Abstract
Background
With crewed deep space exploration on the horizon, preparation for potential astronaut health crises in space missions has become vital. Administration of anaesthesia and analgesia presents many challenges owing to constraints specific to space (physiologic and ergonomic challenges associated with microgravity) and nonspecific factors (isolation and lack of supplies). Regional anaesthesia can be the safest option; however, we hypothesised that the ergonomics of microgravity would compromise ease and accuracy of nerve blocks.
Methods
We evaluated the feasibility of regional anaesthesia in a simulated microgravity environment (free-floating underwater conditions) using a meat (bovine muscle) model. Forty meat models were randomised for injection under simulated microgravity or normal gravity conditions. Success rates were determined by blinded assessors after injection. Parameters assessed included time to block, ease of image acquisition, and ease of needle placement.
Results
The median time to block in normal gravity was 27 (interquartile range 21–69) s vs 35 (interquartile range 22–48) s in simulated microgravity (P=0.751). Ease of image acquisition was similar in both conditions, as was ease of needle placement. There was no significant difference in the rate of accidental intraneural injections (5% vs 5%), with block success rates comparable in both scenarios (80% normal gravity vs 85% microgravity, P>0.999).
Conclusions
Regional anaesthesia appears feasible for experts in simulated microgravity despite the ergonomic challenges. Although our model has limitations and might not fully capture the complexities of actual space conditions, it provides a foundation for future research into anaesthesia and analgesia during deep space missions.
Keywords: microgravity, nerve block, regional anaesthesia, simulation, space medicine
Editor's key points.
-
•
Regional anaesthesia could be the most practical and safest option for anaesthesia and analgesia in space, but the ergonomics of microgravity might compromise ease and accuracy of nerve blocks.
-
•
The feasibility of ultrasound-guided regional anaesthesia in a simulated microgravity environment was compared with normal gravity conditions using a bovine muscle model.
-
•
The median time to block, ease of image acquisition, and ease of needle placement were similar in simulated microgravity and normal gravity conditions, with no difference in the rate of accidental intraneural injections or block success rate.
-
•
Regional anaesthesia appears feasible for experts in simulated microgravity despite the ergonomic challenges and might thus be useful for anaesthesia and analgesia during deep space missions.
The science and technology developed through the Mars Exploration Program and missions, so far only involving robotic exploration (e.g. Perseverance), pave the path for future crewed Mars missions.1 The Artemis programme, initiated by the US National Aeronautics and Space Administration (NASA), aspires to lunar exploration as early as 2025.2,3 Concurrently, private sector companies have set ambitious targets for crewed spaceflight and colonisation within this decade. As these missions push the boundaries of human presence beyond traditional orbital stations and low Earth orbit (LEO; ∼400 km), preparing for possible astronaut illnesses or injuries becomes paramount.4 Although the overall risk is low because of careful mission planning, severe traumatic injury is possible through multiple mechanisms. Even in microgravity, the mass of large objects is maintained, and so is their kinetic energy. Astronauts work with heavy equipment, vehicles, and accelerants, so crush injuries, fractures, lacerations, and burns are all possible. Although evacuating injured astronauts in LEO back to Earth might be feasible, the same cannot be said for deep space missions. Moreover, evacuation from LEO might not be possible depending on injury severity, patient stability, ability to enter the confined space of the re-entry vehicle safely, and other factors.5,6
Given these complexities, the focus is now on devising in situ medical intervention strategies for astronauts in the event of medical emergencies to avoid or before evacuation. Dealing with health crises in the vastness of space will require a limited crew to administer treatments, which might include anaesthesia and pain management, under the constraints of scarce resources and the challenges presented by the space environment.
Among the myriad challenges associated with administering general anaesthesia during spaceflight, microgravity emerges as a particularly daunting obstacle. Microgravity induces a spectrum of physiological changes that significantly complicate the safety and efficacy of the procedure.7, 8, 9, 10, 11, 12 For instance, cephalad fluid shifts can lead to heightened intracranial pressure and airway oedema.7,9,12 Altered vascular autonomic responses to stress, potentially diminished cardiac output, volume redistribution, and hypovolaemia complicate haemodynamic patterns in microgravity.10,11 Reduced gastric emptying escalates the risk of aspiration during airway management.9 Furthermore, microgravity undermines the ergonomics of numerous procedures, including tracheal intubation, rendering even expert practitioners challenged in free-floating positions.13,14 Even lower success rates would be expected among less experienced practitioners.15,16 The safety of general anaesthesia hinges on the utilisation of heavy and bulky equipment. This apparatus must withstand the stress of heavy G-forces during launch, and batteries face vulnerability in space because of radiation exposure. In addition, the delivery of pure oxygen within a confined environment poses a significant fire hazard.17 Given that during space flight, medical supplies are constrained by payload and fiscal considerations, and developed medical expertise might be sparse, it is imperative to simplify medical procedures.
