Abstract
AIM
To evaluate the relationship between monocyte to high-density lipoprotein cholesterol ratio (MHR) and the disease activity of thyroid-associated ophthalmopathy (TAO).
METHODS
A total of 87 patients were classified into two groups based on clinical activity score (CAS) scoring criteria: high CAS group (n=62, the CAS score was ≥3); low CAS group (n=25, the CAS score was <3). In addition, a group of healthy people (n=114) were included to compared the MHR. Proptosis, MHR, average signal intensity ratio (SIR), average lacrimal gland (LG)-SIR, average extraocular muscles (EOM) area from 87 patients with TAO were calculated in magnetic resonance imaging (MRI), and compared between these two groups. Correlation testing was utilized to evaluate the association of parameters among the clinical variables.
RESULTS
Patients in high CAS group had a higher proptosis (P=0.041) and MHR (P=0.048). Compared to the healthy group, the MHR in the TAO group was higher (P=0.001). Correlation testing declared that CAS score was strongly associated with proptosis and average SIR, and MHR was positively associated with CAS score, average SIR, and average LG-SIR. The area under the receiver operating characteristic curve (AUC) of MHR was 0.6755.
CONCLUSION
MHR, a novel inflammatory biomarker, has a significant association with CAS score and MRI imaging (average SIR and LG-SIR) and it can be a new promising predictor during the active phase of TAO.
Keywords: thyroid-associated ophthalmopathy, monocyte to high-density lipoprotein cholesterol ratio, disease activity
INTRODUCTION
Thyroid-associated ophthalmopathy (TAO), also known as Graves' ophthalmopathy, the most common orbital disease in adults, is a systemic autoimmune disease related to thyroid disease characterized as periorbital edema, eyelid retraction, proptosis, strabismus, exposure keratopathy, and compressive neuropathy[1]–[2]. TAO is a self-limiting disease, and few accurate reports of the incidence of TAO were reported before[3].
The natural process of TAO involves two stages: the active stage usually manifests as inflammation and tissue remodeling of orbital fat and extraocular muscles (EOMs); in the succeeding inactive stage, the inflammatory resolution and fibrosis of EOMs occur, eventually leading to EOM dysfunction and impaired vision[1]–[4].
Currently, TAO is still a highly blinding disease, and its clinical treatment remains a major challenge. Clinically, in the active phase, anti-inflammatory therapies achieve a good effect, whereas surgical treatments show a better prognosis than medical therapies in the inactive phase[5]–[7]. Thus, an accurate assessment of the degree of disease activity and severity of TAO is crucial to guide appropriate and effective treatments. Nowadays, the clinical activity score (CAS) score is the most widely used clinically in selecting the treatment methods as well as evaluating the treatment effect. The CAS score is obtained according to the assessment of clinical symptoms and signs (pain, swelling, and redness of the eyelid, conjunctival hyperemia, and edema). TAO is considered to be in the active stage of when the CAS score is more than 3[8]–[9]. However, some studies have demonstrated that the validity and the accuracy for monitoring changes in disease status are insufficient using CAS alone because of its subjectivity and artificial. Therefore, other parameters combined with CAS score are required to improve its accuracy[10]–[11].
Several magnetic resonance imaging (MRI) researches have suggested some quantitative metrics [average signal intensity ratie (SIR), lacrimal gland signal intensity ratio (LG-SIR) average and EOM area] are reported to be related to disease activity of TAO[12]–[13]. As generally known, the active phase of TAO is an inflammatory-dominated process in which systemic inflammatory markers may be novel, potentially economical, and convenient predictors and prognostic factors. Monocyte to high-density lipoprotein cholesterol ratio (MHR), one of the novel inflammatory biomarkers and oxidative stress, was noted to be relevant in the disease-status of cardiovascular disease, metabolic disease, and some tumors[14]–[17].
Presently, only Yılmaz Tuğan et al[18] reported a small sample size research on the relationship between MHR and the disease activity of TAO. Here, our large sample research was conducted to examine and verify the possible correlation.
