Abstract
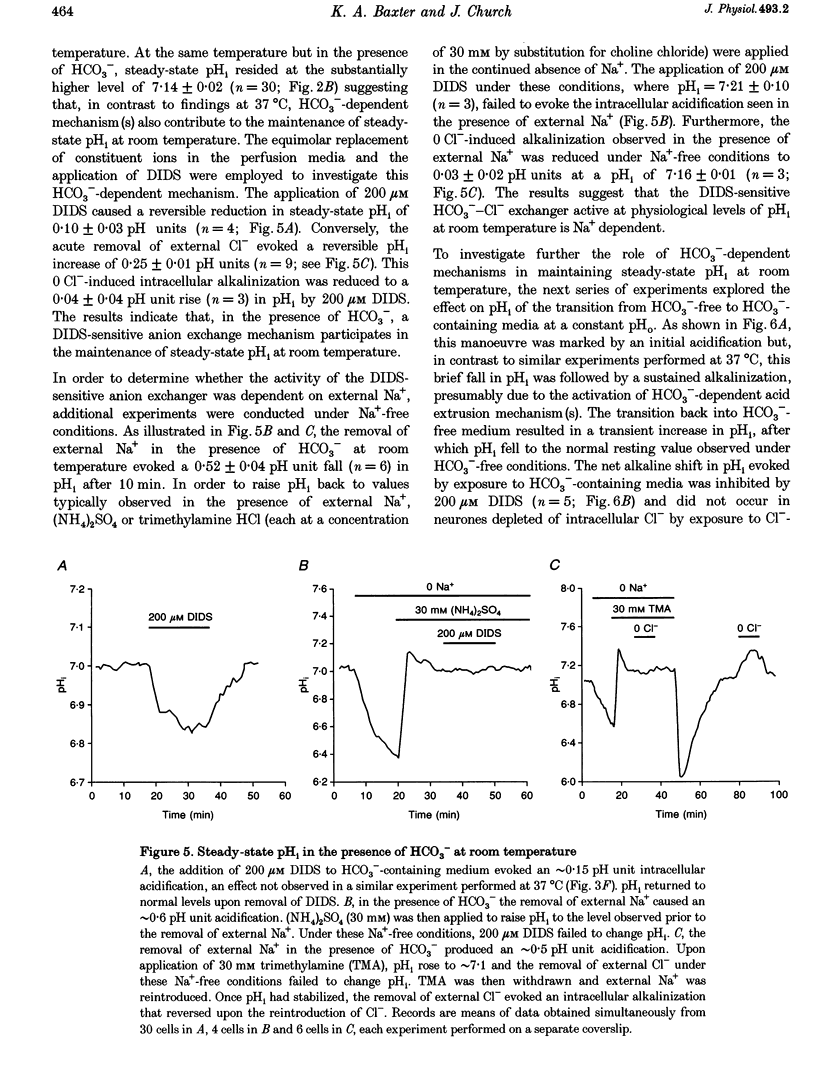
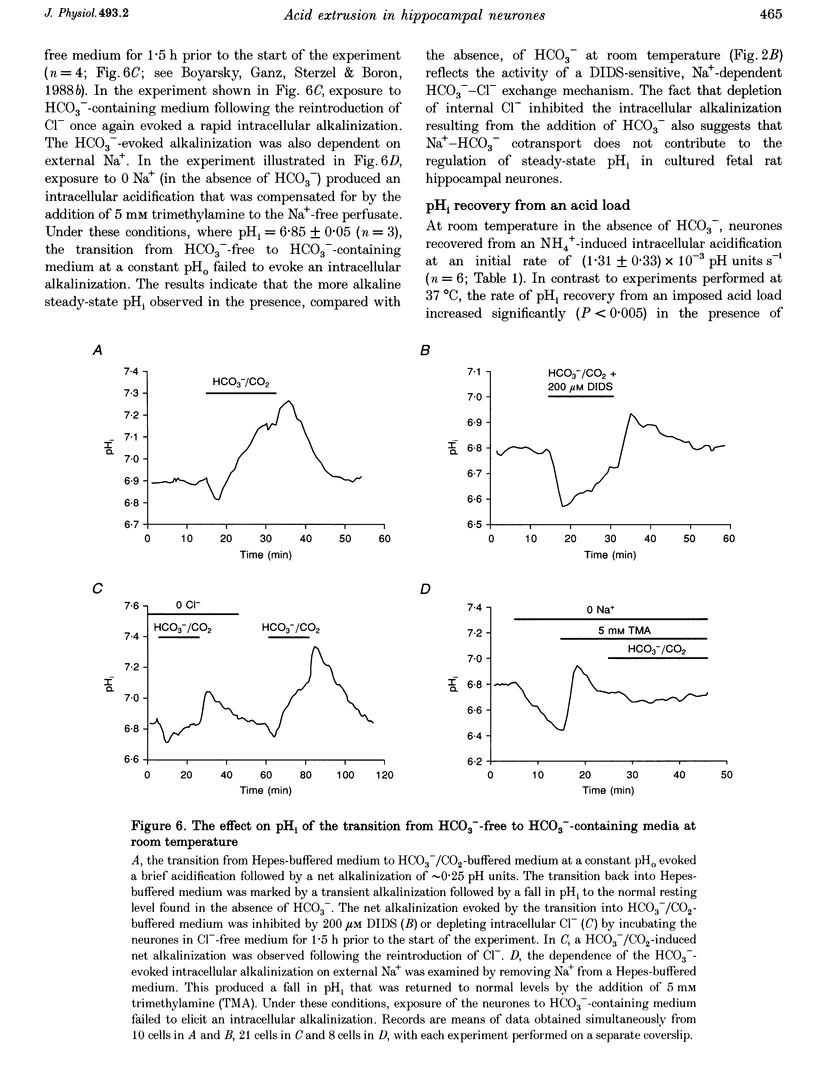
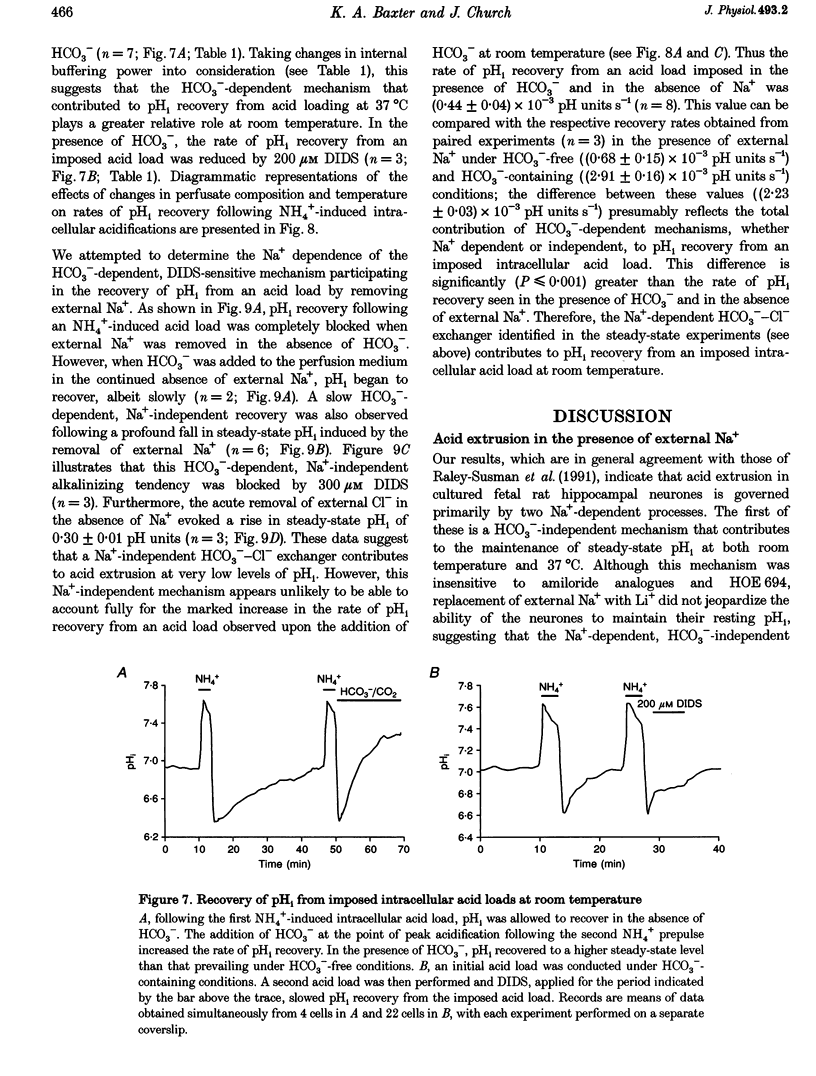
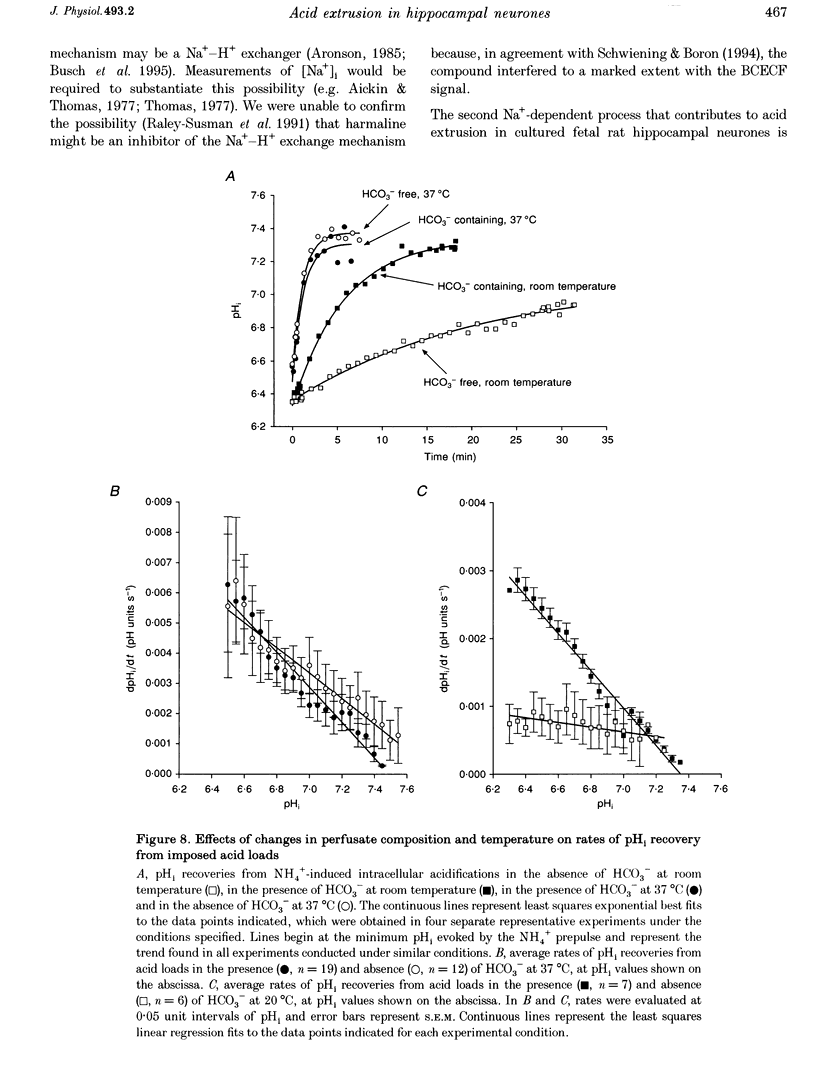
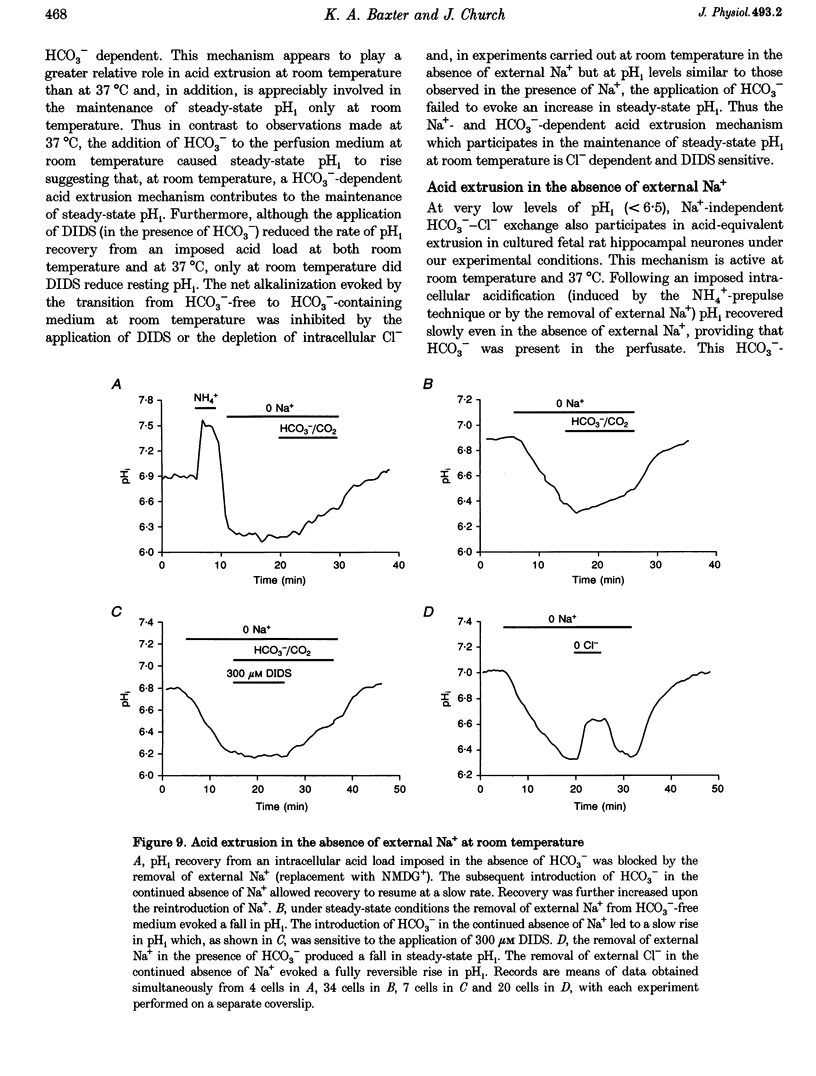
1. We investigated the mechanisms regulating acid extrusion in cultured fetal rat hippocampal neurones loaded with 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein. 2. In the absence of HCO3-, removal of external Na+ by substitution with N-methyl-D-glucamine caused a sustained intracellular acidification that was not observed when Na+ was replaced by Li+, but neither steady-state intracellular pH (pHi) nor the rate of pHi recovery from an imposed acid load were influenced by amiloride analogues or HOE 694, inhibitors of Na(+)-H+ exchange in other cell types. In the presence of HCO3-, removal of external Na+ or Cl- evoked an intracellular acidification and a 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid-sensitive (DIDS-sensitive) intracellular alkalinization, respectively. Applied alone, however, DIDS elicited a fall in steady-state pHi at room temperature but not at 37 degrees C. The DIDS-evoked fall in steady-state pHi and the 0 Cl(-)-evoked intracellular alkalinization observed in the presence of HCO3- at room temperature were dependent on external Na+. 