for HBs, HBc, and HBe Ag expression. The values shown are percentages of the value obtained for the HBV-transfected neo clone. They were calculated as the mean of the optical density of each experiment normalized to the β-galactosidase activity, as described in Materials and Methods. Bars show the standard errors of at least three independent experiments.
MxA protein reduces the synthesis of HBV replicative intermediates.
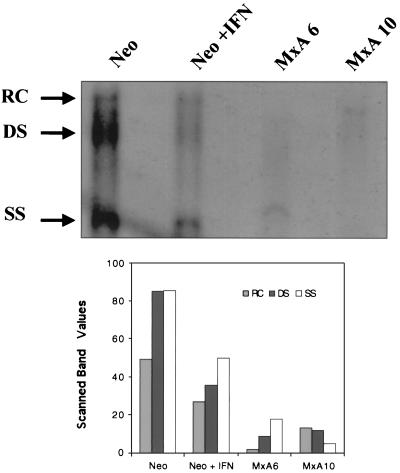
In order to determine whether the decrease in viral protein expression was associated with a change to HBV DNA replicative capacity, the amount of encapsidated viral DNA was measured in the different HBV-transfected clones 3 days posttransfection. As shown in Fig. 3, HBV DNA replicative intermediates were seen to have disappeared almost entirely for the two MxA-expressing clones (MxA6 and MxA10). IFN treatment of the neo clone also led to a significant reduction in DNA replicative forms. Scanning revealed a two- to eightfold reduction. Thus, in addition to viral protein synthesis, MxA protein downregulates viral DNA replication.
FIG. 3.
MxA inhibits HBV DNA replication. Southern blot analysis of core-extracted HBV DNA after transient transfection of untreated (Neo) or IFN-treated (Neo + IFN) neo clone and MxA clones (MxA6 and MxA10) with the pTHBV1.1 plasmid. The DNA was loaded onto a 0.8% agarose gel according to the transfection efficiency and then blotted to nylon membranes. Blots were hybridized with a 32P-labeled HBV probe. The arrows indicate relaxed circular (RC), linear double-stranded (DS), and single-stranded (SS) HBV DNA forms. Histograms express the values of the scanned bands as described in Materials and Methods.
Cytoplasmic HBV RNAs are selectively reduced in MxA-expressing clones.
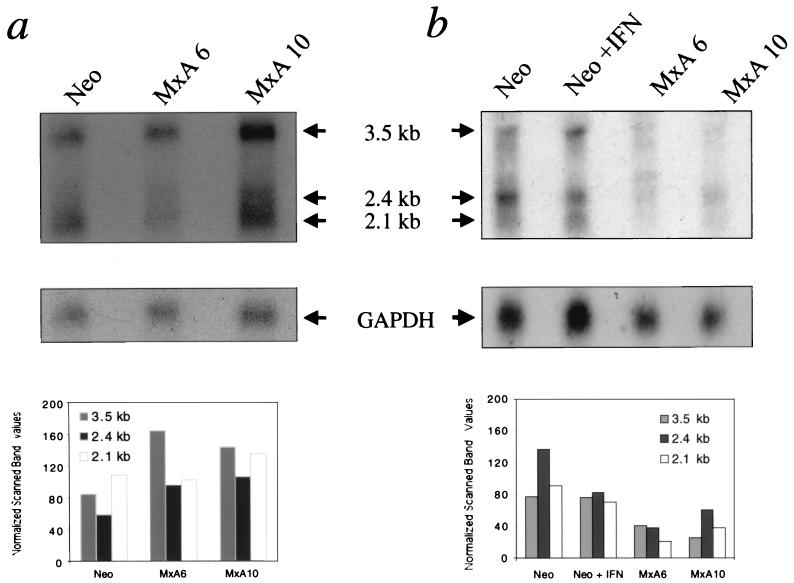
The reduction in HBV protein synthesis and DNA replicative forms in MxA-expressing cells could be due to a modulation of viral transcript synthesis. To investigate this possibility, Northern blot analysis of the HBV-transfected clones was performed for the detection of total and cytoplasmic HBV RNAs. As shown in Fig. 4a, there were no major changes in the expression of total viral transcripts in either MxA clone or the neo clone. By contrast, a two- to fourfold reduction in all intracytoplasmic viral transcripts was observed in MxA-positive clones but not in the negative neo clone (Fig. 4b). Taken together, these results are consistent with MxA protein acting at a posttranscriptional level through the blockade of viral RNA transport from the nucleus to the cytoplasm.
