Abstract
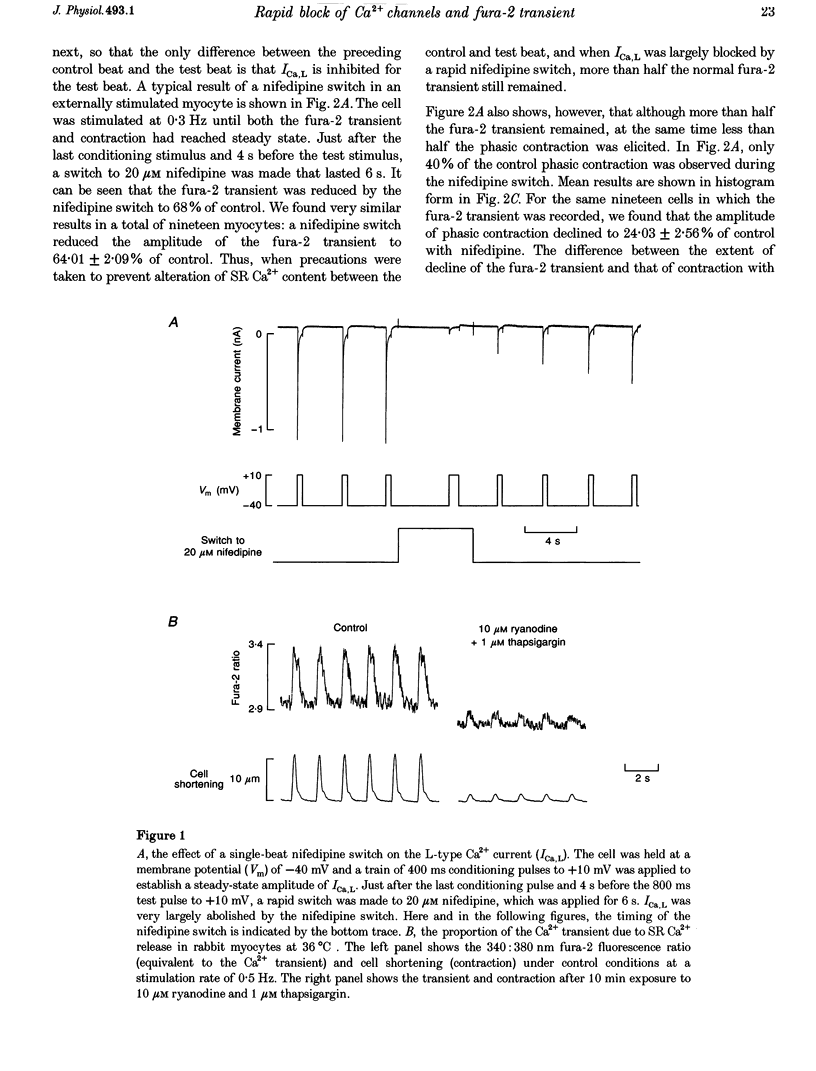
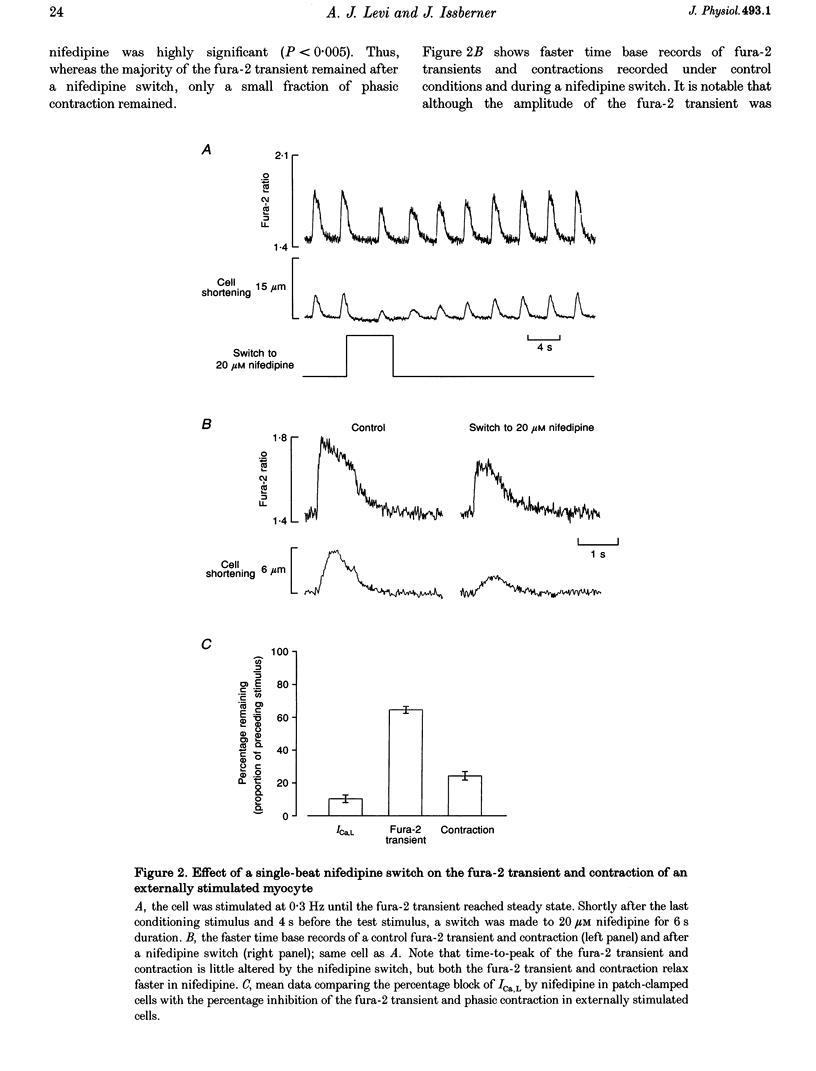
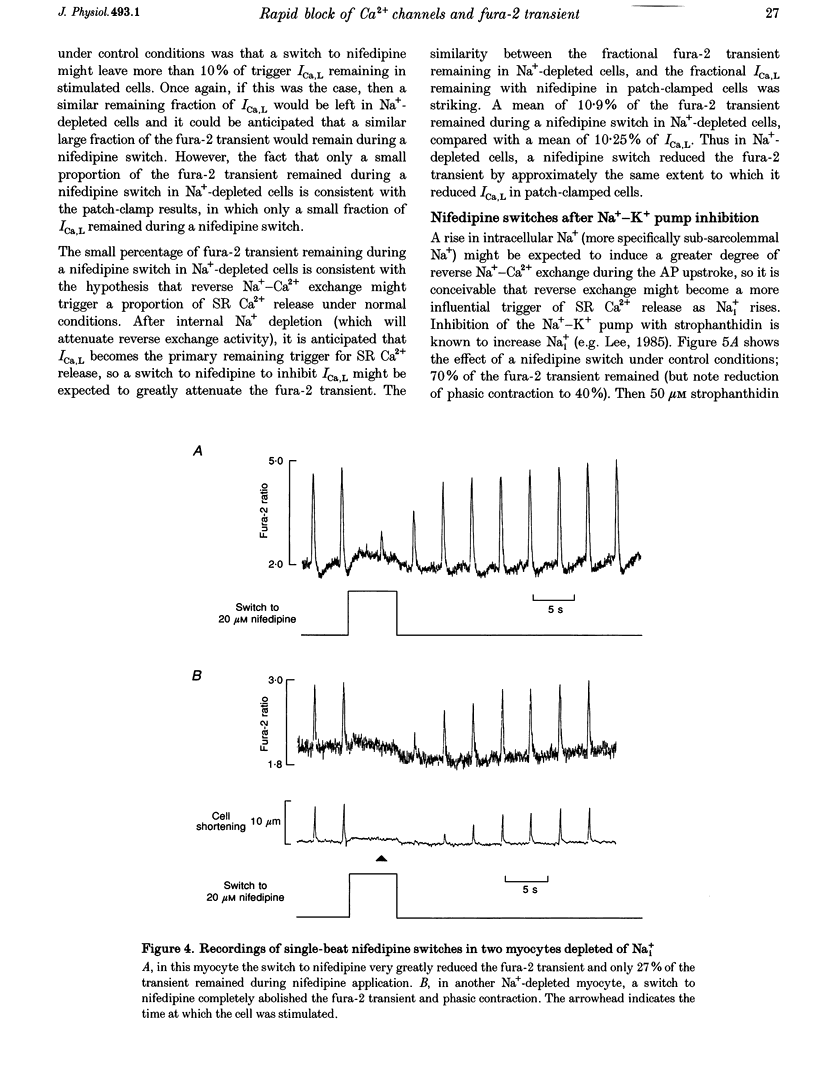
1. We used a rapid solution switcher technique to investigate mechanisms that might trigger intracellular Ca2+ release in rabbit ventricular myocytes. The study was carried out at 36 degrees C, intracellular Ca2+ (Ca2+i) was monitored with fura-2, and myocytes were electrically stimulated. 2. In patch-clamped cells, using the switcher to apply 20 microM nifedipine (an L-type Ca2+ current (ICa,L) blocker) 4 s before a depolarization to +10 mV reduced the amplitude of ICa,L to 10.25 +/- 2.25% of control (mean +/- S.E.M., n = 7 cells). 3. In externally stimulated cells, a rapid switch to 20 microM nifedipine 4 s before a stimulus reduced the amplitude of the fura-2 transient to 64.01 +/- 2.09% of control (mean +/- S.E.M., n = 19 cells). Using an in vivo calibration curve for fura-2, this was equivalent to a reduction in the Ca2+ transient to 50% during nifedipine application. Since an identical nifedipine switch reduced ICa,L to 10.25%, it would seem that blocking a large fraction of ICa,L inhibited only half the Ca2+ transient. 4. The Na(+)-Ca2+ exchanger is inhibited by 5 mM nickel. Switching to 20 microM nifedipine +5 mM nickel 4 s before a stimulus abolished the fura-2 transient completely, consistent with the hypothesis that Ca2+ entry via reverse Na(+)-Ca2+ exchange might trigger a fraction of the fura-2 transient that remained during nifedipine. 5. After the Na(+)-K+ pump was inhibited by strophanthidin to increase intracellular Na+ (Na+i), a switch to 20 microM nifedipine became progressively less effective in reducing the fura-2 transient. This suggests that as Na+i rose, other mechanisms (perhaps reverse Na(+)-Ca2+ exchange) appeared able to substitute for ICa,L in triggering the Ca2+ transient. 