Abstract
AIM
To study functional brain abnormalities in patients with eye trauma (ET) and to discuss the pathophysiological mechanisms of ET.
METHODS
Totally 31 ET patients and 31 healthy controls (HCs) were recruited. The age, gender, and educational background characteristics of the two groups were similar. After functional magnetic resonance imaging (fMRI) scanning, the subjects' spontaneous brain activity was evaluated with the functional connectivity (FC) method. Receiver operating characteristic (ROC) curve analysis was used to classify the data. Pearson's correlation analysis was used to explore the relationship between FC values in specific brain regions and clinical behaviors in patients with ET.
RESULTS
Significantly increased FC between several regions was identified including the medial prefrontal cortex (MPFC) and left hippocampus formations (HF), the MPFC and left inferior parietal lobule (IPL), the left IPL and left medial temporal lobe (MTL), the left IPL and right MTL, and the right IPL and left MTL. No decreased region-to-region connectivity was detected in default mode network (DMN) sub-regions in patients with ET. Compared with HCs, ET patients exhibited significantly increased FC between several paired DMN regions, as follows: posterior cingulate cortex (PCC) and right HF (HF.R, t=2.196, P=0.032), right inferior parietal cortices (IPC.R) and left MTL (MTL.L, t=2.243, P=0.029), and right MTL (MTL.R) and HF.R (t=2.236, P=0.029).
CONCLUSION
FC values in multiple brain regions of ET patients are abnormal, suggesting that these brain regions in ET patients may be dysfunctional, which may help to reveal the pathophysiological mechanisms of ET.
Keywords: eye trauma, functional connectivity, brain region
INTRODUCTION
Eye trauma (ET) is one of the most common factors causing blindness. Epidemiological studies indicate that the incidence of ET in North China is 1.6%±0.2%[1], and it accounts for 16%–35% of ophthalmic inpatients[2]. Eye injuries are often accompanied by multiple types of ocular tissue damage, such as suprachoroidal hemorrhage, retinal detachment, traumatic cataract, and lens dislocation, among others[3]–[6]. In addition, most patients with ET also have damage to the extraocular muscles, and symptoms such as pain, blindness, or loss of vision, and eye movement disorders[7]. At present, patients with ET are generally treated using surgery[8]–[9]; however, due to damage to nerves, muscles, and other structures, caused by ocular trauma, the effects of ET on various regions of the brain are challenging to assess, and the therapeutic effects following surgical treatment difficult to determine. Changes in various brain regions can be determined by resting-state functional magnetic resonance imaging (rs-fMRI) and quantified using various algorithms, to analyze the possible effects of disease states on the brain.
The default mode network (DMN) is an organized functional network that spans several anatomic brain regions, including the medial temporal lobe (MTL), posterior cingulate cortex (PCC), medial prefrontal cortex (MPFC), bilateral inferior parietal cortices (IPC), and precuneus[10]–[11]. When the brain activity was first measured in individuals with undirected mental states, researchers made interesting discoveries relating to the DMN. Subsequently, researchers have compared activity in the DMN during tasks when the brain is quiet or active, using positron emission tomography[12]. With more in-depth understanding of the DMN and the brain, and more frequent use of fMRI, study of changes in cerebral cortex DMN features associated with disease has become a hot research topic.
Given the unique tracking and positioning ability of rs-fMRI and its flexibility, this method is often used to explore neuron activity in the brain, with the aim of understanding disease pathophysiology[13]. Assessment of functional connectivity (FC), is an rs-fMRI technique that allows unbiased analysis[14]. There has been some research exploring the changes in specific brain areas in patients who have experienced ocular trauma; for example, blindness caused by ET is associated with increased homogeneity in the cerebral occipital region[15]. Further, another study showed that patients with blindness caused by ET had significant changes in the primary somatosensory area and the primary visual cortex[16]. Other research on the DMN is ongoing, including in heroin users, individuals with schizophrenia, and people experiencing sleep deprivation, social phobia, sensorineural hearing loss, aging and dementia, cognitive impairment, and subthreshold depression, among other conditions[17]–[23]; however, few studies have focused on patients with eye diseases. Researchers have also reported that visual distractors caused larger pre-responsive interference with auditory processing, and vice versa, demonstrating visual dominance at the pre-responsive level. Nevertheless, the correlation of specific regional pathological changes in the cerebral cortex DMN features of ET patients has not been evaluated. In this study, we explored alterations in brain function following ET by evaluating changes in region-to-region seed-based FC.