FIG. 4.
MxA selectively reduces cytoplasmic HBV RNAs. Northern blot analysis of total (a) and cytoplasmic (b) RNAs after transient transfection of MxA clones (MxA6 and MxA10) and untreated (Neo) or IFN-treated (Neo + IFN) neo clones with the pTHBV1.1 plasmid. Bands corresponding to the 3.5-, 2.4-, and 2.1-kb viral RNAs are indicated (arrows). The blots were stripped and rehybridized with a 32P-labeled GAPDH probe for normalization. Histograms express the values of the scanned specific HBV bands, normalized to the corresponding scanned GAPDH bands and β-galactosidase values.
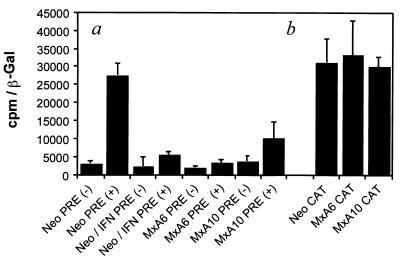
MxA inhibits the nuclear export of RNA mediated by the HBV PRE sequence.
It has been shown that the nucleocytoplasmic export of HBV RNA is mediated by an RNA cis element of about 600 bases termed the posttranscriptional regulatory element (PRE) (18, 19, 39, 48). A pRSV138PRE-CAT construct was used to assay for possible interference between MxA protein expression and the export of HBV RNA (39). pRSV138PRE-CAT expresses a transcript containing both the HBV PRE sequence and the coding sequence for CAT, as described in Materials and Methods. Control and MxA-expressing clones were transfected with pRSVPDM138-CAT, pRSV138PRE-CAT, or pRSV-CAT vector (Fig. 5). In the transfected neo clone, the presence of the PRE sequence in the same intron as the CAT gene enabled the efficient nuclear export of unspliced RNA, resulting in CAT expression [Fig. 5a, Neo PRE(+)]. In contrast, CAT expression was reduced three- to eightfold in the IFN-induced neo clone and in MxA-expressing clones [Fig. 5a, Neo/IFN PRE(+), MxA6 PRE(+), and MxA10 PRE(+)]. This effect was not due to the degradation of CAT transcripts or proteins by MxA protein, since no modification was observed in MxA clones transfected with the pRSV-CAT plasmid (Fig. 5b). These results establish that the MxA protein acts at a posttranscriptional level by inhibiting the nuclear export of viral RNAs by a mechanism(s) which has yet to be clarified.
FIG. 5.
MxA inhibition of the nuclear export of RNA mediated by the HBV PRE sequence. (a) neo clone, untreated (Neo) or treated with IFN (Neo/IFN), and MxA-positive clones (MxA6 and MxA10) were transfected with pRSVPDM138-CAT [PRE(−)] and with pRSVPRE-CAT [PRE(+)]. (b) neo, MxA6, and MxA10 clones were transfected with the pRSV-CAT (CAT) plasmid. Histograms represent the values of CAT activity (counts per minute) normalized to the transfection efficiency (β-galactosidase [β-Gal] activity). Bars show the standard errors of at least two independent experiments, each performed in duplicate.
MxA protein does not interact with the HBV nucleocapsid.
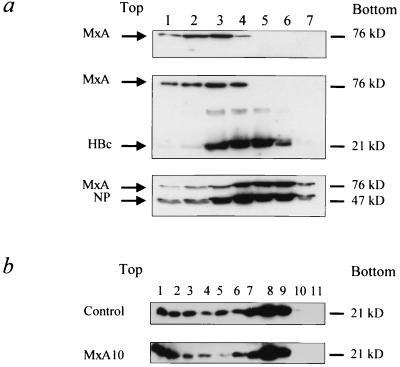
It has recently been demonstrated that MxA interacts with the nucleocapsid of THOV and prevents transport of the nucleocapsid into the nucleus. Our aim was to investigate whether MxA would target HBV nucleocapsid protein and participate in the antiviral effect of MxA against HBV. Previous studies had shown that GTP binding is critical to the antiviral action of the MxA association with THOV nucleocapsid (24). Thus, the in vitro interaction between MxA and HBV capsid was assessed in a cosedimentation assay using a discontinuous glycerol gradient in the presence of GTP-γS, analyzed by Western blot (Fig. 6a). As expected, a shift of MxA sedimentation from lower to higher density glycerol fractions was observed when MxA-expressing and THOV-infected cell lysates were mixed (Fig. 6a, compare upper and lower panels). A similarly modified distribution of MxA protein was not observed in MxA-HBV nucleocapsid mixed lysates (Fig. 6a, middle panel). These results indicate a lack of interaction between the two proteins under these experimental conditions.
