Abstract
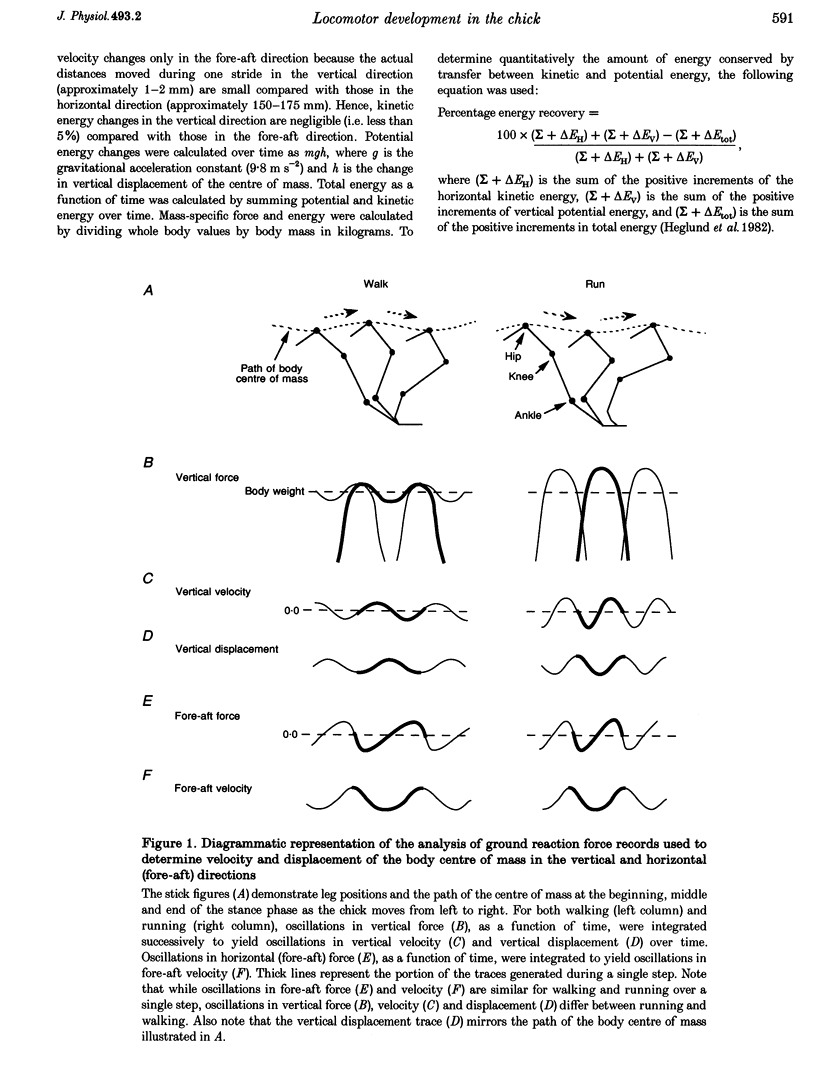
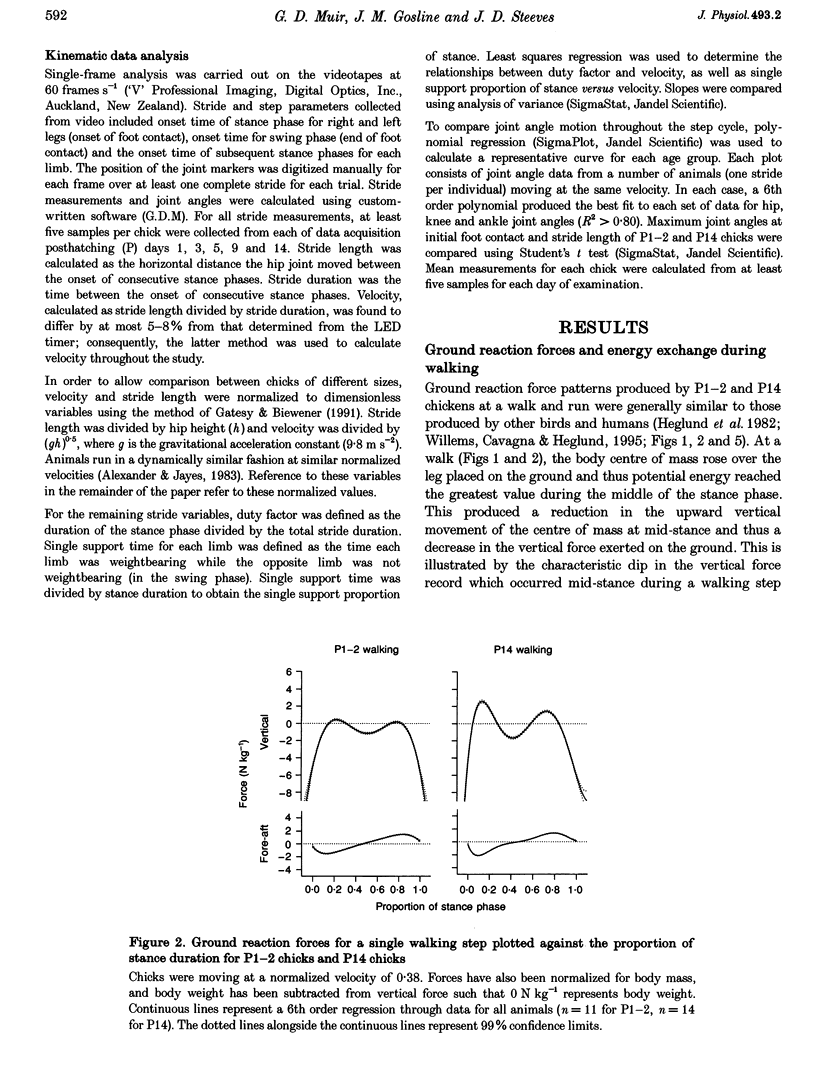
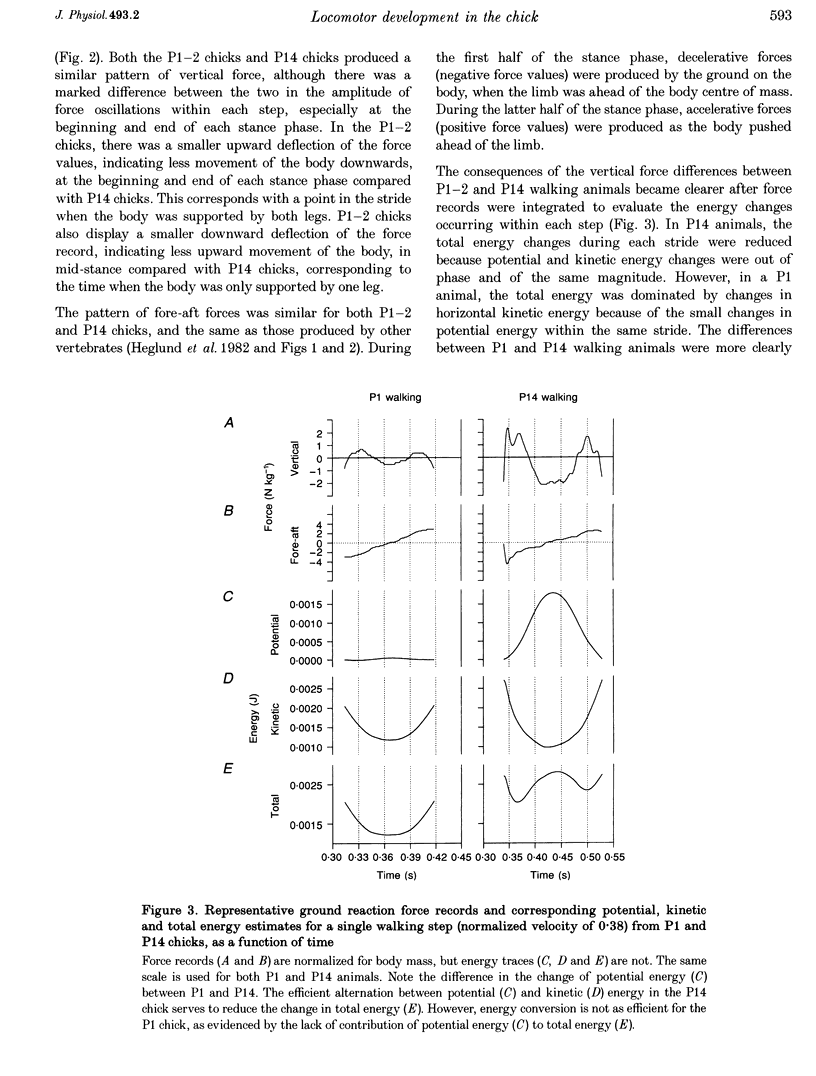
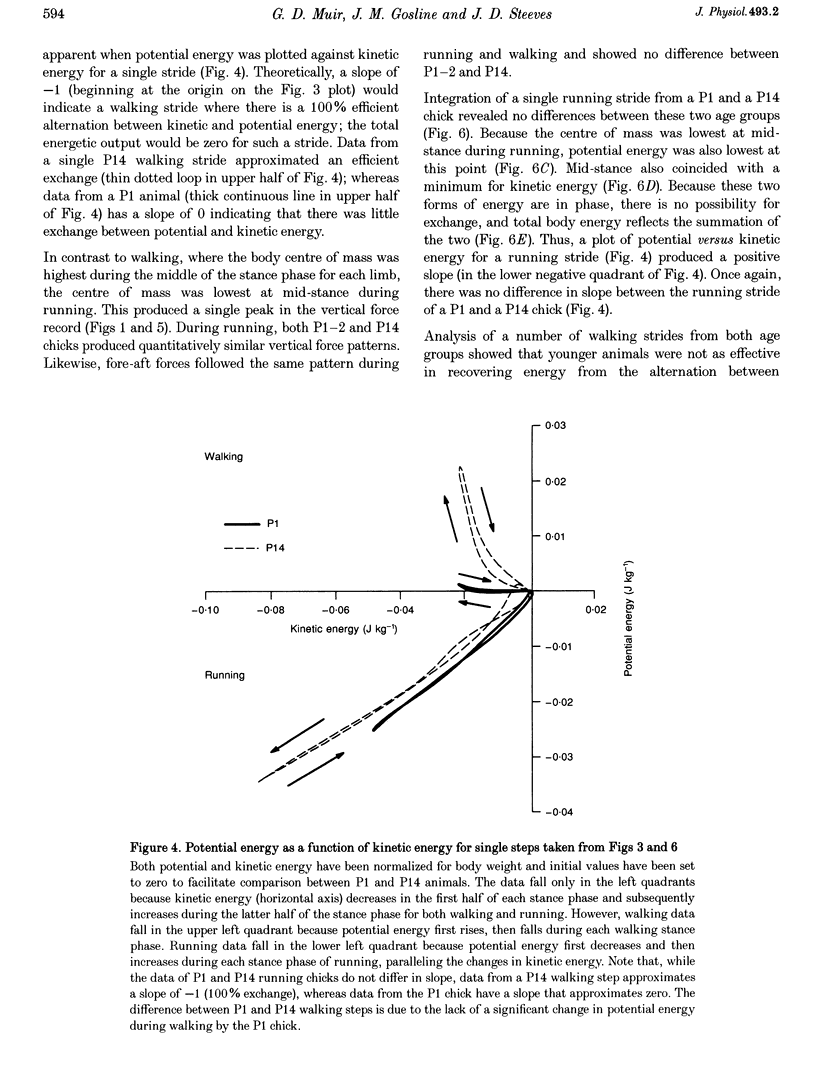
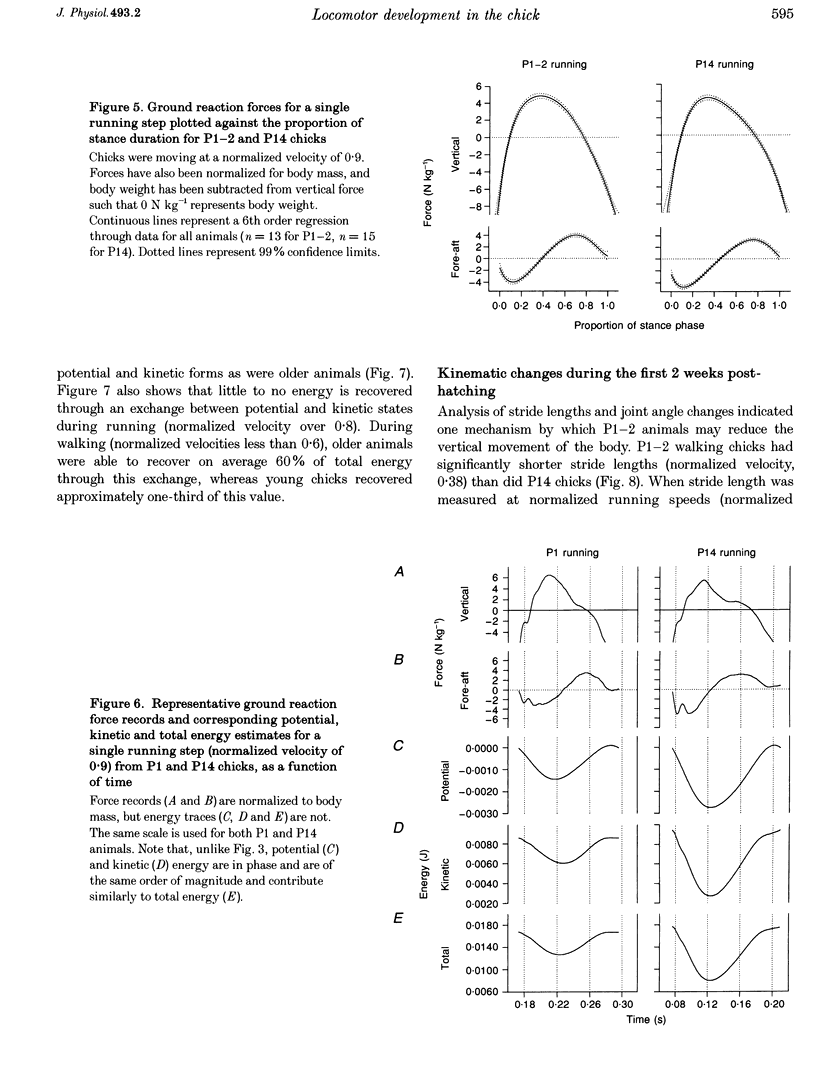
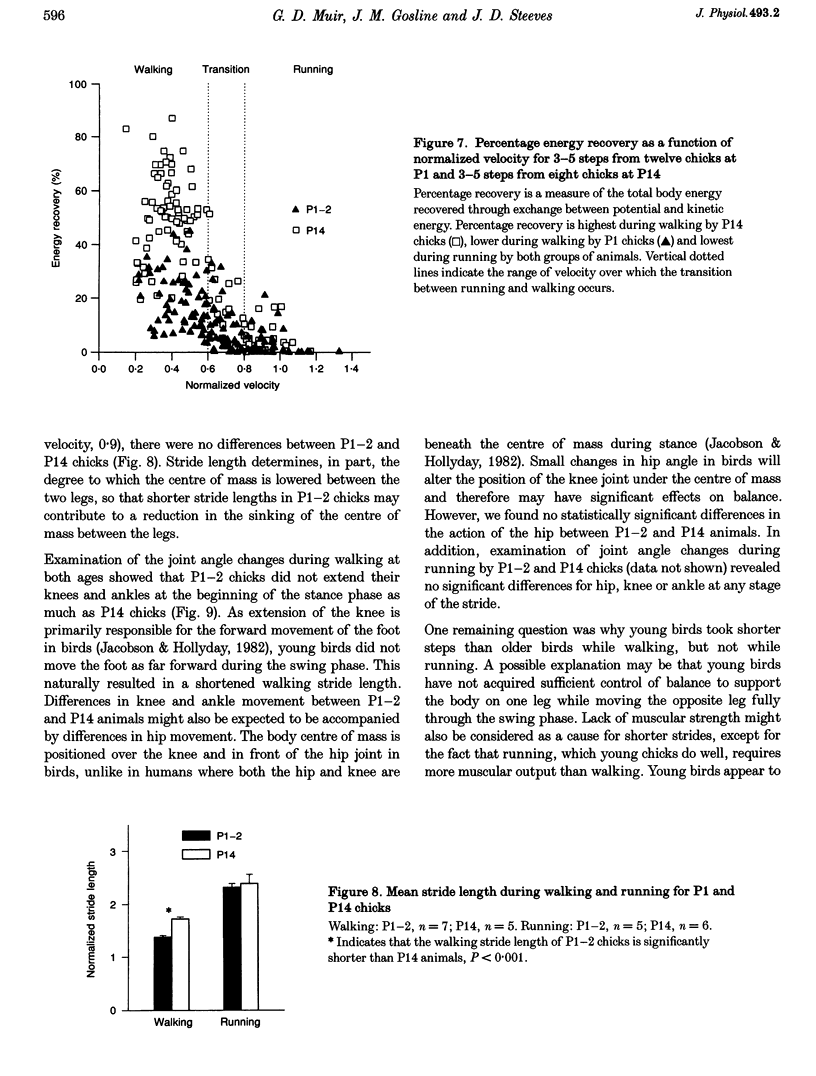
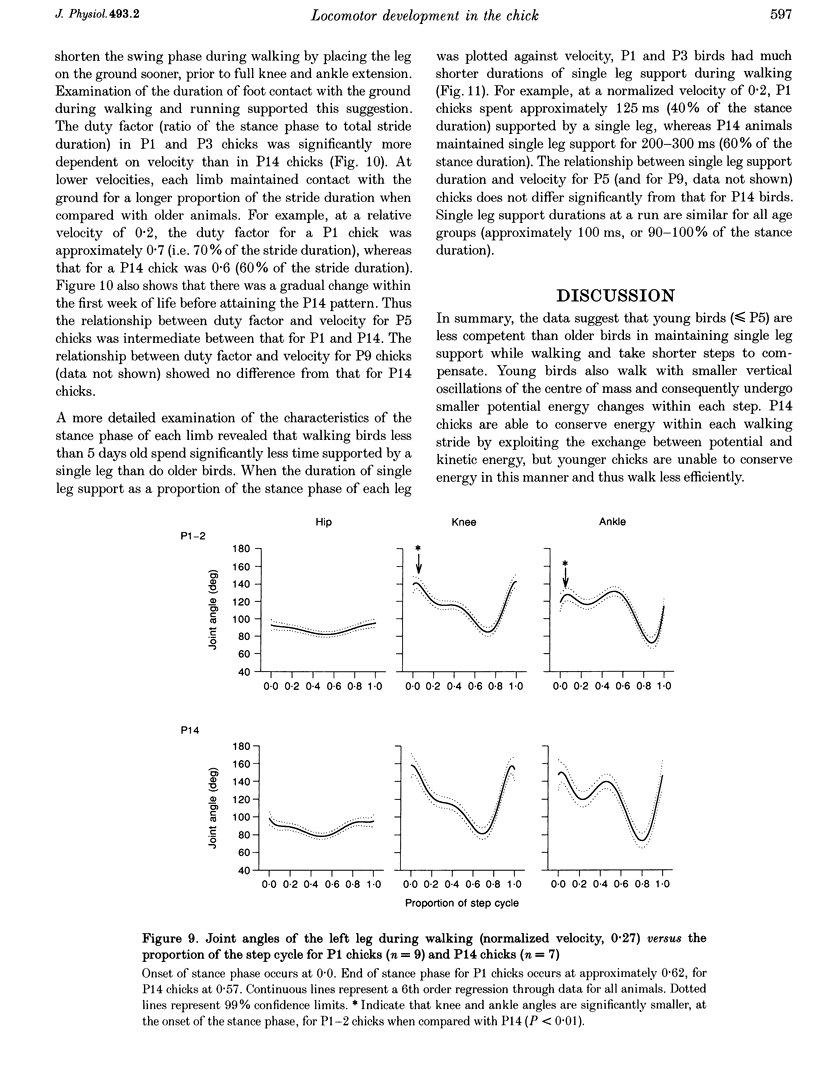
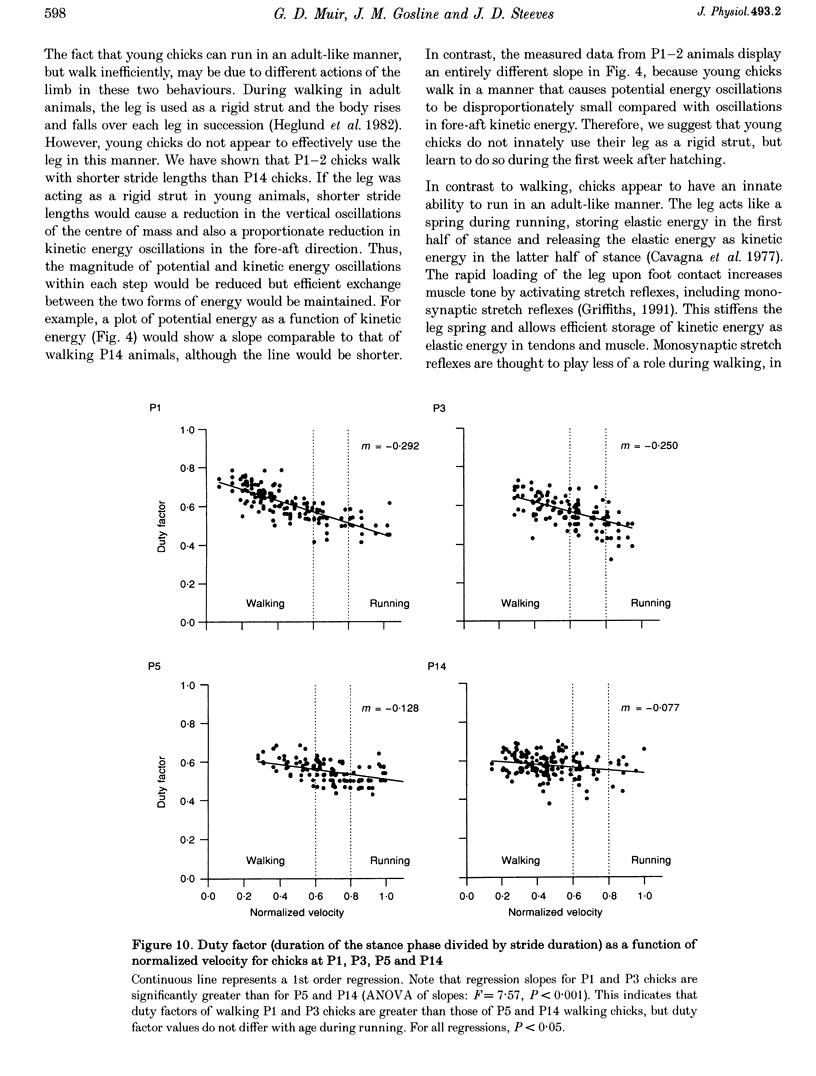
1. The purpose of this study was to determine whether the production of an energy-efficient bipedal walk is an innate attribute of a precocial bird. 2. The locomotor characteristics of hatchling chicks were quantified using kinetic (ground reaction forces) and kinematic (stride length, leg support duration) measurements as the animals moved overground unrestrained. All measurements were made over a range of velocities and at regular intervals throughout the first 2 weeks of life. 3. Ground reaction force records showed that, like all terrestrial walking vertebrates, chicks undergo cyclical increases and