Abstract
Objective
To evaluate the efficacy of Compound Danshen Dripping Pills (CDDP) combined with isosorbide mononitrate (ISMN) versus ISMN alone for treating angina pectoris in patients.
Methods
The PubMed, Web of Science, Cochrane Library, Embase China National Knowledge Infrastructure, China Biomedical Literature Service System, Chinese Medical Journal Database, and Wan Fang MED databases were searched from inception to November 2022. Randomized controlled trials (RCTs) and cohort studies were included. The primary outcomes were angina symptom and electrocardiography (ECG) efficacy, angina symptom efficacy, and ECG efficacy. The protocol was registered with PROSPERO No. CRD42022314774.
Results
Our study included 7 245 patients with angina (59 RCTs, 11 cohort studies). When ISMN was combined with CDDP, the efficacy of angina symptom and ECG [odds ratio (OR) = 4.824, 95% confidence interval (CI) = 3.636–6.401, P = 0.000], the efficacy of angina symptom (OR = 4.347, 95% CI = 3.635–5.198, P = 0.000), the efficacy of ECG (OR = 3.364, 95% CI = 2.767–4.089, P = 0.000) were better than that of patients treated with ISMN alone. CDDP combined with ISMN was superior to ISMN alone in reducing triglyceride (TG) [mean difference (MD) = −35.176, 95% CI = −37.439 to −32.912, P = 0.000], total cholesterol (TC) (MD = −24.296, 95% CI = −26.429 to −22.163, P = 0.000), the duration of angina attack (MD = −1.991, 95% CI = −2.349 to −1.633, P = 0.000), and the frequency of angina attack [standardized MD (SMD) = −2.840, 95% CI = −3.416 to −2.265, P = 0.000]. There was no increase in adverse events between CDDP combined with ISMN and ISMN alone (OR = 0.513, 95% CI = 0.421–0.626, P = 0.000).
Conclusion
CDDP combined with ISMN improved treatment efficacy and was well tolerated. Therefore, this combination could be used as an alternative treatment. However, clinical and patient conditions should be considered.
Keywords: angina pectoris, Compound Danshen Dripping Pills, isosorbide mononitrate, Meta-analysis, systematic review
1. Introduction
Coronary heart disease (CHD), short for coronary atherosclerotic heart disease, is caused by myocardial ischemia, hypoxia, or necrosis attributed to coronary atherosclerosis, which narrows, spasms, or blocks the lumen of the coronary arteries. CHD is the most common clinical cardiovascular diseases (Zhu and Jia, 2005, Xiong et al., 2015, Tobin, 2010). According to the 2020 China Cardiovascular Health and Disease Report, the prevalence of CHD in individuals aged ≥ 15 years was 12.3‰ (National Center for Cardiovascular Disease, 2021). Further, the 2021 China Cardiovascular Disease Medical Quality Report reported that the prevalence of CHD in hospitalized patients was 53.4% (5.31 million cases). The most common clinical manifestations of CHD were angina pectoris (37.8%) and myocardial infarction (14.9%); the mortality rate of angina pectoris (AP) was 0.1%; and the average hospital stay was 7.5 days with a total cost of 18 110.6 yuan (Ma et al., 2021). Based on the 2013–2016 NHANES data, it is estimated that 18.2 million Americans over the age of 20 had CHD. The overall prevalence of CHD, AP, and myocardial infarction was 6.7%, 3.6%, and 3.0%, respectively (Virani et al., 2020). These data suggest that CHD is common among cardiovascular diseases. Since AP is common in patients with CHD, it is essential to consider its clinical treatment.
Currently, the main drugs used to treat AP in patients with CHD are nitrates, beta-blockers, calcium antagonists, lipid-lowering drugs, and antiplatelet drugs. Nitrates can dilate blood vessels, reduce myocardial oxygen consumption, improve myocardial perfusion, and relieve symptoms (Chinese Society of Cardiology, 2010). Nitrates can be classified as short- or long-acting [e.g., isosorbide mononitrate (ISMN)]. ISMN is not suitable for acute AP attack but is suitable for the long-term treatment of chronic AP (National Health and Family Planning Commission Expert Committee on Rational Drug Use, 2018). In China, AP of CHD belongs to the “chest and heart pain category” in traditional Chinese medicine (TCM) (Mao, Wu, & Shi, 2021). Treatment is primarily based on the promotion of blood circulation and the removal of blood stasis. With the development of integrative medicine, the treatment of AP combined with TCM has drawn increasing attention (Lin et al., 2017, Shi et al., 2020). Compound Danshen Dripping Pills (CDDP) is a representative TCM. Among its prescribed components, Salviae Miltiorrhizae Radix et Rhizoma (Danshen in Chinese) promotes blood circulation and removes blood stasis; Notoginseng Radix et Rhizoma (Sanqi in Chinese) disperses blood stasis and alleviates pain; and Borneolum Syntheticum (Bingpian in Chinese) has a diverging effect, which can promote the active components of Salviae Miltiorrhizae Radix et Rhizoma and Notoginseng Radix et Rhizoma into the body (Cheng et al., 2017). The preparation of CDDP involves a dropping pill. This formulation can help the drug dissolve and release quickly, giving it a quick effect. It is also suitable for sublingual ingestion and facilitates rapid relief for emergency patients. Expert consensus indicates that CDDP is safe and effective for the treatment of AP (Mao et al., 2021, Cheng et al., 2017). All drugs were combined to remove blood stasis, clear arteries, promote blood circulation, and relieve pain (Wang, 2014a). CDDP has been widely used in the prevention, treatment, and first aid of CHD for myocardium protection, blood vessel protection, and microcirculation improvement (Ji, 2013, Liu et al., 2022, Wang et al., 2023a, Wang et al., 2022). Thus, CDDP can sufficiently compensate for the limitations of Western medicine. Currently, the TCM and Western medical guidelines recommend drug grades in their respective fields. However, there are very few recommended drug grades for integrated TCM and Western medicine. Therefore, this Meta-analysis compared the efficacy and safety of CDDP combined with ISMN vs. ISMN alone for the treatment of AP in CHD. A comprehensive understanding of the use of CDDP in combination with ISMN is expected to provide an additional clinical reference for the treatment of AP using a combination of TCM and Western medicine.
2. Materials and methods
2.1. Systematic review registration
This Meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009) and was registered with PROSPERO (No. CRD42022314774) (Wang, Hu, Li, & Yin, 2022).
2.2. Search method and data extraction
We searched PubMed, Web of Science (SCI), the Cochrane Library, Embase, the China National Knowledge Infrastructure, the China Biomedical Literature Service System, Wan Fang MED Online, and the Chinese Medical Journal Database. The search period ranged from the establishment of the database to November 2022. The following search terms were used: CDDP, ISMN, coronary artery disease, angina, angina (unstable), angina (stable), AP (variant), microvascular angina, and chest pain. Two authors independently screened the abstracts and full texts.
All search records were imported into Endnote X8, where they were double-checked and filtered by two researchers. After reading the abstract, articles were excluded if they did not meet the inclusion criteria. All eligible materials were extracted after reading the full text. Disagreements were resolved through discussion. The data extraction table included the basic information, treatment measures, and evaluation metrics. The included studies had to have been published in the original literature to ensure the reliability and authenticity of the data extraction. Otherwise, the authors were contacted via e-mail for the raw data.
2.3. Inclusion and exclusion criteria
All randomized controlled trials (RCTs) and cohort studies were included in our study. Studies with the same data published later were excluded. Patients with typical symptoms of AP who met the diagnostic criteria of the Practice Guidelines for AP of CHD were included (Fihn et al., 2012). Studies comparing treatment with ISMN alone and CDDP combined with ISMN reported that angina symptom or electrocardiogram (ECG) efficacy was included. Patients treated with ISMN in combination with additional drugs for AP were excluded. Additional duplicate publications, review articles, and conference papers were excluded from the analysis.
2.4. Evaluation criteria
The primary outcomes were the efficacy of angina symptom and ECG. The efficacy of angina symptom was classified as marked response, moderate response, and no response (Evaluation criteria of curative effect on AP of CHD and electrocardiogram (Kan, 2016, Wang, 2005). A marked response indicated that the same amount of exertion did not trigger an angina attack or that the number of angina attack or nitroglycerin level was reduced by more than 80%. A moderate response was defined as a 50%−80% reduction in the frequency of angina attack or nitroglycerin consumption. No response was defined as a reduction of less than 50% in both the number of angina attack and the amount of nitroglycerin used.
The efficacy of ECG was also classified as a marked response, moderate response, and no response (Fan and Wang, 2014, Wang, 2005). A marked response indicated that the resting ECG had returned to normal. A moderate response refers to 1) the recovery of the ST segment by more than 0.05 mm after treatment and 2) the T-wave becoming shallow or straight on a flat surface. No response indicated that the ECG was essentially the same as before.
Secondary outcomes included lipid levels, the duration and frequency of angina attack, and adverse drug reactions (ADRs). Lipid levels included triglyceride (TG) (mg/dL) and total cholesterol (TC) (mg/dL). The duration of an angina attack was defined as the average duration of an angina attack over a specified period (min). The frequency of angina attack refers to the number of angina attack in a specified period (e.g., per day or week). The total number of ADRs that occurred during treatment was evaluated.
2.5. Quality assessment
The Cochrane risk-bias assessment tool was used to assess the methodological quality of the included studies (Higgins et al., 2011). The tool took into consideration whether the random method was appropriate, whether the allocation was hidden, whether researchers and subjects were double-blinded, whether the results of the study were evaluated using the blind method, whether the resulting data were complete, whether the study avoided selective result reporting, and whether the study avoided creating further problems. The risk of bias was assessed as low risk of bias, unclear risk of bias, or high risk of bias by individually scoring each item (Higgins et al., 2011). The Newcastle-Ottawa Quality Assessment Scale was used to evaluate the methodological quality of the included studies (Luchini et al., 2021). The evaluation was conducted based on the aspects of selection (4 scores), comparability (2 scores), and outcome (3 scores). The higher the research evaluation score is, the better the quality is. Any differences were resolved by consensus.
2.6. Statistical analysis
Data analysis was performed using Review Manager 5.4 and Stata 14. The odds ratio (OR) or relative risk (RR) was used to assess binary variables. The continuous variables were the mean difference (MD) or standardized mean difference (SMD) as the effect quantity. The 95% confidence interval (CI) was used for analysis. We analyzed the heterogeneity of the included studies. If the results showed a P-value < 0.10 and I2 > 50%, the study was considered to be highly heterogeneous, and a random effect model was selected. If the results showed a P-value > 0.10 and I2 ≤ 50%, the heterogeneity among the studies was considered small, and a fixed-effect model was selected (Andrade, 2020). If heterogeneity was detected, a sensitivity analysis was performed. Sensitivity analysis was performed using one-to-one elimination. We also used subgroup analysis to analyze the factors influencing heterogeneity. Funnel plots and Egger’s test were used to analyze the risk of publication bias. A P-value of < 0.05 indicated significant publication bias. The trim-and-fill method was used to analyze the publication bias. The age and disease course of patients were expressed as the combined mean and standard deviation (Yin, Zhou, & Chen, 2010) and the data were analyzed using Microsoft Excel 2019 and SPSS version 26.
