Abstract
1. Trains of action potentials in motoneurones frequently commence with an initial doublet; i.e. a uniquely short interspike interval. Previous authors have speculated on the functional importance of initial doublets. Here we test the hypotheses that these doublets are associated with particular classes of motoneurones or particular physiological conditions. 2. Discharges of inspiratory motoneurones were recorded extracellularly in the thoracic ventral horn of anaesthetized, paralysed cats. Seventy units (35 each with axons in the internal and external intercostal nerves) were classified on the basis of their maximum firing rates, start times in the respiratory cycle and axonal destination. 3. Initial doublets were defined by an interspike interval < 14 ms. Of seventeen units firing initial doublets, fifteen had axons in the external intercostal nerve and two had axons in the internal intercostal nerve. Neither maximum firing rate nor start time during the respiratory cycle predicted the occurrence of doublets. 4. The chemical drive to breathe was manipulated by altering the CO2 content of the inspired gas or by briefly stopping the respiratory pump. Varying the chemical drive to breathe had no consistent effect on the occurrence of initial doublets. 5. These results support the view that initial doublets are part of the normal pattern of discharge of motoneurones. However, because the incidence of doublets does not consistently support previous functional hypotheses, we argue that the occurrence of doublets may not necessarily be dictated by the CNS, but in some circumstances it is an epiphenomenon dependent on the state of the motoneurone, in particular on the statistical properties of its synaptic inputs.
Full text
