Abstract
The objective of this opinion was to determine if any wild caught fish species, originating from specific fishing grounds and consumed in the EU/EFTA could be considered free of zoonotic parasites. In this Opinion the term ‘fishery products’ only refers to fresh finfish. As there are multiple fish species and numerous potential parasites, Anisakis sp. was used as an indicator of zoonotic parasites in marine areas. This parasite species is particularly suited as it is common in marine environments, capable of infecting multiple fish species and is the subject of the majority of published studies. On the rare occasion where Anisakis sp. data were not available, or all tests were negative, other parasites such as Contracaecum osculatum (s.l.) and/or Phocanema spp. were considered. In freshwater systems, all zoonotic parasites were investigated. Consumption, import and landing data were used to determine the most relevant fish species and, where possible, the source fishing areas were identified. The most commonly consumed wild caught fish species in the EU/EFTA include tuna, cod, Alaskan pollock, hake, herring, sardines, mackerel, trout and saithe. Although the majority of these fish are caught in the Atlantic Ocean, the Mediterranean and the Black Sea (37) as well as several areas in the Indian Ocean, imported fish may originate from any global fishing areas, with the exception of Antarctica. Based on the data, at least one zoonotic parasite has been reported in at least one fish species in each of the FAO marine fishing areas. Thus, due to relative low fish host specificity of the zoonotic parasites, the panel concluded that all wild caught fish species may be exposed to and infected with zoonotic parasites. The same applies to freshwater fishing areas, with many areas having multiple studies reporting the presence of zoonotic parasites in the wild caught fish species.
Keywords: Anisakidae, epidemiology, finfish, fishery products, food safety, zoonotic parasites
SUMMARY
The objective of this opinion was to determine if any wild caught fish species, originating from specific fishing grounds, and consumed in the EU/EFTA could be considered free of zoonotic parasites. Fish, an important part of European diets, may be infected with parasites that cause disease in humans. These zoonotic parasites belong to the Nematoda, Trematoda and Cestoda taxa, and infect both farmed and wild caught fish. Relevant parasites in farmed fish are described in the previously published EFSA Scientific Opinion (Part 1) arising from the same EC Mandate. However, wild caught fish are exposed to a greater range of zoonotic parasites including species in the Acanthocephala and Myxozoa taxa, all of which are described in this opinion.
The ‘EU fish market’ report is a comprehensive analysis of the EU fisheries and the aquaculture industry made by the European Market Observatory for Fisheries and Aquaculture (EUMOFA). According to the report for 2023, the most consumed wild caught fish species in the EU include tuna, cod, Alaskan pollock, hake, herring, sardines, mackerel, trout and saithe. Overall, data from the EUMOFA database suggests that the most consumed species are salmon, gilthead bream, hake, cod and sardines, although this data does not distinguish between farmed and caught fish and it is likely that the majority of salmon and gilthead seabream are supplied by aquaculture.
According to Eurostat (2022) the main fish species caught by European fishing vessels in 2021 included skipjack tuna, yellowfin tuna, bigeye tuna, mackerel, cod, halibut, herring, blue whiting, hake, swordfish, blue sharks, anchovy and pilchards. The EU catch was 3.5 million tonnes by live weight and the main fishing areas were in the Atlantic and Indian Oceans as well as in the Mediterranean and the Black Sea, specifically the Atlantic northeast (FAO area 27) (70%), Atlantic northwest (FAO area 21) (1%), Atlantic southeast (FAO area 47) (1%), Atlantic southwest (FAO area 41) (4%), Atlantic eastern central (FAO area 34) (7%), Mediterranean and Black Sea (FAO area 37) (10%) and Indian ocean western (FAO areas 51, 57 & 58) (7%).
Fish is also imported and the main countries exporting finfish products into the EU, based on volumes in 2023, were Norway (EFTA country), China, Ecuador, Morocco and Iceland (EFTA country) while based on value were Norway, Morocco, China, Ecuador and United Kingdom (EU Fish Market 2023).
The FAO major fishing areas include freshwater and marine areas. The former includes African inland waters (area 01), North America inland waters (02), South America inland waters (03), Asia inland waters (04), Europe inland waters (05), Oceania inland waters (06) and Antarctica inland waters (08). The marine areas are divided as follows: Arctic ocean (18), Northwest Atlantic (21), Northeast Atlantic (27), Western Central Atlantic (31), Eastern Central Atlantic (34), Mediterranean & Black Sea (37), Southwest Atlantic (41), Southeast Atlantic (47), Atlantic Antarctic (48), Western Indian Ocean (51), Eastern Indian Ocean (57), Indian Ocean Antarctic (58), Northwest Pacific (61), Northeast Pacific (67), Western Central Pacific (71), Eastern Central Atlantic (77), Southwest Pacific (81), Southeast Pacific (87) and Pacific Antarctic (88).
Anisakis sp. were reported in multiple wild caught fish species in all FAO marine fishing areas except for Indian Ocean Antarctic (58). However, Phocanema decipiens (s.l.) and Contracaecum osculatum were detected in multiple fish species caught in this area. Many different zoonotic parasites including Dibothriocephalus spp., Opisthorchis spp., Metorchis spp., Haplorchis spp., Centrocestus spp., Gnathostoma spp., Clonorchis spp., Metagonimus spp., Cryptocotyle spp., Cyathocotylid spp. and Holostephanus spp. were found in multiple fish species from all freshwater fishing areas, except from Antarctica inland waters (8), for which data is missing, probably because there are few if any fish in Antarctica inland waters and none that are imported into the EU.
It was therefore concluded that there are no particular species of wild caught fish originating from specific marine or freshwater fishing grounds, where the fish consumed in the EU/EFTA can be considered to be free of parasites of public health importance.
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
In 2010 EFSA published a scientific opinion on risk assessment of parasites in fishery products. 1 EFSA was requested in particular to analyse three aspects:
Assessment of food safety concerns due to possible allergic reactions from parasites in fishery products;
Alternative treatments for killing viable parasites and comparison with freezing method;
Criteria for when fishing grounds (wild‐farmed) fishery products do not present a health hazard (Atlantic salmon in particular). EFSA conclusions were taken into account for modifying part D of Annex III, Section VIII, Chapter III to Regulation (EC) No 853/2004 (Commission Regulation (EU) No 1276/2011).
In fact, part D of Annex III, Section VIII, Chapter III to Regulation (EC) No 853/2004 establishes that:
- Food business operators placing on the market the following fishery products derived from finfish or cephalopod molluscs:
- fishery products intended to be consumed raw; or
- marinated, salted and any other treated fishery products, if the treatment is insufficient to kill the viable parasite; must ensure that the raw material or finished product undergo a freezing treatment in order to kill viable parasites that may be a risk to the health of the consumer.
The freezing treatment is not carried out for fishery products:
that have undergone or are intended to undergo before consumption a heat treatment that kills the viable parasite. In the case of parasites other than trematodes the product is heated to a core temperature of 60°C or more for at least 1 min;
that have been preserved as frozen fishery products for a sufficiently long period to kill the viable parasites;
from wild catches, provided that: (i) there are epidemiological data available indicating that the fishing grounds of origin do not present a health hazard with regard to the presence of parasites; and (ii) the competent authority so authorises;
- derived from fish farming, cultured from embryos and have been fed exclusively on a diet that cannot contain viable parasites that present a health hazard, and one of the following requirements is complied with:
- have been exclusively reared in an environment that is free from viable parasites; or
- the food business operator verifies through procedures, approved by the competent authority, that the fishery products do not represent a health hazard with regard to the presence of viable parasites.
Before placing on the market fishery products which have not undergone the freezing treatment or which are not intended to undergo before consumption a treatment that kills viable parasites that present a health hazard, a food business operator must ensure that the fishery products originate from a fishing ground or fish farming which complies with the specific conditions referred to in one of those points. This provision may be met by information in the commercial document or by any other information accompanying the fishery products.
The ParaFishControl is an EU H2020‐funded project that aims at increasing the sustainability and competitiveness of the European aquaculture industry by improving our understanding of fish‐parasite interactions and by developing innovative solutions and tools for the prevention, control and mitigation of the most harmful parasitic species affecting the main European farmed fish species. The project started in 2015 and finished in 2020 and was involving a consortium of 29 partners (public and private) from 13 countries. Research related to that project demonstrated in farmed seabass, farmed seabream, turbot, and sea caged rainbow trout that no zoonotic parasites were found, concluding that the risk related to zoonotic Anisakidae appeared as negligible. The authors suggested that there is groundwork for amending the current legislation.
Other studies demonstrated that farmed fish were found to be less infected in comparison with wild fish (2%) but not Anisakis free. Farmed fish is in general reported to be considerably less infected and therefore representing a very limited food safety risk, but guaranteeing nematode free fish remains impossible.
In addition, the EFSA 2010 Opinion concluded that “no sea fishing grounds can be considered free of A. simplex larvae”, and that “all wild caught seawater and freshwater fish must be considered at risk of containing viable parasites of human health hazard if these products are to be eaten raw or almost raw”. Furthermore, the BIOHAZ Panel recommended the collection of systematic data on the complete life cycle, geographical and seasonal distribution, prevalence, intensity, and anatomical location of parasites of public health importance in wild caught fishery products.
The European Union One Health 2020 Zoonoses report elaborated by EFSA and ECDC reports that in 2020, Anisakis caused two outbreaks, both reported by Spain, involving six individuals. No outbreaks were reported in 2019. The causative agent was not identified at the species level.
TERMS OF REFERENCE
EFSA is asked to update certain aspects of its Scientific Opinion of April 2010 on risk assessment of parasites in fishery products based on any new scientific evidence that may have become available since then. In particular, EFSA is requested to review and assess:
The occurrence of parasites of public health importance in fishery products derived from the most relevant farmed fish species in the EU (in particular, but not limited to, Atlantic salmon, seabass, farmed seabream and turbot).
Diagnostic methods for the detection of parasites of public health importance in fishery products from such farmed fish species.
Technical developments and new scientific data available in relation to killing viable parasites of public health importance in fishery products, in particular treatments other than freezing.
Whether any particular species of wild caught fish originating from specific fishing grounds could be regarded as not representing a health hazard with regards to the presence of parasites of public health importance.
TORs 1–3 (Part 1) are addressed in EFSA BIOHAZ Panel (EFSA BIOHAZ Panel, 2024), while TOR 4 is addressed in this opinion (Part 2).
1.2. Interpretation of the Terms of Reference
ToR 4 was translated into the following assessment question (AQ):
AQ4: Are there any particular species of wild caught fish originating from specific fishing grounds, where fish consumed in the EU/EFTA are caught, that are free of parasites of public health importance?
To answer AQ4, the following was clarified with the requestor:
While the previous EFSA scientific opinion from 2010 (EFSA BIOHAZ Panel, 2010) is considered, this opinion focuses on information and data generated since then. Accordingly, the information/literature/data to be revised covered the period from 2010 (inclusive) to 2024.
Allergies are not covered by the current assessment.
The FAO 2 categorisation of fishing grounds is used in this opinion which includes marine, brackish and freshwater.
Although the legal definition for ‘fishery products’ covers all marine water or freshwater animals (except for live bivalve molluscs, live echinoderms, live tunicates and live marine gastropods, and all mammals, reptiles and frogs) whether wild or farmed and including all edible forms, parts and products of such animals (EC 853/2004), for the purposes of this opinion only wild caught finfish species are covered. Moreover, only fresh fish were considered and frozen fish were excluded as freezing in an effective method for killing parasites.
Only zoonotic parasites that infect finfish are considered. Parasites that do not naturally infect finfish but are found in contaminated waters (e.g. Cryptosporidium, Giardia, Toxoplasma, Blastocystis) and may be present in their gastrointestinal tract and subsequently cross‐contaminating fish during processing, are excluded.
Most of the parasites considered in this opinion infect multiple fish species but exceptions exist. Thus, most fish species are considered susceptible to some extent. Therefore, it was considered that the presence of at least one parasite of public health importance, in at least one fish species, indicated that the fishing areas in which that fish was caught were not parasite‐free and all fish species from that fishing area could potentially be infected with zoonotic parasites.
As the vast majority of studies to date in the marine environment have focused on Anisakis, this was used as an ‘indicator’ parasite and the focus of the literature search was to identify and review scientific papers that examined wild caught fish for this parasite. If detected, it was considered that all fish from the fishing ground where this parasite occurs, represented a hazard with regards to the presence of parasites of public health importance. If there was no record of occurrence of Anisakis in fish from a fishing ground or all tests were negative, other parasites, including Contracaecum osculatum (s.l.) and/or Phocanema sp. were then considered. If there were still no records of parasite detection, all of the parasites described in Section 1.3.2 were considered.
As the entire life cycles of zoonotic Anisakis are usually not completed in freshwater ecosystems, these parasites are not used as an indicator for the presence of zoonotic parasites in freshwater fishing areas. Instead, the literature search was extended to cover other zoonotic parasites in all fish species caught in freshwater areas (see Section 1.3.2, Table 1).
TABLE 1.
Overview of the parasite species of public health importance found in wild caught fish.
| Parasite phylum/class | Parasite family | Zoonotic parasite species |
|---|---|---|
| Nematoda/Chromadorea | Anisakidae | Anisakis pegreffii |
| Anisakis simplex (s.s.) | ||
| Anisakis typica sp. A | ||
| Anisakis typica sp. B | ||
| Phocanema decipiens (s.s.) | ||
| Phocanema krabbei | ||
| Phocanema bulbosa | ||
| Phocanema azarasi | ||
| Phocanema cattani | ||
| Phocanema decipiens sp. E | ||
| Contracaecum osculatum (s.s.) | ||
| Contracaecum osculatum A | ||
| Contracaecum osculatum B | ||
| Nematoda/Secernentea | Gnathostomatidae | Gnathostoma spinigerum |
| Gnathostoma binucleatum | ||
| Gnathostoma hispidum | ||
| Gnathostoma doloresi | ||
| Gnathostoma nipponicum | ||
| Nematoda/Enoplea | Capillaridae | Paracapillaria philippinensis |
| Dioctophymatidae | Dioctophyme renale | |
| Eustrongylides excisus | ||
| Platyhelminthes/Trematoda | Opisthorchiidae | Opisthorchis viverrini |
| Opisthorchis felineus | ||
| Clonorchis sinensis | ||
| Amphimerus pseudofelineus | ||
| Metorchis conjunctus | ||
| Metorchis orientalis | ||
| Pseudamphistomum truncatum | ||
| Heterophyidae | Heterophyes heterophyes | |
| Heterophyes nocens | ||
| Metagonimus yokogawai | ||
| Metagonimus takahashii | ||
| Metagonimus miyatai | ||
| Centrocestus formosanus | ||
| Haplorchis taichui | ||
| Haplorchis pumilio | ||
| Haplorchis yokogawai | ||
| Pygidiopsis summa | ||
| Cryptocotyle lingua | ||
| Clinostomidae | Clinostomum complanatum | |
| Echinostomatidae | Echinostoma hortense | |
| Echinochasmus perfoliatus | ||
| Echinochasmus japonicus | ||
| Echinochasmus liliputanus | ||
| Echinochasmus fujianensis | ||
| Nanophyetidae | Nanophyetus salmincola | |
| Cyathocotylidae | Paracoenogonimus ovatus | |
| Platyhelminthes/Cestoda | Bothriocephalidae | Dibothriocephalus latus |
| Dibothriocephalus dendriticus | ||
| Dibothriocephalus nihonkaiensis | ||
| Dibothriocephalus lanceolatus | ||
| Adenocephalus pacificus (syn Diphyllobothrium pacificum) | ||
| Rotifera/Acanthocephala | Polymorphidae | Bolbosoma nipponicum |
| Bolbosoma capitatum | ||
| Corynosoma strumosum | ||
| Corynosoma validum | ||
| Corynosoma villosum | ||
| Cnidaria/Myxozoa | Kudoidae | Kudoa hexapunctata |
| Kudoa septempunctata |
1.3. Additional information
1.3.1. Update to EFSA BIOHAZ 2010 scientific opinion
In the previous BIOHAZ Panel opinion (EFSA BIOHAZ Panel, 2010) data on the prevalence of zoonotic parasites was provided for several species of wild caught fish. Given that all tested fish from various fishing grounds were found to be positive for A. simplex, it was concluded that no sea fishing grounds could be confirmed as parasite‐free.
However, it is important to note that to the best of our knowledge there is currently only one long term (since 2006) fish‐parasite surveillance programme in Europe. 3 This is in operation in Norway where commercially important pelagic fish species (Atlantic cod, tusk, blue whiting, herring and Atlantic mackerel) are routinely tested for A. simplex.
While climate change (primarily increasing surface water temperatures) is driving changes in the geographical distribution of specific parasites in marine ecosystems, changes in occurrence seem to be largely associated with changes in definitive host population abundance (Selstad Utaaker & Robertson, 2015; van Weelden et al., 2021). The impact of climate change on fish‐borne parasites in freshwater systems appears to be more complex and, thus, more difficult to assess.
Some widely distributed fish‐borne zoonotic parasites such as the trematode Opisthorchis viverrini and the cestode Dibothriocephalus latus, for example, are both characterised by a two intermediate host life cycle and may benefit from higher ambient water temperatures which enhances their transmission efficiency (Poulin, 2006; Wicht et al., 2009). However, long‐lasting droughts and loss of wetlands, events expected to occur in many parts of the world, may result in reduced freshwater snail and fish population size and distribution. This too may lower the transmission rate of some important zoonotic trematode species (e.g. O. viverrini) to humans due to the reduced availability of freshwater fish as part of the daily diet in some regions of southern Asia (Marks, 2011).
There is also evidence that the increasing population of marine mammals in the Baltic Sea, due to conservation efforts protecting these animals, is positively correlated with an observed increase of anisakid infections of various fish stocks (Buchmann, 2023; Zuo et al., 2018).
1.3.2. Parasites in wild caught fish
As reported in EFSA BIOHAZ Panel (2010), the zoonotic parasites of public health relevance in fishery products include species belonging to nematodes, trematodes and cestodes. Information on their life cycle and the distribution of the parasites in the fish body/muscles, was included in that scientific opinion. An update of the information currently available on the parasites infecting farmed fish tested in European waters is provided by the EFSA BIOHAZ Panel in (EFSA BIOHAZ Panel, 2024) including the nematodes Anisakidae. Other relevant species belonging to the acanthocephalan genus Bolbosoma (i.e. B. capitatum and B. nipponicum) and the genus Corynosoma (i.e. C. villosum and C. validum), as well as the relevant species of myxosporidian genus Kudoa (i.e. K. septempunctata and K. hexapunctata) are also described in that document. Parasites of public health to which wild fish are exposed are summarised in Table 1. Information on these parasites, if not already covered in part 1 of this opinion, is provided below.
1.3.2.1. Nematodes
The phylum Nematoda includes the families Anisakidae, Gnathostomatidae, Capillaridae and Dioctophymatidae. Gnathostoma nematodes are heteroxenous parasites. The adult worms live and spawn in a tumour‐like mass in the stomach of the definitive mammalian host. The eggs are released by the host's faeces into the freshwater environment, where they develop and hatch into the first‐stage larvae (L1). These larvae are then ingested by the first intermediate host, a freshwater copepod, where they develop into the second‐stage larvae (L2). When the infected copepods are preyed upon by the second intermediate host such as a fish or tadpole, L2 migrate into the host's muscular tissue where they develop into third‐stage larvae (L3). Finally, L3 in the second intermediate, transport or paratenic host are ingested by a definitive host, where they migrate to the liver and the abdominal cavity after penetrating the gastric wall. Later they return to the gastric wall and develop into adults. The development from L3 to the adult stage usually takes 6–8 months. When L3 are eaten by paratenic hosts such as frogs, snakes, birds and mammals as well as humans, which may act as accidental host, they migrate through host tissues and remain encysted in their muscles. Human gnathostomiasis can occur through the ingestion of raw or undercooked meat of intermediate hosts, such as fish, frogs, snakes or poultry, which contains infective L3. Oral infection can also occur through drinking water, contaminated with infected copepods. Human gnathostomiasis is considered an emerging global zoonosis (Liu et al., 2020). To date, approximately 5000 cases of human gnathostomiasis have been reported worldwide, mainly from endemic areas in Japan and China, Thailand and other parts of Southeast Asia, as well as Mexico, Colombia and Peru in South America. Gnathostomiasis has also been reported, albeit less frequently, in travellers who have visited endemic areas. The most common clinical signs of the disease are migratory cutaneous swellings and eosinophilia. In severe cases, L3 also invades internal organs such as the liver, eyes, nerves, spinal cord and brain, resulting in blindness, nerve pain, paralysis, coma and even death (Nogrado et al., 2023).
Paracapillaria philippinensis, a pathogenic species originally isolated from human patients, is presumed to have a sylvatic life cycle, which involves birds that feed on fish, but the definitive natural host of this species remains unknown (Arumugam et al., 2024).
The most relevant genus of the Dioctophymatidae family are the Eustrongylides, which have a global distribution (Northern and Southern America, Europe, Asia) (Honcharov et al., 2022) and an indirect life cycle, involving a wide range of freshwater fish species and fish‐eating birds. These act as definitive hosts, while oligochaetes, mainly annelids, act as first intermediate hosts, and benthic and planktivorous fish are the second intermediate hosts. In the second intermediate host the third‐stage larva moults into the fourth stage (L4) and remains as L4 until ingestion by the definitive host (Moravec, 2013). L4 larvae can also be accumulated in predatory fish species, serving as paratenic hosts. Some fish species have been found infected in Europe, mainly in lakes from Italy (Castiglione et al., 2023). To date, there have been very few reports of human infection attributed to larval Eustrongylides and those that have occurred are from the USA and Sudan (Eberhard et al., 1989; Eberhard & Ruiz‐Tiben, 2014). In the USA, cases have been characterised by abdominal pain within 24 h of eating either bait minnows or after the consumption of fresh fish used to prepare sushi and sashimi. In Sudan, the cutaneous finding of Eustrongylides in two patients was based on morphological identification (Eberhard & Ruiz‐Tiben, 2014).
1.3.2.2. Trematodes
There are at least 29 different fish‐borne trematode species that infect humans. These belong to several genera including Opistorchis, Clonorchis, Metagonimus, Heterophyes, Haplorchis, Centrocestus and Cryptocotyle (C. lingua) and infect an estimated 7 million people annually (Chai & Jung, 2017). These digeneans are contracted by humans ingesting raw or improperly cooked freshwater or brackish water fish (Chai & Jung, 2024). Human infections are reported globally, but the major endemic areas are in Asian countries, including Korea, China, Taiwan, Vietnam, Laos, Thailand, Malaysia, Indonesia, the Philippines and India (Chai & Jung, 2017). In the case of Opistorchis, several outbreaks have been reported in Europe, especially Italy (Orlando et al., 2008).
Clinical manifestations may include abdominal pain, diarrhoea, lethargy, anorexia and weight loss (Chai & Jung, 2024). However, the severity of symptoms is variable, and it depends on the intensity of infection, immune status of the patient and previous history of infection with flukes (Chai & Jung, 2024). In addition, in immunocompromised patients the infection may have a severe clinical course, including ectopic migration of the flukes (i.e. extraintestinal heterophyiasis) in the heart and brain (Chai et al., 2000). H. heterophyes and H. nocens, for example, may cause cerebral damage, inducing epilepsy and brain cysts (Zhang, 1990).