PARTICIPANTS AND METHODS
Ethical Approval
All research methods were approved by the Ophthalmic Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. The purpose, method, and potential risks of the study were explained to all subjects, who provided signed informed consent.
ET patients (n=31; 24 males and 7 females; mean age, 45.26±13.62y) were recruited from the Ophthalmology Department of our Hospital. Healthy controls (HCs, n=31; 24 males and 7 females; mean age, 46.07±11.59y), who were age, sex, and education status-matched to the ET patients, were also recruited. The diagnostic criteria for ET were: 1) history of ocular trauma; 2) incomplete orbital wall examination by orbital CT or MRI scan; 3) significant changes in vision occurring after ocular trauma; 4) decreased intraocular pressure; 5) rupture of the cornea and sclera. Inclusion criteria for the HC group were: 1) head MRI showed no significant changes in brain parenchyma; 2) no history of eye diseases, such as strabismus, neuritis, diabetic retinopathy, glaucoma, cataract, or dry eye; 3) best corrected visual acuity (BCVA) >1.0; 4) no history of mental illness, such as depression; 5) able to undergo MRI scans, hence individuals with metal implants or cardiac pacemakers were excluded; 6) no systemic immune disease, such as systemic lupus erythematosus.
MRI Parameters
MRI scanning was conducted using a 3-Tesla MR scanner (Trio, Siemens, Erlangen, Germany) to collect the following data, using the indicated parameters:
1) T1-weighted images (n=176): a) a three-dimensional spoiled gradient-recalled sequence; b) thickness=1.0 mm; c) repetition time=1900ms; d) gap=0.5 mm; e) echo time=2.26ms.
2) 240 functional images: a) gradient-recalled echo-planar imaging pulse sequence; b) thickness=4.0 mm; c) repetition time=2000ms; d) gap=1.2 mm; e) echo time=30ms.
Data Preprocessing
Data quality filtering was conducted using MRIcro software (www.MRIcro.com), as previously described[24]–[32]. The following measures were applied: 1) functional images for the first ten time points were discarded; 2) data with x, y, or z motion rotation with maximum translation >1.5 mm were rejected; 3) head-motion effects were removed using the Friston six head-motion parameters; 4) sources of spurious covariates were removed using linear regression; 5) head-motion correction; 6) resampling: resolution of 3×3×3 mm; 7) data were linearly detrended and the time series filtered (bandpass 0.01–0.08 Hz).
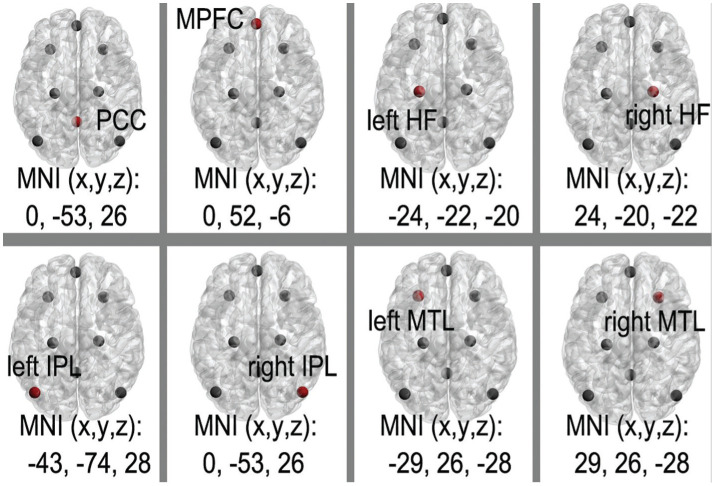
Definition of DMN seed regions and FC analysis
The eight canonical core DMN regions are: PCC; MPFC; and the bilateral hippocampus formations (HF), MTL, and IPC (Table 1, Figure 1)[33]–[35]. By placing spherical seeds (r=6 mm), the average time courses for each of the eight regions from each subject were defined and extracted. Paired connectivity data points (n=28) were yielded by computing the correlation coefficients (r scores) between DMN regions. Fisher's r-to-Z was used to transform and standardize the statistical analysis, after conversion of correlation coefficients to Z-values.