Regional anaesthesia offers a viable solution to these challenges by providing pain relief or anaesthesia to specific body parts, circumventing the need for airway interventions and maintaining stable physiological conditions. Analgesia could be provided in the case of traumatic injury to the trunk (e.g. rib fractures) without reducing respiratory drive. Regional anaesthesia is particularly well suited for analgesia after limb trauma without compromising the cognitive function of the injured astronaut. As each crew member's role is pivotal for mission success, regional anaesthesia ensures their cognitive participation.
Studies suggest that ultrasound-guided brachial plexus blocks can be mastered relatively quickly, potentially even by nonmedical professionals.18 The tools required for regional anaesthesia are compact, with the ultrasound machine, a vital component, already present in the International Space Station's medical kit.19 However, the feasibility of regional anaesthesia in microgravity remains unverified. Given the difficulty of intubation in microgravity by free-floating airway experts,13,14 it is unclear to what degree the ergonomic challenges of microgravity will impact the performance of regional anaesthetic techniques.
To bridge this knowledge gap, this study was designed to assess the feasibility of regional anaesthesia in microgravity conditions compared with normal gravity. We hypothesised that the ergonomic challenges of simulated microgravity would significantly worsen the ease and accuracy of simulated peripheral nerve block performance.
Methods
This within-subjects cohort study assessed the ease and success rate of regional anaesthesia using a meat model in a microgravity analogue. The models were injected under two conditions, a microgravity environment analogue (free-floating underwater) and a normal earth gravity environment. Four investigators injected five models under each of the two conditions. The investigators had varying levels of experience in regional anaesthesia. One investigator was a fellowship-trained regional anaesthesiologist (>1000 blocks), two were senior residents at the time of the study (>50 blocks), and one was a consultant anaesthesiologist with moderate experience (>500 blocks). Later, blinded assessors scored the location of the injectate in the meat model to determine whether the injection was successful. This study was deemed not to require ethical review or ethics waiver by both Nova Scotia Health and Dalhousie University Research Ethics Boards because the research did not involve human participants, their data, or biological samples.
Meat model
The creation and validation of the models are described (Fig. 1).20 The meat models were prepared using a technique similar to that of Naraghi and colleagues.21 This involved using bovine muscle cut to ∼10×10×3 cm pieces with a single tendon to simulate a nerve. This technique had been shown to provide the highest fidelity of regional anaesthesia models currently available, except for cadavers, which were not feasible for our study purposes.22,23 The meat model was encased in ballistics gel, as described by Morrow and colleagues.24
Fig 1.
Meat model created from beef inside round and beef tendon and then encased in ballistics gel. (a) Lean beef about to be wrapped around the tendon. (b) Meat model encased in ballistics gel. (c) Study identifier numbers encased in gel. The Department of Anesthesia, Pain Management & Perioperative Medicine, Dalhousie University, Halifax, NS, Canada, holds the copyright to this image, and it is reproduced here with permission.
Randomisation and assignment
Each of the models was assigned a study identifier and then randomly assigned to one of the gravity conditions and to one of four study investigators based on a computer-generated random number. Each of the four investigators injected five models under normal gravity conditions and five models in a free-floating underwater (microgravity) environment. Two investigators performed the normal gravity injections before the microgravity condition, whereas the other two performed the microgravity injections first.
Injection procedure
Each study investigator aimed to deposit injectate in a ‘perineural’ position, defined as an interfascial injection adjacent to the tendon, resulting in staining of the outer edge of the tendon (Fig. 2). In both conditions, a wireless high-frequency (4–13 MHz) linear transducer (Clarius, Vancouver, BC, Canada) was used along with a phone inside of a diving case. These were secured together using a custom 3D-printed clamp. Using a 22G 50 mm echogenic needle (Pajunk, Geisingen, Germany), the investigator injected 1–2 ml of water mixed with methylene blue dye in the ‘perineural’ position.