The family Clinostomidae, genus Clinostomum includes digenean species with a complex life cycle. Freshwater gastropods act as first intermediate hosts, fish and amphibian species as second intermediate hosts harbouring the metacercariae, while fish‐eating birds are the definitive hosts. Humans can be accidental hosts, after consumption of raw or undercooked freshwater fish (in some cases even brackish fish, such as mullet) infected with metacercariae, causing a fish‐borne zoonosis responsible for a rare disease known as Halzoun syndrome. In human infections, C. complanatum metacercaria excysts in the stomach and then migrates and attaches to the throat causing pharyngitis or laryngitis. Ectopic migration may also occur. Tiewchaloern et al. (1999) reported a case of eye infection. Human clinostomiasis has been reported in America, Southeast Asia and Eastern Europe. The main source of infection are commercially important fish, such as European perch (Perca fluviatilis). Menconi, Manfrin, et al. (2020) reported a 18.75% prevalence in fish from a subalpine lake (Lake Endine) in North Italy, while a prevalence of 13.1% was reported in fish in Türkiye (Çolak, 2013). Kadlec et al. (2003) reported a 15% prevalence in a study undertaken in the Morava River basin, Czech Republic. Soylu (2015) suggested that fish prevalence correlated with migratory routes of fish‐eating birds hosting Clinostomum complanatum.
1.3.2.3. Cestodes
Cestodes of the genus Dibothriocephalus (syn. Diphyllobothrium) include at least 13 species that infect humans. These cestodes are widely distributed in fish, mammal and bird hosts (EFSA BIOHAZ Panel, 2024) from temperate and cold‐water environments. The species D. latus is the main species infecting humans in Europe (Kuchta et al., 2015). Human diphyllobothriosis due to D. latus has re‐emerged in the subalpine region of Italy (Lago Maggiore and Lago di Como), Lake Geneva and from France, while it shows a decrease in other historically endemic areas, such as the Baltic countries (Kuchta et al., 2015). Raw fillets of perch (P. fluviatilis) are a major source of human infection. Sporadic cases of human diphyllobothriosis have also been reported in central and western European countries. As the latter countries are free of these parasites, the cases were attributed to the consumption of imported fish (Kuchta et al., 2015).
The Pacific broad tapeworm A. pacificus (syn. D. pacificum) is the causative agent of the third most common fish‐borne cestodosis in humans. Although most of the nearly 1000 cases have been reported in South America (Peru, Chile and Ecuador), recently reported cases in Europe demonstrate the potential for the spread of this tapeworm globally, as a result of global trade of fresh or chilled marine fish, and travel or migration of humans. Moreover, these parasites have re‐emerged in marine fish species, such as hake (Cantatore et al., 2023) that may now serve as a source of human infection when eaten raw or undercooked (Mondragón‐Martínez et al., 2020).
1.3.2.4. Acanthocephalans
Acanthocephalans of the genus Corynosoma use marine mammals (pinnipeds) as definitive hosts, in which the adult parasite attaches to the gastro‐intestinal mucosa. Fish acts as paratenic hosts carrying the juvenile stage in mesenteries of the gastrointestinal tract. Human consumption of raw or semi‐raw fish dishes may lead to infection and disease (Buchmann & Karami, 2024). A total of 50 species within the genus are recognised, but only few have been recovered from human patients. The first report of a human infected with a C. strumosum was from Alaska (Schmidt, 1971). Several other human infections were caused by C. validum (Takahashi et al., 2016) and/or C. villosum (Fujita et al., 2016) in Japan. The infections were all related to the consumption of raw marine fish.
1.3.3. Consumption of wild caught finfish in the EU
Apparent consumption [AC = (catches + aquaculture production + imports) – exports, EUMOFA] of fishery and aquaculture products in the EU increased in 2021 as compared to 2020 by 10.60 million tonnes Live weight equivalent (LWE), 4 or by 2%, mainly linked to a growth in farmed production (European seabass) and imports (blue whiting and salmon).
The most consumed species in the EU (LWE per capita per year) are tuna (2.86 kg), salmon (2.60 kg), cod (1.75 kg), Alaskan pollock (1.68 kg), hake (1.02 kg), herring (1.00 kg), sardines (0.54 kg), mackerel (0.53 kg), trout (0.49 kg) and saithe (0.36 kg) (EUMOFA EU Fish Market, 2023).
According to EUMOFA database (2023) 5 the most consumed species by households as a percentage of total volume are salmon, gilthead seabream, hake, cod and sardine. Although no distinction between wild caught and aquaculture is provided, the majority of salmon and gilthead seabream were supplied by aquaculture (Figure 1).
FIGURE 1.
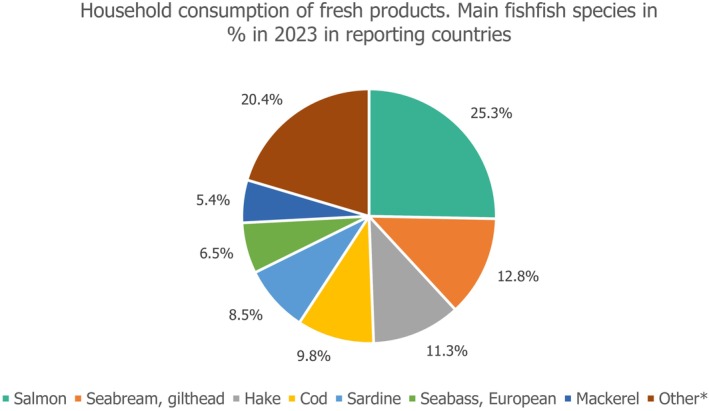
Household consumption of fish, in % of total volume, in 2023, for reporting countries. Graph built by EFSA based on EUMOFA database (https://eumofa.eu/household‐consumption‐of‐fresh‐products). *The category ‘other’ includes categories ‘sole, other’ (3,24%); ‘trout’ (2,80%); “tuna, miscellaneous (2,75%); ‘monkfish’ (2,01%); ‘anchovy’ (1,71%); ‘saithe (also known as coalfish)’ (1,71%); ‘swordfish’ (1,57%); ‘carp’ (1,05%); ‘herring’ (0,76%); ‘other freshwater fish’ (0,76%); ‘whiting’ (0,59%); ‘Allskan pollock’ (0,38%); ‘pangasius’ (0,29%); ‘scabbardfish’ (0,29%); ‘plaice, other’ (0,19%); ‘haddock’ (0,16%); ‘flounder, other’ (0,10%); ‘other salmonids’ (0,02%); ‘halibut, other’ (0,01%); ‘pike‐perch’ (0,01%); ‘dab’ (0,00%).
1.3.4. Landings of finfish in the EU
The total EU catch 6 in 2022 was an estimated 3.4 million tonnes live weight. The fishing fleets of Spain, France, Denmark and the Netherlands accounted for a about half of the total amount of aquatic organisms caught by Member States in 2022.
About 70% of all EU catches in the seven marine areas covered by EU statistics were taken in the Atlantic, Northeast area (FAO area 27). The key species caught in this area in 2022 were herring (19% of the live weight caught in this region), sprat (14%), blue whiting (11%) and mackerel (10%).
About 10% of the total EU catch was taken in the Mediterranean and Black Sea (FAO area 37), where the main species caught were sardines (22% of the EU catch in the area) and anchovies (18%).
About 7% of the total EU catch was taken in the Atlantic, Eastern Central area (FAO area 34), where the main catches were mackerel and yellowfin tuna.
About 7% of the total EU catch was taken in the Indian Ocean, Western area (FAO areas 51, 57 & 58), where the main catch was tuna, particularly skipjack, yellowfin and bigeye tuna.
Only 6% of the total EU catch was taken in three remaining marine areas. The main species caught in these areas were the following: hake in the Atlantic, Southwest area (FAO area 41) (4%); blue sharks and skipjack tuna in the Atlantic, Southeast area (FAO area 47) (1%); and redfish, cod and halibut in the Atlantic, Northwest area (FAO area 21) (1%).
The quantity of all aquatic organisms landed in the EU in 2022 is estimated to have been about 3.2 million tonnes product weight, representing no change from the estimated 3.2 million tonnes landed in 2021 (Figure 2).
FIGURE 2.
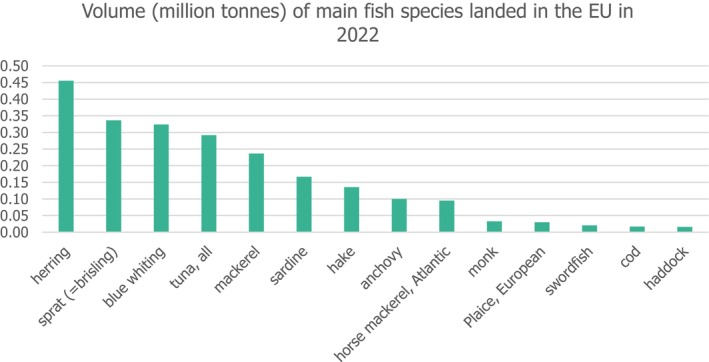
The main fish species landed in the EU by volume in 2022. The category ‘tuna, all’ includes categories ‘tuna, albacore’; ‘tuna, bigeye’; ‘tuna, bluefin’; ‘tuna, miscellaneous’; ‘tuna, skipjack’; ‘tuna, yellowfin’. Graph built by EFSA based on EUMOFA database (https://eumofa.eu/landings).
1.3.5. Import of finfish into the EU
The volume of EU imports in 2022 is shown in Figure 3. The five top importing member states (MS) by volume were Spain, Denmark, Sweden, the Netherlands and France. The five top countries exporting finfish products into the EU were Norway, China, Ecuador, Morocco and Iceland, based on volume recorded in 2023 (EC DG‐MARE, 2023). It should be noted that this data includes both farmed and caught fish.
FIGURE 3.
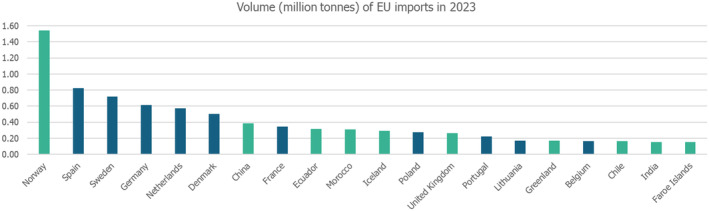
Volume (million tonnes) of fishery products imports by countries into the EU, 2023. Graph built by EFSA based on EUMOFA database (https://eumofa.eu/import‐export).
Intra‐EU trade reached 6 million tonnes in 2023. The most imported fish species were salmon (mostly from aquaculture) from Norway (FAO sub area 27.2), cod also mainly from Norway, Alaska pollock (a large amount was imported as frozen fillets, originating from China), tuna skipjack tuna and yellowfin tuna being the most commonly imported tunas, originating mostly from Ecuador (FAO area 87) and the Seychelles (FAO area 51), respectively. The most important exporting countries were harvesting fish from geographically dispersed locations that make it difficult to define the original fishing areas.
A summary of the available information is provided in Appendix A.
1.3.6. Fishing grounds that are the source of fish imported, landed and/or consumed in the EU
The FAO ‘Major Fishing Areas for Statistical Purposes’ is the world largest and complete mapping systems of the marine and freshwater areas for fishing purposes. It includes 8 inland waters (freshwater fishing areas) and 19 major marine fishing areas Figure 4.
FIGURE 4.
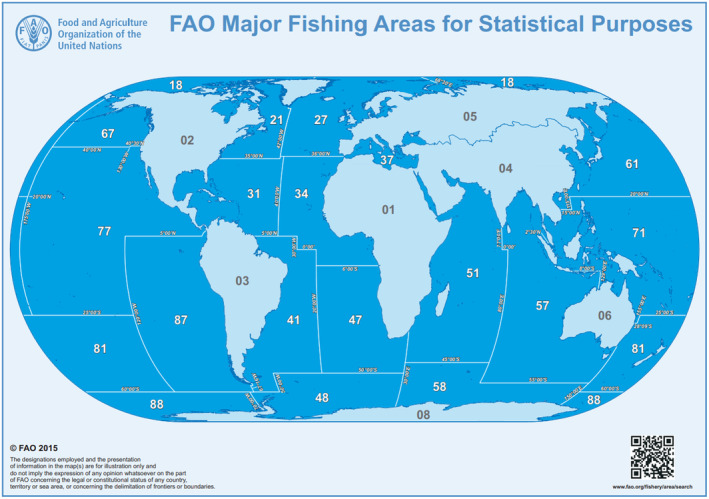
FAO major fishing areas. 7 Source: Food and Agriculture Organization of the United Nations. Reproduced with permission.
The main fishing grounds for the EU fishing fleet are located in FAO fishing areas 27 and 37 as detailed in Table 2. However, fish consumed in the EU may be caught and imported from a wide range of different fishing grounds as shown in Table 3.
TABLE 2.
Main fishing ground for EU fishing vessels.
| FAO area | Description | Fleet country of origin |
|---|---|---|
| 27 | North Sea and Eastern Arctic | Mostly Denmark, the Netherlands and Germany |
| 27 | Baltic Sea | Estonia, Finland, Latvia, Poland, Denmark, Germany, Lithuania, Sweden |
| 27 | North Western Waters | Mainly France and Ireland, followed by Belgium, Denmark, Spain and the Netherlands |
| 27 | South Western Waters | Spain, France, Portugal |
| 37 | Mediterranean Sea | Cyprus, Croatia, Greece, Italy, Malta and Slovenia |
| 37 | Black Sea | Bulgaria and Romania |
TABLE 3.
The FAO fishing areas where the main fish species imported into the EU are caught.
| Fish | Imports to EU (million tonnes, 2023)* | FAO fishing area** |
|---|---|---|
| Tuna, all | 0.915 | Area 21 – Atlantic, Northwest |
| Area 27 – Atlantic, Northeast | ||
| Area 31 – Atlantic, Western Central | ||
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Area 41 – Atlantic, Southwest | ||
| Area 47 – Atlantic, Southeast | ||
| Area 51 – Indian Ocean, Western | ||
| Area 57 – Indian Ocean, Eastern | ||
| Area 61 – Pacific, Northwest | ||
| Area 67 – Pacific, Northeast | ||
| Area 71 – Pacific, Western Central | ||
| Area 77 – Pacific, Eastern Central | ||
| Area 81 – Pacific, Southwest | ||
| Area 87 – Pacific, Southeast | ||
| Cod | 0.639 | Area 21 – Atlantic, Northwest |
| Area 27 – Atlantic, Northeast | ||
| Area 31 – Atlantic, Western Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Area 41 – Atlantic, Southwest | ||
| Herring | 0.521 | Area 2 – America, North ‐ Inland waters |
| Area 21 – Atlantic, Northwest | ||
| Area 27 – Atlantic, Northeast | ||
| Area 31 – Atlantic, Western Central | ||
| Area 5 – Europe – Inland waters | ||
| Mackerel | 0.359 | Area 21 – Atlantic, Northwest |
| Area 27 – Atlantic, Northeast | ||
| Area 31 – Atlantic, Western Central | ||
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Hake | 0.281 | Area 27 – Atlantic, Northeast |
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Saithe (=coalfish) | 0.164 | Area 18 – Arctic Sea |
| Area 21 – Atlantic, Northwest | ||
| Area 27 – Atlantic, Northeast | ||
| Area 31 – Atlantic, Western Central | ||
| Sardine | 0.162 | Area 1 – Africa – Inland waters |
| Area 27 – Atlantic, Northeast | ||
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Area 4 – Asia – Inland waters | ||
| Area 5 – Europe – Inland waters | ||
| Seabream, gilthead | 0.126 | Area 27 – Atlantic, Northeast |
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Area 5 – Europe – Inland waters | ||
| Seabass, European | 0.0847 | Area 1 – Africa – Inland waters |
| Area 27 – Atlantic, Northeast | ||
| Area 34 – Atlantic, Eastern Central | ||
| Area 37 – Mediterranean and Black Sea | ||
| Area 4 – Asia – Inland waters | ||
| Area 5 – Europe – Inland waters |
Sources
The distribution of fish species per fishing area was taken from https://fish‐commercial‐names.ec.europa.eu/fish‐names/home_en (species distribution and habitat).
2. DATA AND METHODOLOGIES
2.1. Literature search
The evidence on the presence of zoonotic parasites in wild caught fish from any fishing grounds was retrieved from studies published between 2010 and 2023 (inclusive).
Under the grant agreement GP/EFSA/BIOHAW/2023/05, 8 the Pathogens in Food (PIF) consortium performed a systematic review on anisakids and other zoonotic parasites. PIF conducted in DistillerSR a literature screening and data extraction (in Excel format) on anisakids and then, on anisakids/ other parasites in wild caught fish, from all countries and covering studies published in Spanish, English, Portuguese and French, between January 2010 and September 2023. The search protocol for the occurrence of anisakids and other zoonotic parasites in wild caught finfish was as described by Kooh et al. (2023). These data will be incorporated in the PIF database accessible through a web application (https://pif.esa.ipb.pt/). Overall, data from 274 studies/papers were extracted for anisakids and from 166 papers for other parasites.
In addition, a non‐systematic literature search was performed to retrieve specific missing data for fishing areas without search results. The relevance of the records was assessed by screening the title, keywords and the abstract. The review included scientific review papers, book chapters, peer‐review papers and other documents known by the experts or retrieved through non‐systematic searches.
2.2. Uncertainty analysis
As recommended by the EFSA guidance and related principles and methods on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018a; EFSA Scientific Committee, 2018b), an uncertainty analysis was undertaken as described in the protocol which is available in the Supporting Information section of Part 1 of this opinion (EFSA BIOHAZ Panel, 2024, Annex A). The sources of the main uncertainties related to ToR4 were identified by the experts in the WG and their impact on the uncertainty of the answers to the AQ4 were discussed. Consensus expert judgement within the WG, informed by the collected evidence and expert knowledge, was used to assess the certainty of the answer to the AQ4.
3. ASSESSMENT
3.1. Assessment of the presence of zoonotic parasites in caught finfish in marine fishing areas
Anisakis sp. were reported to be detected in many wild caught fish species in all FAO marine fishing areas except area 58 (Indian Ocean, Antarctic), for which there was no data on the occurrence of Anisakis sp. (Table 4). However, P. decipiens (s.l.) and C. osculatum (s.l.) were found in multiple fish species from fishing area 58 (Lukashanets et al., 2021).
TABLE 4.