PDF

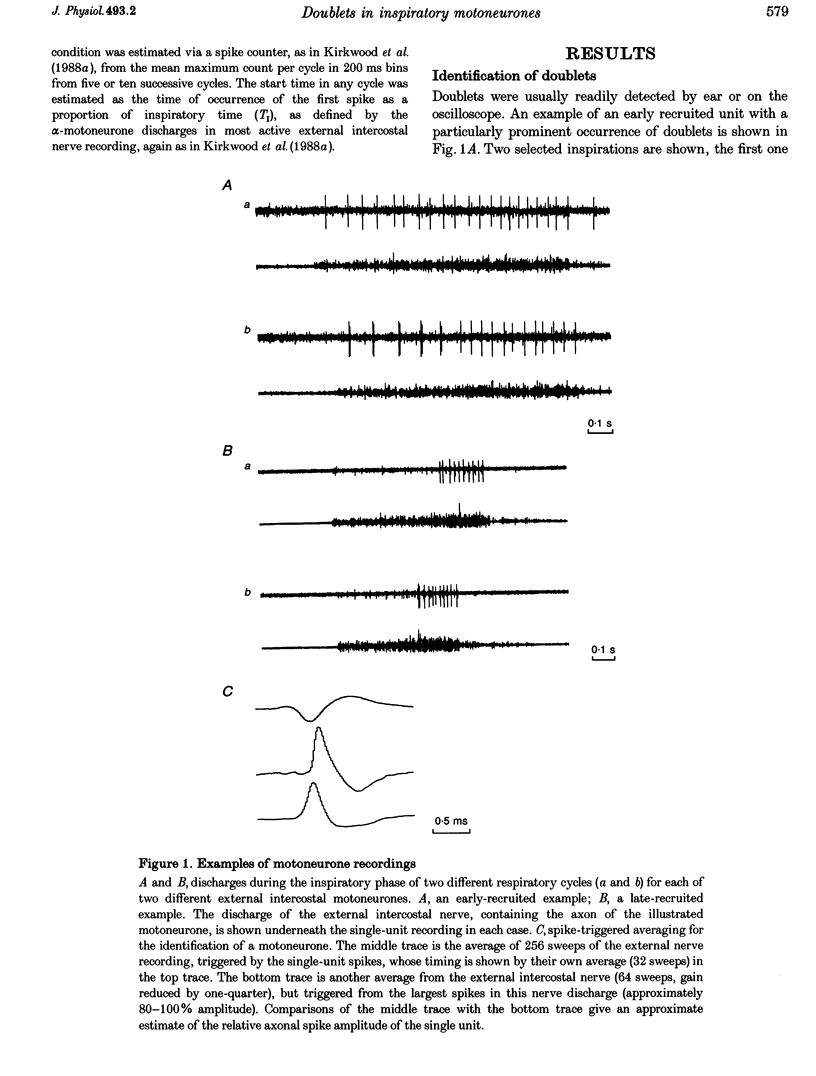
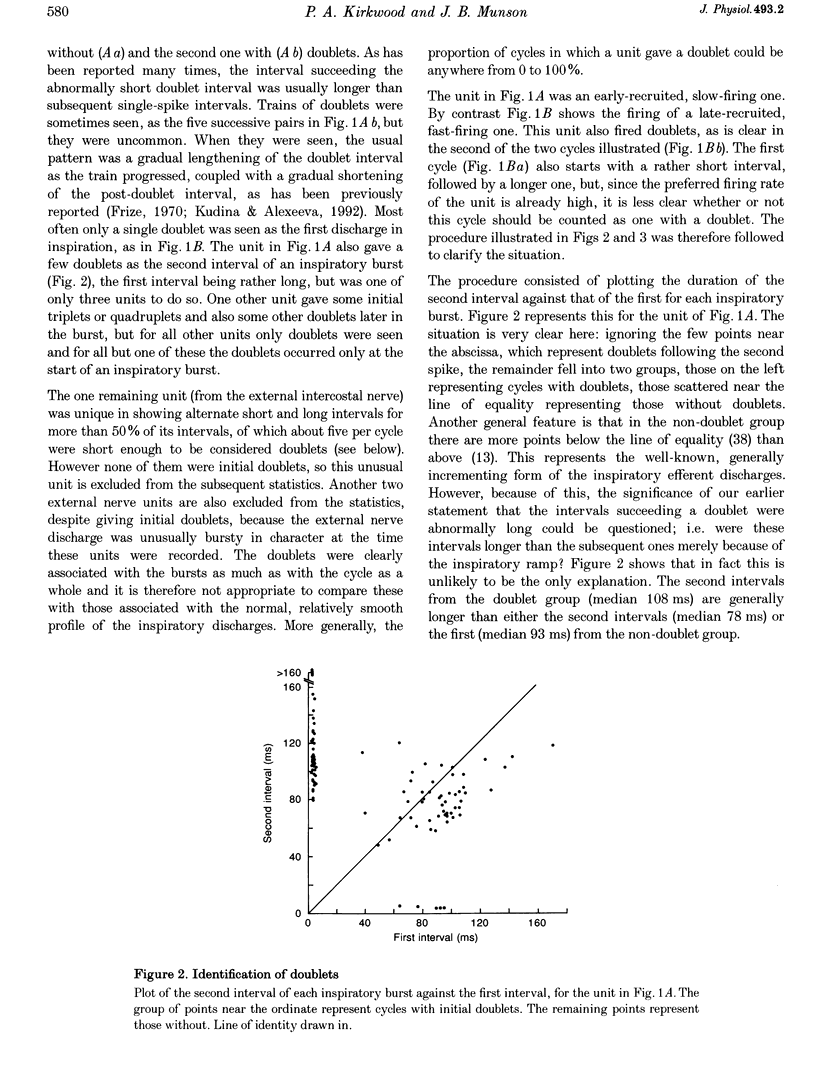
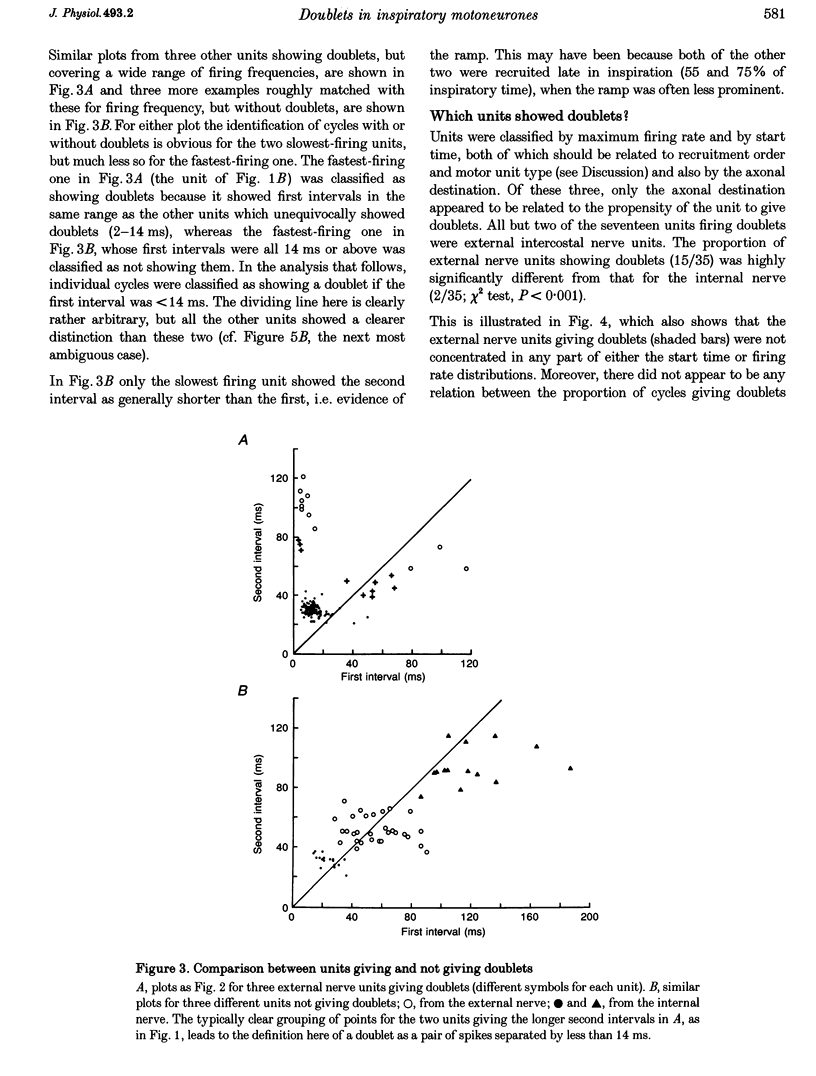
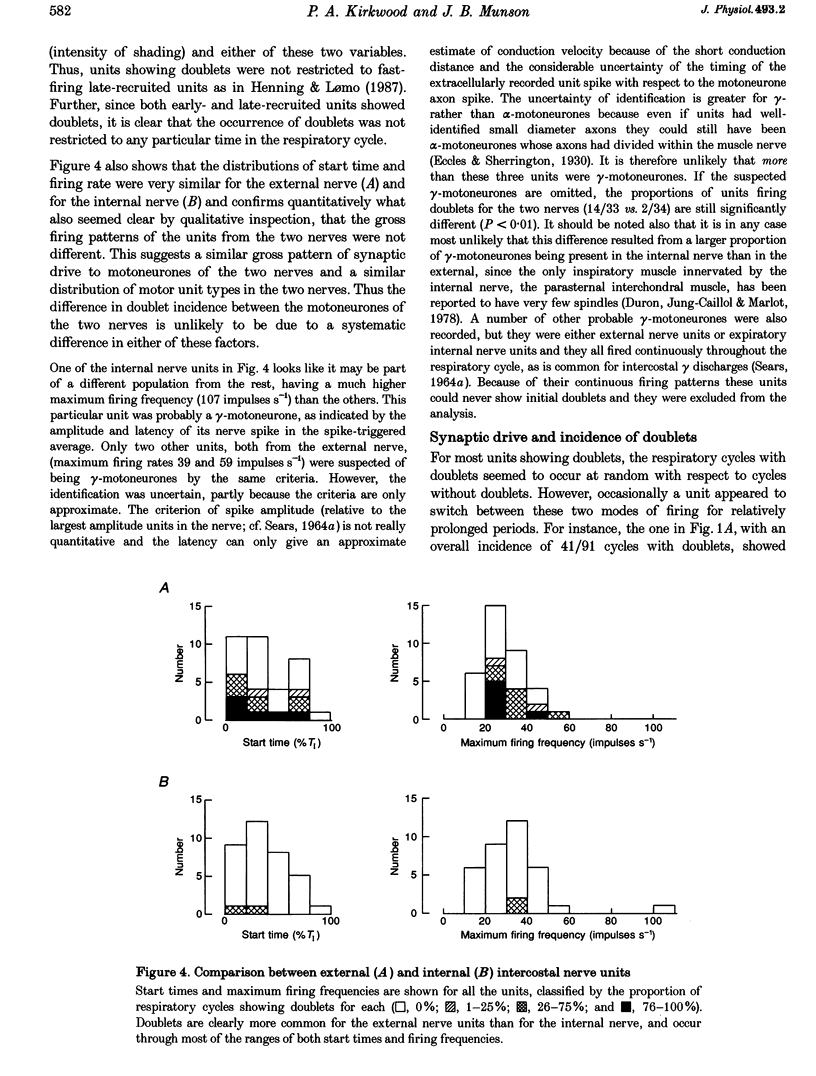




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L., Datta A. K., Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol. 1989 Jun;413:213–231. doi: 10.1113/jphysiol.1989.sp017650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Bronk D. W. The discharge of impulses in motor nerve fibres: Part II. The frequency of discharge in reflex and voluntary contractions. J Physiol. 1929 Mar 20;67(2):i3–151. [PMC free article] [PubMed] [Google Scholar]
- Baker J. R., Davey N. J., Ellaway P. H., Friedland C. L. Short-term synchrony of motor unit discharge during weak isometric contraction in Parkinson's disease. Brain. 1992 Feb;115(Pt 1):137–154. doi: 10.1093/brain/115.1.137. [DOI] [PubMed] [Google Scholar]
- Bawa P., Calancie B. Repetitive doublets in human flexor carpi radialis muscle. J Physiol. 1983 Jun;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan L., Laouris Y., Reinking R. M., Stuart D. G. The effect of the stimulation pattern on the fatigue of single motor units in adult cats. J Physiol. 1992 Apr;449:85–108. doi: 10.1113/jphysiol.1992.sp019076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Generation of spike trains in CNS neurons. Brain Res. 1975 Jan 24;84(1):1–22. doi: 10.1016/0006-8993(75)90796-9. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Farmer S. F., Stephens J. A. Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol. 1991 Jan;432:401–425. doi: 10.1113/jphysiol.1991.sp018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Macefield G., Nail B. S. Laryngeal motoneurone activity in the rabbit during asphyxic gasping. Respir Physiol. 1987 Dec;70(3):327–342. doi: 10.1016/0034-5687(87)90014-4. [DOI] [PubMed] [Google Scholar]