decreases in the body's potential and kinetic energy with each step. The out-of-phase exchange of potential with kinetic energy is an efficient mechanism for the conservation of energy during walking. However, comparisons between chicks at posthatching (P) days 1-2 and P14 revealed that P1-2 chicks are unable to conserve energy because they walk with disproportionately small potential energy oscillations. During running, however, the oscillations between potential and kinetic energy are similar for both P1-2 and P14 animals. 4. P1-2 chicks also walk with a shorter stride length than P14 chicks. Examination of limb support durations shows that younger animals (P1-2, P3) spend less time in single limb support than P14 animals during walking but not running. 5. The results show that even highly precocial bipeds need to acquire the ability to walk in a controlled and energy efficient manner, although they can innately run as well as an adult. This disparity could be due to the distinct actions of the legs in these two behaviours, and the requirement for longer durations of single leg support during walking. These differences relate to constraints inherent to bipedal locomotion and many of the locomotor changes occurring in the first weeks after hatching may therefore be analogous to similar changes seen during human locomotor development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniss A. M., Gandevia S. C., Burke D. Reflex responses in active muscles elicited by stimulation of low-threshold afferents from the human foot. J Neurophysiol. 1992 May;67(5):1375–1384. doi: 10.1152/jn.1992.67.5.1375. [DOI] [PubMed] [Google Scholar]
- Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Res. 1976 Apr 23;106(2):271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- Cavagna G. A. Force platforms as ergometers. J Appl Physiol. 1975 Jul;39(1):174–179. doi: 10.1152/jappl.1975.39.1.174. [DOI] [PubMed] [Google Scholar]