Occurrence of zoonotic Anisakis species in finfish caught in FAO marine fishing areas. Full details are provided in Thébault et al., 2024
| FAO marine fishing areas | Fish species | References |
|---|---|---|
| Area 18: Arctic Sea | Boreogadus saida | Køie (2009) |
| Area 21: Northwest Atlantic | Merluccius bilinearis | Fuentes, Madrid, Cuesta, et al. (2022) |
| Gadus morhua | McClelland and Melendy (2011), Mouritsen et al. (2010), Münster et al. (2015), Severin et al. (2020) | |
| Gadus ogac | Mouritsen et al. (2010) | |
| Area 27: Atlantic, Northeast | Belone belone | Rolbiecki et al. (2020) |
| Clupea harengus | Bao et al. (2017), Levsen and Lunestad (2010), Levsen, Svanevik, et al. (2018), Mattiucci, Giulietti, et al. (2018), Shevchuk et al. (2020), Unger et al. (2014) | |
| Dicentrarchus labrax | Bernardi et al. (2011) | |
| Engraulis encrasicolus | Dessier et al. (2016), Domingo‐Hernandez et al. (2023), Rodríguez, Abollo, et al. (2018), Rodríguez, González, et al. (2018) | |
| Gadus morhua | Gay et al. (2018), Hauksson et al. (2012), Karami et al. (2022), Klapper et al. (2018), Levsen et al. (2022), Mehrdana et al. (2014), Mercken et al. (2021), Münster et al. (2015), Nadolna‐Altyn et al. (2022), Nadolna and Podolska (2014), Najda et al. (2018), Sobecka et al. (2011) | |
| Helicolenus dactylopterus | Sequeira et al. (2010) | |
| Lepidorhombus boscii | Levsen, Svanevik, et al. (2018), Rodríguez, Abollo, et al. (2018) Rodríguez, González, et al. (2018) | |
| Lophius budegassa, Lophius piscatorius | Rodríguez, Abollo, et al. (2018), Rodríguez, González, et al. (2018) | |
| Mallotus villosus | Levsen et al. (2016), Rolbiecki and Izdebska (2023) | |
| Melanogrammus aeglefinus | Levsen et al. (2022), Pierce et al. (2018) | |
| Merlangius merlangus | Gay et al. (2012), Pierce et al. (2018) | |
| Merluccius merluccius | Casti et al. (2017), Ceballos‐Mendiola et al. (2010), Cipriani et al. (2015), Diez et al. (2022), Fuentes, Madrid, Cuesta, et al. (2022), Pascual et al. (2018), Rodríguez, Abollo, et al. (2018), Santos et al. (2022), Tejada et al. (2014) | |
| Micromesistius potassou | Dezfuli et al. (2021), Elena Ahuir‐Baraja et al. (2021), Gómez‐Mateos et al. (2016), Levsen, Svanevik, et al. (2018), Llarena‐Reino et al. (2012), Madrid et al. (2012), Roca‐Geronès et al. (2020), Rodríguez, Abollo, et al. (2018)) | |
| Platichtys flesus | Marques et al. (2010) | |
| Pleuronectes platessa | Levsen, Svanevik, et al. (2018) | |
| Pollachius virens | Levsen et al. (2022) | |
| Reinhardtius hippoglossoides | Najda et al. (2018) | |
| Salmo salar | Kent et al. (2020), Mo et al. (2021), Noguera et al. (2015) | |
| Salmo trutta | Urquhart et al. (2010) | |
| Sardina pilchardus | Caballero‐Huertas et al. (2023), Dessier et al. (2016), Fuentes, Madrid, Elena, et al. (2022), Molina‐Fernández et al. (2015), Rodríguez, Abollo, et al. (2018) | |
| Scomber colias | Levsen, Svanevik, et al. (2018), Rodríguez, Abollo, et al. (2018) | |
| Scomber scombrus | Levsen, Cipriani, et al. (2018), Levsen, Svanevik, et al. (2018), Llarena‐Reino et al. (2012), Madrid et al. (2016), Pekmezci (2014), Rodríguez, Abollo, et al. (2018) | |
| Sebastes mentella | Klapper et al. (2016), Klapper et al. (2015), Najda et al. (2018) | |
| Sprattus sprattus | Kleinertz, Klimpel, and Palm (2012) | |
| Trachurus picturatus | Vasconcelos et al. (2017) | |
| Trachurus trachurus | Debenedetti et al. (2020), Lopes et al. (2020), Roca‐Geronès et al. (2020) | |
| Area 31: Atlantic, Western‐Central | Mugil incilis | Jaramillo‐Colorado et al. (2015) |
| Area 34: Atlantic, Eastern Central | Auxis thazard | Martin‐Carrillo et al. (2022) |
| Dicologlossa cuneata | Buzo‐Dominguez et al. (2021) | |
| Engraulis encrasicolus | Bouzid et al. (2023) | |
| Helicolenus dactylopterus | Sequeira et al. (2010) | |
| Katsuwonus pelamis | Hermida et al. (2018) | |
| Merluccius merluccius | Martin‐Carrillo et al. (2022) | |
| Mugil cephalus | Dione et al. (2014) | |
| Sardinella maderensis | Ogbon et al. (2023) | |
| Scomber colias | Martin‐Carrillo et al. (2022), Abattouy et al. (2011) | |
| Scomber scombrus | Biary et al. (2021), Martin‐Carrillo et al. (2022) | |
| Trachurus picturatus | Costa et al. (2012), Costa et al. (2013), Vasconcelos et al. (2017) | |
| Trachurus trachurus | Abattouy et al. (2014) | |
| Zeus faber | Pekmezci (2019) | |
| Area 37: Mediterranean and Black sea | Alosa immaculata | Shevchuk et al. (2020) |
| Conger conger | Akmirza (2012), Graci et al. (2017) | |
| Dicentrarchus labrax | Abouzaid et al. (2018) | |
| Diplodus annularis | Chaligiannis et al. (2012) | |
| Engraulis encrasicolus | Casti et al. (2017), Cavallero et al. (2015), Chaligiannis et al. (2012), Cipriani, Sbaraglia, Palomba, et al. (2018), Costa et al. (2016), De Liberato et al. (2013), Ferrer‐Maza et al. (2016), Gazzonis et al. (2017), Goffredo et al. (2019), Guardone et al. (2017), Meloni et al. (2011), Mladineo and Poljak (2013), Ozuni et al. (2021), Pulleiro‐Potel et al. (2015), Roca‐Geronès et al. (2020), Serracca et al. (2014) | |
| Epinephelus aeneus | Bouderbala et al. (2022) | |
| Epinephelus marginatus | Bouderbala et al. (2022), De Benedetto et al. (2021) | |
| Lophius budegassa | Pulleiro‐Potel et al. (2015) | |
| Lophius piscatorius | Costa et al. (2016) | |
| Merlangius merlangus | Mladineo and Poljak (2013) | |
| Merluccius merluccius | Casti et al. (2017), Chaligiannis et al. (2012), Cipriani, Sbaraglia, Paoletti, et al. (2018), Cipriani et al. (2015), Costa et al. (2016), Ferrer‐Maza et al. (2014), Fuentes, Madrid, Cuesta, et al. (2022), Goffredo et al. (2019), Graci et al. (2017), Meloni et al. (2011), Mladineo and Poljak (2013) Ozuni et al. (2021), Pekmezci et al. (2014), Sharif and Negm‐Eldin (2013) | |
| Micromesistius poutassou | Chaligiannis et al. (2012), Goffredo et al. (2019), Levsen, Svanevik, et al. (2018), Madrid et al. (2012), Meloni et al. (2011), Molina‐Fernández et al. (2018), Pekmezci et al. (2014), Pulleiro‐Potel et al. (2015), Roca‐Geronès et al. (2020) | |
| Mullus barbatus | Chaligiannis et al. (2012), Goffredo et al. (2019), Graci et al. (2017), Ozuni et al. (2021), Pekmezci et al. (2014) | |
| Mullus surmuletus | Goffredo et al. (2019), Meloni et al. (2011), Pulleiro‐Potel et al. (2015) | |
| Pagellus acarne | Benamara et al. (2020), Chaligiannis et al. (2012), Pulleiro‐Potel et al. (2015) | |
| Pagellus erithrinus | Chaligiannis et al. (2012), Pulleiro‐Potel et al. (2015) | |
| Sardina pilchardus | Bušelić et al. (2018), Caballero‐Huertas et al. (2023), Cavallero et al. (2015), Chaligiannis et al. (2012), Costa et al. (2016), Fuentes, Madrid, Elena, et al. (2022), Goffredo et al. (2019), Graci et al. (2017), Levsen, Svanevik, et al. (2018), Meloni et al. (2011), Mladineo and Poljak (2013), Pulleiro‐Potel et al. (2015) | |
| Sardinella aurita | Chaligiannis et al. (2012), Ozuni et al. (2021) | |
| Scomber colias | Casti et al. (2017), Gazzonis et al. (2017), Levsen, Svanevik, et al. (2018) Abattouy et al. (2011) Abdelsalam et al. (2020) Chaligiannis et al. (2012), Goffredo et al. (2019), Mladineo and Poljak (2013), Ozuni et al. (2021), Pekmezci et al. (2014) | |
| Scomber scombrus | Chaligiannis et al. (2012), Costa et al. (2016), Goffredo et al. (2019), Graci et al. (2017), Gutiérrez‐Galindo et al. (2010), Levsen, Cipriani, et al. (2018), Levsen, Svanevik, et al. (2018), Madrid et al. (2016), Meloni et al. (2011), Ozuni et al. (2021), Pekmezci et al. (2014) | |
| Scorpaena scrofa | Aydin and Pekmezci (2023) | |
| Sphyraena sphyraena | Boussellaa et al. (2018) | |
| Sphyraena viridensis | De Benedetto et al. (2021) | |
| Spicara smaris | Chaligiannis et al. (2012) | |
| Thunnus thynnus | Mladineo et al. (2011) | |
| Trachurus mediterraneus | Casti et al. (2017), Meloni et al. (2011), Pekmezci et al. (2014), Tepe and Oguz (2013) | |
| Trachurus trachurus | Abattouy et al. (2014), Chaligiannis et al. (2012), Costa et al. (2016), Debenedetti et al. (2020), Eissa et al. (2018), Graci et al. (2017), Gutiérrez‐Galindo et al. (2010), Macchioni et al. (2021), Meloni et al. (2011), Menconi et al. (2022), Ozuni et al. (2021), Roca‐Geronès et al. (2020), Tantanasi et al. (2012)) | |
| Trachurus picturatus | Meloni et al. (2011) | |
| Trisopterus minutus | Pulleiro‐Potel et al. (2015) | |
| Trisopterus minutus capelanus | Goffredo et al. (2019) | |
| Xiphias gladius | Mattiucci et al. (2014) | |
| Area 41: Atlantic, Southwest | Cynoscion guatucupa | Crosi Martínez et al. (2022), Fontenelle et al. (2013) |
|
Lutjanus analis Lutjanus jocu Lutjanus synagris Lutjanus vivanus |
Alves et al. (2020) | |
| Lutjanus purpureus | Cavalcanti et al. (2013) | |
| Macrodon ancylodon | Crosi Martínez et al. (2022) | |
| Merluccius hubbsi | Mattiucci, (2006) | |
| Micropogonias furnieri | Di Azevedo and Iñiguez (2018) | |
| Pagrus pagrus | Soares and Luque (2015) | |
| Plagioscion squamosissimus | Chagas de Souza et al. (2020), Rodrigues et al. (2015) | |
| Zenopsis conchifer | Lanfranchi et al. (2016), Lanfranchi et al. (2018) | |
| Area 47: Atlantic, Southeast |
Brama brama Trachurus capensis Thyrsites atun Lophius vomerini Merluccius capensis Helicolenucs dactylopterus Lepidopus caudatus |
Mackintosh et al. (2018), Mattiucci, Cipriani, et al. (2018) |
| Area 48: Atlantic, Antarctic | Gymnoscopelus nicholsi | Klimpel, (2010) |
| Area 51: Indian Ocean, Western | Trichiurus lepturus, Saurida undosquamis | Cipriani et al. (2022) |
| Merluccius merluccius lessepsianus | Abou‐Rahma et al. (2016) | |
| Scomberomorus commerson | Adel et al. (2013) | |
| Area 57: Indian Ocean, Eastern | Epinephelus areolatus | Kleinertz, Damriyasa, et al. (2012) |
| Eupleurogrammus muticus | Bao et al. (2022) | |
| Johnius belangerii | Yun et al. (2022) | |
| Lutjanus malabaricus | Setyobudi (2011) | |
| Rastrelliger spp. | Setyobudi et al. (2019) | |
| Selar crumenophthalmus | Bao et al. (2022) | |
| Trichiurus lepturus | Bao et al. (2022), Setyobudi (2011) | |
| Area 61: Pacific, Northwest | Clupea pallasii | Gordeev and Sokolov (2023) |
| Conger myriaster | Chen et al. (2018) | |
| Coryphaenoides acrolepis | Kumagai and Nishino (2023) | |
| Gadus chalcogrammus Gadus macrocephalus | Gomes et al. (2020) | |
| Katsuwonus pelamis | Murata et al. (2021), Takano et al. (2021) | |
| Oncorhynchus gorbuscha | Gordeev and Sokolov (2023) | |
| Oncorhynchus keta | Gomes et al. (2020), Gordeev and Sokolov (2023), Setyobudi et al. (2011), Setyobudi et al. (2019) | |
| Sardinops sagax melanostictus | Gomes et al. (2020) | |
| Scomber australasicus | Chen and Shih (2015), Chou et al. (2011), Gomes et al. (2020), Ohnishi et al. (2023), Sonko et al. (2020) | |
| Scomber japonicus | Bak et al. (2014), Chen et al. (2015), Gomes et al. (2020), Hidano et al. (2021), Ohnishi et al. (2023), Suzuki et al. (2010) | |
| Scomberomorus niphonius | Zhao et al. (2016) | |
| Seriola quinqueradiata | Cho et al. (2012) | |
| Trachurus japonicus | Chen et al. (2015) | |
| Trichiurus lepturus | Chen et al. (2015), Sonko et al. (2020) | |
| Area 67: Pacific, Northeast | Gadus macrocephalus Sebastes alutus | Oguz et al. (2021) |
| Oncorhynchus gorbuscha Oncorhynchus keta Oncorhynchus nerka | Karl et al. (2011) | |
| Area 71: Pacific, Western central |
Auxis thazard Caranx sp. Katsuwonus pelamis Rastrelliger kanagurta |
Anshary et al. (2014) |
|
Carangoides malabaricus Lutjanus johnii Megalaspis cordyla Decapterus macarellus |
Hien et al. (2021) | |
| Decapterus macrosoma | See et al. (2022) | |
| Euthynnus affinis | Anshary et al. (2014), Pambudi et al. (2021) | |
| Liza vaigiensis | Jabbar et al. (2012) | |
| Trichiurus lepturus | Hien et al. (2021), Setyobudi et al. (2023) | |
| Area 77: Pacific, Eastern central | Caranx caballus | Violante‐González et al. (2016) |
|
Clupea pallasii Engraulis mordax Merluccius productus Oncorhynchus kisutch Oncorhynchus tshawytscha Sardinops sagax Trachurus symmetricus |
Jacobson et al. (2012) | |
| Scorpaena guttata | Rodriguez‐Santiago et al. (2020) | |
| Sebastes miniatus | Rodríguez‐Santiago et al. (2014) | |
| Area 81: Pacific, Southwest central | Chrysophrys auratus | Hossen et al. (2021) |
| Scomber australasicus | Hossen et al. (2023) | |
| Area 87: Pacific, Southeast | Brama australis | Munoz‐Caro et al. (2022), Oliva et al. (2016) |
| Merluccius australis | Gonzalez‐Poblete et al. (2022), Torres‐Frenzel and Torres (2014) | |
| Mugil cephalus | Castellanos et al. (2017) | |
|
Sarda chilensis Scomber japonicus Trachurus murphyi |
Aco Alburqueque et al. (2020) | |
|
Scomber japonicus peruanus Trachurus symmetricus murphyi Merluccius gayi peruanus Brama japonica |
Martinez‐Rojas et al. (2021) | |
| Area 88: Pacific, Antarctic | Dissostichus eleginoides | Brickle, (2005) |
3.2. Assessment of the presence of zoonotic parasites in caught finfish in freshwater fishing areas
In freshwater fishing areas D. latus parasites were detected in Pseudotolithus spp. (ray‐finned fish belonging to the family Sciaenidae) in area 1 (African inland waters) (Table 5). Dibothriocephalus spp. were reported in Arctic char (Salvelinus alpinus) caught in area 2 (North America inland waters) while Dibothriocephalus spp., including D. dendriticus and Di. latus, were detected in a range of fish species (Oncorhynchus mykiss, Salmo trutta, Salvelinus fontinalis, Percichthys trucha, Salmo salar, Odontesthes mauleanum, Basilichthys australis, Oncorhynchs kisutch) in area 3 (South America inland waters). Multiple fish species from fishing area 4 (Asia inland waters) were infected with a range of different zoonotic parasites including Opisthorchis spp. Opisthorchis felineus, O. viverrini, Metorchis spp., M. orientalis, H. taichui, H. pumilio, H. yokogawai, Centrocestus spp., C. formosanus, H. yokogawai, Gnathostoma sp., Clonorchis spp., Metagonimus spp., D. latus, D. dendriticus, Cryptocotyle sp., C. sinensis, M. yokogawai, cyathocotylid trematode (Trematoda: Digenea: Cyathocotylidae) and Holostephanus sp. Fish in area 5 (Europe inland waters) were infected with Dibothriocephalus spp., D. endriticus, O. felineus, D. latus and Corynosoma sp., while Contracaecum bancrofti, Echinostoma sp., Clinostomum sp. were reported in fish in area 6 (Oceania inland waters). There was no data for fishing area 8, possibly because there are few if any fish in Antarctica inland waters and none that are imported into the EU.
TABLE 5.
Occurrence of zoonotic parasites in finfish caught in freshwater fishing areas. For presentation purposes the fish species have been omitted from this table but full details are available in Kooh et al., 2024.
| FAO freshwater fishing areas | Parasites | References |
|---|---|---|
| Area 1: Africa – Inland waters | Dibothriocephalus latus | Bakare et al. (2023) |
| Clinostomum sp. | Tesfaye et al. (2023) | |
| Area 2: America, North – Inland waters | Dibothriocephalus spp. | Gallagher and Dick (2010) |
| Area 3: America, South – Inland waters | Dibothriocephalus spp. | Kuchta et al. (2019), Rauque et al. (2018), Torres and Puga (2011) |
| Dibothriocephalus dendriticus | Rozas et al. (2012), Semenas et al. (2021), Yamasaki et al. (2023) | |
| Dibothriocephalus latus | Semenas et al. (2021), Torres et al. (2012), Yamasaki et al. (2023) | |
| Area 4: Asia – Inland waters | Opisthorchis spp. | Labony et al. (2020), Marcus et al. (2012) |
| Opisthorchis felineus | Aubakirov et al. (2022), Bonina et al., 2024, Karmaliyev et al. (2023), Liberman (2019), Liberman and Voropaeva (2018) | |
| Opisthorchis viverrini | Chai et al. (2019), Chai et al. (2020), Chai et al. (2014), Charoensuk et al. (2022), Dao et al. (2017), Eom et al. (2015), Kiatsopit et al. (2014), Laoprom et al. (2021), Manpratum et al. (2017), Namsanor et al. (2020), Nithikathkul et al. (2014), Nithikathkul et al. (2012), Ong et al. (2016), Onsurathum et al. (2016), Phyo Myint et al. (2020), Pinlaor et al. (2013), Pitaksakulrat et al. (2017), Prakobwong et al. (2017), Pumhirunroj et al. (2020), Rim et al. (2013), Sanpool et al. (2018), Sohn et al. (2012), Sohn, Choi, Jung, Hong, Ryoo, Chang, Lee, et al. (2021), Sohn, Jung, et al. (2019) | |
| Metorchis spp. | Bonina et al. (2024), Labony et al. (2020), Liberman (2019) | |
| Metorchis orientalis | Qiu et al. (2017), Sohn, Na, Cho, Ju, Kim, Hwang, No, and Park (2021), Yang et al. (2019) | |
| Haplorchis spp. | Hung et al. (2015) | |
| Haplorchis taichui | Chai et al. (2019), Chai et al. (2020), Nithikathkul et al. (2014), Phyo Myint et al. (2020), Rim et al. (2013), Sripa et al. (2016), Wongsawad et al. (2013) | |
| Haplorchis pumilio | Chai et al. (2019), Chai et al. (2020), Chai et al. (2014), Phyo Myint et al. (2020), Sripa et al. (2016) | |
| Haplorchis yokogawai | Abattouy et al. (2014), Chai et al. (2019), Chai et al. (2020), Chai et al. (2014), Rim et al. (2013) | |
| Centrocestus spp. | Chai et al. (2020) | |
| Centrocestus formosanus | Chai et al. (2019), Chai et al. (2014), Hung et al. (2015), Rim et al. (2013), Sripa et al. (2016) | |
| Gnathostoma sp. | Chai et al. (2020) | |
| Clonorchis spp. | Labony et al. (2020), Marcus et al. (2012) | |
| Metagonimus spp. | Labony et al. (2020), Sohn, Na, et al. (2019), Sohn et al. (2015) | |
| Dibothriocephalus latus | Liberman et al. (2019) | |
| Dibothriocephalus dendriticus | Nikulina and Polyaeva (2020) | |
| Clonorchis sinensis | Hung et al. (2015), Sohn and Na (2019), Sohn, Na, Cho, Lee, Ju, Lee, Lim, et al. (2021), Sohn, Na, Cho, Lee, Ju, Lee, Park, and Ahn (2021), Sohn et al. (2017), Wang et al. (2022), Yang et al. (2019) | |
| Metagonimus yokogawai | Qiu et al. (2017) | |
| Area 5: Europe – Inland waters | Dibothriocephalus spp. | Bielat et al. (2015), Kuhn et al. (2017) |
| Dibothriocephalus ditremum | Henriksen et al. (2016) | |
| Dibothriocephalus dendriticus | Borgstrøm et al. (2021), Henriksen et al. (2016), Kuhn et al. (2016) | |
| Dibothriocephalus latus | Dupouy‐Camet and Yera (2015), Gustinelli et al. (2016), Menconi, Pastorino, et al. (2020), Menconi et al. (2021), Radačovská et al. (2019) | |
| Opisthorchis felineus | De Liberato et al. (2011), Drozdova et al. (2022), Simakova et al. (2022), Simakova et al. (2019), Simakova et al. (2021) | |
| Corynosoma sp. | Pilecka‐Rapacz et al. (2017) | |
|
Echinostoma sp. Clinostomum sp. Eustrongylides sp. |
Wilson et al. (2019) | |
| Area 8: Antarctica – Inland waters | No data available | No data available |
Uncertainty
There are multiple sources of uncertainty associated with the data provided above. These are summarised in Table 6 including the impact they may have on the conclusions.
TABLE 6.
Sources of uncertainty and their potential impact on the conclusions.
| Sources of uncertainty | Impact of the uncertainty on the conclusions |
|---|---|
|
It is assumed that the presence of anisakids or other zoonotic parasites in one fish species in a given marine fishing area is indicative that all fish species in that area may be infected. The absence of data covering the range of different zoonotic fish parasites that are found in all of the different fish species in marine fishing areas. |
Minimum impact, because anisakids can infect all of the wild caught fish species that are consumed in the EU/EFTA. In the marine fishing areas where there were no reports of aniskaids in fish (area 58), other zoonotic parasites have been detected in the fish and reported. It is generally accepted, based on the data published to date that there is no evidence that any fish species consumed in the EU/EFSA is free of zoonotic parasites (i.e. all fish species are susceptible to infection with at least one zoonotic parasite). |
|
It is assumed that the presence of any zoonotic parasite in one fish species in a given freshwater fishing area is indicative that all fish species in that area may be infected with that or other zoonotic parasites. The absence of data covering the range of different zoonotic fish parasites that are found in all of the different freshwater. |
Minimum impact, as there is data available for all freshwater fishing areas and no studies to suggest that any particular fish species cannot be infected with at least one zoonotic parasite. |
| Although there are multiple studies reported for most fishing areas there are a few where the number of studies is limited. In the marine areas for Anisakis, for example Area 47 (Atlantic Southwest) had only one reported study, while Areas 67 (Pacific Northeast) and 71 (Pacific western central) had 2. There are considerably less data on Anisakis from areas 18, 31, 48 and 88), with each reliant on one published study. A similar issue arises for the freshwater areas with all except Area 5 (Europe – Inland waters) having one to three studies. | Minimum impact, as when the evidence is considered in its entirety and there are multiple publications covering freshwater systems and conditions are not sufficiently different between these bodies of water to suggest that zoonotic parasites are absent from one or more. |
4. CONCLUSIONS
AQ4: Are there any particular species of wild caught fish originating from specific fishing grounds, where fish consumed in the EU/EFTA are caught, that are free of parasites of public health importance?
It was concluded to be 99%–100% certain (almost certain) that there are no particular species of wild caught fish originating from specific marine fishing areas, where the fish consumed in the EU/EFTA are free of parasites of public health importance. Based on the data reported in the peer reviewed literature, at least one zoonotic parasite has been reported in at least one fish species in each of the FAO marine fishing areas (Anisakis spp. in all fishing areas, except area 58, but in which P. decipiens (s.l.) and C. osculatum (s.l.) were reported in multiple fish species). Thus, due to relative low fish host specificity of the zoonotic parasites, the panel concluded that all relevant fish species may be exposed to and infected with zoonotic parasites.
The same applies to freshwater fishing areas from which fish are consumed in the EU/EFTA, as all fishing areas, except for Antarctica, have some or multiple studies reporting the presence of a range of different zoonotic parasites in the caught fish species.
The conclusion on marine fishing areas is consistent with that of the 2010 opinion which stated that no sea fishing grounds could be considered to be free of A. simplex (s.l.) larva.
5. RECOMMENDATIONS
It is recommended to implement routine surveillance of zoonotic parasites in wild caught fish, including niche products that are imported into the EU, using the most effective detection methods, as described in Part 1 of this Opinion.
It is also recommended that research be undertaken to address the dearth of surveillance data, especially for the most consumed wild caught fish in EU and EFTA countries, and that the effect of climate change on the zoonotic parasites' geographical distribution and risk to the consumer be assessed to evaluate the need for revised risk management practices in the future.
It is recommended that research is carried out to estimate the disease burden associated with zoonotic parasites in wild caught fish.
GLOSSARY AND/OR ABBREVIATIONS
- AC
apparent consumption
- AQ
assessment question
- BIOHAZ
biological hazards
- DV
deliverable
- ECDC
European Centre for Disease Prevention and Control
- EFTA
European Food Trade Association
- EUMOFA
European market observatory for fisheries and aquaculture products
- EUROSTAT
Statistical office of the European Union
- FAO
Food and Agriculture Organization of the United Nations
- LWE
Live weight equivalent means weight calculated based on conversion factors from the weight recorded on landing, or from the net weight of the various processed products
- PIF
pathogens in food
- ToR
Term of Reference
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00172
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Ana Allende, Avelino Alvarez‐Ordóñez, Valeria Bortolaia, Sara Bover‐Cid, Alessandra De Cesare, Wietske Dohmen, Laurent Guillier, Lieve Herman, Liesbeth Jacxsens, Maarten Nauta, Lapo Mughini‐Gras, Jakob Ottoson, Luisa Peixe, Fernando Perez‐Rodriguez, Panagiotis Skandamis and Elisabetta Suffredini.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: Rubén Barcia‐Cruz as well as the Pathogens in Food Database consortium (EFSA grant agreement GP/EFSA/BIOHAW/2023/05).
APPENDIX A. Data on import, landings and consumption of finfish in the EU
A.1.
The Tables A.1, A.2 below presents a summary of the data collated on finfish landings, imports and consumption in the EU.
TABLE A.1.
Data on import, landings and consumption of freshwater finfish species in the EU.
|
Freshwater fish Common names |
Latin names | Landings volume (kg), 2021 | Imports to EU (kg, 2023) | Per capita consumption (kg, LWE) EU, 2021 | Household consumption of fresh products, EUMOFA 2021 |
|---|---|---|---|---|---|
| Carp | Cyprinus carpio | 160,800 | 14,377,080 | – | 6,342,911 |
| Catfish | Eg.: Ictalurus puntactus, I. furcatus; Clarias spp., C. gariepinus, C. batrachus | 4710 | 70,624,813 | – | – |
| Pike | Esox lucius | 281,800 | – | – | – |
| Pikeperch | Sander lucioperca | 714,590 | – | – | 36,271 |
TABLE A.2.