Fig 2.
Meat and ballistics gel model ultrasound image and gross appearance after bisection. (a) Ultrasound image of perineural injection. (b) Gross appearance of perineural injection. (c) Perineural injection after removal from the gel. The Department of Anesthesia, Pain Management & Perioperative Medicine, Dalhousie University, Halifax, NS, Canada, holds the copyright to this image, and it is reproduced here with permission.
The investigator was allowed as much time as needed to acquire an image of the tendon inside the meat model. Time started when the ultrasound transducer touched the model and stopped when the operator removed the needle from the model. Because speaking was not possible in the underwater (microgravity) condition, the investigators were not permitted to speak during scanning or injection in the normal gravity condition either. Once the investigator was ready, they signalled their assistant to inject the dye using finger signals. The models were stored in a refrigerator for a minimum of 24 h to dry the models and allow the ink to settle.
Gravity conditions
An underwater environment was used to mimic the effect of microgravity.25 Underwater training using scuba diving techniques has been instrumental in preparing astronauts for space missions, providing a valuable simulation of microgravity conditions. This method offers a realistic environment for practicing tasks and manoeuvres in a buoyant, gravity-reduced setting. Although parabolic flight represents a superior option for simulating true microgravity, it is constrained by its high cost and limited time in free fall. Nonetheless, underwater training remains a cost-effective and practical alternative and has been used in studies evaluating airway techniques and cardiopulmonary resuscitation in microgravity.25,26
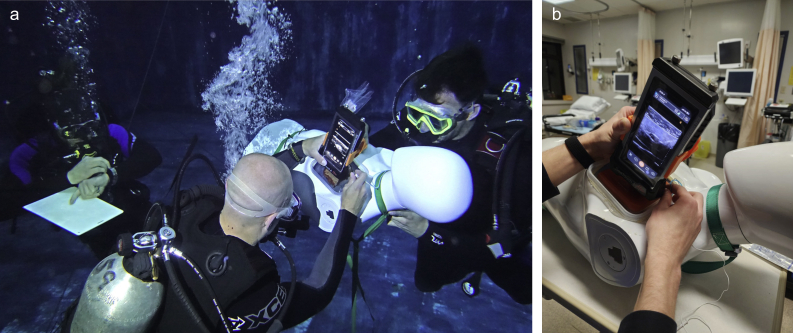
A mannikin was made more buoyant with the addition of internal rigid foam and tethered ∼2 m from the bottom (Fig. 3). The prepared meat model was positioned in the upper chest of the mannikin within a container. The investigators placing the block were free-floating, as was their assistant. The assistant's role was to stabilise the mannikin and the model in the mannequin, inject methylene blue when the injector gave the signal, and then signal that the injection was complete. The Injector obtained the ultrasound image, positioned the needle, signalled to inject, and removed the needle. The assistant was responsible for stabilising the mannikin to reduce the cognitive and physical demands on the person performing the simulated block. During two dives, each of the investigators performed five ultrasound-guided injections. They worked in teams of two to stabilise the mannikin and place the injectate in the meat model (Fig. 3).
Fig 3.
Free-floating underwater (microgravity) condition. (a) Investigators worked in teams of two to stabilise the mannikin and inject dye. (b) A buoyant mannikin was tethered ∼2 m from the pool floor. The Department of Anesthesia, Pain Management & Perioperative Medicine, Dalhousie University, Halifax, NS, Canada, holds the copyright to this image, and it is reproduced here with permission.
All investigators who placed the blocks were certified as divers by the Professional Association of Diving Instructors (PADI). The investigators were not expert scuba divers. During the dives, a dive master was present to supervise and ensure safety. Before each dive, a pre-dive briefing was conducted to review the study plan, safety plan, and hand signals.
In the normal gravity condition, the meat model was placed inside the same mannikin, which was positioned on a table (Fig. 4). The investigator was in a seated position. The ultrasound, phone, and dive case were the same as in the microgravity condition.
Fig 4.
Normal gravity condition. (a) Mannikin resting on a table with the meat model placed flush with chest wall. (b) Investigator in a seated position. The Department of Anesthesia, Pain Management & Perioperative Medicine, Dalhousie University, Halifax, NS, Canada, holds the copyright to this image, and it is reproduced here with permission.