FIG. 6.
MxA protein does not interact with HBV nucleocapsid. (a) MxA protein interactions with HBV core protein were analyzed by cosedimentation assay. Lysates from 3T3 cells stably expressing the MxA protein alone (upper panel) or mixed with purified HBV core protein (middle panel) or with THOV-infected 3T3 cell lysate (lower panel) were subjected to glycerol gradient ultracentrifugation as described in Materials and Methods. The seven fractions collected were analyzed by Western blot using polyclonal antibodies against MxA protein (MxA), HBV core protein (HBc), and THOV nucleoprotein (NP). (b) The influence of the MxA protein on HBV capsid self-assembly into particles was analyzed by sucrose gradient assay. HuH7 cells (control) and MxA10 clone (MxA10) were transiently transfected with a vector expressing the capsid protein. Cleared lysates were subjected to ultracentrifugation through a discontinuous (15 to 60%) sucrose gradient as described in Materials and Methods. Eleven fractions were collected and analyzed for HBcAg expression by western blot using a polyclonal rabbit anti-HBe/c antibody. The size of each protein is indicated.
We then tried to establish whether MxA protein could interfere with capsid self-assembly into particles. To address this question, cytoplasmic lysates from HuH7 cells and MxA10 clone transfected with a capsid-expressing vector were subjected to ultracentrifugation through a discontinuous sucrose gradient and analyzed using Western blot (Fig. 6b). In this assay, nonparticulate capsid protein would be found in low-density fractions, while nucleocapsid-like particles would be found in high-density fractions (27). No major modifications were observed to capsid distribution in the two cell lines, indicating that MxA protein does not interfere with capsid self-assembly.
DISCUSSION
This report offers the first direct evidence for the antiviral activities of IFN-α-inducible human MxA protein against HBV. Our results show that MxA protein induced a marked reduction in the synthesis of envelope and capsid proteins and of HBV DNA replicative intermediates. This was associated with an unmodified expression of total viral RNAs, suggesting that MxA inhibits HBV replication at a posttranscriptional stage. However, the cytoplasmic RNAs were diminished in the MxA-positive clones. Indeed, using the well-characterized PRE reporter system, a considerable reduction in the nucleocytoplasmic export of RNA from MxA-positive clones was evidenced. Until now, the inhibitory effect of MxA had only been reported against certain RNA viruses (12, 13, 28, 31–33, 43, 44, 46, 57). For these viruses, MxA may act at different steps of the virus replication cycle, according to the virus species and the cellular models used. Interestingly, viral glycoprotein synthesis has been found to be inhibited for measles virus in a human monocytic cell line constitutively expressing MxA protein, while the viral RNA level remains unchanged (44). Similarly, the antiviral effects of MxA protein against influenza virus replication do not occur at the viral RNA synthesis level but at an unidentified posttranscriptional step (32).
Several groups have studied the global effect of IFN-α against HBV, using different hepatoma cell lines which stably sustain complete (HepG2 clones 2.2.15, HB3-5 and HB107) (4, 7, 17, 21) or partial (PLC/PRF/5 cell line) HBV replication (2, 26, 55). In line with our findings, reductions in HBsAg and pre-S2 secretion were observed in the IFN-treated PLC/PRF/5 cell line (2, 26, 55). In contrast, in IFN-treated HepG2 cell lines, no change or a moderate reduction in HBsAg and HBeAg secretion was evidenced, even when high doses of IFN-α were employed (4, 21); this probably reflects differences in cell sensitivity to IFN treatment (23, 35). As shown by our data, IFN treatment of HepG2-HBV clones induces a reduction in HBV DNA replicative intermediates (7, 17, 21). Furthermore, no change was seen to the expression of total viral RNA in either IFN-treated HepG2 HB107 or PLC/PRF/5 cells (17, 26), and a reduction in the steady-state level of cytoplasmic viral RNA in the IFN-treated HepG2.2.15 cell line was reported (4). Thus, as in our model, posttranscriptional IFN activity, based on the inhibited nuclear export of viral RNAs, could be suggested. Taken together, the published data and our results indicate that IFN-α and MxA have comparable inhibitory effects on HBV. It is therefore likely that MxA protein plays a key role in the antiviral action of IFN against HBV.