3. Results
3.1. Screening results
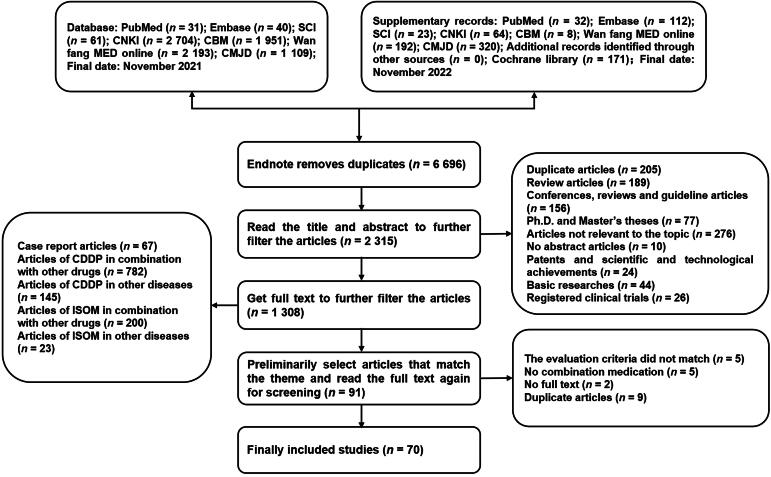
A total of 9 011 articles were retrieved from the databases, and 6 696 duplicates were excluded. In the remaining articles, 2 245 did not meet the inclusion criteria; thus, they were excluded after reading the abstracts and full texts. Finally, 59 RCTs and 11 cohort studies were included (Chen, 2021, Cui et al., 2013, Diao, 2016, Dong, 2019a, Dong, 2019b, Du and Song, 2019, Du, 2021, Fan and Wang, 2014, Gao, 2015, Gu, 2014, Guo, 2013, Huang and Cao, 2010, Jia and Zhu, 2005, Jiang, 2016, Jing, 2014, Kan, 2016, Kong, 2021, Li, 2017, Li and Xie, 2013, Li, 2021, Li, 2012, Li, 2010, Li, 2016, Liang, 2013, Lin and Wu, 2010, Lin, 2012, Liu, 2004, Liu, 2021a, Liu, 2022, Lu, 2017, Lv, 2021, Lv et al., 2011, Peng, 2018, Qin, 2022, Qu, 2017, Ren, 2018, Ren, 2020, Shang, 2013, Shao, 2013, Shen, 2020, Sheng, 2015, Shi and Xu, 2013, Song et al., 2019, Sun, 2016, Wang, 2017, Wang, 2007, Wang, 2014b, Wang, 2012, Wang, 2005, Wu, 2021a, Wu and Wang, 2016, Wu, 2018, Wu, 2021b, Xu, 2020, Xu, 2018, Yan and Jiang, 2016, Yan, 2016, Zeng, 2014, Zhang and Wang, 2011, Zhang, 2021, Zhang, 2012, Zhang, 2016, Zhang et al., 2013, Zhang, 2022, Zhang et al., 2017, Zhao and Guan, 2017, Zheng and Wei, 2006, Zhu, 2020, Ma, 2012, Wu, 2019). The screening process was illustrated in Fig. 1.
Fig. 1.
Flow diagram of study selection.
Seventy studies were written in Chinese, and 7 245 Chinese patients were enrolled. Sixty-one studies included detailed data on patient sex, with 1 654 men and 1 307 women in the experimental group and 1 650 men and 1 292 women in the control group. There was no significant sex difference between the two groups (χ2 = 0.030 a, P = 0.862). Forty-five studies included detailed data on the age of the patients; the mean age ± standard deviation (years) was 63.2 ± 8.7 in the experimental group and 62.7 ± 8.7 in the control group. Twenty-six studies included detailed data on the patient’s course of disease [experimental group, (6.8 ± 5.2) years; control group, (6.8 ± 5.3) years]. Forty-one articles mentioned routine care, including rest, smoking cessation, a light diet, anti-lipid medication, and antiplatelet therapy. Fifty studies involved the treatment of AP, and 20 studies involved the treatment of unstable angina pectoris (UAP). The median daily ISMN dose was 40 mg [Q1 (first quartile): 40 mg, Q3 (third quartile): 50 mg]. The CDDP dose was 270 mg (10 pills), three times a day. The median duration of medication use was 30 d (Q1, 28 d; Q3, 56 d). Table 1 summarized the basic characteristics of the included studies.
Table 1.
Basic characteristics of studies.
| Author/Year | Study types | Samples (Number of cases) |
Interventions |
Dosage and frequency |
Angina symptom and ECG efficacy |
Angina symptom efficacy |
ECG efficacy |
Other effect indicators |
Types | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/T | C | T | C | T | C/S | T/S | C/S | T/S | C/S | T/S | C | T | ||||
| Chen, 2021 | RCT | 31/31 | ①,4 w | ⑤; ①,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | 25/31 | 30/31 | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | AP | ||
| Cui, Zhang, & Zhu, 2013 | RCT | 80/80 | ②,2 m | ⑤; ②,2 m | 20 mg,bid | 10 P,tid | 20 mg,bid | 63/80 | 75/80 | – | – | – | – | – | – | AP |
| Diao, 2016 | cohort study | 40/40 | ②,8 w | ⑤; ②,8 w | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 38/40 | 40/40 | 29/40 | 38/40 | – | – | AP |
| Dong, 2019a | RCT | 48/48 | ⑥,2 m | ⑤; ⑥,2 m | 20 mg,bid | 10 P,tid | 20 mg,tid | 38/48 | 45/48 | – | – | – | – | ⑪ | ⑪ | AP |
| Dong, 2019b | RCT | 48/48 | ①,2 m | ⑤; ①,2 m | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 39/48 | 46/48 | – | – | ⑪ | ⑪ | AP |
| Du & Song, 2019 | cohort study | 63/63 | ①,4 w | ⑤; ①,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | 53/63 | 61/63 | – | – | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | AP |
| Du, 2021 | RCT | 45/45 | ①,1 m | ⑤; ①,1 m | 20 mg,bid | 10P,tid | 20 mg,bid | 37/45 | 44/45 | – | – | – | – | ⑦;⑧ | ⑦;⑧ | AP |
| Fan & Wang, 2014 | RCT | 90/90 | ②,8 w | ⑤; ②,8 w | 20 mg,bid | 10 P,tid | 10 mg,bid | 70/90 | 85/90 | – | – | – | – | ⑪ | ⑪ | AP |
| Gao, 2015 | RCT | 42/42 | ①,1 m | ⑤; ①,1 m | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 30/42 | 39/42 | 28/42 | 40/42 | ⑪ | ⑪ | AP |
| Huang & Cao, 2010 | RCT | 46/52 | ②,2 m | ⑤; ②,2 m | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 34/46 | 49/52 | 22/46 | 36/52 | – | – | AP |
| Jia & Zhu, 2005 | RCT | 30/34 | ⑥,2 w | ⑤; ⑥,2 w | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 19/30 | 31/34 | – | – | ⑨;⑩ | ⑨;⑩ | AP |
| Jiang, 2016 | RCT | 40/40 | ①,28 d | ⑤; ①,28 d | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 32/40 | 39/40 | 32/40 | 38/40 | – | – | AP |
| Kan, 2016 | RCT | 60/60 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 41/60 | 56/60 | – | – | ⑪ | ⑪ | AP |
| Kong, 2021 | RCT | 52/52 | ⑥,8 w | ⑤; ⑥,8 w | 10–20 mg, tid |
10 P,tid | 10–20 mg, tid |
42/52 | 50/52 | – | – | – | – | ⑨;⑩ | ⑨;⑩ | AP |
| Li, 2017 | RCT | 65/65 | ①,4 w | ⑤; ①,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 47/65 | 61/65 | 45/65 | 56/65 | ⑪ | ⑪ | AP |
| Li & Xie, 2013 | RCT | 80/80 | ①,8 w | ⑤; ①,8 w | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 67/80 | 69/80 | 42/80 | 64/80 | ⑪ | ⑪ | AP |
| Li, 2010 | RCT | 40/45 | ⑥,1 m | ⑤; ⑥,1 m | 10 mg,tid | 10 P,tid | 10 mg,tid | – | – | 27/40 | 40/45 | 25/40 | 36/45 | – | – | AP |
| Liang, 2013 | RCT | 54/53 | ①,4 w | ⑤; ①,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 29/54 | 44/53 | 26/54 | 41/53 | – | – | AP |
| Lin & Wu, 2010 | RCT | 39/39 | ②,4 w | ⑤; ②,4 w | – | 10 P,tid | – | – | – | 31/39 | 36/39 | 25/39 | 32/39 | ⑪ | ⑪ | AP |
| Lin, 2012 | RCT | 41/41 | ②,8 w | ⑤; ②,8 w | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 31/41 | 39/41 | 29/41 | 38/41 | – | – | AP |
| Liu, 2021a | RCT | 43/43 | ②,6 w | ⑤; ②,6 w | 20 mg,bid | 10 P,tid | 20 mg,tid | – | – | – | – | 34/43 | 41/43 | ⑦;⑧;⑨;⑩ | ⑦;⑧;⑨;⑩ | AP |
| Lu, 2017 | RCT | 43/43 | ②,3 m | ⑤; ②,3 m | half or one tablet,bid/tid | 10 P,tid | half or one tablet,bid/tid | – | – | 35/43 | 42/43 | 31/43 | 41/43 | ⑨ | ⑨ | AP |
| Lv, 2021 | RCT | 18/18 | ⑥,4 w | ⑤; ⑥,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 13/18 | 17/18 | – | – | ⑨;⑩ | ⑨;⑩ | AP |
| Lv, Liang, & Liang, 2011 | RCT | 80/84 | ⑥,2 m | ⑥; ②,2 m | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 63/80 | 79/84 | 50/80 | 71/84 | – | – | AP |
| Peng, 2018 | RCT | 47/47 | ①,8 w | ⑤; ①,8 w | 30 mg,bid | 10 P,tid | 30 mg,bid | 39/47 | 45/47 | – | – | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | AP |
| Qin, 2022 | RCT | 60/60 | ①,3 m | ⑤; ①,3 m | 40–80 mg, qd |
10 P,tid | 40–80 mg, Qd |
51/60 | 59/60 | – | – | – | – | ⑪ | ⑪ | AP |
| Qu, 2017 | cohort study | 63/63 | ①,2 m | ⑤; ①,2 m | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 50/63 | 58/63 | – | – | ⑪ | ⑪ | AP |
| Ren, 2018 | RCT | 44/44 | ②,14 d | ⑤; ②,14 d | 20 mg, tid | 10 P,tid | 20 mg,tid | – | – | 33/44 | 40/44 | – | – | ⑦;⑧;⑨;⑩ | ⑦;⑧;⑨;⑩ | AP |
| Ren, 2020 | cohort study | 60/60 | ②,1 m | ⑤; ②,1 m | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 46/60 | 58/60 | – | – | ⑨;⑩ | ⑨;⑩ | AP |
| Shang, 2013 | RCT | 34/34 | ②,4 w | ⑤; ②,4 w | – | 10 P,tid | – | – | – | 27/34 | 31/34 | 22/34 | 28/34 | ⑪ | ⑪ | AP |