Table 1. The coordinates of the definition of the DMN subregions.
| Region | Abbreviation | MNI |
||
| X | Y | Z | ||
| Posterior cingulate cortex | PCC | 0 | -53 | 26 |
| Medial prefrontal cortex | MPFC | 0 | 52 | -6 |
| Hippocampal formation | HF.L | -24 | -22 | -20 |
| HF.R | 24 | -20 | -22 | |
| Inferior parietal cortices | IPC.L | -47 | ||
| IPC.R | 47 | -57 | 20 | |
| Medial temporal lobe | MTL.L | -29 | 26 | -28 |
| MTL.R | 29 | 26 | -28 | |
The coordinate of the eight canonical core regions within the DMN. DMN: Default mode network; MNI: Montreal Neurological Institute; R: Right; L: Left; PCC: Posterior cingulate cortex; MPFC: Medial prefrontal cortex; HF: Hippocampus formations; IPC: Inferior parietal cortices; MTL: Medial temporal lobe.
Figure 1. The different functional connectivity of the DMN between the eye trauma and healthy controls group.
The different colored dots represent different nodes; The eight canonical core DMN regions are: PCC, MPFC, and the bilateral HF, MTL, and IPL. DMN: Default mode network; MNI: Montreal neurology Institute; PCC: Posterior cingulate cortex; MPFC: Medial prefrontal cortex; HF: Hippocampus formations; IPL: Inferior parietal lobule; MTL: Medial temporal lobe.
Correlation Analysis
To investigate the relationships between the clinical measures and region-to-region FC strength within the DMN in patients with ET, Z-values of the temporal correlation coefficients of the different paired connectivity values between the ET and HC groups were analyzed, based on data from clinical questionnaires, using Pearson correlation analysis. The significance level threshold was set at P<0.05.
Statistical Analysis
For evaluation of clinical data, the two-sample Student's t-test (homoscedasticity) and Mann-Whitney U-test (heteroscedasticity) were used for analysis of continuous data. All calculated P-values were two-tailed. P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 20.0 statistical software.
RESULTS
Demographic and Clinical Measurements
There were no significant differences in age (P=0.978), best-corrected visual acuity of the contralateral eye (P=0.921), or intraocular pressure (P=0.849) between the ET and HC groups (Table 2).
Table 2. Demographics and behavioral results of ET and HC groups.
| Parameters | ETs | HCs | t | P |
| Male/female | 24/7 | 24/7 | N/A | N/A |
| Age (y) | 45.26±13.62 | 46.07±11.59 | 0.964 | 0.978 |
| Handedness | 31 right | 31 right | N/A | N/A |
| Duration (d) | 0.18±0.06 | N/A | N/A | N/A |
| BCVA-contralateral eye | 0.80±0.11 | 0.92±0.17 | 0.912 | 0.921 |
| IOP-contralateral eye | 14.90±2.76 | 16.12±2.88 | 0.957 | 0.849 |
Independent t-tests comparing the two groups (P<0.05 represented statistically significant differences). Data shown as mean±standard deviation or n. ET: Eye trauma; HC: Healthy control; N/A: Not applicable; BCVA: Best-corrected visual acuity; IOP: Intraocular pressure.
Functional Connectivity Results
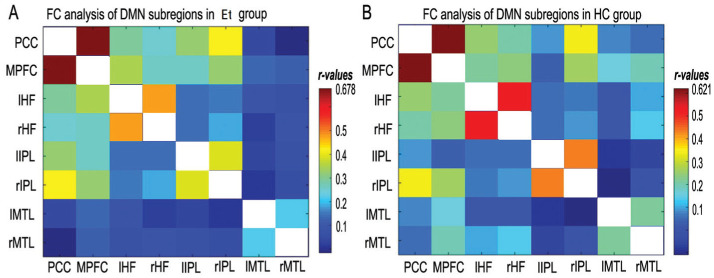
All 28 groups of DMN regions exhibited strong connections to one another in both the ET and HC groups (Figure 2). Compared with HCs, ET patients exhibited significantly increased FC between several paired DMN regions, as follows: PCC and right HF (HF.R, t=2.196, P=0.032), right IPC (IPC.R) and left MTL (MTL.L, t=2.243, P=0.029), and right MTL (MTL.R) and HF.R (t=2.236, P=0.029, Figure 3).
Figure 2. The correlation matrix of subregions of the mean time series of DMN.
A: The correlation matrix of the subregions in the mean time series of the ET group; B: The correlation matrix of the subregions in the mean time series of the HC group. The photo represents DMN as the result of the subregions' FC. Different colors represent different connection coefficients. HC: Healthy control; FC: Functional connectivity; ET: Eye trauma; HC: Healthy control; DMN: Default mode network; PCC: Posterior cingulate cortex; MPFC: Medial prefrontal cortex; L: Left; R: Right; HF: Hippocampus formations; IPL: Inferior parietal lobule; MTL: Medial temporal lobe.
Figure 3. The different functional connectivity of the DMN between the ET and HC group.