PARTICIPANTS AND METHODS
Ethical Approval
This research was authorized by the Ethics Committee of Nanjing Drum Tower Hospital (the approval number is 2022-285-03). All participants received a thorough explanation of the study design and aims followed by a signed informed consent. The study was conducted in compliance with the tenets of the Declaration of Helsinki.
Participants
Participants first diagnosed with TAO were enrolled through Affiliated Drum Tower Hospital of Nanjing University Medical School from March 2022 to August 2023. Participants who had a history of prior injuries, optic neuropathy, other inflammatory disorders of uncertain cause, past orbital irradiation or surgery, and prior immunosuppressing therapies with steroids were excluded from this study. After that, a sum of 87 participants were in the study. An ophthalmological, endocrinological, and radiographic examination of the orbit was performed on each patient. In addition, we included a group of healthy people to compared the MHR.
Clinical and Laboratory Data Collection
Basic clinical assessments include age, gender, smoking history, TAO duration, body mass index (BMI), metabolic syndrome and CAS. TAO activity was graded according to the CAS, and cases with CAS ≥3 were considered active. Analysis of peripheral blood hematology was conducted using Mindray BC-5000. C-reaction protein (CRP) was detected using the method of transmission turbidimetry.
Serum alanine aminotransferase (ALT), urea nitrogen (UN), uric acid (UA), creatinine (Crea), total cholesterol (TC), high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), fasting blood glucose (FBG) were examined by an automatic analyzer (Beckman AU5400). These were the results of a 10-hour fasting blood sample. MHR was determined by dividing the absolute monocyte number by the HDLc.
Serum TSH, free thyroxine and free triiodothyronine, were measured by electrochemical luminescence assays using Cobas Eless 601 (Roche). The reference interval of thyroid stimulating hormone (TSH) was 0.27–4.2 mIU/L. Thyrotrophin receptor antibody (TRAb) was detected by the third-generation TBII assay with the automated Cobas electrochemiluminescence immunoassay (Roche). The reference ranges were 0–1.75 IU/L.
MRI Technique
Orbital MRI imaging was conducted utilizing a 3.0 T scanner (Achieva, Philips Medical Systems, the Netherlands). The participants were asked to remain in the supine position and close their eyes. Comprehensive MRI data included the following: 3 mm slice thickness axial T1-weighted turbo spin-echo; axial T2-weighted turbo spin-echo spectral presaturation with inversion recovery (SPIR) series; coronal T2W DRIVE; coronal DWI sequences.
On T2 SPIR and SE T1-weighted post-contrast pictures, manual separation of the whole muscle region in the coronal segment with the greatest apparent signal change was performed, and signal strengths were then recorded.
T2-SIR was measured by the next formula: SIR=SIEOM/SI ipsilateral temporal muscle; lacrimal gland (LG)-SIR=SILG/SI ipsilateral temporal muscle.
On the coronal T2 mapping images, regions of interest (ROIs) were manually recorded on the superior rectus levator complex, inferior, medial, and lateral EOMs, respectively, at the biggest site of EOM cross-section. The average EOM area was calculated. Proptosis was measured by estimating the perpendicular length between the corneal edge and the interzygomatic border on axial scanning at the level of the optical nerve based on the axial T2-weighted images. The images were assessed by one radiologist who was blinded to the diagnosis and another clinical status of the patients, as well as laboratory results. Within each patient, the clinically more severely affected eye was designated as the study eye, and the average EOM area and SIR of the study eye were calculated and used in the final analysis.
Statistical Analysis
Statistical assessments in this research were performed using SPSS19.0. P<0.05 was deemed nominally significant. Descriptions of data were expressed as mean±standard deviations (SDs) and categorical variables as numbers with percentages. Student's t-test and nonparametric test (Mann-Whitney or Kruskal-Wallis) were used to analyze normally distributed continuous variables and variables with non-normal distribution, respectively. Categorical variables were examined utilizing Chi-squared testing. The correlation was assessed using Spearman or Pearson correlation test. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were applied to assess the diagnostic value of MHR on TAO activity.