Several studies have demonstrated the importance of the PRE region to HBV RNA nuclear export. The PRE is a cis RNA element, encompassing nucleotides 1151 to 1684 and present in viral transcripts (19). The PRE has been shown to act posttranscriptionally to achieve the efficient expression of HBV surface proteins (18, 19). We report here that one of the mechanisms involved in the antiviral effect of MxA protein against HBV is inhibition of the PRE function. Since MxA is a cytoplasmic protein, its action is probably exerted via modulation of one or more of the cellular factors involved in the PRE function. Indeed, MxA protein has a leucine zipper motif in its primary sequence, which is involved in protein-protein interaction (16). The cellular proteins that interact with the PRE sequence are under investigation. A recent report has identified GAPDH as one of the cellular proteins that binds to the PRE, and it is probably involved in the posttranscriptional regulation of HBV expression (56). One might therefore hypothesize that cytoplasmic MxA may act by blocking GAPDH and thus preventing the nucleocytoplasmic transport of viral RNA. Alternatively, MxA protein may induce IκBα expression, which has been shown to inhibit HBV RNA export when it is overexpressed (39).
Since we observed the nearly complete disappearance of replicative intermediates, a further mechanism for HBV inhibition by MxA may be suggested, involving MxA interference with the pregenome encapsidation process. In this respect, recent studies in duck and HBV transgenic models suggest that IFN-α/β may act by inhibiting the formation of pregenome-containing capsids, by preventing their assembly or accelerating their degradation (45, 54). In addition, it is interesting that Kochs et al. demonstrated that MxA protein was able to interact with the THOV nucleocapsid, impairing normal viral replication (25). We were not able to provide evidence for a direct interaction between MxA and capsid proteins. However, the existence of weak interactions cannot be excluded, although they were not detected under our experimental conditions.
We previously demonstrated that HBV defective particles, generated by the reverse transcription of encapsidated spliced RNA, were associated with a chronic course of HBV infection and gave rise to the cytoplasmic accumulation of capsid protein (37). We also showed that expression of the defective genome led to an inhibition of MxA protein and that the capsid protein was implicated in this inhibition. Indeed, we observed an inverse correlation between the amount of intracellular capsid protein and MxA protein expression (38). This would fit with in vivo and in vitro reciprocal interactions between IFN and HBV and further argues in favor of a major role for MxA against HBV. In addition, naturally occurring mutations in the HBV precore promoter and precore open reading frame have been described in patients with severe liver disease (34, 40, 50). These mutations may enhance viral replication and/or core protein accumulation (1, 3, 34, 41). In this context, it is plausible that the relative levels of capsid and MxA proteins expression may contribute to the failure of IFN treatment in such patients.
The present results clearly demonstrate that the antiviral activity of MxA is not restricted to RNA viruses but also includes a DNA virus. We provide evidence for a major antiviral role of MxA protein against HBV. Additional studies are required to further define the precise mechanism(s) involved in the antiviral effect of MxA against HBV and how MxA might be used to develop new diagnostic and therapeutic approaches to the management of chronic HBV infection.
ACKNOWLEDGMENTS
We thank M. A. Petit (INSERM U131, Clamart, France) for providing anti-HBe/c antibodies and purified HBV capsid protein; M. A. Horisberger (Novartis Pharma Inc.) for providing anti-MxA antibodies; O. Haller and G. Kochs (Institute for Medical Microbiology & Hygiene, University of Freiburg, Freiburg, Germany) for providing MxA polyclonal antibodies and for assistance in cosedimentation experiments; B. Matlinger and J. Loutonda and the Blood Transfusion Center for performing immunoenzymatic analyses; and H. Sirma and F. Demaugre for helpful discussions.
Grants from the Fondation pour la Recherche Médicale (FRM), the Institut National de la Sante et de la Recherche Médicale (INSERM), and the Région Guadeloupe supported this work.