| Shen, 2020 | RCT | 31/32 | ①,4 w | ⑤; ①,4 w | 60 mg,qd | 10 P,tid | 60 mg,qd | – | – | 20/31 | 28/32 | 22/31 | 30/32 | – | – | AP |
| Song, Li, & Lu, 2019 | cohort study | 40/40 | ①,2 m | ⑤; ①,2 m | 30–60 mg,qd | 10 P,tid | 30–60 mg,qd | – | – | 30/40 | 38/40 | – | – | ⑨;⑩ | ⑨;⑩ | AP |
| Sun, 2016 | RCT | 68/68 | ③,4 w | ⑤; ③,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 49/68 | 64/68 | 51/68 | 65/68 | ⑪ | ⑪ | AP |
| Wang, 2017 | cohort study | 55/55 | ④,30 d | ⑤; ④,30 d | 25 mg*,qd | 10 P,tid | 25 mg,qd | – | – | 44/55 | 51/55 | – | – | ⑪ | ⑪ | AP |
| Wang, 2007 | RCT | 46/52 | ②,2 m | ⑤; ②,2 m | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 36/46 | 48/52 | 20/46 | 33/52 | – | – | AP |
| Wang, 2012 | RCT | 60/60 | ④,1 m | ⑤; ④,1 m | 20 mL*,bid | 10 P,tid | 20 mL*,bid | – | – | 51/60 | 59/60 | – | – | – | – | AP |
| Wang, 2005 | RCT | 30/30 | ②,8 w | ⑤; ②,8 w | 20 mg,bid | 10 P,tid | 20 mg,bid | 20/30 | 28/30 | 21/30 | 29/30 | 14/30 | 23/30 | – | – | AP |
| Wu & Wang, 2016 | RCT | 48/48 | ①,6 m | ⑤; ①,6 m | 10 mg,tid | 10 P,tid | 10 mg,tid | 27/48 | 38/48 | – | – | – | – | ⑪ | ⑪ | AP |
| Wu, 2018 | cohort study | 40/40 | ①,6 m | ⑤; ①,6 m | 10 mg,tid | 10 P,tid | 10 mg,tid | 31/40 | 39/40 | – | – | – | – | ⑪ | ⑪ | AP |
| Wu, 2021b | RCT | 42/42 | ①,8 w | ⑤; ①,8 w | 20–40 mg, qd |
10 P,tid | 20–40 mg, qd |
– | – | 32/42 | 39/42 | 28/42 | 37/42 | ⑨;⑩;⑪ | ⑨;⑩;⑪ | AP |
| Wu, 2019 | RCT | 51/51 | ①,8 w | ⑤; ①,8 w | 40 mg,qd | 10 P,tid | 40 mg,qd | 36/51 | 48/51 | – | – | – | – | ⑦;⑧;⑨;⑩;⑪ | ⑦;⑧;⑨;⑩;⑪ | AP |
| Xu, 2020 | RCT | 45/45 | ④,1 m | ⑤; ④,1 m | 20 mg*,qd | 10 P,tid | 20 mg*,qd | 36/45 | 43/45 | – | – | – | – | ⑦;⑧;⑩ | ⑦;⑧;⑩ | AP |
| Yan & Jiang, 2016 | RCT | 82/82 | ④,12 d | ⑤; ④,12 d | 20 mg*,qd | 10 P,tid | 20 mg*,qd | 58/82 | 78/82 | – | – | – | – | ⑦;⑧;⑨;⑩;⑪ | ⑦;⑧;⑨;⑩;⑪ | AP |
| Yan, 2016 | RCT | 36/37 | ①,4 w | ⑤; ①,4 w | 60 mg,qd | 10 P,tid | 60 mg,qd | – | – | 27/36 | 34/37 | 27/36 | 33/37 | – | – | AP |
| Zhang, 2021 | RCT | 50/50 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | 40/50 | 47/50 | – | – | – | – | ⑦;⑧;⑩;⑪ | ⑦;⑧;⑩;⑪ | AP |
| Zhang, 2012 | RCT | 20/20 | ⑥,4 w | ⑤; ⑥,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | – | – | 16/20 | 18/20 | 13/20 | 17/20 | – | – | AP |
| Zhang, 2022 | cohort study | 35/36 | ①,1 m | ⑤; ①,1 m | 40 mg,qd | 10 P,tid | 40 mg,qd | 25/35 | 33/36 | – | – | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | AP |
| Zhao & Guan, 2017 | RCT | 24/25 | ①,30 d | ⑤; ①,30 d | 10 mg,tid | 8 P,tid | 10 mg,tid | – | – | 16/24 | 23/25 | – | – | ⑪ | ⑪ | AP |
| Zheng & Wei, 2006 | RCT | 42/42 | ⑥,15 d | ⑤; ⑥,15 d | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 34/42 | 40/42 | 34/42 | 40/42 | – | – | AP |
| Zhu, 2020 | RCT | 60/60 | ①,8 w | ⑤; ①,8 w | 30 mg, bid | 10 P,tid | 30 mg, bid | – | – | 50/60 | 57/60 | – | – | ⑪ | ⑪ | AP |
| Gu, 2014 | RCT | 74/76 | ⑥,10 d | ⑤; ⑥,10 d | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 62/74 | 71/76 | 58/74 | 69/76 | – | – | UAP |
| Guo, 2013 | RCT | 107/129 | ④,4 w | ⑤; ④,4 w | 20 mg*,qd | 10 P,tid | 20 mg*,qd | – | – | 81/107 | 117/129 | 94/107 | 118/129 | – | – | UAP |
| Jing, 2014 | RCT | 84/84 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | 61/84 | 75/84 | – | – | – | – | ⑪ | ⑪ | UAP |
| Li, 2021 | RCT | 40/40 | ①,1 m | ⑤; ①,1 m | 20 mg,bid | 10 P,tid | 20 mg,bid | – | – | 29/40 | 36/40 | – | – | ⑨;⑩ | ⑨;⑩ | UAP |
| Li, 2012 | RCT | 52/60 | ④,4 w | ⑤; ④,4 w | 20 mg*,qd | 10 P,tid | 20 mg*,qd | – | – | 38/52 | 54/60 | – | – | – | – | UAP |
| Li, 2016 | RCT | 42/43 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 30/42 | 40/43 | – | – | ⑪ | ⑪ | UAP |
| Liu, 2004 | RCT | 32/33 | ②,4 w | ⑤; ②,4 w | 20 mg,tid | 10 P,tid | 20 mg,tid | – | – | 24/32 | 29/33 | 19/32 | 25/33 | – | – | UAP |
| Liu, 2022 | RCT | 56/56 | ②,4 w | ⑤; ②,4 w | 30 mg,bid | 10 P,tid | 30 mg,bid | 44/56 | 53/56 | – | – | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | UAP |
| Ma, 2012 | RCT | 70/70 | ①,1 m | ⑤; ①,1 m | 30 mg,bid | 10 P,tid | 30 mg,bid | – | – | 53/70 | 65/70 | 51/70 | 64/70 | – | – | UAP |
| Shao, 2013 | cohort study | 40/40 | ①,4 w | ⑤; ①,4 w | 10 mg,tid | 10 P,tid | 10 mg,tid | – | – | 32/40 | 38/40 | – | – | ⑪ | ⑪ | UAP |
| Sheng, 2015 | RCT | 40/40 | ①,4 w | ⑤; ①,4 w | 10 mg,tid | 10P,tid | 10 mg,tid | – | – | 30/40 | 37/40 | – | – | – | – | UAP |
| Shi & Xu, 2013 | cohort study | 40/40 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 32/40 | 38/40 | – | – | ⑪ | ⑪ | UAP |
| Wang, 2014b | RCT | 75/75 | ①,4 w | ⑤; ①,4 w | 50 mg,qd | 10 P,tid | 50 mg,qd | – | – | 59/75 | 72/75 | – | – | ⑪ | ⑪ | UAP |
| Wu, 2021a | RCT | 39/39 | ②,1 m | ⑤; ②,1 m | 30 mg,bid | 10 P,tid | 30 mg,bid | – | – | 27/39 | 38/39 | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | UAP |
| Xu, 2018 | cohort study | 40/40 | ①,1 m | ⑤; ①,1 m | 30 mg, bid | 10 P,tid | 30 mg, bid | – | – | 30/40 | 37/40 | – | – | ⑨;⑩;⑪ | ⑨;⑩;⑪ | UAP |
| Zeng, 2014 | RCT | 50/50 | ①,8 w | ⑤; ①,8 w | 20 mg,bid | 10 P,tid | 20 mg,bid | 34/50 | 46/50 | – | – | – | – | ⑪ | ⑪ | UAP |
| Zhang & Wang,2011 | RCT | 95/98 | ②,2 w | ⑤; ②,2 w | 20 mg,tid | 10 P,tid | 20 mg,tid | – | – | 60/95 | 88/98 | 52/95 | 81/98 | – | – | UAP |
| Zhang, 2016 | RCT | 45/45 | ①,1 m | ⑤; ①,1 m | 10 mg,tid | 10 P,tid | 10 mg,tid | – | – | 27/45 | 42/45 | – | – | ⑪ | ⑪ | UAP |
| Zhang, Zhang, & He, 2013 | RCT | 48/48 | ②,6 w | ⑤; ②,6 w | 20 mg,tid | 10 P,tid | 20 mg,tid | – | – | 31/48 | 42/48 | – | – | ⑨;⑩ | ⑨;⑩ | UAP |
| Zhang, Wu, & Fang, 2017 | RCT | 90/90 | ①,4 w | ⑤; ①,4 w | 40 mg,qd | 10 P,tid | 40 mg,qd | 66/90 | 83/90 | – | – | – | – | ⑪ | ⑪ | UAP |
Note: –: Not mentioned in studies. 1: C: control group; T: treatment group; S: sample; P: pills; T: tablets; d: day; m: month; w: week; *: Injection. 2: ① ISMN release tablets; ② ISMN tablets; ③ ISMN dispersible tablets; ④ ISMN injection; ⑤ CDDP; ⑥ unclear; ⑦ Triglyceride (mg/dL); ⑧ Total cholesterol (mg/dL); ⑨ The duration of angina attack (min); ⑩ The frequency of angina attack (one day or one week); ⑪ Adverse drug reactions. 3: tid: three times a day; bid: twice a day; qd: once a day. 4: AP: angina pectoris; UAP: unstable angina pectoris; RCT: randomized controlled trial; ECG, electrocardiogram.
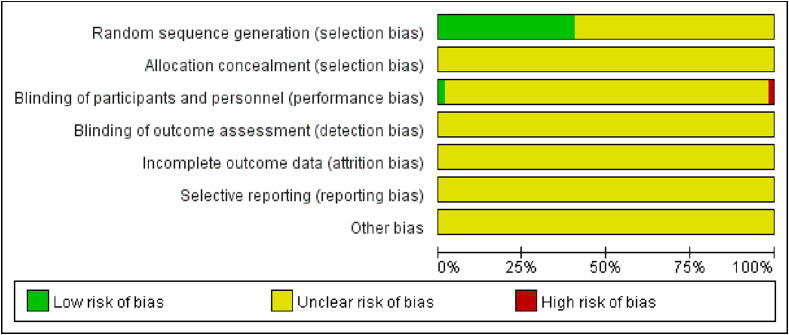
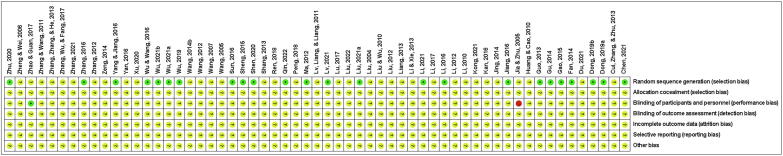
3.2. Risk of bias
The risk of bias for the RCTs was shown in Fig. 2, Fig. 3. Thirty-five studies referred to “randomization into two groups”, and the risk of selection bias was unknown. Twenty-four studies referred to a “random number table”, which was assessed as low-risk. One study was double-blinded; thus, it was considered to have a low risk of performance bias. One study that used a single-blind was evaluated as high-risk. Fifty-seven studies did not specify whether the investigators and participants were double-blinded, and 59 studies did not specify whether the outcomes were assessed in a blinded manner, whether selective reporting of results was avoided, or whether additional issues were avoided. A quality assessment table for the cohort studies was provided in Table 2. The scores in these two studies were 7 (Ren, 2020, Wu, 2018). The mean score for the eight studies was 6 (Diao, 2016, Du and Song, 2019, Qu, 2017, Song et al., 2019, Shao, 2013, Shi and Xu, 2013, Wang, 2017, Zhang, 2022;). The score in one study was 5 (Xu, 2018). Seven studies did not describe the duration or adequacy of follow-up.