Data on import, landings and consumption of marine water finfish species in the EU.
| Marine fishes common names | Latin names (family/species) | Landings volume (kg), 2021 | Imports to EU (kg, 2023) | Per capita consumption of wild fish (kg, LWE) EU, 2021 | Household consumption of fresh products, EUMOFA 2021 |
|---|---|---|---|---|---|
| Anchovy | Engraulidae | 104,776,120 | 52,565,535 | – | 10,323,964 |
| Brill | Scophthalmus rhombus | 1,828,600 | – | – | |
| Cod | Gadidae | 16,489,680 | 648,257,344 | 1.75 | 59,113,954 |
| Dab | Limanda limanda | 4,354,410 | – | – | 13,763 |
| Eel, Cusk | Genypterus blacodes | 1,012,340 | 4,825,508 | – | – |
| Eel | Anguillidae | 770,200 | 4,722,064 | – | – |
| Flounder, European | Platichthys flesus | 18,000,580 | 1,732,435 | – | – |
| Grenadier | Macrouridae | 1,112,270 | 15,869,999 | – | – |
| Gurnard | Triglidae | 10,011,030 | – | – | – |
| Haddock | Melanogrammus aeglefinus | 14,563,780 | 35,270,529 | – | 946,662 |
| Hake | Merlucciidae | 143,063,080 | 280,808,552 | 1.02 | 68,160,374 |
| Halibut, Atlantic | Hippoglossus hippoglossus | 299,420 | 3,808,625 | – | – |
| Halibut, Greenland | Reinhardtius hippoglossoides | 7,318,170 | 46,902,426 | – | – |
| Herring | Clupeidae | 492,290,670 | 520,769,903 | 1.00 | 4,601,847 |
| Horse mackerel, Atlantic | Trachurus trachurus | 76,929,490 | 26,109,454 | – | – |
| John dory | Zeus faber | 2,740,000 | – | – | – |
| Ling | Lotidae | 3,181,310 | 10,879,583 | – | – |
| Mackerel | Scombridae | 222,009,970 | 355,396,879 | 0.53 | 32,731,456 |
| Megrim | Lepidorhombus whiffiagonis | 15,074,920 | 10,825,031 | – | – |
| Monk | Lophiidae | 35,459,700 | 41,128,258 | – | 12,110,118 |
| Mullet, Red | Mullidae | 14,985,760 | – | – | – |
| Picarel | Spicara smaris | 1,988,110 | – | – | – |
| Plaice, European | Pleuronectes platessa | 37,230,580 | 32,294,527 | – | – |
| Pollack | Pollachius pollachius | 2,874,530 | 3,773,009 | – | – |
| Pounting (=Bib) | Trisopterus luscus | 5,606,340 | – | – | – |
| Ray | Rajidae | 15,533,030 | 5,346,570 | – | – |
| Redfish | Berycidae, Sebastidae | 11,625,700 | 66,081,879 | – | – |
| Saithe (=Coalfish) | Pollachius virens | 12,707,860 | 163,051,674 | 0.36 | 10,315,553 |
| Salmon | Salmonidae | – | – | 0.15 | |
| Sardine | Sardina pilchardus | 170,590,560 | 162,945,428 | 0.54 | 51,112,532 |
| Scabbardfish | Trichiuridae | 5,028,800 | – | – | 1,750,880 |
| Seabass, European | Dicentrarchus labrax | 4,485,310 | 84,739,788 | – | 39,034,591 |
| Seabream, Gilthead | Sparus aurata | 3,397,350 | 124,167,733 | – | 77,233,743 |
| Smelt | Osmeridae | 5,294,750 | – | – | – |
| Sole, common | Solea solea | 17,122,230 | 13,437,914 | – | – |
| Sprat | Sprattus spp. | 319,583,330 | 60,477,577 | – | – |
| Swordfish | Xiphias gladius | 20,686,800 | 48,265,115 | – | 9,475,634 |
| Toothfish | Dissostichus mawsoni | 140,240 | 2,348,213 | – | – |
| Trout | Salmonidae | – | – | 0.01 | |
| Tuna all species | Scombridae | 297,007,240 | 912,412,724 | 2.84 | – |
| Turbot | Scophthalmus maximus | 3,729,710 | 10,230,750 | – | – |
| Weever | Trachinidae | 1,113,320 | – | – | – |
| Whiting | Merlangius merlangus | 15,737,430 | 8,088,909 | – | 3,585,404 |
| Amberjack, Greater | Seriola dumerili | – | – | – | – |
| Wolffish, Atlantic | Anarhichas lupus | – | – | – | – |
| Barracuda | Sphyraena | – | – | – | – |
| Blue butterfish | Stromateus fiatola | – | – | – | – |
| Capellin | Mallotus villosus | – | – | – | – |
| Croaker | Argyrosomus regius | – | – | – | – |
| Cusk = tusk | Brosme brosme | – | – | – | – |
| Dentex, Large‐eye | Dentex macrophthalmus | – | – | – | – |
| Dogfish | Squalus acanthias | – | – | – | – |
| Eel, Conger | Conger conger | – | – | – | – |
| Flounder, Arrowtooth | Atheresthes stomas | – | – | – | – |
| Greater Argentine | Argentina silus | – | – | – | – |
| Groupers | Epinephelidae | – | – | – | – |
| Hairtail, Largehead | Trichiurus lepturus | – | – | – | – |
| Horse mackerel, other | Carangidae | – | – | – | – |
| Kingklip | Genypterus hubbsi | – | – | – | – |
| Ling, Blue | Molva dypterygia | – | – | – | – |
| Ling, Common | Molva molva | – | – | – | – |
| Megrim, Four‐spotted | Lepidorhombus boscii | – | – | – | – |
| Pollock, Alaska | Gadus chalcogrammus | – | – | – | – |
| Porgy, Red | Pagrus pagrus | – | – | – | – |
| Scorpionfish, black | Scorpaena porcus | – | – | – | – |
| Scorpionfish, Red | Scorpaena scrofa | – | – | – | – |
| Seabass, Argentine | Acanthistius brasiliensis | – | – | – | – |
| Seabream | Pagrus pagrus | – | – | – | – |
| Sole, Senegalese Common | Solea senegalensis | – | – | – | – |
| Weever, Greater | Trachinus draco | – | – | – | – |
| Whiting, Blue | Gadidae | – | – | – | – |
Abbreviation: –, no data.
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Allende, A. , Alvarez‐Ordóñez, A. , Bortolaia, V. , Bover‐Cid, S. , De Cesare, A. , Dohmen, W. , Guillier, L. , Herman, L. , Jacxsens, L. , Nauta, M. , Mughini‐Gras, L. , Ottoson, J. , Peixe, L. , Perez‐Rodriguez, F. , Skandamis, P. , Suffredini, E. , Buchmann, K. , Levsen, A. , Mattiucci, S. , … Bolton, D. (2024). Re‐evaluation of certain aspects of the EFSA Scientific Opinion of April 2010 on risk assessment of parasites in fishery products, based on new scientific data. Part 2. EFSA Journal, 22 (11), e9090. 10.2903/j.efsa.2024.9090
Adopted: 23 October 2024
The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch
Notes
FAO. FAO Major Fishing Areas. [Cited 1 November 2020]. https://www.fao.org/fishery/en/area/search.
Live weight equivalent (LWE) means weight calculated based on conversion factors from the weight recorded on landing, or from the net weight of the various processed products.
European Market Observatory for Fisheries and Aquaculture Products database. Available at: https://eumofa.eu/data.
Fish catches cover fish, molluscs, crustaceans and other aquatic animals, residues and aquatic plants that are taken for all purposes, by all types and class of vessel, gear and fishermen, operated in all the seven marine areas legally covered by EU statistical regulations.
FAO fishing areas: Marine areas: 18‐Arctic ocean, 21‐Northwest Atlantic, 27‐Northeast Atlantic, 31‐Western Central Atlantic, 34‐Eastern Central Atlantic, 37‐Mediterranean & Black Sea, 41‐Southwest Atlantic, 47‐Southeast Atlantic, 48‐Atlantic, Antarctic, 51‐Western Indian Ocean, 57‐Eastern Indian Ocean, 58‐Indian Ocean, Antarctic, 61‐Northwest Pacific, 67‐Northeast Pacific, 71‐Western Central Pacific, 77‐Eastern Central Atlantic, 81‐Southwest Pacific, 87‐Southeast Pacific, 88‐Pacific Antarctic.
Freshwater areas: 01‐African inland waters, 02‐North America inland waters, 03‐South America inland waters, 04‐Asia inland waters, 05 Europe inland waters, 06 Oceania inland waters, 08‐Antarctica inland waters The fishing area 07 (‘Former USSR area – Inland waters’) referred to the area that was formerly the Union of Soviet Socialist Republics. Starting with the data for 1988, information for each new independent Republic is shown separately. The new independent Republics are: Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan (statistics assigned to the fishing area ‘Asia – Inland waters’) and Belarus, Estonia, Latvia, Lithuania, Republic of Moldova, Russian Federation, Ukraine (statistics assigned to the fishing area ‘Europe – Inland waters’). Source: https://www.fao.org/fishery/en/area (last accessed 22/10/2024).
EFSA's grant agreement GP/EFSA/BIOHAW/2023/05 (Pathogens in foods database: extension with Vibrio and parasites) with as beneficiaries Instituto Politécnico de Bragança (IPB) as coordinator and Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (Anses) as co‐beneficiary.
REFERENCES
- Abattouy, N. , López, A. V. , Maldonado, J. L. , Benajiba, M. H. , & Martín‐Sánchez, J. (2014). Epidemiology and molecular identification of Anisakis pegreffii (Nematoda: Anisakidae) in the horse mackerel Trachurus trachurus from northern Morocco. Journal of Helminthology, 88(3), 257–263. 10.1017/S0022149X13000102 [DOI] [PubMed] [Google Scholar]
- Abattouy, N. , Valero, A. , Benajiba, M. H. , Lozano, J. , & Martín‐Sánchez, J. (2011). Anisakis simplex s.l. parasitization in mackerel (Scomber japonicus) caught in the north of Morocco‐prevalence and analysis of risk factors. International Journal of Food Microbiology, 150(2–3), 136. 10.1016/j.ijfoodmicro.2011.07.026 [DOI] [PubMed] [Google Scholar]
- Abdelsalam, M. , Attia, M. M. , & Mahmoud, M. A. (2020). Comparative morphomolecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Regional Studies in Marine Science, 39, 101469. 10.1016/j.rsma.2020.101469 [DOI] [Google Scholar]
- Abou‐Rahma, Y. , Abdel‐Gaber, R. , & Kamal Ahmed, A. (2016). First record of Anisakis simplex third‐stage larvae (Nematoda, Anisakidae) in European hake Merluccius merluccius lessepsianus in Egyptian water. Journal of Parasitology Research, 2016, 9609752. 10.1155/2016/9609752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abouzaid, A. , Kamal, E. , Desouky, A. , & Abou‐Rawash, A.‐R. (2018). Metazoan parasite fauna of wild sea bass; Dicentrarchus labrax (Linnaeus, 1758) in Egypt. Life Science Journal, 15(6), 48–60. 10.7537/marslsj150618.06 [DOI] [Google Scholar]
- Aco Alburqueque, R. , Palomba, M. , Santoro, M. , & Mattiucci, S. (2020). Molecular identification of zoonotic parasites of the genus Anisakis (Nematoda: Anisakidae) from fish of the southeastern Pacific Ocean (off Peru coast). Pathogens, 9(11), 910. 10.3390/pathogens9110910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adel, M. , Azizi, H. , & Nematolahi, A. (2013). Scomberomorus Commerson, a new Paratenic host of Contracaecum sp. and Anisakis sp. (Nematoda: Anisakidae) from Persian gulf. World Journal of Fish and Marine Sciences, 5, 310–314. 10.5829/idosi.wjfms.2013.05.03.71232 [DOI] [Google Scholar]
- Akmirza, A. (2012). Metazoan parasite Fauna of conger eel (Conger conger L.) near Gökçeada, Northeasten Aegean Sea, Turkey. Kafkas Universitesi Veteriner Fakultesi Dergisi, 18(5), 10.9775/kvfd.2012.6624 [DOI] [Google Scholar]
- Alt, K. G. , Cunze, S. , Kochmann, J. , & Klimpel, S. (2022). Parasites ofthree closely related Antarctic fish species (Teleostei: Nototheniinae) from Elephant Island. Acta Parasitologica, 67(1), 218–232. 10.1007/s11686-021-00455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, V. L. S. , Souza, G. T. R. , Takemoto, R. M. , Melo, C. M. , Madi, R. R. , & Jeraldo, V. L. S. (2020). Anisakidae Skrjabin & Karokhin, 1945 and Raphidascarididae Hartwich, 1954 nematodes in lutjanidae (pisces: Perciformes) from the Brazilian northeast coast. Brazilian Journal of Biology, 80, 255–265. 10.1590/1519-6984.190350 [DOI] [PubMed] [Google Scholar]
- Anshary, H. , Sriwulan, F. , & Mark, A. (2014). Occurrence and molecular identification of Anisakis Dujardin, 1845 from marine fish in southern Makassar Strait, Indonesia. The Korean Journal of Parasitology, 52(1), 9–19. 10.3347/kjp.2014.52.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam, U. , Pandian, R. G. , Jayasimhan, P. , Sudarsanan, G. B. , & Sornappan, G. (2024). First report of Paracapillaria philippinensis infection in flowerhorn cichlid in India. Parasitology Research, 123(3), 150. 10.1007/s00436-024-08175-4 [DOI] [PubMed] [Google Scholar]
- Aubakirov, M. Z. , Abdybekova, A. M. , Khassanova, M. A. , Issabayev, A. Z. , Kaumenov, N. S. , Tegza, A. A. , Sapa, V. A. , Domatsky, V. N. , Erenko, E. N. , & Namazbai, K. N. (2022). Epizootology and epidemiology of Opisthorchiasis in northern Kazakhstan. OnLine Journal of Biological Sciences, 22(3), 340–346. 10.3844/ojbsci.2022.340.346 [DOI] [Google Scholar]
- Aydin, C. , & Pekmezci, G. Z. (2023). Molecular identification and infection levels of Anisakis species (Nematoda: Anisakidae) in the red scorpionfish Scorpaena scrofa (Scorpaenidae) from the Aegean Sea. Parasitology International, 92, 102691. 10.1016/j.parint.2022.102691 [DOI] [PubMed] [Google Scholar]
- Bak, T.‐J. , Jeon, C.‐H. , & Kim, J.‐H. (2014). Occurrence of anisakid nematode larvae in chub mackerel (Scomber japonicus) caught off Korea. International Journal of Food Microbiology, 191, 149. 10.1016/j.ijfoodmicro.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Bakare, O. C. , Agboola, O. A. , & Adegbehingbe, K. O. (2023). Gastrointestinal helminth parasites of Pseudotolithus species from Lagos lagoon, Lagos, Nigeria. Nigerian . Journal of Parasitology, 44(2), 338–346. 10.4314/njpar.v44i2.6 [DOI] [Google Scholar]
- Bao, M. , Cipriani, P. , Giulietti, L. , Alam, M. A. , Palomba, M. , Mattiucci, S. , & Levsen, A. (2022). Ascaridoid nematodes infecting commercially important marine fish and squid species from Bangladesh waters in the bay of Bengal. Food and Waterborne Parasitology, 27, e00157. 10.1016/j.fawpar.2022.e00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, M. , Strachan, N. J. C. , Hastie, L. C. , MacKenzie, K. , Seton, H. C. , & Pierce, G. J. (2017). Employing visual inspection and magnetic resonance imaging to investigate Anisakis simplex s.l. infection in herring viscera. Food Control, 75, 40–47. 10.1016/j.foodcont.2016.12.030 [DOI] [Google Scholar]
- Benamara, M. B. , Hassani, M. M. , Baaloudj, A. , & Kerfouf, A. (2020). The parasitic fauna of pagellus acarne (Risso, 1827) (teleostei: Sparidae) of Béni Saf 's bight in the west coast of Algeria. Egyptian Journal Of Aquatic Biology And Fisheries, 24(7‐Special issue), 593–605. 10.21608/EJABF.2020.122968 [DOI] [Google Scholar]
- Bernardi, C. , Gustinelli, A. , Fioravanti, M. L. , Caffara, M. , Mattiucci, S. , & Cattaneo, P. (2011). Prevalence and mean intensity of Anisakis simplex (sensu stricto) in European sea bass (Dicentrarchus labrax) from Northeast Atlantic Ocean. International Journal of Food Microbiology, 148(1), 55–59. 10.1016/j.ijfoodmicro.2011.04.027 [DOI] [PubMed] [Google Scholar]
- Biary, A. , Berrouch, S. , Dehhani, O. , Maarouf, A. , Sasal, P. , Mimouni, B. , & Hafid, J. (2021). Prevalence and identification of Anisakis nematodes in fish consumed in Marrakesh, Morocco. Molecular Biology Reports, 48(4), 3417–3422. 10.1007/s11033-021-06323-y [DOI] [PubMed] [Google Scholar]
- Bielat, I. , Legierko, M. , & Sobecka, E. (2015). Species richness and diversity of the parasites of two predatory fish species ‐ perch (Perca fluviatilis Linnaeus, 1758) and zander (Sander lucioperca Linnaeus, 1758) from the Pomeranian Bay. Annals of Parasitology, 61(2), 85–92. [PubMed] [Google Scholar]
- Bonina, O. , Efremova, E. , Udaltsov, E. , Zubareva, I. M. , & Bortsova, M. (2024). Opisthorchids in Novosibirsk urban ecosystem. Biology Bulletin, 50, 2804–2812. 10.1134/S1062359023100333 [DOI] [Google Scholar]
- Borgstrøm, R. , Mestrand, Ø. , Brittain, J. , & Lien, L. (2021). The helminth fauna of brown trout (Salmo trutta) from a sub‐alpine lake revisited after 40 years with introduced European minnow (Phoxinus phoxinus). Fauna Norvegica, 41, 15–26. 10.5324/fn.v41i0.3952 [DOI] [Google Scholar]
- Bouderbala, K. , Rangel, L. F. , Santos, M. J. , & Bahri, S. (2022). Molecular and pathological data of Anisakis spp. larvae (Nematoda: Anisakidae) in commercial fish Epinephelus aeneus and Epinephelus marginatus (Serranidae) caught off northern Tunisia (western Mediterranean Sea). Cahiers de Biologie Marine, 63(3), 213–224. 10.21411/CBM.A.9381E8F5 [DOI] [Google Scholar]
- Boussellaa, W. , Neifar, L. , Goedknegt, M. A. , & Thieltges, D. W. (2018). Lessepsian migration and parasitism: Richness, prevalence and intensity of parasites in the invasive fish Sphyraena chrysotaenia compared to its native congener Sphyraena sphyraena in Tunisian coastal waters. PeerJ, 6, e5558. 10.7717/peerj.5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid, S. , Saouini, H. E. , Chiaar, A. , Lioubi, J. , Chakir, O. , Benomar, M. , Rhattas, C. , Chbani, I. , & Er‐Raioui, H. (2023). Preliminary data on the occurrence of PAH and Anisakis spp. in Moroccan anchovies: Environmental parasitology and human health risk. Marine Pollution Bulletin, 194(A), 115269. 10.1016/j.marpolbul.2023.115269 [DOI] [PubMed] [Google Scholar]
- Brickle, P. , MacKenzie, K. , & Pike, A. (2005). Parasites of the Patagonian toothfish, Dissostichus eleginoides Smitt 1898, in different parts of the Subantarctic. Polar Biol, 28, 663–671. 10.1007/s00300-005-0737-2 [DOI] [Google Scholar]
- Buchmann, K. (2023). Seals, fish, humans and parasites in the Baltic: Ecology, evolution and history. Folia Parasitologica (Praha), 70,Article 2023.011 10.14411/fp.2023.011 [DOI] [PubMed] [Google Scholar]
- Buchmann, K. , & Karami, A. M. (2024). Fish acanthocephalans as potential human pathogens. Current Clinical Microbiology Reports, 11(2), 99–106. 10.1007/s40588-024-00226-9 [DOI] [Google Scholar]
- Bušelić, I. , Botić, A. , Hrabar, J. , Stagličić, N. , Cipriani, P. , Mattiucci, S. , & Mladineo, I. (2018). Geographic and host size variations as indicators of Anisakis pegreffii infection in European pilchard (Sardina pilchardus) from the Mediterranean Sea: Food safety implications. International Journal of Food Microbiology, 266, 126–132. 10.1016/j.ijfoodmicro.2017.11.021 [DOI] [PubMed] [Google Scholar]
- Buzo‐Dominguez, S. , Morales‐Yuste, M. , Maria Domingo‐Hernandez, A. , Benitez, R. , & Javier Adroher, F. (2021). Molecular epidemiology of Anisakis spp. in wedge sole, Dicologlossa cuneata (Moreau, 1881), from Fishmarkets in Granada (southern Spain), caught in two adjacent NE and CE Atlantic areas. Pathogens, 10(10), 1302. 10.3390/pathogens10101302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero‐Huertas, M. , Palomba, M. , Frigola‐Tepe, X. , Munoz, M. , Mattiucci, S. , & Vinas, J. (2023). Ascaridoid parasites in European sardine throughout the annual cycle: Variability in parasitic load according to host stock features. International Journal for Parasitology‐Parasites and Wildlife, 20, 1–11. 10.1016/j.ijppaw.2022.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore, D. M. P. , Lanfranchi, A. L. , Canel, D. , Levy, E. , & Timi, J. T. (2023). Plerocercoids of Adenocephalus pacificus in Argentine hakes: Broad distribution, low zoonotic risk. International Journal of Food Microbiology, 391‐393, 110142. 10.1016/j.ijfoodmicro.2023.110142 [DOI] [PubMed] [Google Scholar]
- Castellanos, J. A. , Tangua, A. R. , & Salazar, L. (2017). Anisakidae nematodes isolated from the flathead grey mullet fish (Mugil cephalus) of Buenaventura, Colombia. International Journal for Parasitology. Parasites and Wildlife, 6(3), 265–270. 10.1016/j.ijppaw.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casti, D. , Scarano, C. , Piras, M. C. , Merella, P. , Muglia, S. , Piras, F. , Garippa, G. , Spanu, C. , & De Santis, E. P. L. (2017). Occurrence of nematodes of the genus Anisakis in Mediterranean and Atlantic fish marketed in Sardinia. Italian Journal of Food Safety, 6(1), 6185. 10.4081/ijfs.2017.6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione, D. , Di Maggio, M. , Guardone, L. , Ricci, E. , Tinacci, L. , Guglielmone, G. , Coltraro, M. , Susini, F. , & Armani, A. (2023). Eustrongylides excisus in fish species caught in the Massaciuccoli Lake (Northwest Tuscany, Italy): Implications for freshwater fish quality and public health. Food Control, 153, 109894. 10.1016/j.foodcont.2023.109894 [DOI] [Google Scholar]
- Cavalcanti, E. T. S. , Alves, L. C. , & Chellappa, S. (2013). Occurrence of endoparasites in the southern red snapper, Lutjanus purpureus (Osteichthyes: Lutjanidae) from the coastal waters of Rio Grande do Norte, Brazil. Animal Biology Journal, 4(2), 129. https://www.proquest.com/openview/fc0a09abf2c11404b2ea01c160823b1d/1.pdf?pq‐origsite=gscholar&cbl=2034892 [Google Scholar]
- Cavallero, S. , Magnabosco, C. , Civettini, M. , Boffo, L. , Mingarelli, G. , Buratti, P. , Giovanardi, O. , Fortuna, C. M. , & Arcangeli, G. (2015). Survey of Anisakis sp. and Hysterothylacium sp. in sardines and anchovies from the North Adriatic Sea. International Journal of Food Microbiology, 200, 18–21. 10.1016/j.ijfoodmicro.2015.01.017 [DOI] [PubMed] [Google Scholar]
- Ceballos‐Mendiola, G. , Valero, A. , Polo‐Vico, R. , Tejada, M. , Abattouy, N. , Karl, H. , De las Heras, C. , & Martín‐Sánchez, J. (2010). Genetic variability of Anisakis simplex s.s. parasitizing European hake (Merluccius merluccius) in the little sole Bank area in the Northeast Atlantic. Parasitology Research, 107(6), 1399–1404. 10.1007/s00436-010-2009-5 [DOI] [PubMed] [Google Scholar]