3. At room temperature (18-22 degrees C), but not at 37 degrees C, the transition from HCO3(-)-free to HCO3(-)-containing medium at a constant pHo produced a net alkalinization that was dependent on external Na+ and was inhibited by DIDS or the depletion of internal Cl-. 4. Recovery of pHi from an acid load imposed in the absence of HCO3- was dependent on external Na+. Addition of HCO3- to the perfusion medium increased the rate of pHi recovery from an acid load at room temperature but not at 37 degrees C. In the presence of HCO3-, DIDS slowed the rate of recovery of pHi from an acid load at both room temperature and at 37 degrees C. 5. Recovery of pHi following an imposed intracellular acidification to pH < 6.5 could occur in the absence of external Na+, providing that HCO3- was present in the perfusate. This slow, Na(+)-independent recovery of pHi from very low levels of intracellular pH was sensitive to DIDS. 6. The results indicate that acid extrusion in cultured fetal rat hippocampal neurones involves primarily two Na(+)-dependent mechanisms, one HCO3- dependent (a HCO3(-)-Cl- exchanger) and the other HCO3- independent (possibly a Na(+)-H+ exchanger). Although both mechanisms participate in the maintenance of steady-state pHi at room temperature, only the HCO3(-)-independent mechanism does so at 37 degrees C.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Cotman C. W. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 1989 Aug 7;494(1):65–74. doi: 10.1016/0006-8993(89)90144-3. [DOI] [PubMed] [Google Scholar]
- Busch S., Burckhardt B. C., Siffert W. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflugers Arch. 1995 Apr;429(6):859–869. doi: 10.1007/BF00374811. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation of intracellular pH in reticulospinal neurones of the lamprey, Petromyzon marinus. J Physiol. 1986 Dec;381:241–261. doi: 10.1113/jphysiol.1986.sp016325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. D., Limbird L. E. Na(+)-H+ exchanger subtypes: a predictive review. Am J Physiol. 1991 Dec;261(6 Pt 1):C945–C953. doi: 10.1152/ajpcell.1991.261.6.C945. [DOI] [PubMed] [Google Scholar]