REFERENCES
- 1.Baumert T F, Marrone A, Vergalla J, Liang T J. Naturally occurring mutations define a novel function of the hepatitis B virus core promoter in core protein expression. J Virol. 1998;72:6785–6795. doi: 10.1128/jvi.72.8.6785-6795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthillon P, Crance J M, Leveque F, Jouan A, Petit M A, Deloince R, Trepo C. Inhibition of the expression of hepatitis A and B viruses (HAV and HBV) proteins by interferon in a human hepatocarcinoma cell line (PLC/PRF/5) J Hepatol. 1996;25:15–19. doi: 10.1016/s0168-8278(96)80322-9. [DOI] [PubMed] [Google Scholar]
- 3.Buckwold V E, Xu Z, Chen M, Yen T S, Ou J H. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996;70:5845–5851. doi: 10.1128/jvi.70.9.5845-5851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caselmann W H, Meyer M, Scholz S, Hofschneider P H, Koshy R. Type I interferons inhibit hepatitis B virus replication and induce hepatocellular gene expression in cultured liver cells. J Infect Dis. 1992;166:966–971. doi: 10.1093/infdis/166.5.966. [DOI] [PubMed] [Google Scholar]
- 5.Chang C, Jeng K-S, Hu C-P, Lo S, Su T-S, Ting L-P, Chou C-K, Han S-H, Pfaff E, Salfeld J, Schaller H. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in hepatoma cell line. EMBO J. 1987;6:675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis M G, Jansen R W. Inhibition of hepatitis B virus in tissue culture by alpha interferon. Antimicrob Agents Chemother. 1994;38:2921–2924. doi: 10.1128/aac.38.12.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Maeyer E, De Maeyer-Guignard J. Type I interferons. Int Rev Immunol. 1998;17:53–73. doi: 10.3109/08830189809084487. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag J L, Perrillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez M, Quiroga J A, Martin J, Cotonat T, Pardo M, Horisberger M A, Carreno V. Impaired interferon induction of human MxA protein in chronic hepatitis B virus infection. J Med Virol. 1997;51:332–337. [PubMed] [Google Scholar]
- 11.Foster G R, Acrkrill A M, Goldin R D, Kerr I M, Thomas H C, Stark G R. Expression of the terminal protein region of hepatitis B virus inhibits cellular responses to interferons and double-stranded RNA. Proc Natl Acad Sci USA. 1991;88:2888–2892. doi: 10.1073/pnas.88.7.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70:915–923. doi: 10.1128/jvi.70.2.915-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frese M, Kochs G, Meier-Dieter U, Sielber J, Haller O. Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus. J Virol. 1995;69:3904–3909. doi: 10.1128/jvi.69.6.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotti L G, Matzke B, Schaller H, Chisari F V. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunning P, Leavitt J, Muscat G, Ng S Y, Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci USA. 1987;84:4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller O, Frese M, Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev Sci Technol. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Koike K. Interferon inhibits hepatitis B virus replication in a stable expression system of transfected viral DNA. J Virol. 1989;63:2936–2940. doi: 10.1128/jvi.63.7.2936-2940.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Liang T J. A novel hepatitis B virus (HBV) genetic element with Rev response element-like properties that is essential for expression of HBV gene products. Mol Cell Biol. 1993;13:7476–7486. doi: 10.1128/mcb.13.12.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z-M, Yen T. Role of the hepatitis B virus posttranslational regulatory element in export of intronless transcripts. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakschies D, Zachoval R, Müller R, Manns M, Nolte K-U, Hochkeppel H K, Horisberger M-A, Deicher H, Von Wussow P. Strong transient expression of the type I interferon-induced MxA protein in hepatitis A but not in acute hepatitis B and C. Hepatology. 1993;19:857–865. [PubMed] [Google Scholar]