Fig. 2.
Risk of bias.
Fig. 3.
Summary of risk of bias.
Table 2.
A quality assessment table for cohort studies.
| Studies | Selection |
Comparability |
Outcome |
Scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Outcome of interest was not present at start of study | Comparability of cohorts on basis of design or analysis controlled for confounders | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | ||
| Diao, 2016 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Du & Song, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Qu, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Ren, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | / | 7 |
| Song, Li, & Lu, 2019 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Wang, 2017 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Wu, 2018 | 1 | 1 | 1 | 1 | 2 | 1 | / | / | 7 |
| Zhang, 2022 | 1 | 1 | 1 | 1 | 1 | 1 | / | / | 6 |
| Shao, 2013 | / | 1 | 1 | 1 | 2 | 1 | / | / | 6 |
| Shi & Xu, 2013 | / | 1 | 1 | 1 | 2 | 1 | / | / | 6 |
| Xu, 2018 | / | 1 | 1 | 1 | 1 | 1 | / | / | 5 |
3.3. Primary outcomes
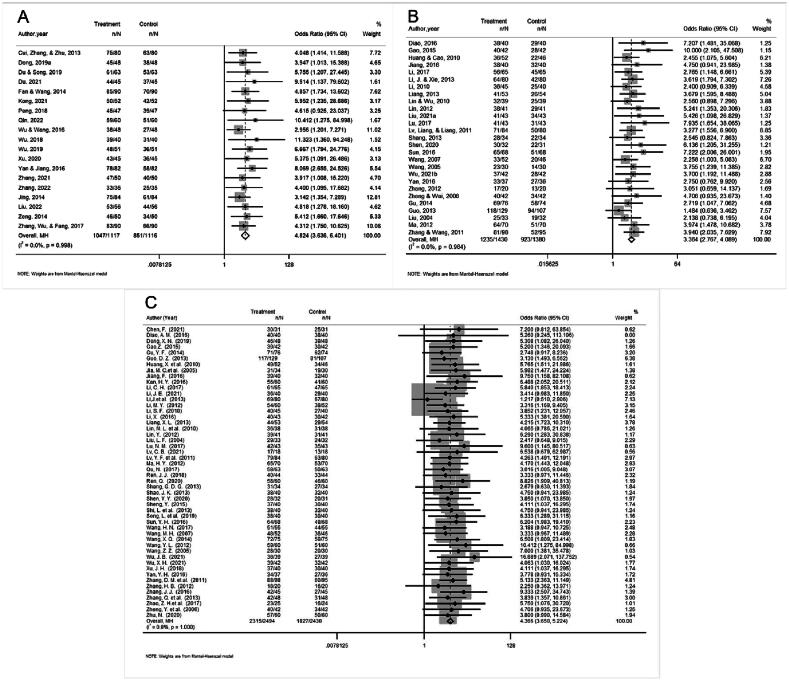
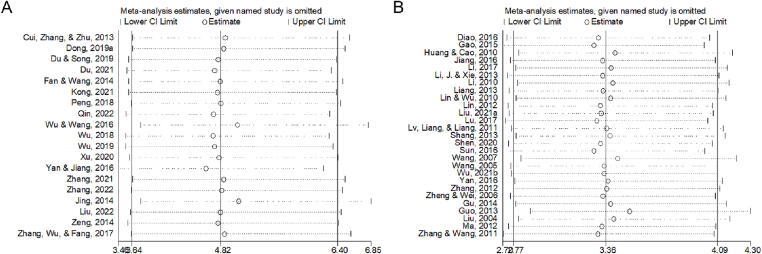
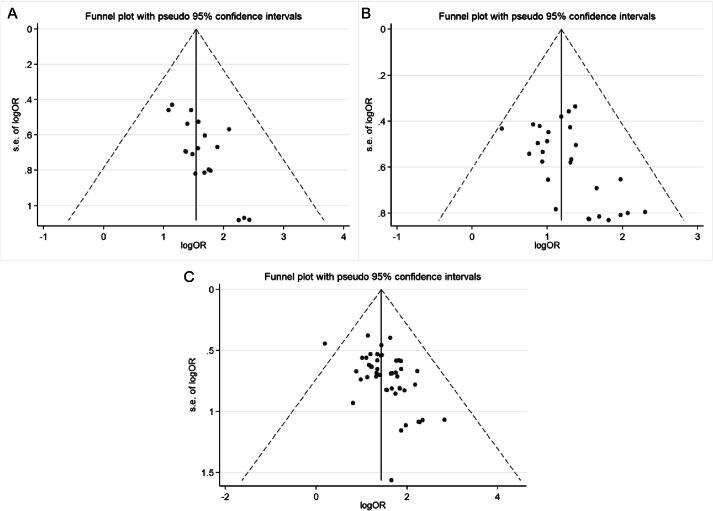
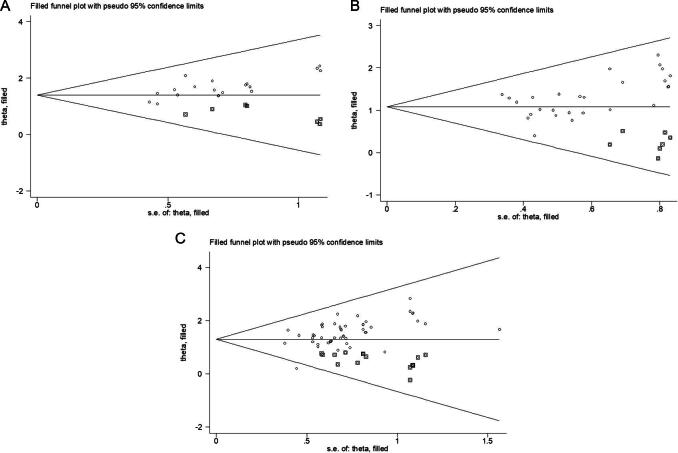
Nineteen studies reported the efficacy of angina symptom combined with ECG. The efficacy of angina symptom combined with ECG was assessed in 2 233 patients. The curative effect on angina symptom combined with ECG in 1 117 patients in the treatment group was better than that in 1 116 patients in the control group. (OR = 4.824, 95% CI = 3.636–6.401, P = 0.000, Fig. 4A). The heterogeneity test result was low (I2 = 0.0%, P = 0.998), and a fixed-effect model was selected. Sensitivity analysis showed that the combined results were stable after excluding each study (Fig. 5A). The funnel plot (Fig. 6A) and Egger’s test (t = 4.08, P = 0.001) showed significant publication bias. The trim-and-filling method was used to analyze the stability of the combined results of the effect indices. After five iterations with the linear method, the number of missing studies in software evaluation was 7. Finally, data from seven virtual studies were included. All the studies were re-analyzed. Compared with the results before trim (OR = 1.542, 95% CI = 1.257–1.828, P = 0.000), the results after filling showed that the curative effect of the treatment group was better than that of the control group (OR = 4.041, 95% CI = 3.125–5.226, P = 0.000). It further indicated that the results of the analysis were stable, as shown in Fig. 7A.
Fig. 4.
Forest plots of angina symptom and ECG efficacy (A), ECG efficacy (B) and angina symptom efficacy (C).
Fig. 5.
Sensitivity analysis of angina symptom and ECG efficacy (A) and ECG efficacy (B).
Fig. 6.
Funnel plots of angina symptom and ECG efficacy (A), ECG efficacy (B) and angina symptom efficacy (C).
Fig. 7.
Trim-and-filling method of angina symptom and ECG efficacy (A), ECG efficacy (B) and angina symptom efficacy (C).
The efficacy of the ECG was reported in 27 studies. The efficacy of ECG was assessed in 2 810 patients; 1 430 patients in the treatment group exhibited better ECG efficacy than 1 380 patients in the control group in the treatment of angina pectoris (OR = 3.364, 95% CI = 2.767–4.089, P = 0.000, Fig. 4B). The heterogeneity test result was low (I2 = 0.0%, P = 0.964), and a fixed-effect model was selected. Sensitivity analysis showed that the final results were significantly different after excluding each study (Fig. 5B). The funnel plot (Fig. 6B) and Egger’s test (t = 2.88, P = 0.008) showed that there was significant publication bias. The trim-and-filling method was used to analyze the stability of the combined results of the effect indices. After five iterations of the linear method, the number of missing studies in software was seven. Finally, data from seven virtual studies were included. All the studies were re-analyzed. Compared with the results before trim (OR = 1.187, 95% CI = 0.989–1.385, P = 0.000), The results after filling showed that the curative effect of the treatment group was better than that of the control group (OR = 2.955, 95% CI = 2.453–3.562, P = 0.000). It further indicated that the results of the analysis are stable, as shown in Fig. 7B.
Fifty studies had reported the efficacy of angina symptom. The treatment efficacy of angina symptom was assessed in 4 926 patients. The curative effect on AP in 2 495 patients in the treatment group was better than that in 2 431 patients in the control group (OR = 4.347, 95% CI = 3.635–5.198, P = 0.000, Fig. 4C). The heterogeneity test result was low (I2 = 0.0%, P = 1.000), and a fixed-effect model was selected. Sensitivity analysis revealed that the final results were significantly different after excluding each study (Table 3). The funnel plot (Fig. 6C) and Egger’s test (t = 3.73, P = 0.001) showed that there was significant publication bias. The trim-and-filling method was used to analyze the stability of the combined results of the effect indices. After six iterations with the linear method, the number of missing studies in software was 16. Finally, data from 16 virtual studies were included. All the studies were re-analyzed. Compared with the results before trim (OR = 1.434, 95% CI = 1.253–1.616, P = 0.000), the results after filling showed that the curative effect of the treatment group was better than that of the control group (OR = 3.639, 95% CI = 3.084–4.294, P = 0.000). It further indicated that the results of the analysis were stable, as shown in Fig. 7C.
Table 3.