- Duron B., Jung-Caillol M. C., Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 1978 Feb 20;152(2):171–192. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Elek J. M., Dengler R., Konstanzer A., Hesse S., Wolf W. Mechanical implications of paired motor unit discharges in pathological and voluntary tremor. Electroencephalogr Clin Neurophysiol. 1991 Aug;81(4):279–283. doi: 10.1016/0168-5597(91)90014-o. [DOI] [PubMed] [Google Scholar]
- Hennig R., Lømo T. Gradation of force output in normal fast and slow muscles of the rat. Acta Physiol Scand. 1987 May;130(1):133–142. doi: 10.1111/j.1748-1716.1987.tb08119.x. [DOI] [PubMed] [Google Scholar]
- Hennig R. Section of fibular nerve affects activity pattern and contractile properties of soleus motor units in adult rats. Acta Physiol Scand. 1987 May;130(1):143–151. doi: 10.1111/j.1748-1716.1987.tb08120.x. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Sugano N., Loeb G. E., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol. 1987 Feb;57(2):530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Jordan L. M. Factors determining motoneuron rhythmicity during fictive locomotion. Symp Soc Exp Biol. 1983;37:423–444. [PubMed] [Google Scholar]
- Kirkwood P. A., Munson J. B., Sears T. A., Westgaard R. H. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988 Jan;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudina L. P., Alexeeva N. L. Repetitive doublets of human motoneurones: analysis of interspike intervals and recruitment pattern. Electroencephalogr Clin Neurophysiol. 1992 Aug;85(4):243–247. doi: 10.1016/0168-5597(92)90112-o. [DOI] [PubMed] [Google Scholar]