RESULTS
Clinical Characteristics
Table 1 shows the clinical features of the high CAS group (n=62) and low CAS group (n=25). This cohort involved a sum of 87 participants. Proptosis in the high CAS group (21.77±2.97) was higher than in the low CAS group (20.52±2.20). Besides, MHR (0.40±0.30 vs 0.26±0.13, P=0.048) was significantly increased in the high CAS group. No significant variation in age, gender, smoking history, TAO duration, TSH level, free thyroxine, free triiodothyronine, TRAb, FBG, CRP, BMI, proportion of metabolic syndrome, average SIR, average LG-SIR, and average EOM area between the two TAO groups (P>0.05). When those patients were enrolled the current medications were methimazole (67 patients, median dose 10 mg), propylthiouracil (1 patient, dose 150 mg), levothyroxine (10 patients, median dose 75 µg), and others were not on antithyroid medications or hormones. And 22 patients were hyperlipemia, among them 16 were on statins and 6 were on fibrates. Table 2 shows the difference of MHR between TAO group and healthy group, the MHR in the TAO group was higher (0.36±0.27 vs 0.24±0.12, P=0.001).
Table 1. Clinical characteristics of the high CAS group and low CAS group.
| Characteristics | Total | High CAS | Low CAS | P |
| No. of patients | 87 | 62 | 25 | |
| Age (y) | 48.6±13.74 | 49.2±13.72 | 47.3±13.97 | 0.563 |
| Male (%) | 39 (44.8) | 26 (41.2) | 13 (52) | 0.477 |
| Smoking (%) | 27 (31.0) | 18 (29.0) | 9 (36.0) | 0.61 |
| TAO duration (mo) | 6.7±0.73 | 7.4±0.85 | 5.1±1.32 | 0.134 |
| CAS | 3.3±0.14 | 3.9±0.13 | 1.8±0.08 | <0.001a |
| TSH level (mIU/L) | 0.03 (0.005, 1.11) | 0.05 (0.005, 1.43) | 0.015 (0.005, 0.07) | 0.073 |
| Free triiodothyronine (pmol/L) | 6.26 (4.81, 14.86) | 6.22 (4.68, 14.6) | 7.01 (5.61, 16.5) | 0.234 |
| Free thyroxine (pmol/L) | 24.5±22.22 | 22.0±21.46 | 30.6±23.31 | 0.102 |
| TRAb (IU/L) | 9.34 (3.29, 21.26) | 9.83 (3.36, 27.6) | 7.56 (2.71, 18.9) | 0.554 |
| FBG (mmol/L) | 5.0±0.78 | 5.1±0.84 | 4.9±0.63 | 0.361 |
| CRP (mg/L) | 4.08±3.22 | 4.16±3.36 | 3.87±2.88 | 0.746 |
| BMI (kg/m2) | 23.0±3.37 | 23.3±3.63 | 22.2±2.47 | 0.142 |
| Metabolic syndrome (%) | 19 (21.8) | 13 (21.0) | 6 (24.0) | 0.757 |
| Average SIR | 3.06±1.82 | 3.27±1.98 | 2.48±1.17 | 0.089 |
| Average LG-SIR | 2.12±1.21 | 2.19±1.26 | 1.94±1.11 | 0.450 |
| Average EOM area | 55.71±20.75 | 57.65±22.40 | 50.33±14.45 | 0.168 |
| Proptosis (mm) | 21.40±2.80 | 21.77±2.97 | 20.52±2.20 | 0.041a |
| MHR | 0.36±0.27 | 0.40±0.30 | 0.26±0.13 | 0.048a |
aP<0.05. CAS: Clinical activity score; TAO: Thyroid-associated ophthalmopathy; TSH: Thyroid stimulating hormone; TRAb: Thyrotrophin receptor antibody; FBG: Fasting blood glucose; CRP: C-reactive protein; BMI: Body mass index; SIR: Signal intensity ratio; LG-SIR: Lacrimal gland signal intensity ratio; EOM: Extraocular muscle; MHR: Monocyte to high-density lipoprotein cholesterol ratio.