- Chagas de Souza, D. , Eiras, J. C. , Adriano, E. A. , & Corrêa, L. L. (2020). Metazoan parasites of Plagioscion squamosissimus (Osteichthyes: Sciaenidae) of two rivers from the eastern Amazon (Brazil). Annals of Parasitology, 66(2), 217–225. 10.17420/ap6602.257 [DOI] [PubMed] [Google Scholar]
- Chai, J. Y. , Han, E. T. , Park, Y. K. , Guk, S. M. , Kim, J. L. , & Lee, S. H. (2000). High endemicity of Metagonimus yokogawai infection among residents of Samchok‐shi, Kangwon‐do. Korean Journal of Parasitology, 38(1), 33–36. 10.3347/kjp.2000.38.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, J.‐Y. , & Jung, B.‐K. (2017). Fishborne zoonotic heterophyid infections: An update. Food and Waterborne Parasitology, 8‐9, 33–63. 10.1016/j.fawpar.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, J.‐Y. , & Jung, B.‐K. (2024). Epidemiology and geographical distribution of human trematode infections. In Toledo R. & Fried B. (Eds.), Digenetic trematodes (pp. 443–505). Springer International Publishing. 10.1007/978-3-031-60121-7_12 [DOI] [PubMed] [Google Scholar]
- Chai, J. Y. , Jung, B. K. , Lee, K. H. , Ryu, J. Y. , Kim, H. S. , Hong, S. J. , Htoon, T. T. , Tin, H. H. , Na, B. K. , & Sohn, W. M. (2020). Larval Gnathostomes and zoonotic trematode Metacercariae in fish from a local market in Yangon City, Myanmar. Korean Journal of Parasitology, 58(6), 701–707. 10.3347/kjp.2020.58.6.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, J.‐Y. , Lee, S.‐H. , Rim, H.‐J. , Sohn, W.‐M. , & Phommasack, B. (2019). Infection status with zoonotic trematode metacercariae in fish from Lao PDR. Acta Tropica, 199, 105100. 10.1016/j.actatropica.2019.105100 [DOI] [PubMed] [Google Scholar]
- Chai, J. Y. , Sohn, W. M. , Na, B. K. , Yong, T. S. , Eom, K. S. , Yoon, C. H. , Hoang, E. H. , Jeoung, H. G. , & Socheat, D. (2014). Zoonotic trematode metacercariae in fish from Phnom Penh and Pursat, Cambodia. The Korean Journal of Parasitology, 52(1), 35–40. 10.3347/kjp.2014.52.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaligiannis, I. , Lalle, M. , Pozio, E. , & Sotiraki, S. (2012). Anisakidae infection in fish of the Aegean Sea. Veterinary Parasitology, 184(2–4), 362. 10.1016/j.vetpar.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Charoensuk, L. , Ribas, A. , Chedtabud, K. , & Prakobwong, S. (2022). Infection rate of Opisthorchis viverrini metacercariae in cyprinoid fish from the markets and its association to human opisthorchiasis in the local community in the Northeast Thailand. Acta Tropica, 225, 106216. 10.1016/j.actatropica.2021.106216 [DOI] [PubMed] [Google Scholar]
- Chen, H.‐X. , Zhang, L.‐P. , Gibson, D. I. , Lü, L. , Xu, Z. , Li, H.‐T. , Ju, H.‐D. , & Li, L. (2018). Detection of ascaridoid nematode parasites in the important marine food‐fish Conger myriaster (Brevoort) (Anguilliformes: Congridae) from the Zhoushan fishery, China. Parasites & Vectors, 11(1), 274. 10.1186/s13071-018-2850-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H.‐Y. , & Shih, H.‐H. (2015). Occurrence and prevalence of fish‐borne Anisakis larvae in the spotted mackerel Scomber australasicus from Taiwanese waters. Acta Tropica, 145, 61–67. 10.1016/j.actatropica.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Chen, Y.‐J. , Wu, C.‐C. , & Yang, J.‐S. (2015). Occurrence of Anisakis larvae in commercial fish along the northern coast of Taiwan. Research Journal of Parasitology, 10(3), 79–91. 10.3923/jp.2015.79.91 [DOI] [Google Scholar]
- Cho, S.‐H. , Lee, S.‐E. , Park, O.‐H. , Na, B.‐K. , & Sohn, W.‐M. (2012). Larval anisakid infections in marine fish from three sea areas of the Republic of Korea. The Korean Journal of Parasitology, 50(4), 295. 10.3347/kjp.2012.50.4.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, Y.‐Y. , Wang, C.‐S. , Chen, H.‐G. , Chen, H.‐Y. , Chen, S.‐N. , & Shih, H.‐H. (2011). Parasitism between Anisakis simplex (Nematoda: Anisakidae) third‐stage larvae and the spotted mackerel Scomber australasicus with regard to the application of stock identification. Veterinary Parasitology, 177(3), 324–331. 10.1016/j.vetpar.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Cipriani, P. , Giulietti, L. , Shayo, S. D. , Storesund, J. E. , Bao, M. , Palomba, M. , Mattiucci, S. , & Levsen, A. (2022). Anisakid nematodes in Trichiurus lepturus and Saurida undosquamis (Teleostea) from the South‐West Indian Ocean: Genetic evidence for the existence of sister species within Anisakis typica (s.l.), and food‐safety considerations. Food and Waterborne Parasitology, 28, e00177. 10.1016/j.fawpar.2022.e00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani, P. , Sbaraglia, G. L. , Palomba, M. , Giulietti, L. , Bellisario, B. , Bušelić, I. , Mladineo, I. , Cheleschi, R. , Nascetti, G. , & Mattiucci, S. (2018). Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy Engraulis encrasicolus from the Mediterranean Sea: Fishing ground as a predictor of parasite distribution. Fisheries Research, 202, 59–68. 10.1016/j.fishres.2017.03.020 [DOI] [Google Scholar]
- Cipriani, P. , Sbaraglia, G. L. , Paoletti, M. , Giulietti, L. , Bellisario, B. , Palomba, M. , Bušelić, I. , Mladineo, I. , Nascetti, G. , & Mattiucci, S. (2018). The Mediterranean European hake, Merluccius merluccius: Detecting drivers influencing the Anisakis spp. larvae distribution. Fisheries Research, 202, 79–89. 10.1016/j.fishres.2017.07.010 [DOI] [Google Scholar]
- Cipriani, P. , Smaldone, G. , Acerra, V. , D'Angelo, L. , Anastasio, A. , Bellisario, B. , Palma, G. , Nascetti, G. , & Mattiucci, S. (2015). Genetic identification and distribution of the parasitic larvae of Anisakis pegreffii and Anisakis simplex (s. s.) in European hake Merluccius merluccius from the Tyrrhenian Sea and Spanish Atlantic coast: Implications for food safety. International Journal of Food Microbiology, 198, 1–8. 10.1016/j.ijfoodmicro.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Çolak, H. S. (2013). Metazoan parasites of fish species from Lake Sýðýrcý (Edirne, Turkey). Turkish Journal of Veterinary and Animal Sciences, 37(2), 200–205. 10.3906/vet-1202-28 [DOI] [Google Scholar]
- Costa, A. , Cammilleri, G. , Graci, S. , Buscemi, M. D. , Vazzana, M. , Principato, D. , Giangrosso, G. , & Ferrantelli, V. (2016). Survey on the presence of a. simplex s.s. and A. Pegreffii hybrid forms in Central‐Western Mediterranean Sea. Parasitology International, 65(6, Part A), 696–701. 10.1016/j.parint.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Costa, G. , Melo‐Moreira, E. , & Pinheiro de Carvalho, M. A. (2012). Helminth parasites of the oceanic horse mackerel Trachurus picturatus Bowdich 1825 (Pisces: Carangidae) from Madeira Island, Atlantic Ocean, Portugal. Journal of Helminthology, 86(3), 368–372. 10.1017/S0022149X11000502 [DOI] [PubMed] [Google Scholar]
- Costa, G. , Santamaria, M. T. G. , Vasconcelos, J. , Perera, C. B. , & Melo‐Moreira, E. (2013). Endoparasites of Trachurus picturatus (Pisces: Carangidae) from the Madeira and Canary Islands: Selecting parasites for use as tags. Scientia Marina, 77(1), 61–68. 10.3989/scimar.03707.07A [DOI] [Google Scholar]
- Crosi Martínez, M. T. , Gonzalo, G. , Federico, V. , Gerardo, C. , Ivette, M. , Valentina, P. , Pablo, V. , Gustavo, G. , Carina, T. , Lucía, D. , Santiago, F. D. K. , Cristina, D. , & José Pedro, A. F. (2022). Primer registro de nematodos zoonóticos de transmisión alimentaria (Nematoda: Anisakidae) en músculo de Cynoscion guatucupa (pescadilla de calada) y Macrodon ancylodon (pescadilla de red) comercializado en Uruguay, Revista FAVE. Sección Ciencias Veterinarias, 21, 9. 10.14409/favecv.2022.0.e0009 [DOI] [Google Scholar]
- Dao, H. T. T. , Dermauw, V. , Gabriël, S. , Suwannatrai, A. , Tesana, S. , Nguyen, G. T. T. , & Dorny, P. (2017). Opisthorchis viverrini infection in the snail and fish intermediate hosts in Central Vietnam. Acta Tropica, 170, 120–125. 10.1016/j.actatropica.2017.02.028 [DOI] [PubMed] [Google Scholar]
- De Benedetto, G. , Arfuso, F. , Ferrara, M. C. , Brianti, E. , & Gaglio, G. (2021). Parasite Fauna of the dusky grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea. Animals, 11(9), 2523. 10.3390/ani11092523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Liberato, C. , Bossù, T. , Scaramozzino, P. , Nicolini, G. , Ceddia, P. , Mallozzi, S. , Cavallero, S. , & Amelio, S. (2013). Presence of Anisakid larvae in the European anchovy, Engraulis encrasicolus, fished off the Tyrrhenian coast of Central Italy. Journal of Food Protection, 76, 1643–1648. 10.4315/0362-028X.JFP-13-092 [DOI] [PubMed] [Google Scholar]
- De Liberato, C. , Scaramozzino, P. , Brozzi, A. , Lorenzetti, R. , Di Cave, D. , Martini, E. , Lucangeli, C. , Pozio, E. , Berrilli, F. , & Bossù, T. (2011). Investigation on Opisthorchis felineus occurrence and life cycle in Italy. Veterinary Parasitology, 177(1–2), 67–71. 10.1016/j.vetpar.2010.11.042 [DOI] [PubMed] [Google Scholar]
- Debenedetti, Á. L. , Codes, F. , Laza, S. , Hernández, S. , Madrid, E. , Trelis, M. , & Fuentes, M. V. (2020). Ascaridoid nematodes in horse mackerel, Trachurus trachurus, sold in Spanish supermarkets–factors able to diminish consumer risk. Fisheries Research, 230, 105669. 10.1016/j.fishres.2020.105669 [DOI] [Google Scholar]
- Dessier, A. , Dupuy, C. , Trancart, T. , Audras, A. , Bustamante, P. , Gérard, C. , Dessier, A. , Dupuy, C. , Trancart, T. , Audras, A. , Bustamante, P. , & Gérard, C. (2016). Low diversity of helminth parasites in Sardina pilchardus and Engraulis encrasicolus (Clupeidae) from the Bay of Biscay. Marine and Freshwater Research, 67(10), 1583–1588. 10.1071/MF15147 [DOI] [Google Scholar]
- Dezfuli, B. S. , Simoni, E. , Bosi, G. , Palomba, M. , Mattiucci, S. , Giulietti, L. , Bao, M. , Levsen, A. , & Cipriani, P. (2021). Immunohistopathological response against anisakid nematode larvae and a coccidian in Micromesistius poutassou from NE Atlantic waters. Journal of Helminthology, 95, e14. 10.1017/S0022149X20000942 [DOI] [PubMed] [Google Scholar]
- Di Azevedo, M. I. N. , & Iñiguez, A. M. (2018). Nematode parasites of commercially important fish from the southeast coast of Brazil: Morphological and genetic insight. International Journal of Food Microbiology, 267, 29–41. 10.1016/j.ijfoodmicro.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Diez, G. , Chust, G. , Andonegi, E. , Santurtun, M. , Abaroa, C. , Bilbao, E. , Maceira, A. , & Mendibil, I. (2022). Analysis of potential drivers of spatial and temporal changes in anisakid larvae infection levels in European hake, Merluccius merluccius (L.), from the north‐East Atlantic fishing grounds. Parasitology Research, 121(7), 1903–1920. 10.1007/s00436-022-07446-2 [DOI] [PubMed] [Google Scholar]
- Dione, E. , Diouf, M. , Fall, J. , & Bâ, C. (2014). Seasonal and spatial distribution of nematode larvae of the genera Anisakis and Contracaecum (Anisakidae) in two populations of Mugil Cephalus (Mugilidae) from Saloum and Senegal Rivers. Journal of Biology and Life Science, 5, 41. 10.5296/jbls.v5i1.4685 [DOI] [Google Scholar]
- Domingo‐Hernandez, A. M. , Morales‐Yuste, M. , Buzo‐Dominguez, S. , Adroher, F. J. , & Benitez, R. (2023). Anisakis infection in anchovies (Engraulis encrasicolus) from Iberian waters, southwestern Europe: Post‐mortem larval migration. Research in Veterinary Science, 157, 26–34. 10.1016/j.rvsc.2023.02.007 [DOI] [PubMed] [Google Scholar]
- Drozdova, L. S. , Shemyakin, G. M. , & Demin, F. A. (2022). Diversity of helminth fauna in teleost fish within the Tver region. Acta Biologica Sibirica, 8, 167–174. 10.5281/zenodo.7700592 [DOI] [Google Scholar]
- Dupouy‐Camet, J. , & Yera, H. (2015). Redécouverte de la diphyllobothriose dans la region des lacs sub‐alpins français. Bulletin de l'Académie Vétérinaire de France, 168, 172. 10.4267/2042/56870 [DOI] [Google Scholar]
- Eberhard, M. L. , Hurwitz, H. , Sun, A. M. , & Coletta, D. (1989). Intestinal perforation caused by larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. The American Journal of Tropical Medicine and Hygiene, 40(6), 648–650. 10.4269/ajtmh.1989.40.648 [DOI] [PubMed] [Google Scholar]
- Eberhard, M. L. , & Ruiz‐Tiben, E. (2014). Cutaneous emergence of Eustrongylides in two persons from South Sudan. The American Journal of Tropical Medicine and Hygiene, 90(2), 315–317. 10.4269/ajtmh.13-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC DG‐MARE . (2023). The EU fish market (2023rd ed.). Publications Office of the European Union. [Google Scholar]
- EC DG‐MARE (European Commission, Directorate‐ General for Maritime Affairs and Fisheries) . (2023). The EU fish market 2023 edition. Publications Office of the European Union. Available online: https://data.europa.eu/doi/10.2771/38507 [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) . (2010). Scientific Opinion on risk assessment of parasites in fishery products. EFSA Journal, 8(4), 1543. 10.2903/j.efsa.2010.1543 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis, K. , Allende, A. , Alvarez‐Ordóñez, A. , Bover‐Cid, S. , Chemaly, M. , De Cesare, A. , Herman, L. , Hilbert, F. , Lindqvist, R. , Nauta, M. , Nonno, R. , Peixe, L. , Ru, G. , Simmons, M. , Skandamis, P. , Suffredini, E. , Buchmann, K. , Careche, M. , … Bolton, D. (2024). Re‐evaluation of certain aspects of the EFSA scientific opinion of April 2010 on risk assessment of parasites in fishery products, based on new scientific data. Part 1: ToRs1–3. EFSA Journal, 22(4), e8719. 10.2903/j.efsa.2024.8719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Younes, M. , Craig, P. , Hart, A. , Von Goetz, N. , … Hardy, A. (2018a). The principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA Journal, 16(1), e05122. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Younes, M. , Craig, P. , Hart, A. , Von Goetz, N. , … Hardy, A. (2018b). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), e05123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa, A. E. , Showehdi, M. L. , Ismail, M. M. , El‐Naas, A. S. , Abu Mhara, A. A. , & Abolghait, S. K. (2018). Identification and prevalence of Anisakis pegreffii and A. pegreffii × A. simplex (s.s.) hybrid genotype larvae in Atlantic horse Mackerel (Trachurus trachurus) from some North African Mediterranean coasts. The Egyptian Journal of Aquatic Research, 44(1), 21–27. 10.1016/j.ejar.2018.02.004 [DOI] [Google Scholar]
- Elena Ahuir‐Baraja, A. , Llobat, L. , & Magdalena Garijo, M. (2021). Effectiveness of gutting blue whiting (Micromesistius poutassou, Risso, 1827), in Spanish supermarkets as an Anisakidosis safety measure. Food, 10(4), 862. 10.3390/foods10040862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom, K. S. , Park, H. S. , Lee, D. , Sohn, W. M. , Yong, T. S. , Chai, J. Y. , Min, D. Y. , Rim, H. J. , Insisiengmay, B. , & Phommasack, B. (2015). Infection status of zoonotic trematode Metacercariae in fishes from Vientiane municipality and Champasak Province in Lao PDR. The Korean Journal of Parasitology, 53(4), 447–453. 10.3347/kjp.2015.53.4.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer‐Maza, D. , Lloret, J. , Muñoz, M. , Elisabeth, F. , Vila, S. , & Sasal, P. (2014). Parasitism, condition and reproduction of the European hake (Merluccius merluccius) in the northwestern Mediterranean Sea. ICES Journal of Marine Science, 71, 1088–1099. 10.1093/icesjms/fst217 [DOI] [Google Scholar]
- Ferrer‐Maza, D. , Lloret, J. , Muñoz, M. , Faliex, E. , Vila, S. , & Sasal, P. (2016). Links between parasitism, energy reserves and fecundity of European anchovy, Engraulis encrasicolus, in the northwestern Mediterranean Sea. Conservation . Physiology, 4(1), cov069. 10.1093/conphys/cov069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenelle, G. , Knoff, M. , Felizardo, N. N. , Lopes, L. M. S. , & São Clemente, S. C. (2013). Nematodes of zoonotic importance in Cynoscion guatucupa (Pisces) in the state of Rio de Janeiro. Revista Brasileira de Parasitologia Veterinária, 22, 281–284. 10.1590/S1984-29612013005000019 [DOI] [PubMed] [Google Scholar]
- Fuentes, M. , Madrid, V. , Elena, M. , Laia, V. , Casan, F. , Saez‐Duran, S. , Trelis, M. , & Debenedetti, A. L. (2022). Nematode parasites of the European pilchard, Sardina pilchardus (Walbaum, 1792): A genuine human Hazard? Animals, 12(15), 1877. 10.3390/ani12151877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes, M. V. , Madrid, E. , Cuesta, C. , Gimeno, C. , Baquedano‐Rodriguez, M. , Soriano‐Sanchez, I. , Bolivar, A. M. , Saez‐Duran, S. , Trelis, M. , & Debenedetti, A. L. (2022). Anisakid nematodes and potential risk of human Anisakiasis through the consumption of hake, Merluccius spp., sold fresh in Spanish supermarkets. Pathogens, 11(6), 622. 10.3390/pathogens11060622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, T. , Waga, E. , Kitaoka, K. , Imagawa, T. , Komatsu, Y. , Takanashi, K. , Anbo, F. , Anbo, T. , Katuki, S. , Ichihara, S. , Fujimori, S. , Yamasaki, H. , Morishima, Y. , Sugiyama, H. , & Katahira, H. (2016). Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitology International, 65(5, Part A), 491–493. 10.1016/j.parint.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Gallagher, C. P. , & Dick, T. A. (2010). Trophic structure of a landlocked Arctic char Salvelinus alpinus population from southern Baffin Island, Canada. Ecology of Freshwater Fish, 19(1), 39–50. 10.1111/j.1600-0633.2009.00387.x [DOI] [Google Scholar]
- Gay, M. , Bao, M. , MacKenzie, K. , Pascual, S. , Buchmann, K. , Bourgau, O. , Couvreur, C. , Mattiucci, S. , Paoletti, M. , Hastie, L. C. , Levsen, A. , & Pierce, G. J. (2018). Infection levels and species diversity of ascaridoid nematodes in Atlantic cod, Gadus morhua, are correlated with geographic area and fish size. Fisheries Research, 202, 90–102. 10.1016/j.fishres.2017.06.006 [DOI] [Google Scholar]
- Gay, M. , Le Fur, B. , Bourgau, O. , Wacogne, D. , & Malle, P. (2012). Localisation et détection des Anisakidae dans deux espèces de poissons: merlan (Merlangus merlangius) et maquereau (Scomber scombrus). Bulletin épidémiologique, santé Animale et Alimentation, 55, 12–17. https://be.anses.fr/sites/default/files/BEP‐mg‐BE55‐Art3.pdf [Google Scholar]
- Gazzonis, A. L. , Cavallero, S. , Zanzani, S. A. , Olivieri, E. , Malandra, R. , Ranghieri, V. , D'Amelio, S. , & Manfredi, M. T. (2017). Anisakis sp. and Hysterothylacium sp. larvae in anchovies (Engraulis encrasicolus) and chub mackerel (Scomber colias) in the Mediterranean Sea: Molecular identification and risk factors. Food Control, 80, 366–373. 10.1016/j.foodcont.2017.05.004 [DOI] [Google Scholar]
- Goffredo, E. , Azzarito, L. , Di Taranto, P. , Mancini, M. E. , Normanno, G. , Didonna, A. , Faleo, S. , Occhiochiuso, G. , D'Attoli, L. , Pedarra, C. , Pinto, P. , Cammilleri, G. , Graci, S. , Sciortino, S. , & Costa, A. (2019). Prevalence of anisakid parasites in fish collected from Apulia region (Italy) and quantification of nematode larvae in flesh. International Journal of Food Microbiology, 292, 159–170. 10.1016/j.ijfoodmicro.2018.12.025 [DOI] [PubMed] [Google Scholar]
- Gomes, T. L. , Quiazon, K. M. A. , Kotake, M. , Itoh, N. , & Yoshinaga, T. (2020). Anisakis spp. in fishery products from Japanese waters: Updated insights on host prevalence and human infection risk factors. Parasitology International, 78, 102137. 10.1016/j.parint.2020.102137 [DOI] [PubMed] [Google Scholar]
- Gómez‐Mateos, M. , Valero, A. , Morales‐Yuste, M. , & Martín‐Sánchez, J. (2016). Molecular epidemiology and risk factors for Anisakis simplex s.l. infection in blue whiting (Micromesistius poutassou) in a confluence zone of the Atlantic and Mediterranean: Differences between a. simplex s.s. And A. Pegreffii . International Journal of Food Microbiology, 232, 111–116. 10.1016/j.ijfoodmicro.2016.05.026 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Poblete, L. , Saavedra, J. C. , Cespedes, R. , & Canales, C. M. (2022). Parasites of Merluccius australis as biological tags to determine the hake ecological stocks in the sea channels of Chilean Patagonia. Estuarine, Coastal and Shelf Science, 278, 108117. 10.1016/j.ecss.2022.108117 [DOI] [Google Scholar]
- Gordeev, I. I. , & Sokolov, S. G. (2023). Helminths of epipelagic fish in the western Bering Sea and southern Sea of Okhotsk. Invertebrate Zoology, 20(2), 140–152. 10.15298/invertzool.20.2.02 [DOI] [Google Scholar]