- 21.Kakumu S, Ito Y, Wakita T, Yoshioka K, Ishikawa T, Takayanagi M, Higashi Y, Yang Z Q. Effects of transforming growth factor-beta 1 against the inhibitory action of interferon on DNA synthesis and viral replication in hepatitis B virus DNA-transfected cell. J Med Virol. 1992;38:62–66. doi: 10.1002/jmv.1890380113. [DOI] [PubMed] [Google Scholar]
- 22.Kann M, Gerlich W. Replication of hepatitis B virus. In: Harrison T J, Zuckerman A J, editors. The molecular medicine of viral hepatitis. London, U.K: Wiley; 1997. pp. 63–87. [Google Scholar]
- 23.Keskinen P, Nyqvist M, Sareneva T, Pirhonen J, Melen K, Julkunen I. Impaired antiviral response in human hepatoma cells. Virology. 1999;263:364–375. doi: 10.1006/viro.1999.9983. [DOI] [PubMed] [Google Scholar]
- 24.Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae) J Biol Chem. 1999;274:4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- 25.Kochs G, Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korba B E, Boumpas D, Mann D, Yoakum G H. Direct modulation of HBV surface antigen in a human, HBsAg-producing hepatocellular carcinoma cell line by alpha, beta, or gamma interferons. J Med Virol. 1990;31:272–276. doi: 10.1002/jmv.1890310406. [DOI] [PubMed] [Google Scholar]
- 27.Koschel M, Oed D, Gerelsaikhan T, Thomssen R, Bruss V. Hepatitis B virus core gene mutations which block nucleocapsid envelopment. J Virol. 2000;74:1–7. doi: 10.1128/jvi.74.1.1-7.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis H, Simon-Jodicke A, Kloti A, Di Paolo C, Schnorr J J, Schneider-Schaulies S, Hefti H P, Pavlovic J. Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins. J Virol. 1998;72:1516–1522. doi: 10.1128/jvi.72.2.1516-1522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nassal M, Schaller H. Hepatitis B virus replication. Trends Microbiol. 1993;1:221–228. doi: 10.1016/0966-842x(93)90136-f. [DOI] [PubMed] [Google Scholar]
- 30.Onji M, Lever A M L, Saito I, Thomas H C. Defective response to interferon in cells transfected with the hepatitis B virus genome. Hepatology. 1989;9:92–96. doi: 10.1002/hep.1840090115. [DOI] [PubMed] [Google Scholar]
- 31.Pavlovic J, Arzet H A, Hefti H P, Frese M, Rost D, Ernst B, Kolb E, Staehli P, Haller O. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J Virol. 1995;69:4506–4510. doi: 10.1128/jvi.69.7.4506-4510.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlovic J, Zurcher T, Haller O, Staehli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pult I, Chouard T, Wieland S, Klemenz R, Yaniv M, Blum H E. A hepatitis B virus mutant with a new hepatocyte nuclear factor 1 binding site emerging in transplant-transmitted fulminant hepatitis B. Hepatology. 1997;25:1507–1515. doi: 10.1002/hep.510250633. [DOI] [PubMed] [Google Scholar]
- 35.Rang A, Gunther S, Will H. Effect of interferon alpha on hepatitis B virus replication and gene expression in transiently transfected human hepatoma cells. J Hepatol. 1999;31:791–799. doi: 10.1016/s0168-8278(99)80279-7. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Lavine J E. Cytokine inhibition of the hepatitis B virus core promoter. Hepatology. 1996;23:17–23. doi: 10.1002/hep.510230103. [DOI] [PubMed] [Google Scholar]
- 37.Rosmorduc O, Petit M-A, Pol S, Capel F, Bortolotti F, Berthelot P, Brechot C, Kremsdorf D. In vivo and in vitro expression of defective hepatitis B virus particles generated by spliced hepatitis B virus RNA. Hepatology. 1995;22:10–19. [PubMed] [Google Scholar]
- 38.Rosmorduc O, Sirma H, Soussan P, Gordien E, Lebon P, Horisberger M, Brechot C, Kremsdorf D. Inhibition of interferon-inducible MxA protein expression by hepatitis B virus capsid protein. J Gen Virol. 1999;80:1253–1262. doi: 10.1099/0022-1317-80-5-1253. [DOI] [PubMed] [Google Scholar]
- 39.Roth J, Dobbelstein M. Export of hepatitis B virus RNA on a Rev-like pathway: inhibition by the regenerating liver inhibitory factor IκBα. J Virol. 1997;71:8933–8939. doi: 10.1128/jvi.71.11.8933-8939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato S, Suzuki K, Akahane Y, Akamatsu K, Akiyama K, Yunomura K, Tsuda F, Tanaka T, Okamoto H, Miyakawa Y, et al. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann Intern Med. 1995;122:241–248. doi: 10.7326/0003-4819-122-4-199502150-00001. [DOI] [PubMed] [Google Scholar]