There were significant differences between ET and HC subjects. The different colored dots represent different nodes; the red lines denote stronger correlations in ET group at the threshold. P<0.05. ET: Eye trauma; HC: Healthy control; DMN: Default mode network; PCC: Posterior cingulate cortex; MPFC: Medial prefrontal cortex; HF: Hippocampus formations; IPL: Inferior parietal lobule; MTL: Medial temporal lobe.
ROC Curve Analysis
The mean FC values of the distinct paired cerebrum areas were analyzed using the ROC curve method to determine whether they could be used to distinguish between the ET and HC groups. The individual area under the curve (AUC) values for analyses of FC between the paired regions exhibiting significant differences were as follows: PCC-HF.R, 0.634 (P=0.070); IPC.R-MTL.L, 0.661 (P=0.030); MTL.R-HF.R, 0.636 (P=0.066; Figure 4).
Figure 4. ROC curve analysis of the FC values in each paired cerebrum areas.
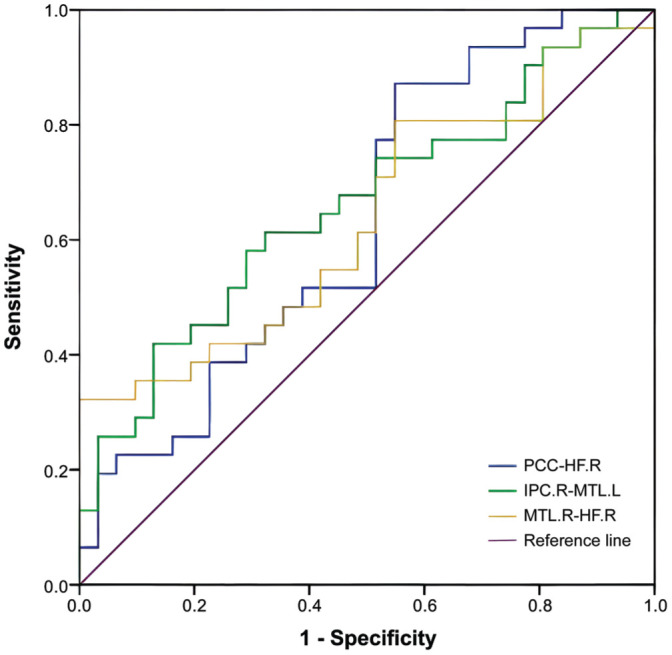
The area under the ROC curve for PCC-HF.R was 0.634 (P=0.070); IPC.R-MTL.L, 0.661 (P=0.030); MTL.R-HF.R, 0.636 (P=0.066). ROC: Receiver operating characteristic; AUC: Area under the curve; FC: Functional connectivity; PCC: Posterior cingulate cortex; MPFC: Medial prefrontal cortex; L: Left; R: Right; IPC: Inferior parietal cortices; HF: Hippocampus formations; MTL: Medial temporal lobe.
DISCUSSION
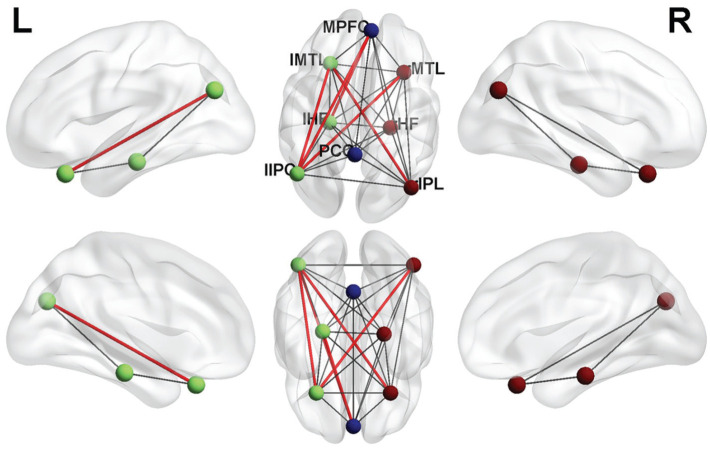
To investigate the differences in FC between DMN subregions in patients with ET, we conducted the first study using the region-to-region FC method, with the aim of improving understanding of the neural mechanisms underlying ET. We identified significantly increased FC between several regions including: the MPFC and the left HF, the MPFC and the left inferior parietal lobule (IPL), the left IPL and the left MTL, the left IPL and the right MTL, and the right IPL and the left MTL (Figure 5). No decreased region-to-region connectivity was detected in DMN sub-regions in patients with ET.