- Graci, S. , Collura, R. , Cammilleri, G. , Buscemi, M. D. , Giangrosso, G. , Principato, D. , Gervasi, T. , Cicero, N. , & Ferrantelli, V. (2017). Mercury accumulation in Mediterranean fish and cephalopods species of Sicilian coasts: Correlation between pollution and the presence of Anisakis parasites. Natural Product Research, 31(10), 1156–1162. 10.1080/14786419.2016.1230119 [DOI] [PubMed] [Google Scholar]
- Guardone, L. , Nucera, D. , Pergola, V. , Costanzo, F. , Costa, E. , Tinacci, L. , Guidi, A. , & Armani, A. (2017). Visceral larvae as a predictive index of the overall level of fish batch infection in European anchovies (Engraulis encrasicolus): A rapid procedure for food business operators to assess marketability. International Journal of Food Microbiology, 250, 12–18. 10.1016/j.ijfoodmicro.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Gustinelli, A. , Menconi, V. , Prearo, M. , Caffara, M. , Righetti, M. , Scanzio, T. , Raglio, A. , & Fioravanti, M. L. (2016). Prevalence of Diphyllobothrium latum (Cestoda: Diphyllobothriidae) plerocercoids in fish species from four Italian lakes and risk for the consumers. International Journal of Food Microbiology, 235, 109–112. 10.1016/j.ijfoodmicro.2016.06.033 [DOI] [PubMed] [Google Scholar]
- Gutiérrez‐Galindo, J. F. , Osanz‐Mur, A. C. , & Mora‐Ventura, M. T. (2010). Occurrence and infection dynamics of anisakid larvae in Scomber scombrus, Trachurus trachurus, Sardina pilchardus, and Engraulis encrasicolus from Tarragona (NE Spain). Food Control, 21(11), 1550–1555. 10.1016/j.foodcont.2010.03.019 [DOI] [Google Scholar]
- Hauksson, E. , Karlsson, H. , & Eliasson, K. (2012). Decreases in Anisakid nematodes abundance and density in cod, kept in On‐growing Sea‐cages. Advanced Studies in Biology, 4, 217–230. http://www.m‐hikari.com/asb/asb2012/asb5‐8‐2012/haukssonASB5‐8‐2012.pdf [Google Scholar]
- Henriksen, E. H. , Knudsen, R. , Kristoffersen, R. , Kuris, A. M. , Lafferty, K. D. , Siwertsson, A. , & Amundsen, P.‐A. (2016). Ontogenetic dynamics of infection with Diphyllobothrium spp. cestodes in sympatric Arctic charr Salvelinus alpinus (L.) and brown trout Salmo trutta L. Hydrobiologia, 783(1), 37–46. 10.1007/s10750-015-2589-2 [DOI] [Google Scholar]
- Hermida, M. , Cavaleiro, B. , Gouveia, L. , & Saraiva, A. (2018). Parasites of skipjack, Katsuwonus pelamis, from Madeira, eastern Atlantic. Parasitology Research, 117(4), 1025–1033. 10.1007/s00436-018-5778-x [DOI] [PubMed] [Google Scholar]
- Hidano, S. , Mizukami, K. , Yahiro, T. , Shirakami, K. , Ito, H. , Ozaka, S. , Ariki, S. , Saechue, B. , Dewayani, A. , Chalalai, T. , Soga, Y. , Goto, M. , Sonoda, A. , Ozaki, T. , Sachi, N. , Kamiyama, N. , Nishizono, A. , Murakami, K. , & Kobayashi, T. (2021). Analysis of the prevalence and species of Anisakis nematode in Sekisaba, Scomber japonicus caught in coastal waters off Saganoseki, Oita in Japan. Japanese Journal of Infectious Diseases, 74(5), 387–391. 10.7883/yoken.JJID.2020.859 [DOI] [PubMed] [Google Scholar]
- Hien, H. V. , Dung, B. T. , Ngo, H. D. , & Doanh, P. N. (2021). First morphological and molecular identification of third‐stage larvae of Anisakis typica (Nematoda: Anisakidae) from marine fishes in Vietnamese water. Journal of Nematology, 53(1), 1–9. 10.21307/jofnem-2021-010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honcharov, S. L. , Soroka, N. M. , Galat, M. V. , Zhurenko, O. V. , Dubovyi, A. I. , & Dzhmil, V. I. (2022). Eustrongylides (Nematoda:Dioctophymatidae): Epizootology and special characteristics of the development biology. Helminthologia, 59(2), 127–142. 10.2478/helm-2022-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen, M. S. , Suthar, J. , Wassens, S. , & Shamsi, S. (2023). Occurrence and molecular identification of nematodes from blue mackerel Scomber australasicus Cuvier in Australian waters. Parasitology International, 92, 102664. 10.1016/j.parint.2022.102664 [DOI] [PubMed] [Google Scholar]
- Hossen, M. S. , Wassens, S. , & Shamsi, S. (2021). Occurrence and abundance of zoonotic nematodes in snapper Chrysophrys auratus, a popular table fish from Australian and New Zealand waters. Food and Waterborne Parasitology, 23, e00120. 10.1016/j.fawpar.2021.e00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, N. M. , Dung Do, T. , Lan Anh, N. T. , Van, P. T. , Thanh, B. N. , Van Ha, N. , Van Hien, H. , & Canh Le, X. (2015). Current status of fish‐borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasites & Vectors, 8, 21. 10.1186/s13071-015-0643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar, A. , Asnoussi, A. , Norbury, L. J. , Eisenbarth, A. , Shamsi, S. , Gasser, R. B. , Lopata, A. L. , Beveridge, I. , Jabbar, A. , Asnoussi, A. , Norbury, L. J. , Eisenbarth, A. , Shamsi, S. , Gasser, R. B. , Lopata, A. L. , & Beveridge, I. (2012). Larval anisakid nematodes in teleost fishes from Lizard Island, northern great barrier reef, Australia. Marine and Freshwater Research, 63(12), 1283–1299. 10.1071/MF12211 [DOI] [Google Scholar]
- Jacobson, K. , Baldwin, R. , & Reese, D. (2012). Parasite communities indicate effects of cross‐shelf distributions, but not mesoscale oceanographic features on northern California current mid‐trophic food web. Marine Ecology Progress Series, 454, 19–36. 10.3354/meps09654 [DOI] [Google Scholar]
- Jaramillo‐Colorado, B. E. , Arroyo‐Salgado, B. , & Ruiz‐Garcés, L. C. (2015). Organochlorine pesticides and parasites in Mugil incilis collected in Cartagena Bay, Colombia. Environmental Science and Pollution Research International, 22(22), 17475–17485. 10.1007/s11356-015-4986-5 [DOI] [PubMed] [Google Scholar]
- Kadlec, D. , Šimková, A. , Jarkovský, J. , & Gelnar, M. (2003). Parasite communities of freshwater fish under flood conditions. Parasitology Research, 89(4), 272–283. 10.1007/s00436-002-0740-2 [DOI] [PubMed] [Google Scholar]
- Karami, A. M. , Marnis, H. , Korbut, R. , Zuo, S. , Jaafar, R. , Duan, Y. , Mathiessen, H. , Al‐Jubury, A. , Kania, P. W. , & Buchmann, K. (2022). Absence of zoonotic parasites in salmonid aquaculture in Denmark: Causes and consequences. Aquaculture, 549, 737793. 10.1016/j.aquaculture.2021.737793 [DOI] [Google Scholar]
- Karl, H. , Baumann, F. , Ostermeyer, U. , Kuhn, T. , & Klimpel, S. (2011). Anisakis simplex (s.s.) larvae in wild Alaska salmon: No indication of post‐mortem migration from viscera into flesh. Diseases of Aquatic Organisms, 94, 201–209. 10.3354/dao02317 [DOI] [PubMed] [Google Scholar]
- Karmaliyev, R. , Nurzhanova, F. , Sidikhov, B. , Murzabaev, K. , Sariyev, N. , Satybayev, B. , & Abirova, I. (2023). Epizootiology of Opisthorchiasis in carnivores, fish and mollusks in the West Kazakhstan region. American Journal of Animal and Veterinary Sciences, 18(2), 147–155. 10.3844/ajavsp.2023.147.155 [DOI] [Google Scholar]
- Kent, A. J. , Pert, C. C. , Briers, R. A. , Diele, K. , & Rueckert, S. (2020). Increasing intensities of Anisakis simplex third‐stage larvae (L3) in Atlantic salmon of coastal waters of Scotland. Parasites and Vectors, 13(1), 62. 10.1186/s13071-020-3942-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatsopit, N. , Sithithaworn, P. , Saijuntha, W. , Pitaksakulrat, O. , Petney, T. N. , Webster, J. P. , & Andrews, R. H. (2014). Analysis of the population genetics of Opisthorchis viverrini sensu lato in the Nam Ngum River wetland, Lao PDR, by multilocus enzyme electrophoresis. Parasitology Research, 113(8), 2973–2981. 10.1007/s00436-014-3959-9 [DOI] [PubMed] [Google Scholar]
- Klapper, R. , Carballeda‐Sangiao, N. , Kuhn, T. , Jensen, H. M. , Buchmann, K. , Gonzalez‐Muñoz, M. , & Karl, H. (2018). Anisakid infection levels in fresh and canned cod liver: Significant reduction through liver surface layer removal. Food Control, 92, 17–24. 10.1016/j.foodcont.2018.04.029 [DOI] [Google Scholar]
- Klapper, R. , Kochmann, J. , O'Hara, R. , Karl, H. , & Kuhn, T. (2016). Parasites as biological tags for stock discrimination of beaked redfish (Sebastes mentella): Parasite infra‐communities vs. limited resolution of cytochrome markers. PLoS One, 11, e0153964. 10.1371/journal.pone.0153964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper, R. , Kuhn, T. , Münster, J. , Levsen, A. , Karl, H. , & Klimpel, S. (2015). Anisakid nematodes in beaked redfish (Sebastes mentella) from three fishing grounds in the North Atlantic, with special notes on distribution in the fish musculature. Veterinary Parasitology, 207(1–2), 72–80. 10.1016/j.vetpar.2014.11.017 [DOI] [PubMed] [Google Scholar]
- Kleinertz, S. , Damriyasa, I. M. , Hagen, W. , Theisen, D. , & Palm, H. (2012). An environmental assessment of the parasite fauna of the reef‐ associated grouper Epinephelus areolatus from Indonesian waters. Journal of Helminthology, 88, 1–14. 10.1017/S0022149X12000715 [DOI] [PubMed] [Google Scholar]
- Kleinertz, S. , Klimpel, S. , & Palm, H. W. (2012). Parasite communities and feeding ecology of the European sprat (Sprattus sprattus L.) over its range of distribution. Parasitology Research, 110(3), 1147–1157. 10.1007/s00436-011-2605-z [DOI] [PubMed] [Google Scholar]
- Klimpel, S. , Busch, M. W. , Kuhn, T. , Rohde, A. , & Palm, H. W. (2010). The Anisakis simplex complex off the South Shetland Islands (Antarctica): endemic populations versus introduction through migratory hosts. Marine Ecology Progress Series, 403, 1–11. [Google Scholar]
- Køie, M. (2009). Boreogadus saida (Lepechin) (Gadidae): a review of its metazoan parasite fauna from Greenland, eastern Canada, Alaska and the Russian Arctic. Polar Biology, 32(10), 1399–1406. 10.1007/s00300-009-0650-1 [DOI] [Google Scholar]
- Kooh, P. , Gonzales‐Barron, U. , Cadavez, V. , Talleu, R. , El Metennani, S. , Gay, M. , & Thébault, A. (2023). Systematic review protocol for the “pathogens in foods” database: Occurrence of parasites of public health importance in fishery products (version 1). Zenodo. 10.5281/zenodo.10270810 [DOI] [Google Scholar]
- Kooh, P. , Carvalho, L. , El Metennani, S. , Reguieg, K. , Cadavez, V. , Gonzales‐Barron, U. , & Thébault, A. (2024). Data on the occurrence on anisakids and other parasites in fishery products from wild and farmed fish in all countries (Jan 2010 – Sept 2023) [Data set]. Zenodo. 10.5281/zenodo.14169622 [DOI] [Google Scholar]
- Kuchta, R. , Radačovská, A. , Bazsalovicsová, E. , Viozzi, G. , Semenas, L. , Arbetman, M. , & Scholz, T. (2019). Host switching of zoonotic broad fish tapeworm (Dibothriocephalus latus) to salmonids, Patagonia. Emerging Infection Disease, 25(11), 2156–2158. 10.3201/eid2511.190792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchta, R. , Scholz, T. , Brabec, J. , & Narduzzi‐Wicht, B. (2015). Chapter 17. Diphyllobothrium, Diplogonoporus and Spirometra. In Xiao L., Ryan U., & Feng Y. (Eds.), Biology of foodborne parasites: Section III important foodborne helminths (1st ed., pp. 299–326). Food Microbiology Series. 10.1201/b18317 [DOI] [Google Scholar]
- Kuhn, J. A. , Frainer, A. , Knudsen, R. , Kristoffersen, R. , & Amundsen, P. A. (2016). Effects of fish species composition on Diphyllobothrium spp. infections in brown trout ‐ is three‐spined stickleback a key species? Journal of Fish Diseases, 39(11), 1313–1323. 10.1111/jfd.12467 [DOI] [PubMed] [Google Scholar]
- Kuhn, J. A. , Knudsen, R. , Kristoffersen, R. , & Amundsen, P. A. (2017). A simplified method to estimate Diphyllobothrium spp. infection in salmonids. Journal of Fish Diseases, 40(7), 863–871. 10.1111/jfd.12566 [DOI] [PubMed] [Google Scholar]
- Kumagai, T. , & Nishino, Y. (2023). Occurrence and prevalence of third‐stage larvae of the Anisakidae family in Macrouridae species captured from bathyal depths of Sagami Bay, Japan. Deep‐Sea Research Part I: Oceanographic Research Papers, 200, 104151. 10.1016/j.dsr.2023.104151 [DOI] [Google Scholar]
- Labony, S. S. , Alim, M. A. , Hasan, M. M. , Hossain, M. S. , Islam, A. , Alam, M. Z. , & Tsuji, N. (2020). Fish‐borne trematode infections in wild fishes in Bangladesh. Pathogens and Global Health, 114(2), 91–98. 10.1080/20477724.2020.1727217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi, A. , Braicovich, P. , Cantatore, D. , Alarcos, A. , Luque, J. , & Timi, J. (2016). Ecotonal marine regions ‐ ecotonal parasite communities: Helminth assemblages in the convergence of masses of water in the southwestern Atlantic Ocean. International Journal for Parasitology, 46, 809–818. 10.1016/j.ijpara.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Lanfranchi, A. L. , Braicovich, P. E. , Cantatore, D. M. P. , Irigoitia, M. M. , Farber, M. D. , Taglioretti, V. , & Timi, J. T. (2018). Influence of confluent marine currents in an ecotonal region of the south‐West Atlantic on the distribution of larval anisakids (Nematoda: Anisakidae). Parasites & Vectors, 11(1), 583. 10.1186/s13071-018-3119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoprom, N. , Prathummang, S. , Chuangchaiya, S. , Navanesan, S. , Munajat, M. B. , Suwannatrai, A. T. , & Idris, Z. M. (2021). Opisthorchis viverrini metacercarial infection in cyprinid fish in Nakhon Phanom Province, Northeastern Thailand. Tropical Biomedicine, 38(2), 25–30. 10.47665/tb.38.2.033 [DOI] [PubMed] [Google Scholar]
- Levsen, A. , Cipriani, P. , Mattiucci, S. , Gay, M. , Hastie, L. C. , MacKenzie, K. , Pierce, G. J. , Svanevik, C. S. , Højgaard, D. P. , Nascetti, G. , González, A. F. , & Pascual, S. (2018). Anisakis species composition and infection characteristics in Atlantic mackerel, Scomber scombrus, from major European fishing grounds – Reflecting changing fish host distribution and migration pattern. Fisheries Research, 202, 112–121. 10.1016/j.fishres.2017.07.030 [DOI] [Google Scholar]
- Levsen, A. , Cipriani, P. , Palomba, M. , Giulietti, L. , Storesund, J. E. , & Bao, M. (2022). Anisakid parasites (Nematoda: Anisakidae) in 3 commercially important gadid fish species from the southern Barents Sea, with emphasis on key infection drivers and spatial distribution within the hosts. Parasitology, 149(14, SI), 1942–1957. 10.1017/S0031182022001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levsen, A. , & Lunestad, B. (2010). Anisakis simplex third stage larvae in Norwegian spring spawning herring (Clupea harengus L.), with emphasis on larval distribution in the flesh. Veterinary Parasitology, 171, 247–253. 10.1016/j.vetpar.2010.03.039 [DOI] [PubMed] [Google Scholar]
- Levsen, A. , Paoletti, M. , Cipriani, P. , Nascetti, G. , & Mattiucci, S. (2016). Species composition and infection dynamics of ascaridoid nematodes in Barents Sea capelin (Mallotus villosus) reflecting trophic position of fish host. Parasitology Research, 115(11), 4281–4291. 10.1007/s00436-016-5209-9 [DOI] [PubMed] [Google Scholar]
- Levsen, A. , Svanevik, C. S. , Cipriani, P. , Mattiucci, S. , Gay, M. , Hastie, L. C. , Bušelić, I. , Mladineo, I. , Karl, H. , Ostermeyer, U. , Buchmann, K. , Højgaard, D. P. , González, Á. F. , Pascual, S. , & Pierce, G. J. (2018). A survey of zoonotic nematodes of commercial key fish species from major European fishing grounds–introducing the FP7 PARASITE exposure assessment study. Fisheries Research, 202, 4–21. 10.1016/j.fishres.2017.09.009 [DOI] [Google Scholar]
- Liberman, E. (2019). Sexual and age characteristics of the parasitofauna of Abramis brama (Cypriniformes, Cyprinidae) of the lower Irtysh (Russia). Biosystems Diversity, 27, 200–204. 10.15421/011927 [DOI] [Google Scholar]
- Liberman, E. , Voropaeva, E. , & Kozlov, S. (2019). Parasitofauna of pike Esox lucius of the lower Tobol (Russia). Biosystems Diversity, 27, 214–220. 10.15421/011929 [DOI] [Google Scholar]
- Liberman, E. L. , & Voropaeva, E. L. (2018). New data on parasitic Fauna of bream Abramis brama (Linnaeus, 1758) of the lower Irtysh (acquired part of the range). Russian Journal of Biological Invasions, 9(3), 232–236. 10.1134/S2075111718030098 [DOI] [Google Scholar]
- Liu, G.‐H. , Sun, M.‐M. , Elsheikha, H. M. , Fu, Y.‐T. , Sugiyama, H. , Ando, K. , Sohn, W.‐M. , Zhu, X.‐Q. , & Yao, C. (2020). Human gnathostomiasis: a neglected food‐borne zoonosis. Parasites & Vectors, 13(1), 616. 10.1186/s13071-020-04494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llarena‐Reino, M. , González, Á. F. , Vello, C. , Outeiriño, L. , & Pascual, S. (2012). The accuracy of visual inspection for preventing risk of Anisakis spp. infection in unprocessed fish. Food Control, 23(1), 54–58. 10.1016/j.foodcont.2011.06.010 [DOI] [Google Scholar]
- Lopes, P. , Vilares, A. , Cacador, T. , Martins, S. , Ferreira, I. , Carvalho, L. M. , & Gargate, M. J. (2020). Occurrence of larval anisakids in horse mackerel (Trachurus trachurus) caught in Portuguese waters. Parasitology Research, 119(9), 2799–2811. 10.1007/s00436-020-06816-y [DOI] [PubMed] [Google Scholar]
- Lukashanets, D. A. , Convey, P. , Borodin, O. I. , Miamin, V. Y. , Hihiniak, Y. H. , Gaydashov, A. A. , Yatsyna, A. P. , Vezhnavets, V. V. , Maysak, N. N. , & Shendrik, T. V. (2021). Eukarya biodiversity in the Thala Hills, East Antarctica. Antarctic Science, 33(6), 605–623. 10.1017/S0954102021000328 [DOI] [Google Scholar]
- Macchioni, F. , Tedesco, P. , Cocca, V. , Massaro, A. , Sartor, P. , Ligas, A. , Pretti, C. , Monni, G. , Cecchi, F. , & Caffara, M. (2021). Anisakid and Raphidascaridid parasites in Trachurus trachurus: Infection drivers and possible effects on the host's condition. Parasitology Research, 120(9), 3113–3122. 10.1007/s00436-021-07200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh, A. L. , Reed, C. C. , Nunkoo, M. A. I. , King, P. H. , & van der Lingen, C. D. (2018). Macroparasites of angelfish Brama brama (Bonnaterre, 1788) in the southern Benguela current ecosystem. African Journal of Marine Science, 40(3), 245–252. 10.2989/1814232X.2018.1499551 [DOI] [Google Scholar]
- Madrid, E. , Galán‐Puchades, M. T. , & Fuentes, M. V. (2012). Risk analysis of human anisakidosis through the consumption of the blue whiting, Micromesistius poutassou, sold at Spanish supermarkets. Foodborne Pathogens and Disease, 9(10), 934–938. 10.1089/fpd.2012.1196 [DOI] [PubMed] [Google Scholar]
- Madrid, E. , Gil, F. , García, M. , Debenedetti, Á. L. , Trelis, M. , & Fuentes, M. V. (2016). Potential risk analysis of human anisakiasis through the consumption of mackerel, Scomber scombrus, sold at Spanish supermarkets. Food Control, 66, 300–305. 10.1016/j.foodcont.2016.02.025 [DOI] [Google Scholar]
- Manpratum, Y. , Kaewkes, W. , Echaubard, P. , Sripa, B. , & Kaewkes, S. (2017). New locality record for Haplorchoides mehrai and possible interactions with Opisthorchis viverrini metacercariae in cyprinid fishes in Northeast Thailand. Parasitology Research, 116(2), 601–608. 10.1007/s00436-016-5324-7 [DOI] [PubMed] [Google Scholar]
- Marcus, S. , Maqbool, A. , Khan, N. , Iqbal, K. , Ashraf, K. , & Ahmad, N. (2012). Food borne parasitic zoonosis with special reference to metacercarial infection in fishes. Journal of Animal and Plant Sciences, 22, 619–621. [Google Scholar]
- Marks, D. (2011). Climate change and Thailand: Impact and response. In Contemporary Southeast Asia (Vol. 33, pp. 229–258). Institute of Southeast Asian Studies. 10.1355/cs33-2d [DOI] [Google Scholar]
- Marques, J. F. , Santos, M. J. , & Cabral, H. N. (2010). Aggregation patterns of macroendoparasites in phylogenetically related fish hosts. Parasitology, 137(11), 1671–1680. 10.1017/S0031182010000491 [DOI] [PubMed] [Google Scholar]
- Martin‐Carrillo, N. , Garcia‐Livia, K. , Baz‐Gonzalez, E. , Abreu‐Acosta, N. , Dorta‐Guerra, R. , Valladares, B. , & Foronda, P. (2022). Morphological and molecular identification of Anisakis spp. (Nematoda: Anisakidae) in commercial fish from the Canary Islands coast (Spain): Epidemiological data. Animals, 12(19), 2634. 10.3390/ani12192634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Rojas, R. , Mondragon‐Martinez, A. , De‐Los‐Santos, E. R. , Cruz‐Neyra, L. , Garcia‐Candela, E. , Delgado‐Escalante, A. , & Sanchez‐Venegas, J. R. (2021). Molecular identification and epidemiological data of Anisakis spp. (Nematoda: Anisakidae) larvae from southeastern Pacific Ocean off Peru. International Journal for Parasitology‐Parasites and Wildlife, 16, 138–144. 10.1016/j.ijppaw.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiucci, S. , & Nascetti, G. (2006). Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845: an update. Parasite, 13(2), 99–113. [DOI] [PubMed] [Google Scholar]