6. In cells depleted of Nai+ to inhibit the triggering of sarcoplasmic reticulum (SR) Ca2+ release by reverse Na(+)-Ca2+ exchange, a nifedipine switch reduced the fura-2 transient to 10.9 +/- 4.19% (mean +/- S.E.M., n = 7; equivalent to 6.5% of the Ca2+ transient). 7. A switch to Na(+)-free (Li+) solution 100 ms before an electrical stimulus caused an increase in the fura-2 transient of 12.2 +/- 1.5% (mean +/- S.E.M., n = 7; equivalent to a 22% increase in the Ca2+ transient). 8. The results confirm that ICa,L is an important trigger for SR Ca2+ release and the resulting Ca2+ transient. However, since 50% of the Ca2+ transient remained when ICa,L was largely inhibited, it would seem likely that other SR trigger mechanisms might exist in addition. These data are consistent with the idea that Ca2+ entry via reverse Na(+)-Ca2+ exchange during the upstroke of the normal cardiac action potential might trigger a fraction of SR Ca2+ release and the resulting Ca2+ transient.
Full text
PDF



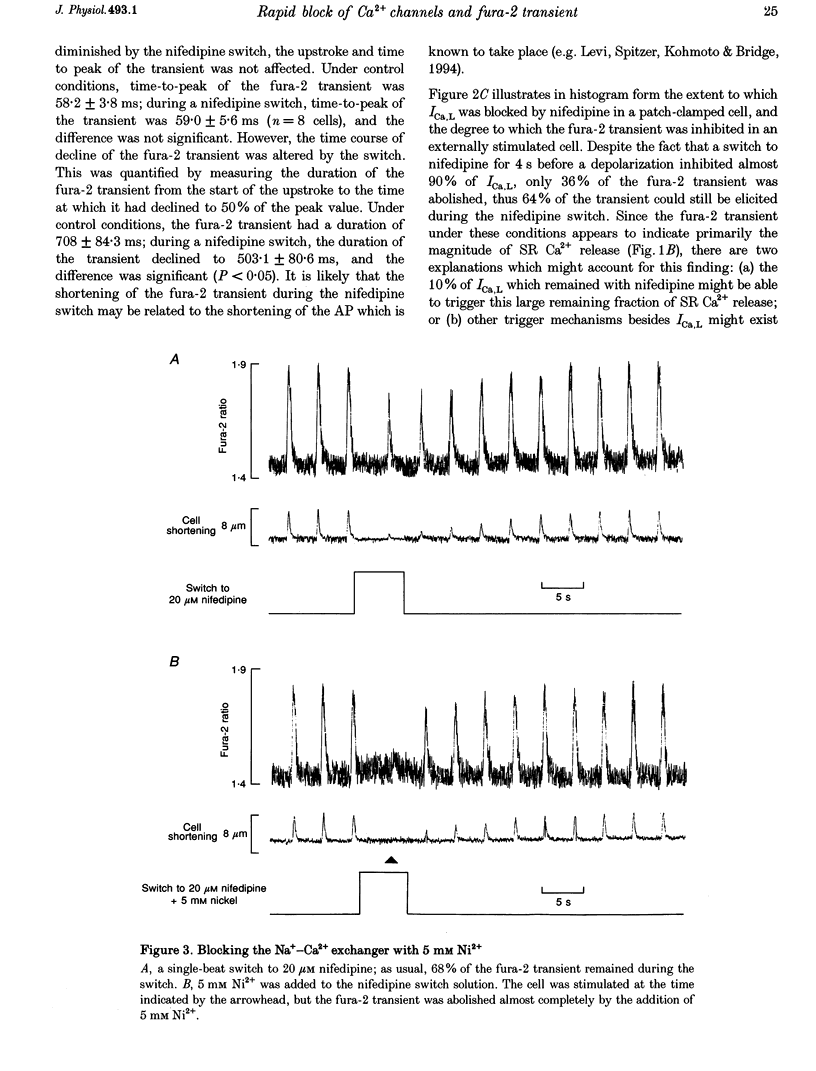







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995 Apr;68(4):1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–H381. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R. A., Clark R. B., Giles W. R. Role of sodium-calcium exchange in activation of contraction in rat ventricle. J Physiol. 1993 Dec;472:391–413. doi: 10.1113/jphysiol.1993.sp019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooksby P., Levi A. J., Jones J. V. Investigation of the mechanisms underlying the increased contraction of hypertrophied ventricular myocytes isolated from the spontaneously hypertensive rat. Cardiovasc Res. 1993 Jul;27(7):1268–1277. doi: 10.1093/cvr/27.7.1268. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992 Aug;262(2):809–817. [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Slow inward current and contraction of sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):367–384. doi: 10.1085/jgp.65.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hancox J. C., Levi A. J., Lee C. O., Heap P. A method for isolating rabbit atrioventricular node myocytes which retain normal morphology and function. Am J Physiol. 1993 Aug;265(2 Pt 2):H755–H766. doi: 10.1152/ajpheart.1993.265.2.H755. [DOI] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohomoto O., Levi A. J., Bridge J. H. Relation between reverse sodium-calcium exchange and sarcoplasmic reticulum calcium release in guinea pig ventricular cells. Circ Res. 1994 Mar;74(3):550–554. doi: 10.1161/01.res.74.3.550. [DOI] [PubMed] [Google Scholar]
- Le Guennec J. V., Noble D. Effects of rapid changes of external Na+ concentration at different moments during the action potential in guinea-pig myocytes. J Physiol. 1994 Aug 1;478(Pt 3):493–504. doi: 10.1113/jphysiol.1994.sp020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee C. O. 200 years of digitalis: the emerging central role of the sodium ion in the control of cardiac force. Am J Physiol. 1985 Nov;249(5 Pt 1):C367–C378. doi: 10.1152/ajpcell.1985.249.5.C367. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Levi A. J. The role of intracellular sodium in the control of cardiac contraction. Ann N Y Acad Sci. 1991;639:408–427. doi: 10.1111/j.1749-6632.1991.tb17329.x. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Brooksby P., Hancox J. C. One hump or two? The triggering of calcium release from the sarcoplasmic reticulum and the voltage dependence of contraction in mammalian cardiac muscle. Cardiovasc Res. 1993 Oct;27(10):1743–1757. doi: 10.1093/cvr/27.10.1743. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Spitzer K. W., Kohmoto O., Bridge J. H. Depolarization-induced Ca entry via Na-Ca exchange triggers SR release in guinea pig cardiac myocytes. Am J Physiol. 1994 Apr;266(4 Pt 2):H1422–H1433. doi: 10.1152/ajpheart.1994.266.4.H1422. [DOI] [PubMed] [Google Scholar]