Table 2. Difference of MHR between the TAO group and the control group.
| Characteristics | TAO | Contral | P |
| Number | 87 | 114 | |
| Age (y) | 48.6±13.74 | 47.9±9.63 | 0.663 |
| Male (%) | 39 (44.8) | 50 (43.9) | 0.891 |
| BMI (kg/m2) | 23.0±3.37 | 23.0±2.99 | 0.920 |
| MHR | 0.36±0.27 | 0.24±0.12 | 0.001a |
aP<0.05. TAO: Thyroid-associated ophthalmopathy; BMI: Body mass index; MHR: Monocyte to high-density lipoprotein cholesterol ratio.
Correlation of MHR with Other Clinical Parameters of TAO
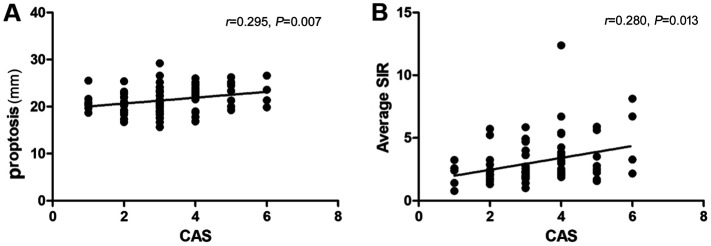
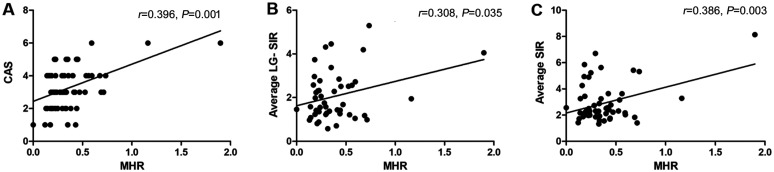
Spearman correlation testing in this research demonstrated that CAS score was strongly connected to proptosis (P=0.007) and average SIR (P=0.013; Figure 1). In addition, according to the results, MHR was found to have moderate associations with CAS score (P=0.001), average LG-SIR (P=0.035), and average SIR (P=0.003; Figure 2, Table 3).
Figure 1. Correlation of CAS with MRI parameters.
A: Correlation between CAS and proptosis; B: CAS and average SIR. CAS: Clinical activity score; SIR: Signal intensity ratio.
Figure 2. Correlation of MHR with other clinical parameters.
A: Correlation between MHR and CAS; B: MHR and average LG-SIR; C: MHR and average SIR. MHR: Monocyte to HDL cholesterol ratio; CAS: Clinical activity score; LG-SIR: Lacrimal gland signal intensity ratio; SIR: Signal intensity ratio; HDL: High-density lipoprotein.
Table 3. Correlation analysis between MHR and various parameters.
| Parameters | MHR |
CAS |
||||
| No. | Correlation coefficient | P | No. | Correlation coefficient | P | |
| CAS | 65 | 0.396 | 0.001a | 87 | 1 | NA |
| MHR | 65 | 1 | NA | 65 | 0.396 | 0.001a |
| Age | 65 | -0.067 | 0.596 | 87 | 0.139 | 0.198 |
| Average SIR | 56 | 0.386 | 0.003a | 78 | 0.280 | 0.013a |
| Average LG-SIR | 47 | 0.308 | 0.035a | 65 | 0.206 | 0.099 |
| Proptosis | 60 | 0.127 | 0.332 | 82 | 0.295 | 0.007a |
| Average EOM area | 57 | -0.137 | 0.309 | 79 | 0.204 | 0.072 |
aP<0.05. MHR: Monocyte to HDL cholesterol ratio; CAS: Clinical activity score; SIR: Signal intensity ratio; LG-SIR: Lacrimal gland signal intensity ratio; EOM: Extraocular muscle.