Sensitivity analysis for AP efficacy.
| Study omitted | Estimate | 95% CI |
|---|---|---|
| Chen, 2021 | 4.3 291 306 | 3.6 177 325–5.1 804 199 |
| Diao, 2016 | 4.3 315 573 | 3.6 218 257–5.1 803 679 |
| Dong, 2019b | 4.3 347 831 | 3.6 205 833–5.1 898 661 |
| Gao, 2015 | 4.3 326 454 | 3.617 193–5.1 896 095 |
| Huang & Cao, 2010 | 4.3 238 649 | 3.6 097 152–5.1 793 027 |
| Jia & Zhu, 2005 | 4.3 242 154 | 3.6 105 504–5.1 789 446 |
| Jiang, 2016 | 4.3 134 732 | 3.6 043 501–5.1 621 103 |
| Kan, 2016 | 4.3 009 396 | 3.5 883 563–5.1 550 298 |
| Li, 2017 | 4.3 129 444 | 3.5 984 087–5.1 693 654 |
| Li & Xie, 2013 | 4.5 858 517 | 3.8 157 866–5.5 113 244 |
| Li, 2010 | 4.3 593 626 | 3.6 371 241–5.225 019 |
| Liang, 2013 | 4.3 52 119 | 3.6 259 079–5.2 237 778 |
| Lin & Wu, 2010 | 4.3 703 308 | 3.64 908–5.2 341 385 |
| Lin, 2012 | 4.3 240 647 | 3.6 115 539–5.1 771 441 |
| Lu, 2017 | 4.3 138 318 | 3.60 465–5.1 625 381 |
| Lv, 2021 | 4.3 347 011 | 3.6 225 963–5.1 867 862 |
| Lv, Liang, & Liang, 2011 | 4.3 494 964 | 3.6 274 302–5.2 152 944 |
| Qu, 2017 | 4.3 888 903 | 3.6 609 557–5.2 615 657 |
| Ren, 2018 | 4.370 914 | 3.6 479 061–5.2372 212 |
| Ren, 2020 | 4.2 934 527 | 3.5 853 322–5.1 414 309 |
| Shang, 2013 | 4.3 781 123 | 3.6 557 534–5.2 432 065 |
| Shen, 2020 | 4.3 568 621 | 3.6 367 407–5.2 195 768 |
| Song, Li, & Lu, 2019 | 4.3 237 486 | 3.611 299–5.176 753 |
| Sun, 2016 | 4.3 048 124 | 3.5 914 569–5.1 598 582 |
| Wang, 2017 | 4.3 76 235 | 3.6 520 975–5.2 439 542 |
| Wang, 2007 | 4.3 704 138 | 3.6 475 167–5.2 365 813 |
| Wang, 2012 | 4.3 070 087 | 3.5 988 126–5.1 545 677 |
| Wang, 2005 | 4.3 194 404 | 3.6 078 334–5.1 714 044 |
| Wu, 2021b | 4.3 520 474 | 3.6 335 893–5.2 125 645 |
| Yan, 2016 | 4.3 568 449 | 3.6 378 064–5.2 180 071 |
| Zhang, 2012 | 4.3 731 022 | 3.6 534 278–5.2 345 424 |
| Zhao & Guan, 2017 | 4.3 327 031 | 3.6 192 646–5.1 867 762 |
| Zheng & Wei, 2006 | 4.342 423 | 3.6 271 231–5.1 987 867 |
| Zhu, 2020 | 4.3 576 851 | 3.638 072–5.2 196 388 |
| Gu, 2014 | 4.399 487 | 3.6 696 892–5.2 744 212 |
| Guo, 2013 | 4.429 368 | 3.6 831 563–5.3 267 636 |
| Li, 2021 | 4.3 682 599 | 3.6 458 206–5.2 338 548 |
| Li, 2012 | 4.3 803 163 | 3.6 528 108–5.2 527 142 |
| Li, 2016 | 4.3 306 074 | 3.6 154 773–5.1 871 877 |
| Liu, 2004 | 4.3 918 653 | 3.6 659 727–5.2 614 903 |
| Ma, 2012 | 4.3 522 701 | 3.6 299 331–5.218 348 |
| Shao, 2013 | 4.3 419 271 | 3.626 725–5.1 981 688 |
| Sheng, 2015 | 4.3 511 095 | 3.6 328 459–5.2 113 843 |
| Shi & Xu, 2013 | 4.3 419 271 | 3.626 725–5.1 981 688 |
| Wang, 2014b | 4.3 069 582 | 3.5 949 044–5.1 600 509 |
| Wu, 2021a | 4.2 797 556 | 3.5 754 974–5.1 227 303 |
| Xu, 2018 | 4.3 511 095 | 3.6 328 459–5.2 113 843 |
| Zhang & Wang, 2011 | 4.3 074 231 | 3.5 841 024–5.1 767 201 |
| Zhang, 2016 | 4.2 768 908 | 3.5 698 328–5.1 239 915 |
| Zhang, Zhang, & He, 2013 | 4.3 625 793 | 3.6 380 978–5.2 313 328 |
| Combined | 4.3 469 529 | 3.6 349 295–5.1 984 501 |
3.4. Secondary outcomes
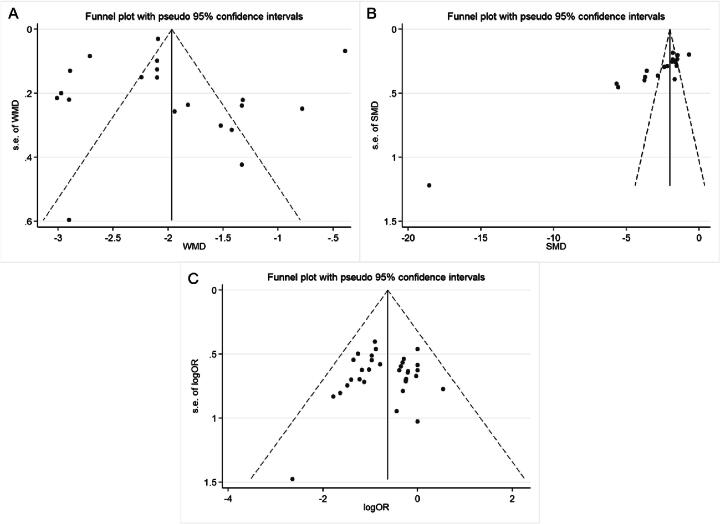
Seven studies had reported the determination of TG levels. In total, 720 patients were enrolled, half of whom belonged to the experimental group. The results showed that CDDP combined with ISMN for the treatment of AP was superior to ISMN alone in reducing TG levels (MD = −35.176, 95% CI = −37.439 to −32.912, P = 0.000, Fig. 8A). The heterogeneity was low (I2 = 0.0%, P = 0.567) and a fixed-effect model was chosen. Sensitivity analysis showed that the final combined results were significantly different after excluding each study (Fig. 9A). Egger’s test (t = −0.37, P = 0.726) showed no significant publication bias.
Fig. 8.
Forest plots of TG levels (A), TC levels (B), duration of angina attack (min) (C), frequency of angina attack (in 1 d or 1 week) (D) and ADRs (E).
Fig. 9.
Sensitivity analysis of TG levels (A), TC levels (B), duration of angina attack (min) (C), frequency of angina attack (in 1 d or 1 week) (D) and ADRs (E).
Seven studies reported TC levels. In total, 720 patients were enrolled, half of whom belonged to the experimental group. The results showed that CDDP combined with ISMN for the treatment of AP was superior to ISMN alone in reducing TC levels (MD = −24.296, 95% CI = −26.429 to −22.163, P = 0.000, Fig. 8B). The heterogeneity was high (I2 = 64.3%, P = 0.010) and a random-effect model was chosen. Sensitivity analysis showed that the study by Liu et al was the source of heterogeneity (MD = −23.268, 95% CI = −24.582 to −21.954, P = 0.000, I2 = 0.0%). The combined results after excluding this study were consistent with the original results (Fig. 9B). No differences were found in sample size, patient age, sex, evaluation criteria, and outcome indicators. Therefore, the relevant literature (Liu, 2021a) was not excluded. Egger’s test (t = −2.35, P = 0.065) showed no significant publication bias.
Twenty studies reported the duration of an angina attack (min). A total of 1 813 patients were enrolled, with 909 patients in the experimental group. The results showed that CDDP combined with ISMN for the treatment of AP was superior to ISMN alone in reducing the duration of angina attack (MD = −1.991, 95% CI = −2.349 to −1.633, P = 0.000, Fig. 8C). The funnel plot (Fig. 10A) and Egger’s test (t = 0.09, P = 0.930) showed no significant publication bias. The heterogeneity was high (I2 = 97.6%, P = 0.000) and a random-effect model was chosen. To examine which factors influenced the heterogeneity, we conducted subgroup analysis according to the disease type (AP or UAP), different drug dosages (≤ 40 mg or > 40 mg), different courses of treatment (≤ 4 weeks or > 4 weeks), and whether conventional treatment was used or not (Fig. S1). The results of the subgroup analysis showed that these factors did not cause the high heterogeneity. Additional sensitivity analyses were also performed. The results showed that the result was consistent with the original results after excluding individual studies (Fig. 9C).
Fig. 10.
Funnel plots of duration of angina attack (min) (A), frequency of angina attack (in 1 d or 1 week) (B) and ADRs (C).
Twenty-one studies reported the frequency of angina attack (1 day or 1 week). A total of 1 921 patients were enrolled, with 961 patients in the experimental group. The results showed that CDDP combined with ISMN for the treatment of AP was superior to ISMN alone in reducing the frequency of angina attack (SMD = −2.840, 95 % CI = −3.416 to −2.265, P = 0.000, Fig. 8D). The funnel plot (Fig. 10B) and Egger’s test (t = −8.30, P = 0.000) indicated significant publication bias. The trim-and-filling method was used to analyze the stability of the combined results of the effect indices. The results showed that there was no need to fill in the virtual study, as consistent with the original merge results (Fig. 11). A subgroup analysis was conducted to explore the factors influencing the heterogeneity. The subgroup analysis showed that different disease types, different drug dosages (≤ 40 mg or > 40 mg), different courses of treatment (≤ 4 weeks or > 4 weeks), and different outcome evaluation criteria (1 d or 1 week) were not the sources of heterogeneity (Fig. S2). Sensitivity analysis confirmed that the combined results were stable (Fig. 9D).
Fig. 11.
Trim-and-filling method of frequency of angina attack (in 1 d or 1 week).
Thirty-six studies reported ADRs. A total of 3 855 patients were enrolled. Compared to ISMN alone, the results showed no increase in ADRs with CDDP combined with ISMN (OR = 0.513, 95% CI = 0.421–0.626, P = 0.000, Fig. 8E). The combination drug was well-tolerated by the patients. The heterogeneity was low (I2 = 0.0%, P = 0.807), and a fixed effect model was chosen. Sensitivity analysis showed that the results were stable after excluding the studies one by one (Fig. 9E). The funnel plot (Fig. 10C) and Egger’s test (t = −0.21, P = 0.832) showed that there was no significant publication bias. ADRs in the experimental and control groups were mainly caused by nervous system damage (headache and dizziness), circulatory system damage (facial flushing), and gastrointestinal system damage (nausea and gastrointestinal discomfort) (Table 4). No serious ADRs were observed.
Table 4.
Comparison of adverse drug reactions between treatment group and control group.
| Systems and organs involved | Clinical manifestations (frequency) |
|
|---|---|---|
| Control group | Treatment group | |
| Central and peripheral nervous system disorders | Headache (115), dizziness (99), vertigo (7), headache or dizziness (5), syncope (1). | Headache (62), dizziness (52), vertigo (3), tongue paralysis (3). |
| Cardiovascular system damage | Facial flushing (88), palpitation (8), hypotension (1). | Facial flushing (52), palpitation (1), hypotension (2). |
| Gastro-intestinal system disorders | Nausea (33), gastrointestinal discomfort (26), vomiting (10), loss of appetite (4), constipation (2). | Gastrointestinal discomfort (28), nausea (6), vomiting (1), loss of appetite (1). |
| Body as a whole-general disorder | Fatigue (18), shortness of breath (2), chest distress (2), chest pain (1), collapse (1). | Fatigue (10), shortness of breath (1), chest pain (1). |
| Others | No detailed clinical presentation (11). | No detailed clinical presentation (7). |
| Skin and appendages disorders | Rash (1). | |
| Total | 435 | 230 |
3.5. Subgroup analysis
Our study included 59 RCTs and 11 cohort studies. Seventy studies were analyzed in subgroups according to the study type (RCTs or cohort studies). Both the TC and TG levels were included in the RCT analysis. Therefore, we did not perform subgroup analyses. Subgroup analyses were performed for other outcomes (Primary outcomes and Secondary outcomes). All subgroup analysis results were consistent with those of the main analyses. (Fig. S3).