- Mattiucci, S. , Cipriani, P. , Levsen, A. , Paoletti, M. , & Nascetti, G. (2018). Molecular epidemiology of Anisakis and Aisakiasis: An ecological and evolutionary road map. Advances in Parasitology, 99, 93–263. 10.1016/bs.apar.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Mattiucci, S. , Garcia, A. , Cipriani, P. , Santos, M. N. , Nascetti, G. , & Cimmaruta, R. (2014). Metazoan parasite infection in the swordfish, Xiphias gladius, from the Mediterranean Sea and comparison with Atlantic populations: Implications for its stock characterization. Parasite (Paris, France), 21, 35. 10.1051/parasite/2014036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiucci, S. , Giulietti, L. , Paoletti, M. , Cipriani, P. , Gay, M. , Levsen, A. , Klapper, R. , Karl, H. , Bao, M. , Pierce, G. J. , & Nascetti, G. (2018). Population genetic structure of the parasite Anisakis simplex (s. s.) collected in Clupea harengus L. from north East Atlantic fishing grounds. Fisheries Research, 202, 103–111. 10.1016/j.fishres.2017.08.002 [DOI] [Google Scholar]
- McClelland, G. , & Melendy, J. (2011). Use of parasites as tags in delineating stocks of Atlantic cod (Gadus morhua) from the southern gulf of St. Lawrence and the cape Breton shelf. Fisheries Research, 107(1), 233–238. 10.1016/j.fishres.2010.10.022 [DOI] [Google Scholar]
- Mehrdana, F. , Bahlool, Q. Z. M. , Skov, J. , Marana, M. H. , Sindberg, D. , Mundeling, M. , Overgaard, B. C. , Korbut, R. , Strøm, S. B. , Kania, P. W. , & Buchmann, K. (2014). Occurrence of zoonotic nematodes Pseudoterranova decipiens, Contracaecum osculatum and Anisakis simplex in cod (Gadus morhua) from the Baltic Sea. Veterinary Parasitology, 205(3–4), 581–587. 10.1016/j.vetpar.2014.08.027 [DOI] [PubMed] [Google Scholar]
- Meloni, M. , Angelucci, G. , Merella, P. , Siddi, R. , Deiana, C. , Orrù, G. , & Salati, F. (2011). Molecular characterization of Anisakis larvae from fish caught off Sardinia. The Journal of Parasitology, 97(5), 908–914. 10.1645/GE-2742.1 [DOI] [PubMed] [Google Scholar]
- Menconi, V. , Manfrin, C. , Pastorino, P. , Mugetti, D. , Cortinovis, L. , Pizzul, E. , Pallavicini, A. , & Prearo, M. (2020). First report of Clinostomum complanatum (Trematoda: Digenea) in European perch (Perca fluviatilis) from an Italian subalpine Lake: A risk for public health? International Journal of Environmental Research and Public Health, 17(4), 1389. 10.3390/ijerph17041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menconi, V. , Pastorino, P. , Canola, S. , Pavoletti, E. , Vitale, N. , Scanzio, T. , Righetti, M. , Mugetti, D. , Tomasoni, M. , Bona, M. C. , & Prearo, M. (2022). Occurrence and spatial variation of Anisakis pegreffii in the Atlantic horse mackerel Trachurus trachurus (Carangidae): A three‐year monitoring survey in the western Ligurian Sea. Food Control, 131, 108423. 10.1016/j.foodcont.2021.108423 [DOI] [Google Scholar]
- Menconi, V. , Pastorino, P. , Momo, I. , Mugetti, D. , Bona, M. C. , Levetti, S. , Tomasoni, M. , Pizzul, E. , Ru, G. , Dondo, A. , & Prearo, M. (2020). Occurrence and spatial distribution of Dibothriocephalus latus (Cestoda: Diphyllobothriidea) in Lake Iseo (northern Italy): An update. International Journal of Environmental Research and Public Health, 17(14), 5070. 10.3390/ijerph17145070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menconi, V. , Zoppi, S. , Pastorino, P. , Di Blasio, A. , Tedeschi, R. , Pizzul, E. , Mugetti, D. , Tomasoni, M. , Dondo, A. , & Prearo, M. (2021). Relationship between the prevalence of Dibothriocephalus latus (Cestoda: Diphyllobothriidea) and the load of Escherichia coli: New findings in a neglected fish‐borne parasitic zoonosis. Zoonoses and Public Health, 68(8), 965–972. 10.1111/zph.12891 [DOI] [PubMed] [Google Scholar]
- Mercken, E. , Van Damme, I. , Soba, B. , Vangeenberghe, S. , Serradell, A. , Lumain, J. P. L. , De Sterck, T. , Lalle, M. , & Gabriel, S. (2021). High occurrence of Anisakidae at retail level in cod (Gadus morhua) belly flaps and the impact of extensive candling. Food and Waterborne Parasitology, 22, e00108. 10.1016/j.fawpar.2020.e00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladineo, I. , & Poljak, V. (2013). Ecology and genetic structure of zoonotic Anisakis spp. from Adriatic commercial fish species. Applied and Environmental Microbiology, 80, 1281–1290. 10.1128/AEM.03561-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladineo, I. , Šegvić, T. , & Petrić, M. (2011). Do captive conditions favor shedding of parasites in the reared Atlantic bluefin tuna (Thunnus thynnus)? Parasitology International, 60(1), 25–33. 10.1016/j.parint.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Mo, T. A. , Fossøy, F. , & Poppe, T. T. (2021). Increasing intensities of Anisakis simplex (Rudolphi, 1809 det. Krabbe, 1878) larvae with weight and sea age in returning adult Atlantic salmon, Salmo salar L., of coastal waters of Norway. Journal of Fish Diseases, 44(8), 1075–1089. 10.1111/jfd.13369 [DOI] [PubMed] [Google Scholar]
- Molina‐Fernández, D. , Malagón, D. , Gómez‐Mateos, M. , Benítez, R. , Martín‐Sánchez, J. , & Adroher, F. J. (2015). Fishing area and fish size as risk factors of Anisakis infection in sardines (Sardina pilchardus) from Iberian waters, southwestern Europe. International Journal of Food Microbiology, 203, 27–34. 10.1016/j.ijfoodmicro.2015.02.024 [DOI] [PubMed] [Google Scholar]
- Molina‐Fernández, D. , Rubio‐Calvo, D. , Adroher, F. J. , & Benítez, R. (2018). Molecular epidemiology of Anisakis spp. in blue whiting Micromesistius poutassou in eastern waters of Spain, western Mediterranean Sea. International Journal of Food Microbiology, 282, 49–56. 10.1016/j.ijfoodmicro.2018.05.026 [DOI] [PubMed] [Google Scholar]
- Mondragón‐Martínez, A. , Martínez‐Rojas, R. , Garcia‐Candela, E. , Delgado‐Escalante, A. , Tantaleán‐Vidaurre, M. , & Cruz‐Neyra, L. (2020). Molecular identification of Adenocephalus pacificus (Cestoda) from three human cases in Lima Province, Peru. The Korean Journal of Parasitology, 58(4), 457–460. 10.3347/kjp.2020.58.4.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravec, F. (2013). Parasitic nematodes of freshwater fishes of Europe. Academia. https://www.academiabooks.com/book/parasitic‐nematodes‐of‐freshwater‐fishes‐of‐europe/ [Google Scholar]
- Mouritsen, K. , Hedeholm, R. , Schack, H. , Møller, L. , Storr‐Paulsen, M. , Dzido, J. , & Rokicki, J. (2010). Occurrence of anisakid nematodes in Atlantic cod (Gadus morhua) and Greenland cod (Gadus ogac), West Greenland. Acta Parasitologica, 55(1), 81–89. 10.2478/s11686-010-0009-3 [DOI] [Google Scholar]
- Munoz‐Caro, T. , Machuca, A. , Morales, P. , Verdugo, J. , Reyes, R. , Garcia, M. , Rutaihwa, L. , Schindler, T. , Poppert, S. , Taubert, A. , & Hermosilla, C. (2022). Prevalence and molecular identification of zoonotic Anisakis and Pseudoterranova species in fish destined to human consumption in Chile. Parasitology Research, 121(5), 1295–1304. 10.1007/s00436-022-07459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster, J. , Klimpel, S. , Fock, H. O. , MacKenzie, K. , & Kuhn, T. (2015). Parasites as biological tags to track an ontogenetic shift in the feeding behaviour of Gadus morhua off west and East Greenland. Parasitology Research, 114(7), 2723–2733. 10.1007/s00436-015-4479-y [DOI] [PubMed] [Google Scholar]
- Murata, R. , Suzuki, J. , Kodo, Y. , Kobayashi, K. , Sadamasu, K. , Takano, T. , Iwaki, T. , Waki, T. , & Ogawa, K. (2021). Probable association between Anisakis infection in the muscle of skipjack tuna (Katsuwonus pelamis) and human anisakiasis in Tokyo, Japan. International Journal of Food Microbiology, 337, 108930. 10.1016/j.ijfoodmicro.2020.108930 [DOI] [PubMed] [Google Scholar]
- Nadolna, K. , & Podolska, M. (2014). Anisakid larvae in the liver of cod (Gadus morhua) L. from the southern Baltic Sea. Journal of Helminthology, 88(2), 237–246. 10.1017/S0022149X13000096 [DOI] [PubMed] [Google Scholar]
- Nadolna‐Altyn, K. , Podolska, M. , Pawlak, J. , & Szostakowska, B. (2022). Distribution of anisakid nematodes in the muscle tissue of cod (Gadus morhua) from the Norwegian Sea. Oceanologia, 64(3), 489–502. 10.1016/j.oceano.2022.03.003 [DOI] [Google Scholar]
- Najda, K. , Kijewska, A. , Kijewski, T. , Plauška, K. , & Rokicki, J. (2018). Distribution of ascaridoid nematodes (Nematoda: Chromadorea: Ascaridoidea) in fish from the Barents Sea. Oceanological and Hydrobiological Studies, 47(2), 128–139. 10.1515/ohs-2018-0014 [DOI] [Google Scholar]
- Namsanor, J. , Kiatsopit, N. , Laha, T. , Andrews, R. H. , Petney, T. N. , & Sithithaworn, P. (2020). Infection dynamics of Opisthorchis viverrini Metacercariae in cyprinid fishes from two endemic areas in Thailand and Lao PDR. The American Journal of Tropical Medicine and Hygiene, 102(1), 110–116. 10.4269/ajtmh.19-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina, Y. , & Polyaeva, K. (2020). Morphology, biology and parasite fauna of the least cisco (Coregonus sardinella) of the Yenisei River. Biosystems Diversity, 28, 230–237. 10.15421/012030 [DOI] [Google Scholar]
- Nithikathkul, C. , Reungsang, P. , Trivanich, A. , Homchumpa, P. , Tongsiri, S. , & Wongsawad, C. (2014). Geographic information of fish‐borne parasitic metacercaria in chi river, Mahasarakham, Thailand. International Journal of Geoinformatics, 10, 25–29. [Google Scholar]
- Nithikathkul, C. , Wongsaroj, T. , Buntilov, V. , & Limsomboon, J. (2012). Geographic information system of fish‐borne parasitic Zoonoses metacercaria from water reservoirs under his Majesty's recommended project, Phitsanulok, Thailand. International Journal of Geoinformatics, 8, 53–57. [Google Scholar]
- Nogrado, K. , Adisakwattana, P. , & Reamtong, O. (2023). Human gnathostomiasis: A review on the biology of the parasite with special reference on the current therapeutic management. Food and Waterborne Parasitology, 33, e00207. 10.1016/j.fawpar.2023.e00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera, P. , Pert, C. , Collins, C. , Still, N. , & Bruno, D. (2015). Quantification and distribution of Anisakis simplex sensu stricto in wild, one sea winter Atlantic salmon, Salmo salar, returning to Scottish rivers. Journal of the Marine Biological Association of the United Kingdom, 95(2), 391–399. 10.1017/S0025315414001374 [DOI] [Google Scholar]
- Ogbon, A. M. , Afoakwah, R. , Mireku, K. K. , Tossavi, N. D. , & MacKenzie, K. (2023). Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and their potential as biological tags for stock identification along the coast of West Africa. Biology‐Basel, 12(3), 389. 10.3390/biology12030389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguz, M. C. , Campbell, A. M. , Bennett, S. P. , & Belk, M. C. (2021). Nematode parasites of rockfish (Sebastes spp.) and cod (Gadus spp.) from waters near Kodiak Island Alaska, USA. Diversity‐Basel, 13(9), 436. 10.3390/d13090436 [DOI] [Google Scholar]
- Ohnishi, T. , Banzai, A. , Hara‐Kudo, Y. , & Sugiyama, H. (2023). Prevalence and abundance of Anisakis larvae in ready‐to‐eat mackerel products in Japan. International Journal of Food Microbiology, 395, 110181. 10.1016/j.ijfoodmicro.2023.110181 [DOI] [PubMed] [Google Scholar]
- Oliva, M. E. , Espinola, J. F. , & Ñacari, L. A. (2016). Metazoan parasites of Brama australis from southern Chile: a tool for stock discrimination? Journal of Fish Biology, 88(3), 1143–1148. 10.1111/jfb.12881 [DOI] [PubMed] [Google Scholar]
- Ong, X. , Wang, Y.‐C. , Sithithaworn, P. , Grundy‐Warr, C. , & Pitaksakulrat, O. (2016). Dam influences on liver fluke transmission: Fish infection and human fish consumption behavior. Annals of the American Association of Geographers, 106(4), 755–772. 10.1080/24694452.2015.1122508 [DOI] [Google Scholar]
- Onsurathum, S. , Pinlaor, P. , Charoensuk, L. , Haonon, O. , Chaidee, A. , Intuyod, K. , Laummaunwai, P. , Boonmars, T. , Kaewkes, W. , & Pinlaor, S. (2016). Contamination of Opisthorchis viverrini and Haplorchis taichui metacercariae in fermented fish products in northeastern Thailand markets. Food Control, 59, 493–498. 10.1016/j.foodcont.2015.06.020 [DOI] [Google Scholar]
- Orlando, A. , Caterini, L. , Marucci, G. , Ferri, F. , Bernardini, G. , Natalini Raponi, G. , Ludovisi, A. , Bossù, T. , Angeles, M. , Morales, G. , & Pozio, E. (2008). Human illnesses caused by Opisthorchis felineus flukes, Italy. Emerging Infectious Diseases, 14, 1902–1905. 10.3201/EID1412.080782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özesen Çolak, S. (2013). Metazoan parasites of fish species from Lake Siǧirci (Edirne, Turkey). Turkish Journal of Veterinary and Animal Sciences, 37, 200–205. 10.3906/vet-1202-28 [DOI] [Google Scholar]
- Ozuni, E. , Vodica, A. , Castrica, M. , Brecchia, G. , Curone, G. , Agradi, S. , Miraglia, D. , Menchetti, L. , Balzaretti, C. M. , & Andoni, E. (2021). Prevalence of Anisakis larvae in different fish species in southern Albania: Five‐year monitoring (2016–2020). Applied Sciences‐Basel, 11(23), 11528. 10.3390/app112311528 [DOI] [Google Scholar]
- Pambudi, M. R. , Sulistiono, T. , & Risa, K. (2021). Infection patterns of helminth parasites in mackerel tuna (Euthynnus affinis Cantor, 1849) from Banten waters, Indonesia. Ilmu Kelautan: Indonesian Journal of Marine Sciences, 26(2), 117–124. 10.14710/IK.IJMS.26.2.117-124 [DOI] [Google Scholar]
- Pascual, S. , Rodríguez, H. , Pierce, G. J. , Hastie, L. C. , & González, A. F. (2018). The NE Atlantic European hake: A neglected high exposure risk for zoonotic parasites in European fish markets. Fisheries Research, 202, 69–78. 10.1016/j.fishres.2017.12.008 [DOI] [Google Scholar]
- Pekmezci, G. Z. (2014). Occurrence of Anisakis simplex sensu stricto in imported Atlantic mackerel (Scomber scombrus) represents a risk for Turkish consumers. International Journal of Food Microbiology, 185, 64–68. 10.1016/j.ijfoodmicro.2014.05.018 [DOI] [PubMed] [Google Scholar]
- Pekmezci, G. Z. (2019). Occurrence of Anisakis pegreffii (Nematoda: Anisakidae) larvae in imported John dory (Zeus faber) from Senegalese coast sold in Turkish supermarkets. Acta Parasitologica, 64(3), 582–586. 10.2478/s11686-019-00078-0 [DOI] [PubMed] [Google Scholar]
- Pekmezci, G. Z. , Onuk, E. E. , Bolukbas, C. S. , Yardimci, B. , Gurler, A. T. , Acici, M. , & Umur, S. (2014). Molecular identification of Anisakis species (Nematoda: Anisakidae) from marine fishes collected in Turkish waters. Veterinary Parasitology, 201(1–2), 82–94. 10.1016/j.vetpar.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Phyo Myint, E. E. , Sereemaspun, A. , Rocklöv, J. , & Nithikathkul, C. (2020). Discovery of carcinogenic liver fluke Metacercariae in second intermediate hosts and surveillance on fish‐borne trematode Metacercariae infections in Mekong region of Myanmar. International Journal of Environmental Research and Public Health, 17(11), 4108. 10.3390/ijerph17114108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, G. J. , Bao, M. , MacKenzie, K. , Dunser, A. , Giulietti, L. , Cipriani, P. , Mattiucci, S. , & Hastie, L. C. (2018). Ascaridoid nematode infection in haddock (Melanogrammus aeglefmus) and whiting (Merlangius merlangus) in Northeast Atlantic waters. Fisheries Research, 202, 122–133. 10.1016/j.fishres.2017.09.008 [DOI] [Google Scholar]
- Pilecka‐Rapacz, M. , Piasecki, W. , Głoćko, M. , Kesminas, V. , Domagała, J. , Wiśniewski, G. , & Czerniawski, R. (2017). Parasitological survey of smelt, (Actinopterygii: Osmeridae), from five estuary sites along the southern coast of the Baltic Sea. Oceanological and Hydrobiological Studies, 46(3), 314–324. 10.1515/ohs-2017-0033 [DOI] [Google Scholar]
- Pinlaor, S. , Onsurathum, S. , Boonmars, T. , Pinlaor, P. , Hongsrichan, N. , Chaidee, A. , Haonon, O. , Limviroj, W. , Tesana, S. , Kaewkes, S. , & Sithithaworn, P. (2013). Distribution and abundance of Opisthorchis viverrini metacercariae in cyprinid fish in northeastern Thailand. The Korean Journal of Parasitology, 51(6), 703–710. 10.3347/kjp.2013.51.6.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaksakulrat, O. , Kiatsopit, N. , Laoprom, N. , Webster, B. L. , Webster, J. P. , Lamberton, P. H. L. , Laha, T. , Andrews, R. H. , Petney, T. N. , Blair, D. , Carlton, E. J. , Spear, R. C. , & Sithithaworn, P. (2017). Preliminary genetic evidence of two different populations of Opisthorchis viverrini in Lao PDR. Parasitology Research, 116(4), 1247–1256. 10.1007/s00436-017-5401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, R. (2006). Global warming and temperature‐mediated increases in cercarial emergence in trematode parasites. Parasitology, 132(Pt 1), 143–151. 10.1017/s0031182005008693 [DOI] [PubMed] [Google Scholar]
- Prakobwong, S. , Gunnula, W. , Chaipibool, S. , Nimala, B. , Sangthopo, J. , Sirivetthumrong, N. , & Ribas, A. (2017). Epidemiology of Opisthorchis viverrini in an endemic area of Thailand, an integrative approach. Helminthologia, 54, 298–306. 10.1515/helm-2017-0036 [DOI] [Google Scholar]
- Pulleiro‐Potel, L. , Barcala, E. , Mayo‐Hernández, E. , & Muñoz, P. (2015). Survey of anisakids in commercial teleosts from the western Mediterranean Sea: Infection rates and possible effects of environmental and ecological factors. Food Control, 55, 12–17. 10.1016/j.foodcont.2015.02.020 [DOI] [Google Scholar]
- Pumhirunroj, B. , Aukkanimart, R. , Boonmars, T. , Sriraj, P. , Boueroy, P. , Artchayasawat, A. , Songsri, J. , Sripan, P. , Chomphumee, K. , Ratanasuwan, P. , Laummaunwai, P. , Khueangchaingkhwang, S. , Suwannatrai, A. , Aunpromma, S. , Eamudomkarn, C. , Khuntikeo, N. , Loilome, W. , Namwat, N. , & Yongvanit, P. (2020). Liver fluke‐infected cyprinoid fish in northeastern Thailand (2016–2017). The Southeast Asian Journal of Tropical Medicine and Public Health, 51, 1–7. [Google Scholar]
- Qiu, J. H. , Zhang, Y. , Zhang, X. X. , Gao, Y. , Li, Q. , Chang, Q. C. , & Wang, C. R. (2017). Metacercaria infection status of Fishborne zoonotic trematodes, except for Clonorchis sinensis in fish from the Heilongjiang Province, China. Foodborne Pathogen Disease, 14(8), 440–446. 10.1089/fpd.2016.2249 [DOI] [PubMed] [Google Scholar]
- Radačovská, A. , Čisovská Bazsalovicsová, E. , Blasco‐Costa, I. , Orosová, M. , Gustinelli, A. , & Králová‐Hromadová, I. (2019). Occurrence of Dibothriocephalus latus in European perch from alpine lakes, an important focus of diphyllobothriosis in Europe. Revue Suisse de Zoologie; Annales de la Société Zoologique Suisse et du Muséum d'histoire Naturelle de Genève, 126, 219–225. 10.5281/zenodo.3463453 [DOI] [Google Scholar]
- Rauque, C. , Viozzi, G. , Flores, V. , Vega, R. , Waicheim, A. , & Salgado‐Maldonado, G. (2018). Helminth parasites of alien freshwater fishes in Patagonia (Argentina). International Journal for Parasitology: Parasites and Wildlife, 7(3), 369–379. 10.1016/j.ijppaw.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim, H. J. , Sohn, W. M. , Yong, T. S. , Eom, K. S. , Chai, J. Y. , Min, D. Y. , Lee, S. H. , Hoang, E. H. , Phommasack, B. , & Insisiengmay, S. (2013). Fishborne trematode metacercariae in Luang Prabang, Khammouane, and Saravane Province, Lao PDR. The Korean Journal of Parasitology, 51(1), 107–114. 10.3347/kjp.2013.51.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca‐Geronès, X. , Segovia, M. , Godínez‐González, C. , Fisa, R. , & Montoliu, I. (2020). Anisakis and Hysterothylacium species in Mediterranean and north‐East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular and morphometric discriminant analysis. International Journal of Food Microbiology, 325, 108642. 10.1016/j.ijfoodmicro.2020.108642 [DOI] [PubMed] [Google Scholar]
- Rodrigues, M. V. , Pantoja, J. C. F. , Guimarães, C. D. O. , Benigno, R. N. M. , Palha, M. D. C. , & Biondi, G. F. (2015). Prevalence for nematodes of hygiene‐sanitary importance in fish from Colares Island and Vigia, Pará, Brasil. Revista Brasileira de Ciência Veterinária, 22(2), 124–128. 10.4322/rbcv.2015.364 [DOI] [Google Scholar]
- Rodríguez, H. , Abollo, E. , González, Á. F. , & Pascual, S. (2018). Scoring the parasite risk in highly‐valuable fish species from southern ICES areas. Fisheries Research, 202, 134–139. 10.1016/j.fishres.2017.06.019 [DOI] [Google Scholar]