- Counillon L., Scholz W., Lang H. J., Pouysségur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol. 1993 Nov;44(5):1041–1045. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Gaillard S., Dupont J. L. Ionic control of intracellular pH in rat cerebellar Purkinje cells maintained in culture. J Physiol. 1990 Jun;425:71–83. doi: 10.1113/jphysiol.1990.sp018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T., Frelin C., Vigne P., Barbry P., Lazdunski M. Biochemical properties of the Na+/H+ exchange system in rat brain synaptosomes. Interdependence of internal and external pH control of the exchange activity. J Biol Chem. 1985 Aug 15;260(17):9678–9684. [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Gillies R. J., Sánchez-Armass S. Regulation of pH in rat brain synaptosomes. II. Role of Cl-. J Neurophysiol. 1994 Jun;71(6):2249–2257. doi: 10.1152/jn.1994.71.6.2249. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Drapeau P. The regulation of cytosolic pH in isolated presynaptic nerve terminals from rat brain. J Gen Physiol. 1988 Feb;91(2):289–303. doi: 10.1085/jgp.91.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-yang Y., Mellergård P., Siesjö B. K. Regulation of intracellular pH in single rat cortical neurons in vitro: a microspectrofluorometric study. J Cereb Blood Flow Metab. 1993 Sep;13(5):827–840. doi: 10.1038/jcbfm.1993.105. [DOI] [PubMed] [Google Scholar]
- Raley-Susman K. M., Cragoe E. J., Jr, Sapolsky R. M., Kopito R. R. Regulation of intracellular pH in cultured hippocampal neurons by an amiloride-insensitive Na+/H+ exchanger. J Biol Chem. 1991 Feb 15;266(5):2739–2745. [PubMed] [Google Scholar]
- Raley-Susman K. M., Sapolsky R. M., Kopito R. R. Cl-/HCO3- exchange function differs in adult and fetal rat hippocampal neurons. Brain Res. 1993 Jun 18;614(1-2):308–314. doi: 10.1016/0006-8993(93)91049-x. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Schwiening C. J., Boron W. F. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(-)-HCO3- exchange. J Physiol. 1994 Feb 15;475(1):59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Armass S., Martínez-Zaguilán R., Martínez G. M., Gillies R. J. Regulation of pH in rat brain synaptosomes. I. Role of sodium, bicarbonate, and potassium. J Neurophysiol. 1994 Jun;71(6):2236–2248. doi: 10.1152/jn.1994.71.6.2236. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]