- Cavagna G. A., Heglund N. C., Taylor C. R. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol. 1977 Nov;233(5):R243–R261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- Dietz V. Role of peripheral afferents and spinal reflexes in normal and impaired human locomotion. Rev Neurol (Paris) 1987;143(4):241–254. [PubMed] [Google Scholar]
- Duysens J., Tax A. A., Trippel M., Dietz V. Increased amplitude of cutaneous reflexes during human running as compared to standing. Brain Res. 1993 Jun 11;613(2):230–238. doi: 10.1016/0006-8993(93)90903-z. [DOI] [PubMed] [Google Scholar]
- Evans A. L., Harrison L. M., Stephens J. A. Maturation of the cutaneomuscular reflex recorded from the first dorsal interosseous muscle in man. J Physiol. 1990 Sep;428:425–440. doi: 10.1113/jphysiol.1990.sp018220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res. 1985;57(3):480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- Full R. J., Tu M. S. Mechanics of six-legged runners. J Exp Biol. 1990 Jan;148:129–146. doi: 10.1242/jeb.148.1.129. [DOI] [PubMed] [Google Scholar]
- Gossard J. P., Brownstone R. M., Barajon I., Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98(2):213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Griffiths R. I. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991 May;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Balaban M., Oppenheim R., Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965 Jun;159(1):1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Oppenheim R. Prehatching motility and hatching behavior in the chick. J Exp Zool. 1967 Nov;166(2):171–203. doi: 10.1002/jez.1401660203. [DOI] [PubMed] [Google Scholar]
- Heglund N. C., Cavagna G. A., Taylor C. R. Energetics and mechanics of terrestrial locomotion. III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals. J Exp Biol. 1982 Apr;97:41–56. doi: 10.1242/jeb.97.1.41. [DOI] [PubMed] [Google Scholar]