Figure 5. FC results of DMN in the eye trauma group.
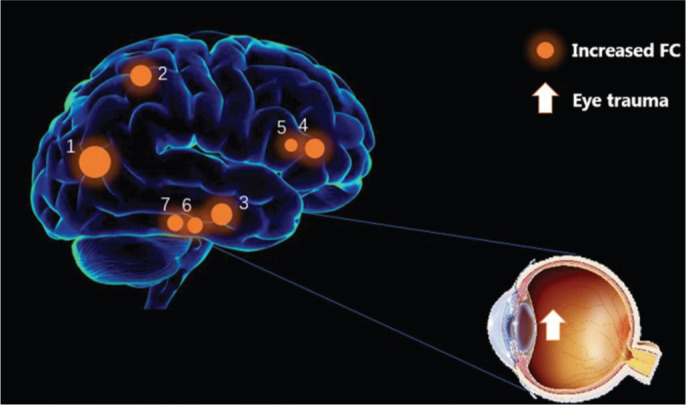
Compared with the HC group, the FC was increased to various extents: the MPFC and the left HF, the MPFC and the left IPL, the left IPL and the left MTL, the left IPL and the right MTL, and the right IPL and the left MTL in eye trauma patients. The sizes of the spots represent the degree of quantitative changes. FC: Functional connectivity; DMN: Default mode network; HC: Healthy control; HF: Hippocampus formations; MPFC: Medial prefrontal cortex; IPL: Inferior parietal lobule; MTL: Medial temporal lobe.
It has been known since the late 19th century that mental activity modulates local blood flow[36]–[38]. Recently, it was determined that the metabolism remains constant when individuals performed an activity[39], indicating that the brain exhibits persistent activity in the resting state[10]. The DMN is part of the brain that exhibits metabolic properties unlike those of other brain systems[40]–[41]. Studies have shown that DMN functions allow flexible mental exploration (i.e., simulations), before an activity occurs, facilitating preparation for upcoming, self-relevant events[42]. Brown pointed out that brain functions are intrinsic, and involve the acquisition and maintenance of information[43]–[44]; therefore, in cognitive neuroscience, particularly attention has been paid to the resting-state networks of the DMN[41],[43].
Using rs-fMRI studies to investigate DMN activity can diagnose the early stages of Alzheimer's disease, where specific regional reductions in the coherence of low-frequency signal fluctuations in the precuneus have been discovered. By evaluating the coherence of low-frequency signal fluctuations using rs-fMRI to study DMN activity, Rektorova et al[44] identified regionally specific reductions in the precuneus. Subsequently, studies of Parkinson's disease (PD), which involves saccadic eye movement, showed that, functional resting-state connectivity was altered between the mid-line regions of the DMN. Further, at different stages of PD, the DMN connectivity patterns relating to visual processes exhibited specific changes localized to the PCC and MTL. In particular, the functional resting-state connectivity of the DMN in the vertical direction is involved in PD-associated saccadic hypometria[34].
Although the DMN has been studied in the context of mental and neurological disorders, little is known about changes in the characteristics of the DMN in patients with ocular diseases. Limited studies have shown that physiological activities of the eyes, such as blinking, fixation, and movement, can lead to characteristic physiological changes in the cerebral cortex. Using resting-state fMRI, Ramot et al[45] detected electro-ocular activity when subjects closed their eyes. Further, Nakano et al[46] found that brain activity in the DMN increased following spontaneous involuntary eye blinks; however, when ET occurs, the physiological activity of the eye is certain to be severely affected, thereby changing the characteristics of the cerebral cortex. One study discovered that the frontal eye field, supplementary eye field, intraparietal sulcus, precuneus, and the anterior and posterior cingulate cortices were activated during changes in physiological eye activities[47]. Subsequently, Fransson employed resting-state functional magnetic imaging to study the relationship between physiological eye and brain activities, and showed that the eyes physiological activities led to changes in the bilateral occipitotemporal cortex, supplementary motor cortex, and frontal eye fields. These findings indicate that ocular trauma damages physiological activities relevant to the eye, causing changes in midline cortical brain regions, located in the posteromedial parietal cortex and MPFC[48]. Intriguingly, brain structures in the DMN include the posteromedial parietal cortex and the MPFC, which are key nodes[42]. Given the visual impairment of patients with ocular trauma, our results may indicate that the MPFC and IPL undergo significant quantitative changes in response to visual impairment. When eye injury also causes damage to the patient's eye-related tissue, it can influence the normal physiological activities of eye-related structures, representing pathological changes that are reflected in variations in the relevant brain regions.