Accuracy of MHR for Disease Activity Assessment of TAO
The cutoff value, sensitivity, and specificity were calculated based on the Youdens index. AUC was 0.6755 (95%CI: 0.528–0.823, P<0.05). The optimal cutoff value of MHR as a predictor for evaluating disease activity of TAO was 0.198, with a reliability of 85.5% and validity of 44.4% (Figure 3).
Figure 3. ROC curve of MHR for assessment of disease activity of TAO.
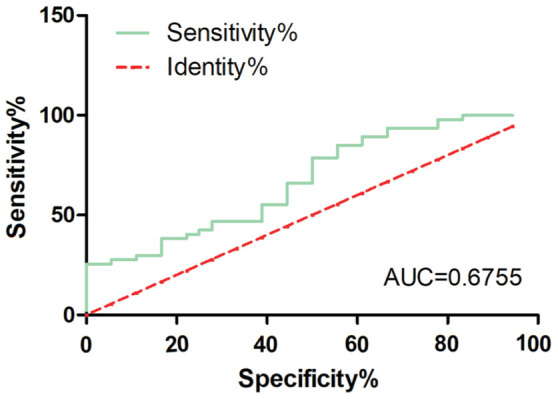
AUC=0.6755 (95%CI: 0.528–0.823, P<0.05). MHR: Monocyte to HDL cholesterol ratio; TAO: Thyroid-associated ophthalmopathy; ROC: Receiver operating characteristic; AUC: Area under the ROC curve; HDL: High-density lipoprotein.
DISCUSSION
As a new biomarker, MHR has been reported in cardiovascular illness, cerebrovascular disorder, peripheral artery disorder, metabolic syndrome, diabetic nephropathy, and several sclerosis in previous reviews[14]–[17],[19]–[24]. However, there are few studies exploring the function of MHR in thyroid disorders. Recently, several reports have declared that MHR is closely linked to papillary thyroid carcinoma as well as thyroid nodule[25]–[26]. So far, only one report on the correlation between MHR and TAO has been published in 2024. Yılmaz Tuğan et al[18] found that Graves' ophthalmopathy patients have higher MHR than healthy controls.
The present research was the largest sample size cohor designed to evaluate the connection between MHR and the disease activity of TAO. These results revealed novel evidence that MHR is positively associated with the disease activity of TAO. In addition, significant variation was observed between the high CAS group and the low CAS group regarding proptosis and MHR. Additionally, there were no differences in the metabolic status, fasting blood glucose, thyroxine levels and TSH levels between the two groups, which excluded the influence of lipid factors on the results. The MHR of TAO group appeared to be higher compared to the healthy group.
This cohort found average SIR was positively related to CAS score, reconfirming that SIR was a valuable MRI indicator of the disease activity of TAO, compensating for the poor objectivity CAS score, which was consistent with previous research. Liu et al[12] proposed that T2 SIRs and normalized apparent diffusion coefficients (n-ADC) values were ideal complementary methods of CAS score to predict the stage of disease. Gagliardo et al[13] found that the measurement of lacrimal gland herniation could be a good predictor of disease activity of TAO. Das et al[27] identified that fat fraction measurement combined with T2-relaxation times through MRI imaging might be useful to monitoring treatment effects. Our results showed that there was a noticeable correlation between MHR and average SIR and LG-SIR, proving the reliability of the assessment of disease activity.