Outcomes
Investigators who performed the blocks rated both ease of image acquisition and block performance immediately after each block in both conditions using a 5-point Likert scale (1 = very difficult, 2 = difficult, 3 = neutral, 4 = easy, and 5 = very easy). We also measured the time to block, from when the transducer contacted the model until removal of the needle.
The study evaluated the success rates of perineural injections in microgravity and normal gravity by assessing the location of the injectate in the model. Two blinded investigators (different from those performing injections) dissected and scored the injections in the models. They bisected the model at the approximate point of injection and removed the meat and tendon from the gel mould to allow for better examination. The injection was deemed successful if there was dye staining on the surface tendon without any staining beneath the surface. The injection was deemed ‘intraneural’ if there was dye staining within the fibres of the tendon beneath the surface. Our group has shown this to be a reliable and accurate method of determining injection location.20
Statistical analysis
Continuous data were tested for normality using the Shapiro–Wilk normality test. Nonparametric testing was used for all non-normal distributed data. Univariate comparisons were analysed using Fisher's exact tests for dichotomous outcomes (success rate and intraneural injection), the Kruskall–Wallis rank sum test for ordinal outcomes (ease of image acquisition and ease of needle placement), and the Wilcoxon signed rank test for continuous data (time to block).
To determine the sample size with an alpha of 0.05 and power of 80% and assuming a normal gravity success rate of 95% and a microgravity success rate of 65%, a total sample size of 36 models was needed. All analyses were performed in R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Feasibility outcomes
There was no significant difference in time to injection between normal and underwater gravity conditions (Table 1). There was also no significant difference in ease of image acquisition or ease of needle placement.
Table 1.
Ease and success of ultrasound-guided injection using meat models in normal gravity and simulated microgravity conditions. IQR, interquartile range.
| Outcome | Gravity condition |
P-value | |
|---|---|---|---|
| Normal |
Underwater |
||
| (N=20) | (N = 20) | ||
| Time to injection (s), median (IQR) | 27.3 (21.0–69.0) | 35.0 (22.3–48.3) | 0.751 |
| Ease of image acquisition, median (IQR) | 4.0 (3.0–5.0) | 5.0 (4.0–5.0) | 0.070 |
| Ease of needle placement, median (IQR) | 4.5 (4.0–5.0) | 4.0 (3.0–4.0) | 0.067 |
| Intraneural injection, n/total N (%) | 1/20 (5) | 1/20 (5) | >0.999 |
| Successful perineural staining, n/total N (%) | 16/20 (80) | 17/20 (85) | >0.999 |
In terms of safety outcomes, there was no significant difference in the incidence of accidental intraneural injection between the two conditions, with one out of 20 cases reported in each scenario. In addition, block success rate was comparable; 16 out of 20 cases were successful with the normal gravity condition vs 17 out of 20 in the underwater scenario.
Subjective assessment
Tethering the mannikin 2 m from the bottom of the pool was used to simulate a free-floating environment (Fig. 5). The mannikin changed depth with gentle pressure necessitating stabilisation from the block assistant. The meat models were also buoyant underwater, which meant they had to be held in place by the block assistant. This increased the difficulty for the assistant but did not appear to change the difficulty for the person performing the block.
Fig 5.
Ultrasound image acquisition and needle placement in free-floating underwater (microgravity) or normal gravity condition. The same ultrasound transducer, phone screen, dive case, and 3D-printed clamp were used in both conditions. (a) Free-floating underwater (microgravity) condition. (b) Normal gravity condition. The Department of Anesthesia, Pain Management & Perioperative Medicine, Dalhousie University, Halifax, NS, Canada, holds the copyright to this image, and it is reproduced here with permission.
During the assessment phase of the study, after the models had been frozen for 24 h the assessors cut open the models to make determination of the accuracy of the injection. When assessing the position of the dye, the assessors found the dye initially very difficult to see. This is because methylene blue is colourless in its deoxygenated state. With exposure to air, it became progressively more blue and easy to identify.