- Rodríguez, H. , González, Á. F. , Abollo, E. , & Pascual, S. (2018). Re‐evaluation of anchovies (Engraulis encrasicolus) as an important risk factor for sensitization to zoonotic nematodes in Spain. Fisheries Research, 202, 49–58. 10.1016/j.fishres.2017.11.013 [DOI] [Google Scholar]
- Rodríguez‐Santiago, M. A. , Rosales‐Casián, J. A. , & Grano‐Maldonado, M. I. (2014). Dynamics of a parasite assemblage of the vermilion rockfish Sebastes miniatus from northwestern Baja California, México. Helgoland Marine Research, 68(2), 299–306. 10.1007/s10152-014-0390-7 [DOI] [Google Scholar]
- Rodriguez‐Santiago, M. A. , Rosales‐Casian, J. A. , Grano‐Maldonado, M. I. , Vazquez‐Caballero, J. A. , Laffon‐Leal, S. M. , & Nunez‐Lara, E. (2020). Parasitological Records of Eight Rockfish Species (Scorpaeniformes: Scorpaenidae) from Pacific Baja California, Mexico. Pacific Science, 74(4), 395–403. [Google Scholar]
- Rolbiecki, L. , & Izdebska, J. N. (2023). Anisakidae nematodes in capelin Mallotus villosus (Müller, 1776) (Actinopterygii: Osmeridae) caught for food purposes. Annals of . Parasitology, 69(1), 43–47. 10.17420/ap6901.506 [DOI] [PubMed] [Google Scholar]
- Rolbiecki, L. , Izdebska, J. N. , & Dzido, J. (2020). The helminthofauna of the garfish Belone belone (Linnaeus, 1760) from the southern Baltic Sea, including new data. Annals of . Parasitology, 66(2), 237–241. https://www.scopus.com/inward/record.uri?eid=2‐s2.0‐85087320846&partnerID=40&md5=e985ca5c8049062ab1d08c1ec19a67a1 [PubMed] [Google Scholar]
- Rozas, M. , Bohle, H. , Sandoval, A. , Ildefonso, R. , Navarrete, A. , & Bustos, P. (2012). First molecular identification of Diphyllobothrium dendriticum Plerocercoids from feral rainbow trout (Oncorhynchus mykiss) in Chile. Journal of Parasitology, 98(6), 1220–1226. 10.1645/JP-GE-3097.1 [DOI] [PubMed] [Google Scholar]
- Sanpool, O. , Aung, W. P. P. , Rodpai, R. , Maleewong, W. , & Intapan, P. M. (2018). Human liver fluke Opisthorchis viverrini (Trematoda, Opisthorchiidae) in Central Myanmar: New records of adults and metacercariae identified by morphology and molecular analysis. Acta Tropica, 185, 149–155. 10.1016/j.actatropica.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Santos, M. J. , Matos, M. , Guardone, L. , Golden, O. , Armani, A. , Caldeira, A. J. R. , & Vieira‐Pinto, M. (2022). Preliminary data on the occurrence of Anisakis spp. in European hake (Merluccius merluccius) caught off the Portuguese coast and on reports of human Anisakiosis in Portugal. Microorganisms, 10(2), 331. 10.3390/microorganisms10020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, G. D. (1971). Acanthocephalan infections of man, with two new records. The Journal of Parasitology, 57(3), 582–584. [PubMed] [Google Scholar]
- See, M. S. , Harison, F. S. , Zakeri, H. A. , & Harun, N. O. (2022). Anisakis typica (DIESING, 1860), DOMINANT ANISAKID NEMATODE PRESENT IN SHORTFIN SCAD, Decapterus macrosoma (BLEEKER, 1851) FROM TERENGGANU WATERS, Malaysia. Journal of Sustainability Science and Management, 17(7), 104–120. 10.46754/jssm.2022.07.008 [DOI] [Google Scholar]
- Selstad Utaaker, K. , & Robertson, L. J. (2015). Climate change and foodborne transmission of parasites: A consideration of possible interactions and impacts for selected parasites. Food Research International, 68, 16–23. 10.1016/j.foodres.2014.06.051 [DOI] [Google Scholar]
- Semenas, L. , Viozzi, G. , & Arbetman, M. (2021). A regional study of the zoonotic broad tapeworm Dibothriocephalus spp. in northwestern Patagonia (Argentina): Origin of fishes and coastal cities as factors affecting infection in fishes. Parasitology Research, 120(7), 2415–2427. 10.1007/s00436-021-07150-7 [DOI] [PubMed] [Google Scholar]
- Sequeira, V. , Gordo, L. S. , Neves, A. , Paiva, R. B. , Cabral, H. N. , & Marques, J. F. (2010). Macroparasites as biological tags for stock identification of the bluemouth, Helicolenus dactylopterus (Delaroche, 1809) in Portuguese waters. Fisheries Research, 106(3), 321–328. 10.1016/j.fishres.2010.08.014 [DOI] [Google Scholar]
- Serracca, L. , Battistini, R. , Rossini, I. , Carducci, A. , Verani, M. , Prearo, M. , Tomei, L. , De Montis, G. , & Ercolini, C. (2014). Food safety considerations in relation to Anisakis pegreffii in anchovies (Engraulis encrasicolus) and sardines (Sardina pilchardus) fished off the Ligurian coast (cinque Terre National Park, NW Mediterranean). International Journal of Food Microbiology, 190, 79–83. 10.1016/j.ijfoodmicro.2014.08.025 [DOI] [PubMed] [Google Scholar]
- Setyobudi, E. (2011). Infection of Anisakis sp. larvae in some marine fishes from the southern coast of Kulon Progo, Yogyakarta. Biodiversitas, Journal of Biological Diversity, 12, 34–37. 10.13057/biodiv/d120107 [DOI] [Google Scholar]
- Setyobudi, E. , Jeon, C. H. , Lee, C.‐H. , Seong, K.‐B. , & Kim, J.‐H. (2011). Occurrence and identification of Anisakis spp. (Nematoda: Anisakidae) isolated from chum salmon (Oncorhynchus keta) in Korea. Parasitology Research, 108, 585–592. 10.1007/s00436-010-2101-x [DOI] [PubMed] [Google Scholar]
- Setyobudi, E. , Murwantoko, U. , & Ria. S., A. M. (2023). Anisakid nematodes from the largehead hairtail fish (Trichiurus lepturus) from the northern coast of Java, Indonesia. Biodiversitas, 24(3), 1560–1568. 10.13057/biodiv/d240328 [DOI] [Google Scholar]
- Setyobudi, E. , Rohmah, I. , Syarifah, R. , Ramatia, L. I. S. A. , Murwantoko, M. , & Sari, D. (2019). Presence of Anisakis nematode larvae in Indian mackerel (Rastrelliger spp.) along the Indian Ocean southern coast of East Java, Indonesia. Biodiversitas, 20, 313–319. 10.13057/biodiv/d200136 [DOI] [Google Scholar]
- Severin, N. L. , Yurchenko, M. , Oslash, J. S. , Rensen, Z. , Shaozhi, K. , Asma, M. K. , & Per, W. B. (2020). Anisakid nematode larvae in the liver of Atlantic cod Gadus morhua L. from West Greenland. Parasitology Research, 119(10), 3233–3242. 10.1007/s00436-020-06807-z [DOI] [PubMed] [Google Scholar]
- Sharif, M. , & Negm‐Eldin, M. (2013). Occurrence of Anisakis sp. larvae in Merluccius merluccius (Teleostei, Gadiformes) of the libyan north coast and evaluation of its zoonotic potential. Institue Scientifique, Rabat, Série ZoologieI, 49, 29–32. http://www.israbat.ac.ma/wp‐content/uploads/2015/02/04 [Google Scholar]
- Shevchuk, T. , Kateryna, V. , Seratko, M. S. , Ovsyemko, M. N. , & Novgorodska, V. (2020). The degree of residual invasion after infection with anisakiasis fish of various culinary processing. Carpathian Journal of Food Science and Technology, 12(2), 125–133. 10.34302/crpjfst/2020.12.2.12 [DOI] [Google Scholar]
- Simakova, A. V. , Babkina, I. B. , Chitnis, N. , Katokhin, A. V. , Babkin, A. M. , & Fedorova, O. S. (2022). The role of non‐commercial cyprinids in maintenance and spread of the opisthorchiasis focus in the middle Ob River basin (Tomsk region, Russia). Food and Waterborne Parasitology, 26, e00146. 10.1016/j.fawpar.2022.e00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakova, A. V. , Babkina, I. B. , Khodkevich, N. E. , Babkin, A. M. , & Interesova, E. A. (2019). Infestation of alien cyprinid fishes with trematode Opisthorchis felineus Rivolta, 1884 in the middle Ob River basin. Russian Journal of Biological Invasions, 10(2), 178–180. 10.1134/S2075111719020115 [DOI] [Google Scholar]
- Simakova, A. V. , Chitnis, N. , Babkina, I. B. , Fedorova, O. S. , Fedotova, M. M. , Babkin, A. M. , & Khodkevich, N. E. (2021). Abundance of Opisthorchis felineus Metacercariae in cyprinid fish in the middle Ob River basin (Tomsk region, Russia). Food and Waterborne Parasitology, 22, e00113. 10.1016/j.fawpar.2021.e00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, I. , & Luque, J. (2015). Seasonal variability of the composition and structure of parasite communities of red porgy, Pagrus pagrus (Perciformes: Sparidae) off Brazil. Helminthologia, 52, 236–243. 10.1515/helmin-2015-0038 [DOI] [Google Scholar]
- Sobecka, E. , Łuczak, E. , Wiecaszek, B. , & Antoszek, A. (2011). Parasite community structure of cod from Bear Island (Barents Sea) and Pomeranian Bay (Baltic Sea). Polish Polar Research, 32, 253–262. 10.2478/v10183-011-0016-6 [DOI] [Google Scholar]
- Sohn, W. M. , Choi, S. H. , Jung, B. K. , Hong, S. , Ryoo, S. , Chang, T. , Lee, K. H. , Na, B. K. , Hong, S. J. , Khieu, V. , & Chai, J. Y. (2021). Prevalence and intensity of Opisthorchis viverrini Metacercarial infection in fish from Phnom Penh, Takeo, and Kandal provinces, Cambodia. The Korean Journal of Parasitology, 59(5), 531–536. 10.3347/kjp.2021.59.5.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Jung, B. K. , Hong, S. J. , Lee, K. H. , Park, J. B. , Kim, H. S. , Cho, S. , Htoon, T. T. , Tin, H. H. , & Chai, J. Y. (2019). Low‐grade Endemicity of Opisthorchiasis, Yangon, Myanmar. Emerging Infection Disease, 25(7), 1435–1437. 10.3201/eid2507.190495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , & Na, B. K. (2019). Infections with digenetic trematode Metacercariae in freshwater fishes from two visiting sites of migratory birds in Gyeongsangnam‐do, Republic of Korea. The Korean Journal of Parasitology, 57(3), 273–281. 10.3347/kjp.2019.57.3.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , Ju, J. W. , Kim, C. H. , Hwang, M. A. , No, K. W. , & Park, J. H. (2021). Prevalence and infection intensity of zoonotic trematode Metacercariae in fish from Soyang‐cheon (stream), in Wanju‐gun, Jeollabuk‐do, Korea. The Korean Journal of Parasitology, 59(3), 265–271. 10.3347/kjp.2021.59.3.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , Lee, H. I. , Ju, J. W. , Lee, M. R. , Lim, E. J. , Son, S. Y. , Ko, E. , & Choi, J. (2021). Survey of zoonotic trematode Metacercariae in fish from Irrigation Canal of Togyo‐jeosuji (reservoir) in Cheorwon‐gun, Gangwon‐do, Republic of Korea. The Korean Journal of Parasitology, 59(4), 427–432. 10.3347/kjp.2021.59.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , Lee, H. I. , Ju, J. W. , Lee, M. R. , Park, J. G. , & Ahn, J. (2021). Endemicity of zoonotic trematode Metacercariae in fish from Deokcheon‐gang (river) in Sancheong‐gun, Gyeongsangnam‐do, Republic of Korea. The Korean Journal of Parasitology, 59(5), 523–529. 10.3347/kjp.2021.59.5.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , & Lee, S. W. (2019). Infection status with digenetic trematode Metacercariae in fishes from Coastal Lakes in Gangwon‐do, Republic of Korea. The Korean Journal of Parasitology, 57(6), 681–690. 10.3347/kjp.2019.57.6.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , Lee, S. W. , Choi, S. B. , & Seok, W. S. (2015). Trematode Metacercariae in freshwater fish from water Systems of Hantangang and Imjingang in Republic of Korea. The Korean Journal of Parasitology, 53(3), 289–298. 10.3347/kjp.2015.53.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W. M. , Na, B. K. , Cho, S. H. , Park, M. Y. , Kim, C. H. , Hwang, M. A. , No, K. W. , Yoon, K. B. , & Lim, H. C. (2017). Prevalence of Clonorchis sinensis Metacercariae in fish from water Systems of Seomjin‐gang (river). The Korean Journal of Parasitology, 55(3), 305–312. 10.3347/kjp.2017.55.3.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, W.‐M. , Yong, T.‐S. , Eom, K. S. , Pyo, K.‐H. , Lee, M. Y. , Lim, H. , Choe, S. , Jeong, H.‐G. , Sinuon, M. , Socheat, D. , & Chai, J.‐Y. (2012). Prevalence of Opisthorchis viverrini infection in humans and fish in Kratie Province, Cambodia. Acta Tropica, 124(3), 215–220. 10.1016/j.actatropica.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Sonko, P. , Chen, S. C.‐C. , Chou, C.‐M. , Huang, Y.‐C. , Hsu, S.‐L. , Barcak, D. , Oros, M. , & Fan, C.‐K. (2020). Multidisciplinary approach in study of the zoonotic Anisakis larval infection in the blue mackerel (Scomber australasicus) and the largehead hairtail (Trichiurus lepturus) in northern Taiwan. Journal of Microbiology, Immunology, and Infection, 53(6), 1021–1029. 10.1016/j.jmii.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Soylu, E. (2015). Metazoan parasites of fish species from Lake gala (Edirne, Turkey) gala Gölü (Edirne)' ndeki balık türlerinin metazoan parazitleri. Ege Journal of Fisheries and Aquatic Sciences, 31, 187–193. 10.12714/egejfas.2014.31.4.03 [DOI] [Google Scholar]
- Sripa, J. , Kiatsopit, N. , & Piratae, S. (2016). Prevalence of trematode larvae in intermediate hosts: Snails and fish in KO ae sub‐district of KHUEANG NAI, UBON RATCHATHANI province, Thailand. The Southeast Asian Journal of Tropical Medicine and Public Health, 47(3), 399–409. [PubMed] [Google Scholar]
- Suzuki, J. , Murata, R. , Hosaka, M. , & Araki, J. (2010). Risk factors for human Anisakis infection and association between the geographic origins of Scomber japonicus and anisakid nematodes. International Journal of Food Microbiology, 137(1), 88–93. 10.1016/j.ijfoodmicro.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , Ito, T. , Sato, T. , Goto, M. , Kawamoto, T. , Fujinaga, A. , Yanagawa, N. , Saito, Y. , Nakao, M. , Hasegawa, H. , & Fujiya, M. (2016). Infection with fully mature Corynosoma cf. validum causes ulcers in the human small intestine. Clinical Journal of Gastroenterology, 9(3), 114–117. 10.1007/s12328-016-0646-7 [DOI] [PubMed] [Google Scholar]
- Takano, T. , Iwaki, T. , Waki, T. , Murata, R. , Suzuki, J. , Kodo, Y. , Kobayashi, K. , & Ogawa, K. (2021). Species composition and infection levels of Anisakis (Nematoda: Anisakidae) in the skipjack tuna Katsuwonus pelamis (Linnaeus) in the Northwest Pacific. Parasitology Research, 120(5), 1605–1615. 10.1007/s00436-021-07144-5 [DOI] [PubMed] [Google Scholar]
- Tantanasi, J. , Diakou, A. , Tamvakis, A. , & Batjakas, I. (2012). Anisakis spp. burden in Trachurus trachurus. Helminthologia, 49(1), 16–20. 10.2478/s11687-012-0003-4 [DOI] [Google Scholar]
- Tejada, M. , Karl, H. , Heras, C. , Vidacek, S. , Solas, M. T. , & Garcia, M. L. (2014). Does the intensity of Anisakis infection affect the quality of hake muscle? Journal of Aquatic Food Product Technology, 23(3), 221–236. 10.1080/10498850.2012.710301 [DOI] [Google Scholar]
- Tepe, Y. , & Oguz, M. (2013). Nematode and acanthocephalan parasites of marine fish of the eastern Black Sea coasts of Turkey. Turkish Journal of Zoology, 37, 753–760. 10.3906/zoo-1206-18 [DOI] [Google Scholar]
- Tesfaye, Z. , Hiko, A. , Belina, D. , & Firdisa, M. (2023). Assessments and identification of selected fish‐borne zoonotic parasites in Nile tilapia and African catfish species in lakes of Haramaya District, Ethiopia. Aquaculture Research, 2023(1), 2638123. 10.1155/2023/2638123 [DOI] [Google Scholar]
- Thébault, A. , El Metennani, S. , Carvalho, L. , Cadavez, V. , Gonzales‐Barron, U. , & Kooh, P. (2024). Data on the occurrence of anisakids in fishery products from wild caught fish in all countries (Jan 2010 – Sept 2023) [Data set]. Zenodo. 10.5281/zenodo.14047972 [DOI] [Google Scholar]
- Tiewchaloern, S. , Udomkijdecha, S. , Suvouttho, S. , Chunchamsri, K. , & Waikagul, J. (1999). Clinostomum trematode from human eye. The Southeast Asian Journal of Tropical Medicine and Public Health, 30(2), 382–384. [PubMed] [Google Scholar]
- Torres, P. , Leyán, V. , & Puga, S. (2012). Prevalence, intensity, and abundance of infection and pathogenesis caused by diphyllobothriosis in vulnerable, native fish and introduced trout in lake Panguipulli, Chile. Journal of Wildlife Diseases, 48(4), 937–950. 10.7589/2011-08-235 [DOI] [PubMed] [Google Scholar]
- Torres, P. , & Puga, S. (2011). Comparative efficacy of candling and glass plate compression for detection of diphyllobothriosis in rainbow trout (Oncorhynchus mykiss) musculature. Revue Scientifique et Technique, 30(3), 831–837. 10.20506/rst.30.3.2082 [DOI] [PubMed] [Google Scholar]
- Torres‐Frenzel, P. , & Torres, P. (2014). Anisakid parasites in commercial hake ceviche in southern Chile. Journal of Food Protection, 77(7), 1237–1240. 10.4315/0362-028X.JFP-13-538 [DOI] [PubMed] [Google Scholar]
- Unger, P. , Klimpel, S. , Lang, T. , & Palm, H. W. (2014). Metazoan parasites from herring (Clupea harengus L.) as biological indicators in the Baltic Sea. Acta Parasitologica, 59(3), 518–528. 10.2478/s11686-014-0276-5 [DOI] [PubMed] [Google Scholar]
- Urquhart, K. , Pert, C. C. , Fryer, R. J. , Cook, P. , Weir, S. , Kilburn, R. , McCarthy, U. , Simons, J. , McBeath, S. J. , Matejusova, I. , & Bricknell, I. R. (2010). A survey of pathogens and metazoan parasites on wild sea trout (Salmo trutta) in Scottish waters. ICES Journal of Marine Science, 67(3), 444–453. 10.1093/icesjms/fsp271 [DOI] [Google Scholar]
- van Weelden, C. , Towers, J. R. , & Bosker, T. (2021). Impacts of climate change on cetacean distribution, habitat and migration. Climate Change Ecology, 1, 100009. 10.1016/j.ecochg.2021.100009 [DOI] [Google Scholar]
- Vasconcelos, J. , Hermida, M. , Saraiva, A. , González, J. A. , & Gordo, L. S. (2017). The use of parasites as biological tags for stock identification of blue jack mackerel, Trachurus picturatus, in the North‐eastern Atlantic. Fisheries Research, 193, 1–6. 10.1016/j.fishres.2017.03.015 [DOI] [Google Scholar]
- Violante‐González, J. , Gallegos‐Navarro, Y. , Monks, S. , García‐Ibáñez, S. , Rojas‐Herrera, A. A. , Pulido‐Flores, G. , Villerías‐Salinas, S. , & Larumbe‐Morán, E. (2016). Parasites of the green jack Caranx caballus (Pisces: Carangidae) in three locations from Pacific coasts of Mexico, and their utility as biological tags. Revista Mexicana de Biodiversidad, 87(3), 1015–1022. 10.1016/j.rmb.2016.07.010 [DOI] [Google Scholar]
- Wang, Y. , Wang, X. , Gong, P. , Yu, Y. , Zhang, N. , Ren, Y. , Ma, Y. , Zhao, Z. , Zhang, X. , Li, X. , & Li, J. (2022). Prevalence of fish‐borne zoonotic trematode infection in Jilin Province, China. International Journal for Parasitology: Parasites and Wildlife, 18, 52–60. 10.1016/j.ijppaw.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicht, B. , Limoni, C. , Peduzzi, R. , & Petrini, O. (2009). Diphyllobothrium latum (Cestoda: Diphyllobothriidea) in perch (Perca fluviatilis) in three sub‐alpine lakes: Influence of biotic and abiotic factors on prevalence. Journal of Limnology, 68(2), 167–173. 10.4081/jlimnol.2009.167 [DOI] [Google Scholar]
- Wilson, J. R. , Saunders, R. J. , & Hutson, K. S. (2019). Parasites of the invasive tilapia Oreochromis mossambicus: evidence for co‐introduction. Aquatic Invasions, 14(2), 332–349. [Google Scholar]
- Wongsawad, C. , Wongsawad, P. , Anuntalabhochai, S. , Chai, J.‐Y. , & Sukontason, K. (2013). Occurrence and molecular identification of liver and minute intestinal flukes metacercariae in freshwater fish from fang‐Mae Ai Agricultural Basin, Chiang Mai province, Thailand. Asian Biomedicine, 7, 97–104. 10.5372/1905-7415.0701.155 [DOI] [Google Scholar]
- Yamasaki, H. , Sato, M. O. , Kuramochi, T. , & Mercado, R. (2023). Genetic characterization of Dibothriocephalus latus and Dibothriocephalus dendriticus (Cestoda: Diphyllobothriidae) from Chile based on haplotype analysis using mitochondrial DNA markers. Parasitology International, 96, 102767. 10.1016/j.parint.2023.102767 [DOI] [PubMed] [Google Scholar]
- Yang, S. , Pei, X. , Yin, S. , Yu, X. , Mei, L. , Zhao, W. , Liang, H. , Wang, Q. , & Yang, D. (2019). Investigation and research of Clonorchis sinensis metacercariae and Metorchis orientalis metacercariae infection in freshwater fishes in China from 2015 to 2017. Food Control, 104, 115–121. 10.1016/j.foodcont.2019.04.028 [DOI] [Google Scholar]
- Yun, N. Q. , Syarifah, R. F. , & Murwantoko, S. (2022). Anisakis infection of Belanger's croaker (Johnius Belangerii Cuvier 1830) at the Indian Ocean coast of Yogyakarta, Indonesia. Jordan . Journal of Biological Sciences, 15(1), 29–36. 10.54319/jjbs/150105 [DOI] [Google Scholar]
- Zhang, S. L. , & Fan, G. H. (1990). Case report: brain abscess caused by heterophyid eggs. Chinese Journal of Parasitology and Parasitic Diseases, 8(8), 178. [Google Scholar]
- Zhao, W.‐T. , Lü, L. , Chen, H.‐X. , Yang, Y. , Zhang, L.‐P. , & Li, L. (2016). Ascaridoid parasites infecting in the frequently consumed marine fishes in the coastal area of China: A preliminary investigation. Parasitology International, 65(2), 87–98. 10.1016/j.parint.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Zuo, S. , Kania, P. W. , Mehrdana, F. , Marana, M. H. , & Buchmann, K. (2018). Contracaecum osculatum and other anisakid nematodes in grey seals and cod in the Baltic Sea: Molecular and ecological links. Journal of Helminthology, 92(1), 81–89. 10.1017/S0022149X17000025 [DOI] [PubMed] [Google Scholar]