However, our study was the biggest sample ever confirmed the hypothesis that MHR was significantly higher in the high CAS group, and closely related to the disease status of TAO, bringing the possibility to complement the clinical evaluation of disease activity in TAO. Our results told that CRP did not correlate with TAO activity. So the correlation between MHR and TAO activity might be not only related to general inflammation, but also affected by the type of inflammation. In this study, ROC curve evaluation showed the optimal cutoff value of MHR as a predictor for evaluating disease activity of TAO was 0.198, with a sensitivity of 85.5% and a specificity of 44.4%. The obtained sensitivity is pretty strong, while the specificity is obviously poor. Possible reasons for this may be the followings: 1) the sample size was not sufficiently large. 2) CAS score was the only indicator in this study when performing the clinical assessment.
This study still has some weaknesses. This was a retrospective cross-sectional study, so no causative relationships can be established here. Additional longitudinal studies involving a bigger number of participants are needed.
To conclude, the present study is the largest sample size retrospective review that identify the MHR positive association with the disease activity of TAO, supporting the application of MHR as an effective complementary predictor to assess the disease activity of TAO, and consequently adding value to standard CAS scoring parameters.
Footnotes
Authors' contributions: Sun XH and Zhang XW were co-first authors and contributed to the study design, data acquisition, data analysis, data interpretation and drafting the manuscript. Han C, Su MR contributed to the data acquisition. Dou X and He XY contributed to the data interpretation. Jiang F and Yuan ST contributed to the study design, and data interpretation. All authors approved the final version submitted for publication.
Foundation: Supported by the Special Fund for Clinical Research of Nanjing Drum Tower Hospital (No.2023-LCYJ-PY-37).
Conflicts of Interest: Sun XH, None; Zhang XW, None; Han C, None; Dou X, None; He XY, None; Su MR, None; Jiang F, None; Yuan ST, None.
REFERENCES
- 1.Perros P, Kendall-Taylor P. Thyroid-associated ophthalmopathy: pathogenesis and clinical management. Baillière's Clin Endocrinol Metab. 1995;9(1):115–135. doi: 10.1016/s0950-351x(95)80867-1. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735–1748. doi: 10.1167/iovs.14-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012;96(2):311–328. doi: 10.1016/j.mcna.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas RS, Gupta S. The pathophysiology of thyroid eye disease: implications for immunotherapy. Curr Opin Ophthalmol. 2011;22(5):385–390. doi: 10.1097/ICU.0b013e3283499446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soeters MR, van Zeijl CJ, Boelen A, Kloos R, Saeed P, Vriesendorp TM, Mourits MP. Optimal management of Graves orbitopathy: a multidisciplinary approach. Neth J Med. 2011;69(7):302–308. [PubMed] [Google Scholar]
- 6.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM, European Group on Graves' Orbitopathy (EUGOGO) The 2016 European thyroid association/European group on Graves' orbitopathy guidelines for the management of Graves' orbitopathy. Eur Thyroid J. 2016;5(1):9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein A, Schittkowski M, Esser J. Surgical treatment of Graves' ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26(3):339–358. doi: 10.1016/j.beem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana S, Murakami T, Noguchi H, Noguchi Y, Nakashima A, Ohyabu Y, Noguchi S. Orbital magnetic resonance imaging combined with clinical activity score can improve the sensitivity of detection of disease activity and prediction of response to immunosuppressive therapy for Graves' ophthalmopathy. Endocr J. 2010;57(10):853–861. doi: 10.1507/endocrj.k10e-156. [DOI] [PubMed] [Google Scholar]