Discussion
In this study, we show that experts in regional anaesthesia performing simulated blocks on meat models can overcome the sensory motor and ergonomic limitations of weightlessness. Our primary objective was to compare the feasibility of blocks in simulated microgravity with ideal land gravity conditions. We found that the measures of ease (image acquisition, needle placement, and time to injection) and success (tendon staining and intraneural injection) were not different between microgravity and normal gravity conditions.
To our knowledge, this is the first assessment of feasibility of regional anaesthesia in a microgravity environment. As there are numerous physiologic and logistical challenges to providing general anaesthesia in space, regional anaesthesia might circumvent many of the disadvantages of general anaesthesia.10,12,13,27 Further evidence of feasibility can be drawn from the use of regional anaesthesia in other austere environments. Regional anaesthesia is preferred by forward surgical teams; many reasons for its application in war align with arguments for its use in space.28,29 The equipment is portable, it offers stable haemodynamics, effective analgesia, there is minimal need for supplemental oxygen, and patients maintain alertness.28,29 Examples include continuous peripheral nerve block (CPNB) catheters placed at forward bases before hospital transfer.30,31 Wounded soldiers with CPNB catheters placed had lower pain scores during evacuation, which correlated with reduced anxiety and distress.32 Regional anaesthesia has also been used during disaster relief in low-resource settings.33 For example, regional anaesthesia made orthopaedic surgeries possible in the early phase of medical relief following the Haiti earthquake before hospital facilities were re-established.34 The Wilderness Medical Society recommends using regional anaesthesia for similar reasons.35 Aside from limb injuries, fascial plane blocks could be used for analgesia for rib fractures without central nervous system side effects.36 This has been successfully used in combat situations.37
There are additional considerations for space medicine beyond those already recognised for other austere earth-based environments. Microgravity complicates regional anaesthesia. Although no direct measurements have been made, there are some signs suggesting increased intracranial pressure during prolonged space journeys, calling the use of subarachnoid blocks (SABs) into question.8 Moreover, the efficiency of medicines used for SABs, which rely on their specific gravity, might be compromised in microgravity. There are concerns for use of epidurals and nerve catheters owing to infection risks exacerbated by weakened immune function, heightened bacterial virulence, and changes in antibiotic effectiveness.7,38 The increased infection risk of CPNB catheters would have to be weighed against the need for long-acting analgesia for evacuation of injured astronauts. Single-shot medications might exhibit a shorter duration of efficacy in space as a result of the impact of cosmic radiation, necessitating special packaging and more frequent replacement compared with their counterparts on Earth. Injured astronauts in LEO could be returned to Earth to receive treatment. However, a significant injury such as a broken arm could seriously interfere with an astronaut's ability to don an extravehicular mobility unit (or ‘space suit’) or to be loaded into a cramped capsule for re-entry. Effective analgesia with minimal systemic side effects would be essential for safe evacuation. If emergency extraction is not possible, as might be the case during trips to Mars, peripheral nerve blocks could be used to allow closed reduction and splinting of fractures. There might also be instances where space surgery will be necessary, and surgical anaesthesia with peripheral nerve blocks might be the only feasible option.
Other studies have explored medical resuscitation challenges in simulated microgravity, such as tracheal intubation and CPR, using underwater and parabolic flight simulations.13,14,25 Some of the issues are similar to the challenges of regional anaesthesia, such as stabilisation and generation of traction and counter-traction to generate sufficient force. In our study, the assistant needed to support the mannikin to prevent it from sinking or moving away when the operator performed the block. This is analogous to techniques used to stabilise mannikins for adequate force generation during CPR or intubation. We conducted blocks while both the assistant and regional anaesthesiologist were free-floating, and the mannikin was tethered but still mobile. Previous studies found that even experts struggled to intubate mannikins while free-floating,14 but success rates were good in microgravity when experts and an airway mannikin were restrained (direct laryngoscopy: 96%; videolaryngoscopy: 95%).16 Restraining both patients and providers inside a space station or vehicle during performance of a nerve block should decrease difficulty and increase safety by avoiding inadvertent movement. Further, regional anaesthesia would not be an emergency, and therefore, there would be time to optimise positioning and stabilisation. However, the optimal ergonomics for a microgravity environment have yet to be tested.