4. Discussion
AP is an episode of myocardial ischemia caused by an imbalance between myocardial oxygen supply and demand (Manfredi et al., 2022). Myocardial oxygen is delivered through the coronary arteries. The coronary artery is divided into several small arteries. When myocardial oxygen consumption increases, the arterioles dilate in response to nitric oxide, prostaglandins, carbon dioxide, hydrogen ions, adenosine, and other nucleotides. With this modulation, blood flow to the normal heart muscle can be increased four- to five-fold. Coronary artery stenosis, which leads to insufficient blood supply to the myocardium and can cause myocardial ischemia or angina (Jain et al., 2017). Organic nitrates are one of the most commonly used drugs for the treatment of AP. Organic nitrates release nitric acid via cellular metabolism. Nitric oxide activates guanylate cyclase, leading to the conversion of guanosine triphosphate to cyclic guanosine monophosphate, which causes vasodilation (Parker & Parker, 1998). Organic nitrates can relax the veins and arteries, reduce the pre- and post-load on the heart, and reduce oxygen consumption in the heart muscle. Short-acting organic nitrates can also be used to treat acute AP. Long-acting organic nitrates are used for the long-term prophylactic treatment of AP (Chinese Society of Cardiovascular Diseases & Chinese Medical Association, 2007). Most organic nitrates are tolerant of nitrate. ISMN does not induce vascular tolerance and can lead to endothelial dysfunction and increase cardiovascular risk (Münzel, Steven, & Daiber, 2014). Therefore, the development of new treatment options is essential.
Previous studies have confirmed the positive effects of ISMN in combination with TCMs on angina pectoris (Jia et al., 2020). Thus, the combination of TCM and Western medicine provides a new approach for the treatment of angina. CDDP is widely used to treat AP in patients with CHD. CDDP consists of Salviae Miltiorrhizae Radix et Rhizoma, Notoginseng Radix et Rhizoma, and Borneolum Syntheticum. Salviae Miltiorrhizae Radix et Rhizoma, the main prescribed drug, has antimyocardial ischemic effects, increasing cardiac output and coronary blood volume. Salviae Miltiorrhizae Radix et Rhizoma is more effective at dilating the coronary arteries than Notoginseng Radix et Rhizoma. However, Notoginseng Radix et Rhizoma has a stronger protective effect on heart muscle than Salviae Miltiorrhizae Radix et Rhizom. Their combination has both synergistic and complementary effects (Zhang et al., 2003). Network pharmacology studies have shown that CDDP has 65 core targets and 61 active components for the treatment of AP. Quercetin, luteolin, salvianone, and asiatic acid are key chemical components of CDDP used in the treatment of AP. These components regulate lipoproteins and atherosclerosis, as well as the tumor necrosis factor signaling pathway, HIF-1 signaling pathway, and PI3K-Akt signaling pathway through related targets. They play a role in anti-inflammation, the regulation of oxidative stress and lipid metabolism disorders, and the repair of vascular damage. CDDP contains different active ingredients that act on the same target, indicating that CDDP has a multi-component and multi-target mechanism of action in the treatment of AP (Ai, 2022, Li et al., 2023). In summary, the mechanism of CDDP in the treatment of AP is different from that of ISMN, which provides an essential theoretical basis for the treatment of CHD with TCM. Chinese guidelines recommend CDDP during an episode of AP (Society of Cardiovascular Disease and Chinese Association of Traditional Chinese Medicine, 2019); however, there is little evidence supporting the use of CDDP combined with ISMN for the treatment of AP.
The ECG of patients with AP may show ST-segment depression or T-wave inversion. The Chinese criteria for efficacy assessment are angina symptom and ECG efficacy (Wang, 2005, Chinese Society of Cardiovascular Diseases and Chinese Medical Association, 2007). ECG combined with clinical symptom features can improve the accuracy of angina diagnosis and evaluation (Liu, 2021b). Dyslipidemia is a risk factor for atherosclerotic cardiovascular disease (ASCVD) and CHD, and low-density lipoprotein cholesterol (LDL-C) are pathogenic risk factors for ASCVD (Wang et al., 2023b, Xu et al., 2023). The monitoring and management of blood lipids are essential for assessing and preventing ASCVD and CHD. Therefore, TG and TC levels were used as secondary outcomes of angina in our study. Compared with previous Meta-analyses (Xue et al., 2013, Zhang et al., 2018), our study included not only angina symptom and ECG efficacy but also lipid levels, angina attack frequency, and angina attack duration. Thus, our study was more comprehensive than previous studies.
This systematic review included 7 245 angina patients from 70 studies (59 RCTs, 11 cohort studies). The results showed that CDDP combined with ISMN was superior to ISMN alone in treating AP symptoms and improving ECG findings. The combined medication was also beneficial for lowering TG and TC levels. In terms of reducing the frequency and duration of angina attack, drug combinations were more effective than single drugs. For example, a Meta-analysis showed that the clinical efficacy of CDDP plus aspirin was superior to that of aspirin alone in the treatment of AP (Wang et al., 2021). Moreover, a study of CDDP combined with trimetazidine indicated that this treatment could significantly improve clinical efficacy in patients with AP (Zhu & Deng, 2019). Together, these findings suggest that the combination of TCM and Western medicine is beneficial for the treatment of AP. TCM can also be used as a vital supplement to Western medicine for the treatment of cardiovascular diseases.
In terms of safety, there was no significant difference in the ADRs between CDDP combined with ISMN and ISMN alone for AP. The main ADRs were headaches, dizziness, facial flushing, gastrointestinal reactions, palpitations, and other common reactions. Headache and dizziness may also be associated with ISMN. Headache rates decreased with treatment duration (Boettcher et al., 2022). No serious ADRs were observed in the trial group, suggesting there was superior tolerability of AP in patients treated with ISMN in combination with CDDP.
CDDP combined with ISMN was superior to ISMN alone for the treatment of AP, with better efficacy for angina combined with ECG, angina alone, and ECG alone. However, all three evaluated metrics exhibited significant publication bias. The trim-and-filling method showed that the combined results of the three effect indicators did not change, indicating that the results were robust. The stability of the results was confirmed by sensitivity analysis. Meanwhile, quality control in data retrieval and extraction and selection of evaluation criteria were also performed in this study, although there was still publication bias. Although the common databases of the retrieved literature contained both Chinese and English, the search results showed that CDDP mainly focused on China, which could lead to regional publication bias. All studies had positive and no negative results, which could have resulted in publication bias. The low overall quality of the included studies was also responsible for the publication bias.
CDDP combined with ISMN was superior to ISMN alone in reducing the duration and frequency of angina attack. However, the combined results for both metrics showed high heterogeneity. Subgroup analysis was carried out according to the different disease types (AP or UAP), different drug doses (≤ 40 mg or > 40 mg), different courses of treatment (≤ 4 weeks or > 4 weeks), and whether conventional treatment was performed. No source of heterogeneity was found in the subgroup analysis. Of the 70 included studies, 20 reported detailed data on the angina onset time and frequency, with a low data reporting rate. These metrics should be used with caution in combination with specific clinical references.
This study has some limitations. First, the overall quality of the original studies was low. There was a significant publication bias in the primary outcome measures. Significant heterogeneity was observed in the secondary outcome measures. No source of heterogeneity was found based on the available data from the various institutes. The Cochrane Library database was screened simultaneously. Most Chinese literature was not registered, and the experimental design was not published, which reduced the quality of the experiment. Researchers should use standardized test designs to improve the quality of the literature and data reliability (Shi et al., 2015). Secondly, there were insufficient observations of ADRs in the included studies. Chinese patent medicines may cause some known adverse reactions; however, they have not attracted sufficient general attention. The observation and evaluation of ADRs in TCMs require further exploration (Jiang, Xu, & Sun, 2018).
5. Conclusion
CDDP combined with ISMN was superior to ISMN alone in the treatment of AP, with patients showing good tolerance. This combination of TCM and Western medicine can be used as an alternative treatment option for patients. Although the results were positive, publication bias and heterogeneity need to be considered. The results of this study suggested that clinical therapy should be tailored based on the clinical presentation of patients; however, caution should be taken. In future studies, rigorous clinical trial design processes should be considered to improve the quality of studies. More scholars will pay attention to the clinical treatment of TCM to provide additional evidence for TCM diagnosis and treatment.
CRediT authorship contribution statement
Ru Wang: Conceptualization, Validation, Methodology, Formal analysis, Data curation, Resources, Writing – original draft, Writing – review & editing. Jing Hu: Methodology, Formal analysis, Data curation. Yuanyuan Li: Conceptualization, Validation, Methodology, Formal analysis, Writing – review & editing. Hong Yin: Conceptualization, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2023.12.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ai, D. (2022). Visualization analysis and network pharmacology mechanism of traditional Chinese medicine in the prevention and treatment of stable angina pectoris of coronary heart disease. Liaoning University of Traditional Chinese Medicine. Thesis of Master Degree.
- Andrade C. Understanding the basics of meta-analysis and how to read a forest plot: A simple as it gets. The Journal of Cinical Pychiatry. 2020;81(5):21858. doi: 10.4088/JCP.20f13698. [DOI] [PubMed] [Google Scholar]
- Boettcher M., Mikus G., Trenk D., Düngen H.D., Donath F., Werner N.…Becker C. Vericiguat in combination with isosorbide mononitrate in patients with chronic coronary syndromes: The randomized, phase Ib, VISOR study. Clinical and Translational Science. 2022;15(5):1204–1214. doi: 10.1111/cts.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. Effect of Danshen Dripping Pills in combination with isosorbide mononitrate on angina pectoris in coronary heart disease. World Latest Medicine Information (Electronic Version) 2021;21(101):593–594. [Google Scholar]
- Cheng B., Li X.Y., Liu K.Q., Wang L., Wu W.P., Xu H. Chinese expert advice on the clinical application of Compound Danshen Dropping Pills. Chinese Journal of Integrated Traditional and Western Medicine. 2017;37(1):17–22. [Google Scholar]
- Chinese Society of Cardiology. (2010). The expert consensus of standardization of nitrate application in cardiovascular disease. Chinese Journal of Cardiovascular Research, 8(11), 801–807.
- Chinese Society of Cardiovascular Diseases and Chinese Medical Association. (2007). Guidelines for the diagnosis and treatment of chronic stable angina pectoris. Chinese Journal of Cardiology, 35(3), 195–206.