- 41.Scaglioni P P, Melegari M, Wands J R. Biologic properties of hepatitis B viral genomes with mutations in the precore promoter and precore open reading frame. Virology. 1997;233:374–381. doi: 10.1006/viro.1997.8594. [DOI] [PubMed] [Google Scholar]
- 42.Schalm S W, de Man R A, Heijtink R A, Niesters H G. New nucleoside analogues for chronic hepatitis B. J Hepatol. 1995;22:52–56. [PubMed] [Google Scholar]
- 43.Schneider-Schaulies S, Schneider-Schaulies J, Schuster A, Bayer M, Pavlovic J, ter Meulen V. Cell type-specific MxA-mediated inhibition of measles virus transcription in human brain cells. J Virol. 1994;68:6910–6917. doi: 10.1128/jvi.68.11.6910-6917.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnorr J J, Schneider-Schaulies S, Simon-Jodicke A, Pavlovic J, Horisberger M A, ter Meulen V. MxA-dependent inhibition of measles virus glycoprotein synthesis in a stably transfected human monocytic cell line. J Virol. 1993;67:4760–4768. doi: 10.1128/jvi.67.8.4760-4768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz U, Summers J, Staeheli P, Chisari F V. Elimination of duck hepatitis B virus RNA-containing capsids in duck interferon-alpha-treated hepatocytes. J Virol. 1999;73:5459–5465. doi: 10.1128/jvi.73.7.5459-5465.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwemmle M, Weining K C, Richter M F, Schumacher B, Staeheli P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 47.Seeger C, Mason W S. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith G J, 3rd, Donello J E, Luck R, Steger G, Hope T J. The hepatitis B virus post-transcriptional regulatory element contains two conserved RNA stem-loops which are required for function. Nucleic Acids Res. 1998;26:4818–4827. doi: 10.1093/nar/26.21.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terré S, Petit M A, Bréchot C. Defective hepatitis B virus particles are generated by packaging and reverse transcription of spliced viral RNAs in vivo. J Virol. 1991;65:5539–5543. doi: 10.1128/jvi.65.10.5539-5543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tur-Kaspa R, Klein A, Aharonson S. Hepatitis B virus precore mutants are identical in carriers from various ethnic origins and are associated with a range of liver disease severity. Hepatology. 1992;16:1338–1342. doi: 10.1002/hep.1840160606. [DOI] [PubMed] [Google Scholar]
- 51.Tur-Kaspa R, Teicher L, Laub O, Itin A, Dagan D, Bloom B R, Shafritz D A. Alpha interferon suppresses hepatitis B virus enhancer activity and reduces viral gene transcription. J Virol. 1990;64:1821–1824. doi: 10.1128/jvi.64.4.1821-1824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Twu J S, Schloemer R H. Transcription of the human beta interferon gene is inhibited by hepatitis B virus. J Virol. 1989;63:3065–3071. doi: 10.1128/jvi.63.7.3065-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitten T M, Quets A T, Schloemer R H. Identification of the hepatitis B virus factor that inhibits expression of the beta interferon gene. J Virol. 1991;65:4699–4704. doi: 10.1128/jvi.65.9.4699-4704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wieland S F, Guidotti L G, Chisari F V. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74:4165–4173. doi: 10.1128/jvi.74.9.4165-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita Y, Koike K, Takaoki M, Matsuda S. Suppression of HBsAg production in PLC/PRF/5 human hepatoma cell line by interferons. Microbiol Immunol. 1988;32:1119–1126. doi: 10.1111/j.1348-0421.1988.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 56.Zang W Q, Fieno A M, Grant R A, Yen T S. Identification of glyceraldehyde-3-phosphate dehydrogenase as a cellular protein that binds to the hepatitis B virus posttranscriptional regulatory element. Virology. 1998;248:46–52. doi: 10.1006/viro.1998.9255. [DOI] [PubMed] [Google Scholar]
- 57.Zhao H, De B P, Das T, Banerjee A K. Inhibition of human parainfluenza virus-3 replication by interferon and human MxA. Virology. 1996;220:330–338. doi: 10.1006/viro.1996.0321. [DOI] [PubMed] [Google Scholar]