Given that anaesthesiologists are unlikely to be present routinely for deep space mission medical coverage, training should prioritise non-anaesthesiologists. Potential training models could involve using advanced Navy divers as an experimental model for non-expert regionalists rather than those expected to go to space. The current meat model, although a starting point for demonstrating feasibility, falls short in accurately replicating real-world sono-anatomy. Training for non-anaesthesiologists in austere conditions necessitates more comprehensive approaches, with future studies using complex multilayer models to better demonstrate the feasibility of performing real blocks.21 However, this study was not concerned with the ability of anaesthesiologists to identify sono-anatomy but rather with evaluating the ergonomics of injections in microgravity.
There are several limitations of our study. Our model likely presents increased difficulty compared with actual space conditions for technical block performance. Because we were using scuba as our microgravity analogue, this involved maintaining neutral buoyancy that requires breath control, something not needed in space. Wearing masks underwater potentially affected image clarity. Relying on pre-arranged hand signals reduced possible communication. In addition, movement and the ability to stabilise in the underwater environment differ from true microgravity. The mass of the water acts as a source of counter-support, not present in the air. Alternatively, parabolic flight could serve as another model for LEO scenarios. In future studies using the meat model, we recommend substituting methylene blue dye with blue food colouring in the injectate for better visibility.20,39 Lastly, our sample size calculation was based on a 30% difference in success rates, attributed to low intubation success rates in simulated microgravity environments, which limited power to detect smaller differences.13,14 Future studies should calculate power based on smaller effect sizes to enhance precision in outcome assessment.
In conclusion, this study demonstrates the feasibility of nerve blocks in a simulated microgravity environment. Despite study design features contributing to difficulty in the microgravity condition (free-floating, inability to speak, and looking through goggles), the success rate was similar to normal gravity conditions. This model can be used to further test feasibility of more complex blocks and the training of nonspecialist regional anaesthesia providers.
Authors’ contributions
Involved in study design: MBK, RB, JGB
Participated in grant application: MBK, RB, GB, JGB
Participated in ethics application: MBK, RB, JGB
Created models: JGB
Participated in data acquisition (simulated microgravity): MBK, RB, MM, JGB
Participated in data acquisition (scored models): DW, SB
Created the first draft of the manuscript: MBK, JB
Participated in manuscript review: RB, MM, DW, SB, GB, JGB
Approved the final draft: MBK, RB, MM, DW, SB, GB, JGB
Acknowledgements
Our group would like to acknowledge Torpedo Rays, specifically April Weickert, for their assistance in securing a place to undertake the study and for their safety consultation. We also acknowledge Osprey's Roost Butchery & Provisions for kindly consulting on the meat cuts and tendons for our models.
Declaration of interest
The authors declare no conflicts of interest.
Funding
An operating grant was obtained for this project through the Orlando Hung Innovation Fund.
Data availability
All relevant data are available on request.
Handling Editor: Hugh C Hemmings Jr
References
- 1.National Aeronautics and Space Administration (NASA). Mars exploration. Available from https://science.nasa.gov/planetary-science/programs/mars-exploration/ (accessed 3 June 2024).
- 2.National Aeronautics and Space Administration (NASA). Artemis: how. Available from https://www.nasa.gov/specials/artemis/#how (accessed 10 April 2023).
- 3.National Aeronautics and Space Administration (NASA). Lunar living: NASA’s Artemis Base Camp concept. 2020. Available from https://blogs.nasa.gov/artemis/2020/10/28/lunar-living-nasas-artemis-base-camp-concept/ (accessed 10 April 2023).