- Levi A. J. The effect of strophanthidin on action potential, calcium current and contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:1–23. doi: 10.1113/jphysiol.1991.sp018819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994 Feb 1;474(3):439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B., Krueger J. W. Contraction in voltage-clamped, internally perfused single heart cells. J Gen Physiol. 1986 Oct;88(4):475–505. doi: 10.1085/jgp.88.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989 Jun;93(6):1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe M. W., Lemasters J. J., Herman B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):63–73. doi: 10.1016/0143-4160(90)90060-8. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolmillos M., O'Neill S. C., Smith G. L., Eisner D. A. Calcium-induced calcium release activates contraction in intact cardiac cells. Pflugers Arch. 1989 Apr;413(6):676–678. doi: 10.1007/BF00581820. [DOI] [PubMed] [Google Scholar]
- Vornanen M., Shepherd N., Isenberg G. Tension-voltage relations of single myocytes reflect Ca release triggered by Na/Ca exchange at 35 degrees C but not 23 degrees C. Am J Physiol. 1994 Aug;267(2 Pt 1):C623–C632. doi: 10.1152/ajpcell.1994.267.2.C623. [DOI] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F., Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]
- Wrzosek A., Schneider H., Grueninger S., Chiesi M. Effect of thapsigargin on cardiac muscle cells. Cell Calcium. 1992 May;13(5):281–292. doi: 10.1016/0143-4160(92)90063-x. [DOI] [PubMed] [Google Scholar]