- Cui Y.F., Zhang W., Zhu Z.Y. Clinical observation on the treatment of coronary heart disease angina pectoris with Chinese and Western medicine. China Health and Nutrition. 2013;7(23):3463. [Google Scholar]
- Diao A.M. Discussion and analysis of the curative effect of Compound Danshen Dripping Pills on coronary heart disease. China Health Care & Nutrition. 2016;26(3):351–352. [Google Scholar]
- Dong S.J. Efficacy and safety analysis of Compound Danshen Dripping Pills combined with isosorbide mononitrate in the treatment of angina pectoris of coronary heart disease. Journal of Practical Chinese Medicine Internal Medicine. 2019;33(12):8–10. [Google Scholar]
- Dong X.N. Effect of Compound Danshen Dropping Pills in the treatment of coronary heart disease and angina pectoris. China Health Care & Nutrition. 2019;29(23):298. [Google Scholar]
- Du Y.K., Song F.L. Clinical value of isosorbide mononitrate combined with Compound Danshen Dropping Pills in treating angina pectoris. Clinical Medicine Research and Practice. 2019;4(21):106–108. [Google Scholar]
- Du Y.T. Clinical effect of Danshen Dripping Pills in combination with isosorbide mononitrate in the treatment of stable angina pectoris. World Latest Medicine Information (Electronic Version) 2021;21(101):265–266. [Google Scholar]
- Fan Q.C., Wang K. Efficacy analysis of isosorbide mononitrate combined with Compound Danshen Dripping Pills in the treatment of coronary heart disease. Medicine and Health Care. 2014;22(7):87. [Google Scholar]
- Fihn S.D., Gardin J.M., Abrams J., Berra K., Blankenship J.C., Dallas A.P.…Anderson J.L. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- Gao Z. Clinical study on angina pectoris of coronary heart disease treated by Compound Danshen Dripping Pills combined with isosorbide mononitrate. Modern Medicine Application in China. 2015;9(5):100–101. [Google Scholar]
- Gu Y.F. Treatment of unstable angina pectoris with Compound Danshen Dripping Pills combined with isosorbide mononitrate. Medical Information. 2014;27(8):520–521. [Google Scholar]
- Guo D.Z. Effect analysis of compound Danshen Dripping Pills and isosorbide mononitrate injection in treating unstable angina pectoris. Modern Chinese Doctors. 2013;51(4):66–68. [Google Scholar]
- Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D.…Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. British Medical Journal/British Medical Association. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Cao C.M. Report of 52 cases of angina pectoris of coronary heart disease treated by isosorbide mononitrate combined with Compound Danshen Dripping Pills. Aerospace Medicine. 2010;21(3):349. [Google Scholar]
- Jain A., Elgendy I.Y., Al-Ani M., Agarwal N., Pepine C.J. Advancements in pharmacotherapy for angina. Expert Opinion on Pharmacotherapy. 2017;18(5):457–469. doi: 10.1080/14656566.2017.1303483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H.J. Clinical observation of Chinese patent medicine Compound Danshen Dripping Pills in treating 200 cases of angina pectoris of coronary heart disease. Heilongjiang Medicine Journal. 2013;26(6):1049–1050. [Google Scholar]
- Jia M.C., Zhu W. Treating 34 cases of angina pectoris of coronary heart disease with Compound Danshen Dripping Pills and Western medicine. Shanxi Traditional Chinese Medicine. 2005;26(7):630–631. [Google Scholar]
- Jia X., Jia W.R., Nie A.Z., Wang J. Research progress in the treatmen to coronary heart disease and angina pectoris with integrated traditional Chinese and Western medicine. Asia-Pacific Traditional Medicine. 2020;16(2):190–195. [Google Scholar]
- Jiang F. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate on angina pectoris of coronary heart disease. The Channel of Pharmaceutical. 2016;28(8):123–124. [Google Scholar]
- Jiang N., Xu T.T., Sun S.S. Discussion on adverse reactions of Chinese patent medicine for activating blood circulation and removing blood stasis in clinical application. The Northern Pharmaceutical. 2018;15(6):151. [Google Scholar]
- Jing H.Y. Clinical analysis of isosorbide mononitrate combined with Compound Danshen Dripping Pills in treating senile unstable angina pectoris. Medical Frontier. 2014;18:386–387. [Google Scholar]
- Kan H.Y. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate on angina pectoris patients with coronary heart disease. Modern Medicine Application in China. 2016;10(3):169–170. [Google Scholar]
- Kong L. Application value of Compound Danshen Dropping Pills in the treatment of angina pectoris in coronary heart disease. Great Health. 2021;29:137–139. [Google Scholar]
- Li C.H. Clinical analysis of Compound Danshen Dripping Pills combined with isosorbide mononitrate in the treatment of angina pectoris of coronary heart disease. Journal of Practical Chinese Medicine. 2017;33(8):942–943. [Google Scholar]
- Li J.E. Clinical study on Compound Danshen Dripping Pills combined with isosorbide mononitrate for unstable angina pectoris of coronary heart disease. New Chinese Medicine. 2021;53(20):45–49. [Google Scholar]
- Li J., Xie J. Clinical observation on the curative effect of Compound Danshen Dripping Pills on angina pectoris of coronary heart disease. Contemporary Chinese Medicine. 2013;20(4):120–121. [Google Scholar]
- Li M.L., Xu T., Li Q., Deng Y.J., Li P.F. Mechanism of Compound Danshen Dropping Pills in the treatment of coronary artery disease based on network pharmacology. Chinese Journal of Medicinal Guide. 2023;25(2):156–162. [Google Scholar]
- Li M.Y. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate injection on unstable angina pectoris. Chinese Practical Medicine. 2012;7(16):190. [Google Scholar]
- Li S.F. 45 cases of angina pectoris of coronary heart disease treated by Compound Danshen Dripping Pills. The Light of Traditional Chinese Medicine. 2010;25(8):1435–1436. [Google Scholar]
- Li X. Effect of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Journal of Clinical Rational Drug Use. 2016;9(31):54–55. [Google Scholar]
- Liang X.L. A comprehensive evaluation of Compound Danshen Dripping Pills combined with isosorbide mononitrate sustained-release tablets in the treatment of angina pectoris of coronary heart disease. World Traditional Chinese Medicine. 2013;8(10):1169–1171. [Google Scholar]
- Lin M.L., Wu J.Y. Clinical analysis of Compound Danshen Dropping Pills in the treatment of 78 cases of senile coronary artery disease angina. China Medical Herald. 2010;7(7):52–53. [Google Scholar]
- Lin X.D., Tang J.M., Yang J.Y., Zhang L., Cao T., Jiang F.B.…Wang J.N. Tongxinluo combined with betaloc in treating coronary heart disease patients with angina: A meta-analysis. Chinese Journal of Clinical Healthcare. 2017;20(2):166–169. [Google Scholar]
- Lin Y. Clinical effect of Compound Danshen Dripping Pills on coronary heart disease. Journal of Practical Cardio-cerebral Pulmonary Vascular Diseases. 2012;20(11):1764–1765. [Google Scholar]
- Liu L.F. Treatment of unstable angina pectoris with Compound Danshen Dripping Pills combined with Lunan Xinkang. Sichuan Medical. 2004;25(4):419–420. [Google Scholar]
- Liu L.Z. Clinical observation of Compound Danshen Dripping Pills combined with isosorbide mononitrate in treating angina pectoris of coronary heart disease. Forum on Primary Medicine. 2021;25(5):642–644. [Google Scholar]
- Liu Q.Y. Clinical effect analysis on Compound Danshen Dripping Pills combined with isosorbide mononitrate in treatment of unstable angina pectoris. Chinese Community Doctors. 2022;38(3):73–75. [Google Scholar]
- Liu Y. Value analysis of electrocardiogram combined with clinical features in the diagnosis of angina pectoris of coronary heart disease. China Practical Medical. 2021;16(2):16–18. [Google Scholar]
- Liu Y.C., Tian Y., Guo D., Chen W.Q., Fan M.X., Zhao J.S. Fufang Danshen Dripping Pills in treatment of essential hypertension: A systematic review and meta-analysis. Chinese Traditional and Herbal Drugs. 2022;53(10):3111–3124. [Google Scholar]
- Luchini C., Veronese N., Nottegar A., Shin J.I., Gentile G., Granziol U.…Solmi M. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharmaceutical Statistics. 2021;20(1):185–195. doi: 10.1002/pst.2068. [DOI] [PubMed] [Google Scholar]
- Lu N.M. Comparative study on the efficacy of isosorbide mononitrate and Compound Danshen Dripping Pills in patients with coronary heart disease and angina pectoris. Electronic Journal of Cardiovascular Diseases of Integrated Traditional Chinese and Western Medicine. 2017;16(5):55–56. [Google Scholar]
- Lv C.B. Clinical value of isosorbide mononitrate in combination with Compound Danshen Dropping Pills in the treatment of angina pectoris in coronary heart disease. Kang Yi. 2021;15:221. [Google Scholar]
- Lv Y.F., Liang L.J., Liang D. Clinical analysis of Compound Danshen Dripping Pills combined with isosorbide mononitrate in treating 84 cases of angina pectoris of coronary heart disease. Chinese Manipulation & Rehabilitation Mediciqne. 2011;2(4):5–6. [Google Scholar]
- Ma, H. Y. (2012). Clinical analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Journal of Yangtze University Natural Science Edition (Medical Science), 9(11), 1−2+4+5.
- Ma W.J., Ma H.P., Wang Y.H., Wang J., Wang H.J., Wang S.Z.…Shi Y. 2021 Medical quality report of cardiovascular diseases in China: An executive summary. Chinese Circulation Journal. 2021;36(11):1041–1064. [Google Scholar]
- Manfredi R., Verdoia M., Compagnucci P., Barbarossa A., Stronati G., Casella M.…Ciliberti G. Angina in 2022: Current perspectives. Journal of Clinical Medicine. 2022;11(23):6891. doi: 10.3390/jcm11236891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.Y., Wu Y.J., Shi D.Z. Clinical application guidelines for the treatment of coronary heart disease with Chinese patent medicine (2020) Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease. 2021;19(9):1409–1435. [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed]
- Münzel T., Steven S., Daiber A. Organic nitrates: Update on mechanisms underlying vasodilation, tolerance and endothelial dysfunction. Vascul Pharmacol. 2014;63(3):105–113. doi: 10.1016/j.vph.2014.09.002. [DOI] [PubMed] [Google Scholar]
- National Center for Cardiovascular Disease. (2021). Annual report on cardiovascular health and diseases in China 2020. Journal of Cardiovascular and Pulmonary Diseases, 40(9), 885–889.
- National health and family planning commission expert committee on rational drug use. (2018). Guidelines for rational drug use in coronary heart disease (2nd Version). Chinese Journal of the Frontiers of Medical Science (Electronic Version), 10(6), 1–130.