- 4.Swaffield T.P., Neviaser A.S., Lehnhardt K. Fracture risk in spaceflight and potential treatment options. Aerosp Med Hum Perform. 2018;89:1060–1067. doi: 10.3357/AMHP.5007.2018. [DOI] [PubMed] [Google Scholar]
- 5.Nowadly C.D., Trapp B.D., Robinson S.K., Richards J.R. Resuscitation and evacuation from low Earth orbit: a systematic review. Prehosp Disaster Med. 2019;34:521–531. doi: 10.1017/S1049023X19004734. [DOI] [PubMed] [Google Scholar]
- 6.Anderton R., Posselt B., Komorowski M., Hodkinson P. Medical considerations for a return to the Moon. Occup Med (Lond) 2019;69:311–313. doi: 10.1093/occmed/kqz099. [DOI] [PubMed] [Google Scholar]
- 7.Scarpa J., Wu C.L. The role for regional anesthesia in medical emergencies during deep space flight. Reg Anesth Pain Med. 2021;46:919–922. doi: 10.1136/rapm-2021-102710. [DOI] [PubMed] [Google Scholar]
- 8.Baker E.S., Barratt M.R., Sams C.F., Wear M.L. In: Principles of Clinical Medicine for Space Flight. Barratt M.R., Baker E.S., Pool S.L., editors. Springer; New York, NY: 2019. Human response to space flight; pp. 367–411. [Google Scholar]
- 9.Komorowski M., Fleming S., Mawkin M., Hinkelbein J. Anaesthesia in austere environments: literature review and considerations for future space exploration missions. NPJ Microgravity. 2018;4:5. doi: 10.1038/s41526-018-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komorowski M., Thierry S., Stark C., Sykes M., Hinkelbein J. On the challenges of anesthesia and surgery during interplanetary spaceflight. Anesthesiology. 2021;135:155–163. doi: 10.1097/ALN.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 11.Mandsager K.T., Robertson D., Diedrich A. The function of the autonomic nervous system during spaceflight. Clin Auton Res. 2015;25:141–151. doi: 10.1007/s10286-015-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodkinson P.D., Anderton R.A., Posselt B.N., Fong K.J. An overview of space medicine. Br J Anaesth. 2017;119:i143–i153. doi: 10.1093/bja/aex336. [DOI] [PubMed] [Google Scholar]
- 13.Warnecke T., Tochtermann F., Kerkhoff S., Komorowski M., Neuhaus C., Hinkelbein J. Airway management in microgravity: a systematic review. Acta Anaesthesiol Scand. 2019;63:2–7. doi: 10.1111/aas.13251. [DOI] [PubMed] [Google Scholar]
- 14.Thierry S., Jaulin F., Starck C., et al. Evaluation of free-floating tracheal intubation in weightlessness via ice-pick position with a direct laryngoscopy and classic approach with indirect videolaryngoscopy. NPJ Microgravity. 2023;9:73. doi: 10.1038/s41526-023-00314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernhard M., Mohr S., Weigand M.A., Martin E., Walther A. Developing the skill of endotracheal intubation: implication for emergency medicine. Acta Anaesthesiol Scand. 2012;56:164–171. doi: 10.1111/j.1399-6576.2011.02547.x. [DOI] [PubMed] [Google Scholar]
- 16.Starck C., Thierry S., Bernard C.I., et al. Tracheal intubation in microgravity: a simulation study comparing direct laryngoscopy and videolaryngoscopy. Br J Anaesth. 2020;125:e47–e53. doi: 10.1016/j.bja.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Wood M.H., Hailwood M., Koutelos K. Reducing the risk of oxygen-related fires and explosions in hospitals treating Covid-19 patients. Process Saf Environ Prot. 2021;153:278–288. doi: 10.1016/j.psep.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrington M.J., Viero L.P., Kluger R., Clarke A.L., Ivanusic J.J., Wong D.M. Determining the Learning Curve for Acquiring Core Sonographic Skills for Ultrasound-Guided Axillary Brachial Plexus Block. Reg Anesth Pain Med. 2016;41:667–670. doi: 10.1097/AAP.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 19.Taddeo T.A., Gilmore S., Armstrong C.W. In: Principles of Clinical Medicine for Space Flight. Barratt M.R., Baker E.S., Pool S.L., editors. Springer; New York, NY: 2019. Spaceflight medical systems; pp. 69–100. [Google Scholar]
- 20.Brownbridge R.G., Kiberd M.B., Werry D., Bailey J.G. Discriminative ability of dye injected into a meat model to determine accuracy of ultrasound-guided injection. Simul Healthc. 2024, Jun 10 doi: 10.1097/SIH.0000000000000799. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Naraghi L., Lin J., Odashima K., Buttar S., Haines L., Dickman E. Ultrasound-guided regional anesthesia simulation: use of meat glue in inexpensive and realistic nerve block models. BMC Med Educ. 2019;19:1–10. doi: 10.1186/s12909-019-1591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuan A., Lim Y.C., Aneja H., et al. A randomised controlled trial comparing meat-based with human cadaveric models for teaching ultrasound-guided regional anaesthesia. Anaesthesia. 2016;71:921–929. doi: 10.1111/anae.13446. [DOI] [PubMed] [Google Scholar]