ET is also associated with pain, extraocular muscle injury, and dysfunction, as well as neurological abnormalities[49]. Further, ET also inevitably causes damage to various structural eye tissues, seriously affecting the physiological functions of the eye, including movement, vision, and feeling. Damage to various eye structures can result in changes in the DMN region, and patients with ET may also exhibit DMN-specific alterations[50]. Previous studies have linked changes in eye function to alterations in neuronal connections[51], and impacts on eye function can affect the binocular properties of neurons in the primary visual fields; however, relevant research on characteristic cerebral cortex DMN pathological changes in patients with ET are lacking. Our study is the first to evaluate FC between paired DMN subregions with the aim of determining the abnormal changes in the cerebral cortex motor area in patients with ET.
The BA17 area is part of the primary visual cortex, which is the first region to receive visual information in this part of the brain[52]–[53]. Further, the advanced visual cortex can mediate conscious intuition, after synthesizing visual information; however, in our study, we did not detect any decrease in region-to-region connectivity in DMN sub-regions in patients with ET. Hence, there is no reduction of information or enhanced stimulation to the senior cortex in patients with ET, which does not appear to impair the function of various DMN subregions. This abnormal connectivity may underline the decline in emotional or cognitive ability observed in patients with ET. Further, we did not detect any significant correlations of abnormal FC between paired DMN subregions with clinical parameters. Nevertheless, our results reveal alterations in FC between intrinsic DMN subregions, which will assist analysis of the mechanisms underlying ET. Integrative comparisons of the types and degree of ET would provide additional information regarding how ET, particularly extraocular muscle injury caused by ocular trauma, can affect FC in DMN subregions.
This study has some limitations. First, the number of samples used to study DMN in patients with ET was relatively small. Second, we have not excluded the influence of psychological or physiological abnormalities due to ocular trauma on inter-regional FC. Third, the changes in the cerebral cortex motor area in patients with ET are likely to be related to different types of ocular trauma, as well as the course and severity of the condition, which were not considered in our analyses. Future research should conduct a more comprehensive analysis of the DMN with regards to the afore mentioned issues.
In conclusion, changes in the FC between brain areas, including the DMN, can be evaluated using rs-fMRI, and reflect the relationships between various eye diseases and related brain regions, which have been evaluated in other studies (Table 3[34],[44],[46],[52],[54]–[55]). ET damages the normal physiological activit1y of the eye, thereby disrupting FC within DMN subregions.
Table 3. DMN method applied in ophthalmological objects.
| Author | Year | Object of study | The brain areas responding to the object |
| Gorges et al[34] | 2013 | Ocular reactive saccades, smooth pursuit, and executive tests | Medial prefrontal cortex, medial temporal lobe, posterior cingulate cortex, etc. |
| Rektorova et al[44] | 2014 | Visual processing in Parkinson's disease | The bilateral middle temporal/middle occipital gyri, etc. |
| Nakano et al [46] | 2013 | Blink | The hippocampus, the orbitalfrontal cortex, the caudate nucleus, etc. |
| Jorge et al[52] | 2018 | Visual stimulation | Visual cortex, auditory cortex and superior parietal lobule, etc. |
| Zhang et al[54] | 2018 | The resting state and a mental rotation task state | Primary visual area, prefrontal cortex and the medial temporal lobe, etc. |
| Buchweitz et al[55] | 2019 | Dyslexic | Posterior cingulate cortex, anterior cingulate cortex, visual word form area |
Footnotes
Foundations: Supported by National Natural Science Foundation of China (No.82160195; No.82460203); Key R&D Program of Jiangxi Province (No.20223BBH80014); Science and Technology Project of Jiangxi Province Health Commission of Traditional Chinese Medicine (No. 2022B258); Science and Technology Project of Jiangxi Health Commission (No.202210017).
Conflicts of Interest: Xing ZM, None; Song D, None; Hu JY, None; Zhou XM, None; Liao X, None; Chen C, None; Wei H, None; Kang M, None; Ling Q, None; He LQ, None; Liu ZZ, None; Zou J, None; Chen X, None; Wu ZK, None; Shao Y, None.