- Parker J.D., Parker J.O. Nitrate therapy for stable angina pectoris. The New England Journal of Medicine. 1998;338(8):520–531. doi: 10.1056/NEJM199802193380807. [DOI] [PubMed] [Google Scholar]
- Peng W.H. Compound Danshen Dropping Pills combined with isosorbide mononitrate sustained-release tablets in the treatment of coronary heart disease observation of the effect of angina pectoris. Chinese Journal of Clinical Rational Drug Use. 2018;11(11):27–28. [Google Scholar]
- Qin R.Q. Clinical analysis of Compound Danshen Dripping Pills for treating community coronary heart disease. Chinese Community Doctors. 2022;38(15):99–101. [Google Scholar]
- Qu N. Application of Compound Danshen Dropping Pills and isosorbide mononitrate sustained-release tablets in the treatment of coronary heart disease and angina pectoris. Guide of China Medicine. 2017;15(8):199. [Google Scholar]
- Ren J.J. Analysis of curative effect and application advantage of integrated traditional Chinese and Western medicine in treating angina pectoris of coronary heart disease. World Latest Medicine Information (Electronic Version) 2018;18:182. [Google Scholar]
- Ren Q. Clinical efficacy and safety of combined traditional Chinese and Western medicine in the treatment of coronary heart disease and angina pectoris. Home Medicine. 2020;4:134. [Google Scholar]
- Shang G.D.G. Treatment of senile coronary heart disease with Compound Danshen Dropping Pills and isosorbide mononitrate tablets: Analysis of the clinical curative effect of angina pectoris. Chinese and Foreign Medical Research. 2013;11(21):48–49. [Google Scholar]
- Shao J.K. Clinical efficacy and safety analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Chinese Medical Guide. 2013;11(12):518. [Google Scholar]
- Shen Y.Y. Analysis of the curative effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate sustained-release tablets for angina pectoris of coronary heart disease. Capital Food and Medicine. 2020;27(9):69–70. [Google Scholar]
- Sheng Y. Observation on the curative effect of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Electronic Journal of Clinical Medicine literature. 2015;15(2):3117+3120. [Google Scholar]
- Shi E.L., Li Z.D., Sun J., Gou X.R., Wang J.C. Meta-analysis of the efficacy of aspirin alone and combined with Chinese patent medicine in treating coronary heart disease. Chinese Journal of Gerontology. 2015;35(5):1245–1249. [Google Scholar]
- Shi L., Xu H.J. Efficacy and safety analysis of isosorbide mononitrate combined with Compound Danshen in the treatment of senile unstable angina pectoris. China Medical Guide. 2013;15(10):1662–1663. [Google Scholar]
- Shi Z.C., Li F.Y., Wang H.F., Ding T. Effect and feasibility of combined application of Chinese patent medicine and aspirin in the treatment of chronic stable angina. Strait Pharmaceutical Journal. 2020;32(7):80–83. [Google Scholar]
- Society of Cardiovascular Disease and Chinese Association of Traditional Chinese Medicine. (2019). Traditional Chinese medicine guidelines for the diagnosis and treatment of stable angina pectoris in coronary heart disease. Journal of Traditional Chinese Medicine, 60(21), 1880–1890.
- Song L., Li H.L., Lu Y. Effect of Compound Danshen Dripping Pills and isosorbide mononitrate sustained-release tablets on angina pectoris of coronary heart disease. A Collection of Contemporary Medical Essays. 2019;17(13):209–210. [Google Scholar]
- Sun Y.H. Analysis of the curative effect of Compound Danshen Dripping Pills and isilodine in treating angina pectoris of coronary heart disease. Chinese Practical Medicine. 2016;11(27):192–193. [Google Scholar]
- Tobin K.J. Stable angina pectoris: What does the current clinical evidence tell us? The Journal of the American Osteopathic Association. 2010;110(7):364–370. [PubMed] [Google Scholar]
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P.…Tsao C.W. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Wang B. Fifty cases of angina pectoris of coronary heart disease were treated with Compound Danshen Dripping Pills. Chinese Medicine Modern Distance Education of China. 2014;12(11):130–131. [Google Scholar]
- Wang H.N. Treatment of angina pectoris patients with Compound Danshen Dropping Pills and nitrates. Journal of Frontiers of Medicine. 2017;7(35):319. [Google Scholar]
- Wang M.H. Clinical observation of Compound Danshen Dripping Pills and Lunan Xinkang combined treatment of angina pectoris of coronary heart disease. Chinese Journal of Medical Research. 2007;7(9):832–833. [Google Scholar]
- Wang Q., Gao L., Zhao B., Qu X.L., Wei P.F., Wang B., Li M. Systematic evaluation of Compound Danshen Dropping Pills combined with conventional drugs in treatment of diabetes mellitus complicated with asymptomatic myocardial ischemia. Drug Evaluation Research. 2023;46(5):1105–1115. [Google Scholar]
- Wang, R., Hu, J., Li, Y. Y., & Yin, H. (2022) Isosorbide mononitrate combined with Compound Danshen Dripping Pills in the treatment of coronary heart disease angina pectoris: A systematic review and Meta-analysis. Available at: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=314774, 2022.
- Wang T., Qu H.Y., Sha W.J., Lan Z.Z., Yang X.L., Liu W.R., Zhou H. Efficacy and safety of Compound Danshen Dropping Pills combined with aspirinin patients with coronary heart disease: A Meta-analysis. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease. 2021;19(4):533–539. [Google Scholar]
- Wang X.Q. Clinical efficacy and safety analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Journal of Practical Cardio-cerebral Pulmonary Vascular Diseases. 2014;22(6):50–51. [Google Scholar]
- Wang Y., Hao L., Huo Z.P., Liu Y.X., Sun Y.J., Song Z.H. Systematic review and Meta-analysis of 26 randomized controlled clinical trials of Compound Danshen Dripping Pill for non-proliferating diabetic retinopathy. Chinese Herbal Medicines. 2022;14(1):142–153. doi: 10.1016/j.chmed.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L. Clinical observation of Danshen Dropping Pills combined with isosorbide mononitrate in the treatment of 60 cases of coronary heart disease and angina pectoris. China & Foreign Medical Treatment. 2012;31(10):92. [Google Scholar]
- Wang Z.W., Liu J., Li J.J., Wu N.Q., Lu G.P., Chen Z.Y.…Yan S.K. Chinese guidelines for lipid management (2023) Chinese Circulation Journal. 2023;38(3):237–271. [Google Scholar]
- Wang Z.Z. Effect of Compound Danshen Dripping Pills combined with Lunan Xinkang on angina pectoris of coronary heart disease. Chinese Journal of Integrative Medicine. 2005;6(3):238. [Google Scholar]
- Wu J.B. Clinical study of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Shanghai Medical & Pharmaceutical Journal. 2021;42(4):26–28. [Google Scholar]
- Wu S.H., Wang D.W. Clinical effect of Compound Danshen Dripping Pills and isosorbide mononitrate sustained-release tablets in the treatment of angina pectoris. Journal of Chengdu Medical College. 2016;11(4):423–426. [Google Scholar]
- Wu X.F. Clinical effect of Compound Danshen Dripping Pills and isosorbide mononitrate sustained-release tablets in the treatment of angina pectoris. Health Required. 2018;36:32. [Google Scholar]
- Wu X.H. Effect of Chinese and Western medicine combined therapy on angina pectoris of coronary heart disease. A Collection of Contemporary Medical Essays. 2021;19(4):150–151. [Google Scholar]
- Wu, Y. Y. (2019). Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate on angina pectoris of coronary heart disease. Chinese Medical Sciences, 9(24), 69–71+173.
- Xiong X.J., Wang Z., Wang J. Innovative strategy in treating angina pectoris with chinese patent medicines by promoting blood circulation and removing blood stasis: Experience from combination therapy in Chinese medicine. Current Vascular Pharmacology. 2015;13(4):540–553. doi: 10.2174/1570161112666141014153735. [DOI] [PubMed] [Google Scholar]
- Xu J. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate on angina pectoris of coronary heart disease. Higher Medical Education in China. 2020;5:141+143. [Google Scholar]
- Xu J.H. Efficacy and safety evaluation of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. The Northern Pharmaceutical. 2018;15(10):157–158. [Google Scholar]
- Xu Q.Y., Qin Q., Dai S.R., Li F. Detection of blood lipid abnormalities in elderly physical examination population and its correlation with the incidence of coronary heart disease. Chinese Journal of Laboratory Diagnosis. 2023;27(3):276–278. [Google Scholar]
- Xue J.Z., Chen Y., Ma Z.H., Si X., Feng W.Y. Meta-analysis of efficacy comparison of Compound Danshen Dropping Pills and isosorbide mononitrate in treatment for angina pectoris of coronary heart disease. Chinese Traditional Patent Medicine. 2013;35(3):466–471. [Google Scholar]
- Yan P., Jiang L.P. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate on angina pectoris of coronary heart disease. Evaluation and Analysis of Drug Use in Chinese Hospitals. 2016;16(10):1362–1364. [Google Scholar]
- Yan Y.H. Effect of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dropping Pills on angina pectoris of coronary heart disease in 37 cases. Modern Chinese Doctors. 2016;54(35):97–99. [Google Scholar]
- Yin J.H., Zhou Z.H., Chen J. Merging algorithm for mean, standard deviation, and a pass rate of multiple small samples. Journal of Zhaotong Teacher’s College. 2010;32(5):61–64. [Google Scholar]
- Zeng S.Z. Clinical observation of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Hebei Medical. 2014;20(4):554–557. [Google Scholar]
- Zhang, B. L., Gao, X. M., Shang, H. C., Zhao, Y. J., & Wang, Y. Y. (2003). Investigation into the pharmacodynamic constituents and mechanisms of the Compound Danshen Formula. Modernization of Traditional Chinese Medicine and World Science and Technology, 5(5), 14-17+78-79.
- Zhang, D. D., Liu, H. Chen, J. Y., Jin, J., Xiong, Y., & Yang, Y. (2018). Bayesian network Meta-analysis of randomized controlled trials comparing Compound Danshen Dripping Pills, isosorbide nitrate and isosorbide mononitrate in treating coronary heart disease angina. Chinese Journal of Pharmacovigilance, 15(7), 419-428+433.
- Zhang D.M., Wang H.G. Clinical efficacy and safety evaluation of Compound Danshen Dripping Pills combined with isosorbide mononitrate in the treatment of unstable angina pectoris. Chinese Practical Medicine. 2011;6(4):141–142. [Google Scholar]
- Zhang H. Observation on the effect of Compound Danshen Dropping Pills combined with isosorbide mononitrate in patients with coronary heart disease and angina pectoris. Chinese Journal of Modern Drug Application. 2021;15(16):100–102. [Google Scholar]
- Zhang H.B. Compound Danshen Dropping Pills combined with isosorbide mononitrate in the treatment of coronary heart disease is more effective than single use. Inner Mongolia Journal of Traditional Chinese Medicine. 2012;31(18):26. [Google Scholar]
- Zhang J.J. Clinical efficacy and safety analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Chinese and Foreign Women’s Health Study. 2016;12:197+193. [Google Scholar]
- Zhang Q., Zhang J., He C.L. Clinical efficacy and safety analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Medical Frontier. 2013;4:385–386. [Google Scholar]
- Zhang Q.W. Efficacy and safety of isosorbide mononitrate sustained-release tablet combined with Compound Salvia Miltiorrhiza Dropping Pills in the treatment of angina pectoris of coronary heart disease. Guide of China Medicine. 2022;20(8):111–114. [Google Scholar]
- Zhang X.Q., Wu X.L., Fang X.M. Clinical analysis of isosorbide mononitrate sustained-release tablets combined with Compound Danshen Dripping Pills in the treatment of senile unstable angina pectoris. Chinese Medical Guide. 2017;15(34):11–12. [Google Scholar]
- Zhao Z.H., Guan Y.F. Effect evaluation of Compound Danshen Dripping Pills on coronary heart disease patients with angina pectoris. Clinical Medicine Research and Practice. 2017;2(19):31–32. [Google Scholar]
- Zheng Y., Wei Q. Effect of isosorbide mononitrate combined with Compound Danshen Dripping Pills on angina pectoris of coronary heart disease. Jiangxi Traditional Chinese Medicine. 2006;37(1):26–27. [Google Scholar]
- Zhu N. Effect of Compound Danshen Dripping Pills combined with isosorbide mononitrate sustained-release tablets on angina pectoris patients with coronary heart disease. Chinese Minkang Medicine. 2020;32(2):82–83. [Google Scholar]
- Zhu W., Jia M.C. The clinical observation on 60 patients with angina pectoris treated with compound red sage root pill. Journal of Emergency in Traditional Chinese Medicine. 2005;8:742–747. [Google Scholar]
- Zhu X.Y., Deng Z.H. To evaluate the effect of trimetazidine combined with the Compound Danshen Dropping Pills in the treatment of patients with coronary heart disease. Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine. 2019;7(25):29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.