- 23.Sparks S., Evans D., Byars D. A low cost, high fidelity nerve block model. Crit Ultrasound J. 2014;6:12. doi: 10.1186/s13089-014-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow D.S., Cupp J.A., Broder J.S. Versatile, Reusable, and inexpensive ultrasound phantom procedural trainers. J Ultrasound Med. 2016;35:831–841. doi: 10.7863/ultra.15.04085. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz J., Ahlbäck A., Ducanto J., et al. Randomized comparison of two new methods for chest compressions during CPR in microgravity—a manikin study. J Clin Med. 2022;11:646. doi: 10.3390/jcm11030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkelbein J., Ahlbäck A., Antwerber C., et al. Using supraglottic airways by paramedics for airway management in analogue microgravity increases speed and success of ventilation. Sci Rep. 2021;11:9286. doi: 10.1038/s41598-021-88008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman G.L., McCartney C.J. Regional anesthesia for the management of limb injuries in space. Aviat Space Environ Med. 2008;79:620–625. doi: 10.3357/asem.2181.2008. [DOI] [PubMed] [Google Scholar]
- 28.Mathais Q., Montcriol A., Cotte J., et al. Anesthesia during deployment of a military forward surgical unit in low income countries: a register study of 1547 anesthesia cases. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vietor R., Buckenmaier C., 3rd Regional anesthesia in the field for trauma victims. Anesthesiol Clin. 2021;39:337–351. doi: 10.1016/j.anclin.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Carness J.M., Wilson M.A., Lenart M.J., Smith D.E., Dukes S.F. Experiences with regional anesthesia for analgesia during prolonged aeromedical evacuation. Aerosp Med Hum Perform. 2017;88:768–772. doi: 10.3357/AMHP.4760.2017. [DOI] [PubMed] [Google Scholar]
- 31.Buckenmaier C.C., McKnight G.M., Winkley J.V., et al. Continuous peripheral nerve block for battlefield anesthesia and evacuation. Reg Anesth Pain Med. 2005;30:202–205. doi: 10.1016/j.rapm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Buckenmaier C.C., Rupprecht C., McKnight G., et al. Pain following battlefield injury and evacuation: a survey of 110 casualties from the wars in Iraq and Afghanistan. Pain Med. 2009;10:1487–1496. doi: 10.1111/j.1526-4637.2009.00731.x. [DOI] [PubMed] [Google Scholar]
- 33.Dohlman L., Kwikiriza A., Ehie O. Benefits and barriers to increasing regional anesthesia in resource-limited settings. Local Reg Anesth. 2020;13:147–158. doi: 10.2147/LRA.S236550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Missair A., Gebhard R., Pierre E., et al. Surgery under extreme conditions in the aftermath of the 2010 Haiti earthquake: the importance of regional anesthesia. Prehosp Disaster Med. 2010;25:487–493. doi: 10.1017/s1049023x00008645. [DOI] [PubMed] [Google Scholar]
- 35.Russell K.W., Scaife C.L., Weber D.C., et al. Wilderness Medical Society practice guidelines for the treatment of acute pain in remote environments. Wilderness Environ Med. 2014;25:41–49. doi: 10.1016/j.wem.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Thiruvenkatarajan V., Cruz Eng H., Adhikary S.D. An update on regional analgesia for rib fractures. Curr Opin Anaesthesiol. 2018;31:601–607. doi: 10.1097/ACO.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 37.Dmytriiev D., Dîrzu D.S., Melnychenko M., Eichholz R. Erector spinae plane block for affective and safe analgesia in a patient with severe penetrating chest trauma caused by an explosion in the battlefield. Clin Case Rep. 2022;10 doi: 10.1002/ccr3.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick A.W., Ball C.G., Campbell M., et al. Severe traumatic injury during long duration spaceflight: light years beyond ATLS. J Trauma Manag Outcome. 2009;3:4. doi: 10.1186/1752-2897-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsharkawy H., Bajracharya G.R., El-Boghdadly K., Drake R.L., Mariano E.R. Comparing two posterior quadratus lumborum block approaches with low thoracic erector spinae plane block: an anatomic study. Reg Anesth Pain Med. 2019;44:549–555. doi: 10.1136/rapm-2018-100147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available on request.



 @jon_bailey_anes
@jon_bailey_anes