- Heglund N. C., Willems P. A., Penta M., Cavagna G. A. Energy-saving gait mechanics with head-supported loads. Nature. 1995 May 4;375(6526):52–54. doi: 10.1038/375052a0. [DOI] [PubMed] [Google Scholar]
- Jacobson R. D., Hollyday M. A behavioral and electromyographic study of walking in the chick. J Neurophysiol. 1982 Jul;48(1):238–256. doi: 10.1152/jn.1982.48.1.238. [DOI] [PubMed] [Google Scholar]
- Landmesser L. T., O'Donovan M. J. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol. 1984 Feb;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon T. A., Valiant G., Frederick E. C. Groucho running. J Appl Physiol (1985) 1987 Jun;62(6):2326–2337. doi: 10.1152/jappl.1987.62.6.2326. [DOI] [PubMed] [Google Scholar]
- Muir G. D., Steeves J. D. Phasic cutaneous input facilitates locomotor recovery after incomplete spinal injury in the chick. J Neurophysiol. 1995 Jul;74(1):358–368. doi: 10.1152/jn.1995.74.1.358. [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Collins D. F. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993 Sep;70(3):1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Vecchierini-Blineau M. F., Guiheneuc P. Excitability of the monosynaptic reflex pathway in the child from birth to four years of age. J Neurol Neurosurg Psychiatry. 1981 Apr;44(4):309–314. doi: 10.1136/jnnp.44.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchierini-Blineau M. F., Guihneuc P. Lower limb cutaneous polysynaptic reflexes in the child, according to age and state of waking or sleeping. J Neurol Neurosurg Psychiatry. 1982 Jun;45(6):531–538. doi: 10.1136/jnnp.45.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P. A., Cavagna G. A., Heglund N. C. External, internal and total work in human locomotion. J Exp Biol. 1995 Feb;198(Pt 2):379–393. doi: 10.1242/jeb.198.2.379. [DOI] [PubMed] [Google Scholar]