- 10.Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2):168–199. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol. 2001;55(3):283–303. doi: 10.1046/j.1365-2265.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Su Y, Jiang M, Fang S, Huang Y, Li Y, Zhong S, Wang Y, Zhang S, Wu Y, Sun J, Fan X, Zhou H. Application of magnetic resonance imaging in the evaluation of disease activity in Graves' ophthalmopathy. Endocr Pract. 2021;27(3):198–205. doi: 10.1016/j.eprac.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardo C, Radellini S, Morreale Bubella R, Falanga G, Richiusa P, Vadalà M, Ciresi A, Midiri M, Giordano C. Lacrimal gland herniation in Graves ophthalmopathy: a simple and useful MRI biomarker of disease activity. Eur Radiol. 2020;30(4):2138–2141. doi: 10.1007/s00330-019-06570-5. [DOI] [PubMed] [Google Scholar]
- 14.Onoe S, Maeda A, Takayama Y, Fukami Y, Takahashi T, Uji M, Kaneoka Y. The prognostic impact of the lymphocyte-to-monocyte ratio in resected pancreatic head adenocarcinoma. Med Princ Pract. 2019;28(6):517–525. doi: 10.1159/000501017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258(2):301–305. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 16.Inonu Koseoglu H, Pazarli AC, Kanbay A, Demir O. Monocyte count/HDL cholesterol ratio and cardiovascular disease in patients with obstructive sleep apnea syndrome: a multicenter study. Clin Appl Thromb Hemost. 2018;24(1):139–144. doi: 10.1177/1076029616677803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganjali S, Gotto AM, Jr, Ruscica M, Atkin SL, Butler AE, Banach M, Sahebkar A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–9246. doi: 10.1002/jcp.27028. [DOI] [PubMed] [Google Scholar]
- 18.Yılmaz Tuğan B, Ergen A, Özkan B. Monocyte-to-high-density lipoprotein ratio and systemic immune-inflammation index: potential parameters for the evaluation of disease activity and severity in Graves' ophthalmopathy? Int Ophthalmol. 2024;44(1):154. doi: 10.1007/s10792-024-03077-x. [DOI] [PubMed] [Google Scholar]
- 19.Akboga MK, Balci KG, Maden O, Ertem AG, Kirbas O, Yayla C, Acar B, Aras D, Kisacik H, Aydogdu S. Usefulness of monocyte to HDL-cholesterol ratio to predict high SYNTAX score in patients with stable coronary artery disease. Biomark Med. 2016;10(4):375–383. doi: 10.2217/bmm-2015-0050. [DOI] [PubMed] [Google Scholar]
- 20.Bolayir A, Gokce SF, Cigdem B, Bolayir HA, Yildiz OK, Bolayir E, Topaktas SA. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol Neurochir Pol. 2018;52(2):150–155. doi: 10.1016/j.pjnns.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Dincgez Cakmak B, Dundar B, Ketenci Gencer F, Aydin BB, Yildiz DE. TWEAK and monocyte to HDL ratio as a predictor of metabolic syndrome in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(1):66–71. doi: 10.1080/09513590.2018.1490401. [DOI] [PubMed] [Google Scholar]
- 22.Onalan E. The relationship between monocyte to high-density lipoprotein cholesterol ratio and diabetic nephropathy. Pak J Med Sci. 2019;35(4):1081–1086. doi: 10.12669/pjms.35.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaggio S, Abate A, Brugaletta G, et al. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int J Mol Med. 2020;46(3):1210–1216. doi: 10.3892/ijmm.2020.4644. [DOI] [PubMed] [Google Scholar]
- 24.Ulusoy EK, Bolattürk ÖF, Göl MF. Relation Between the novel marker monocyte to high-density lipoprotein cholesterol ratio and severity in multiple sclerosis. Ann Indian Acad Neurol. 2020;23(3):275–279. doi: 10.4103/aian.AIAN_249_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Pang Y, Li X, Zha B, He T, Ding H. Monocyte to high-density lipoprotein cholesterol ratio as an independent risk factor for papillary thyroid carcinoma. J Clin Lab Anal. 2021;35(11):e24014. doi: 10.1002/jcla.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XZ, Wang JM, Ji YX, Zhao DB. Monocyte-to-high-density lipoprotein cholesterol ratio is associated with the presence and size of thyroid nodule irrespective of the gender. Lipids Health Dis. 2020;19(1):36. doi: 10.1186/s12944-020-1196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das T, Roos JCP, Patterson AJ, Graves MJ, Murthy R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye(Lond) 2019;33(2):235–243. doi: 10.1038/s41433-018-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]