- Kudina L. P., Churikova L. I. Testing excitability of human motoneurones capable of firing double discharges. Electroencephalogr Clin Neurophysiol. 1990 Apr;75(4):334–341. doi: 10.1016/0013-4694(90)90111-v. [DOI] [PubMed] [Google Scholar]
- Macefield G., Nail B. Phrenic and external intercostal motoneuron activity during progressive asphyxia. J Appl Physiol (1985) 1987 Oct;63(4):1413–1420. doi: 10.1152/jappl.1987.63.4.1413. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975 Mar;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Nail B. S., Sterling G. M., Widdicombe J. G. Patterns of spontaneous and reflexly-induced activity in phrenic and intercostal motoneurons. Exp Brain Res. 1972;15(3):318–332. doi: 10.1007/BF00235915. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Kirkwood P. A., Munson J. B., Shen E., Sears T. A. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol. 1993 Feb;461:647–665. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmied A., Ivarsson C., Fetz E. E. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res. 1993;97(1):159–172. doi: 10.1007/BF00228826. [DOI] [PubMed] [Google Scholar]
- Spielmann J. M., Laouris Y., Nordstrom M. A., Robinson G. A., Reinking R. M., Stuart D. G. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993 May;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegner A. W., Wierzbicka M. M., Davies L., Young R. R. Discharge properties of single motor units in patients with spinal cord injuries. Muscle Nerve. 1993 Jun;16(6):661–671. doi: 10.1002/mus.880160613. [DOI] [PubMed] [Google Scholar]
- Zajac F. E., Young J. L. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J Neurophysiol. 1980 May;43(5):1221–1235. doi: 10.1152/jn.1980.43.5.1221. [DOI] [PubMed] [Google Scholar]