REFERENCES
- 1.Wang JD, Xu L, Wang YX, You QS, Zhang JS, Jonas JB. Prevalence and incidence of ocular trauma in North China: the Beijing eye study. Acta Ophthalmol. 2012;90(1):e61–7. doi: 10.1111/j.1755-3768.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 2.Cai MM, Zhang J. Epidemiological characteristics of work-related ocular trauma in southwest region of China. Int J Environ Res Public Health. 2015;12(8):9864–9875. doi: 10.3390/ijerph120809864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Li HJ, Ye L, Zhang Y, Wei R, Zhong YL, Peng DC, Shao Y. Altered regional homogeneity in patients with unilateral acute open-globe injury: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2016;12:1901–1906. doi: 10.2147/NDT.S110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nawani N, Vazirani J, Ojha H, Sangwan VS. Conjunctival pedicle flap in management of open globe injury with corneal tissue loss. BMJ Case Rep. 2016;2016:bcr2015213703. doi: 10.1136/bcr-2015-213703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MP, Colyer MH, Weichel ED, Stutzman RD. Traumatic cataracts secondary to combat ocular trauma. J Cataract Refract Surg. 2015;41(8):1693–1698. doi: 10.1016/j.jcrs.2014.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Koksaldi S, Utine CA, Kayabasi M. Management of suprachoroidal hemorrhage during cataract surgery: a case report. Beyoglu Eye J. 2022;7(1):66–70. doi: 10.14744/bej.2021.50455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Négrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiol. 1998;5(3):143–169. doi: 10.1076/opep.5.3.143.8364. [DOI] [PubMed] [Google Scholar]
- 8.Heidari E, Taheri N. Surgical treatment of severely traumatized eyes with no light perception. Retina. 2010;30(2):294–299. doi: 10.1097/IAE.0b013e3181babd75. [DOI] [PubMed] [Google Scholar]
- 9.Feng K, Hu YT, Ma ZZ. Prognostic indicators for no light perception after open-globe injury: eye injury vitrectomy study. Am J Ophthalmol. 2011;152(4):654–662.e2. doi: 10.1016/j.ajo.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 12.Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 13.Bruera MG, Benedetto MM, Guido ME, Degano AL, Contin MA. 16 Glial cell response to constant low light exposure in rat retina. Visual Neuroscience. 2022;39:E005. doi: 10.1017/S0952523822000049. [DOI] [PubMed] [Google Scholar]
- 14.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 15.Burge WK, Griffis JC, Nenert R, Elkhetali A, DeCarlo DK, ver Hoef LW, Ross LA, Visscher KM. Cortical thickness in human V1 associated with central vision loss. Sci Rep. 2016;6:23268. doi: 10.1038/srep23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii T, Tanabe HC, Kochiyama T, Sadato N. An investigation of cross-modal plasticity of effective connectivity in the blind by dynamic causal modeling of functional MRI data. Neurosci Res. 2009;65(2):175–186. doi: 10.1016/j.neures.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr Res. 2011;125(2-3):101–109. doi: 10.1016/j.schres.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 18.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59(2):1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M. Beyond amygdala: default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res Bull. 2009;79(6):409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang GY, Yang M, Liu B, Huang ZC, Chen H, Zhang PP, Li J, Chen JY, Liu LJ, Wang J, Teng GJ. Changes in the default mode networks of individuals with long-term unilateral sensorineural hearing loss. Neuroscience. 2015;285:333–342. doi: 10.1016/j.neuroscience.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JW, Xin SC, Ou YM, et al. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J Psychiatr Res. 2016;76:111–120. doi: 10.1016/j.jpsychires.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papma JM, den Heijer T, de Koning I, et al. The influence of cerebral small vessel disease on default mode network deactivation in mild cognitive impairment. Neuroimage Clin. 2012;2:33–42. doi: 10.1016/j.nicl.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q, Zhou XL. Vision dominates at the preresponse level and audition dominates at the response level in cross-modal interaction: behavioral and neural evidence. J Neurosci. 2013;33(17):7109–7121. doi: 10.1523/JNEUROSCI.1985-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan CG, Cheung B, Kelly C, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, Wang YX. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–2175. doi: 10.2147/NDT.S69681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015;11:207–214. doi: 10.2147/NDT.S73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Zhong YL, Zeng XJ, Zhou FQ, Liu XH, Hu PH, Pei CG, Shao Y, Dai XJ. Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI study. Neuropsychiatr Dis Treat. 2015;11:1877–1883. doi: 10.2147/NDT.S87596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:2015–2020. doi: 10.2147/NDT.S110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2(1):25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai XJ, Gong HH, Wang YX, Zhou FQ, Min YJ, Zhao F, Wang SY, Liu BX, Xiao XZ. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13(6):720–727. doi: 10.1016/j.sleep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Qi RF, Zhang LJ, Xu Q, Liang X, Luo S, Zhang ZQ, Huang W, Zheng L, Lu GM. Abnormal functional connectivity within the default mode network in patients with HBV-related cirrhosis without hepatic encephalopathy revealed by resting-state functional MRI. Brain Res. 2014;1576:73–80. doi: 10.1016/j.brainres.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 34.Gorges M, Müller HP, Lulé D, Ludolph AC, Pinkhardt EH, Kassubek J. Functional connectivity within the default mode network is associated with saccadic accuracy in Parkinson's disease: a resting-state FMRI and videooculographic study. Brain Connect. 2013;3(3):265–272. doi: 10.1089/brain.2013.0146. [DOI] [PubMed] [Google Scholar]
- 35.Zhang DY, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 36.Min YL, Su T, Shu YQ, Liu WF, et al. Altered spontaneous brain activity patterns in strabismus with amblyopia patients using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:2351–2359. doi: 10.2147/NDT.S171462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu YY, Yuan Q, Li B, Lin Q, Zhu PW, Min YL, Shi WQ, Shu YQ, Zhou Q, Shao Y. Altered spontaneous brain activity patterns in patients with retinal vein occlusion indicated by the amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Exp Ther Med. 2019;18(3):2063–2071. doi: 10.3892/etm.2019.7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L, Ma MY, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–257. doi: 10.2147/NDT.S150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest. 1948;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–90;discussion 1097-9. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 41.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 42.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 43.Yuste R, MacLean JN, Smith J, Lansner A. The cortex as a central pattern generator. Nat Rev Neurosci. 2005;6(6):477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- 44.Rektorova I, Krajcovicova L, Marecek R, Novakova M, Mikl M. Default mode network connectivity patterns associated with visual processing at different stages of Parkinson's disease. J Alzheimers Dis. 2014;42(Suppl 3):S217–S228. doi: 10.3233/JAD-132684. [DOI] [PubMed] [Google Scholar]
- 45.Ramot M, Wilf M, Goldberg H, Weiss T, Deouell LY, Malach R. Coupling between spontaneous (resting state) fMRI fluctuations and human oculo-motor activity. NeuroImage. 2011;58(1):213–225. doi: 10.1016/j.neuroimage.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Nakano T, Kato M, Morito Y, Itoi S, Kitazawa S. Blink-related momentary activation of the default mode network while viewing videos. Proc Natl Acad Sci U S A. 2013;110(2):702–706. doi: 10.1073/pnas.1214804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8(4):209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fransson P, Flodin P, Seimyr GÖ, Pansell T. Slow fluctuations in eye position and resting-state functional magnetic resonance imaging brain activity during visual fixation. Eur J Neurosci. 2014;40(12):3828–3835. doi: 10.1111/ejn.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barton JJS, Ranalli PJ. Vision therapy: ocular motor training in mild traumatic brain injury. Ann Neurol. 2020;88(3):453–461. doi: 10.1002/ana.25820. [DOI] [PubMed] [Google Scholar]
- 50.Olavarria JF. Callosal connections correlate preferentially with ipsilateral cortical domains in cat areas 17 and 18, and with contralateral domains in the 17/18 transition zone. J Comp Neurol. 2001;433(4):441–457. doi: 10.1002/cne.1152. [DOI] [PubMed] [Google Scholar]
- 51.Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21(10):3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jorge J, Figueiredo P, Gruetter R, van der Zwaag W. Mapping and characterization of positive and negative BOLD responses to visual stimulation in multiple brain regions at 7T. Hum Brain Mapp. 2018;39(6):2426–2441. doi: 10.1002/hbm.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olavarria JF, Qi HX, Takahata T, Kaas JH. Overall patterns of eye-specific retino-geniculo-cortical projections to layers III, IV, and VI in primary visual cortex of the greater galago (Otolemur crassicudatus), and correlation with cytochrome oxidase blobs. Vis Neurosci. 2022;39:E007. doi: 10.1017/S0952523822000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Zhang DL, Wang ZJ, Li JC, Lin YT, Chang S, Huang RW, Liu M. Intrinsic neural linkage between primary visual area and default mode network in human brain: evidence from visual mental imagery. Neuroscience. 2018;379:13–21. doi: 10.1016/j.neuroscience.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 55.Buchweitz A, Costa AC, Toazza R, et al. Decoupling of the occipitotemporal cortex and the brain's default-mode network in dyslexia and a role for the cingulate cortex in good readers: a brain imaging study of Brazilian children. Dev Neuropsychol. 2019;44(1):146–157. doi: 10.1080/87565641.2017.1292516. [DOI] [PubMed] [Google Scholar]