Abstract
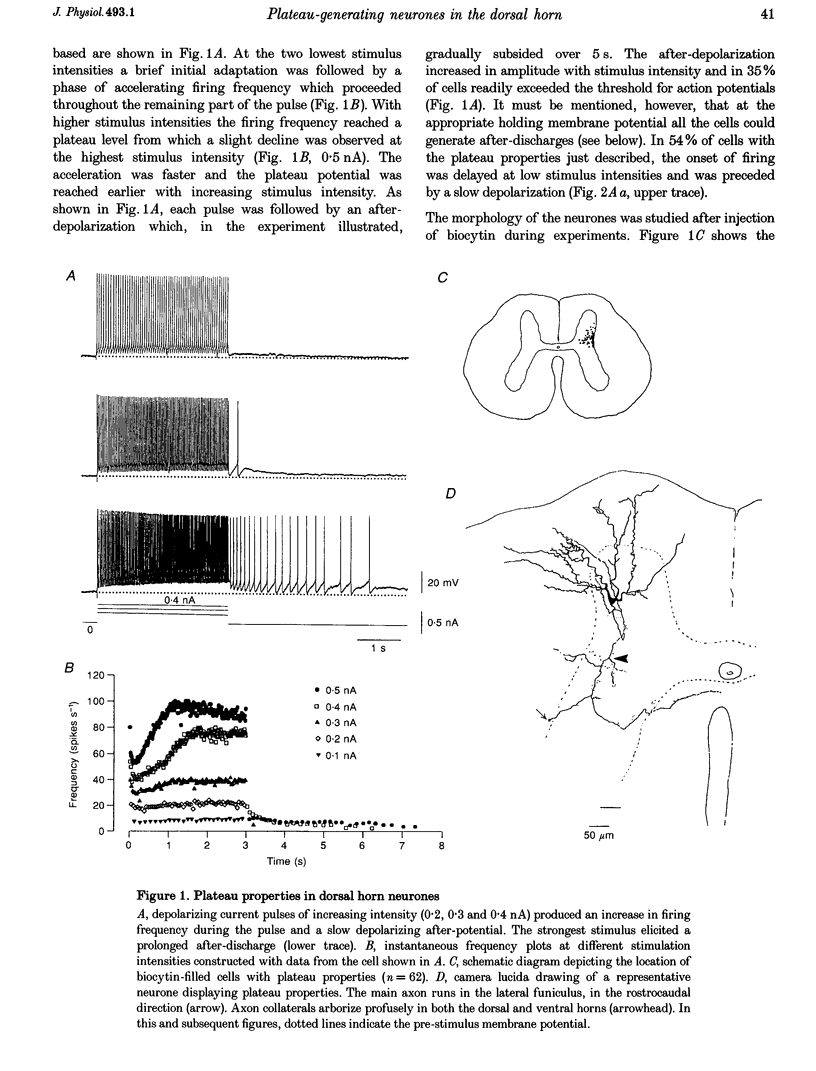
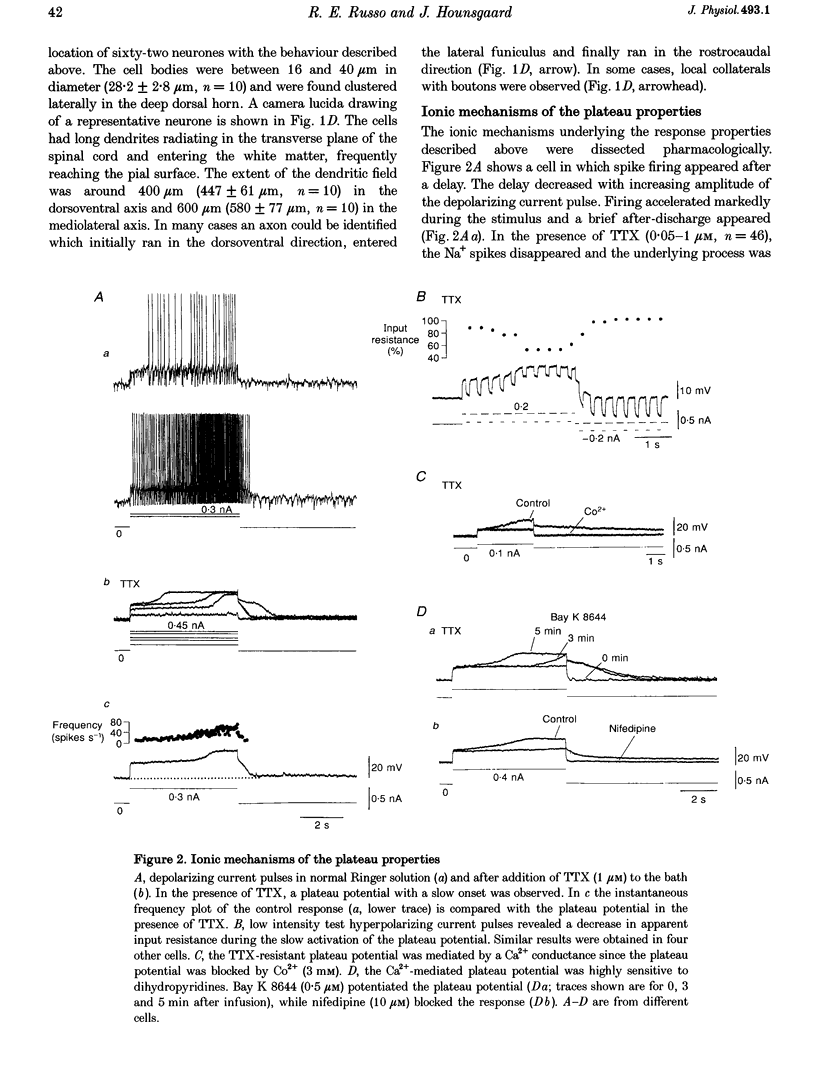
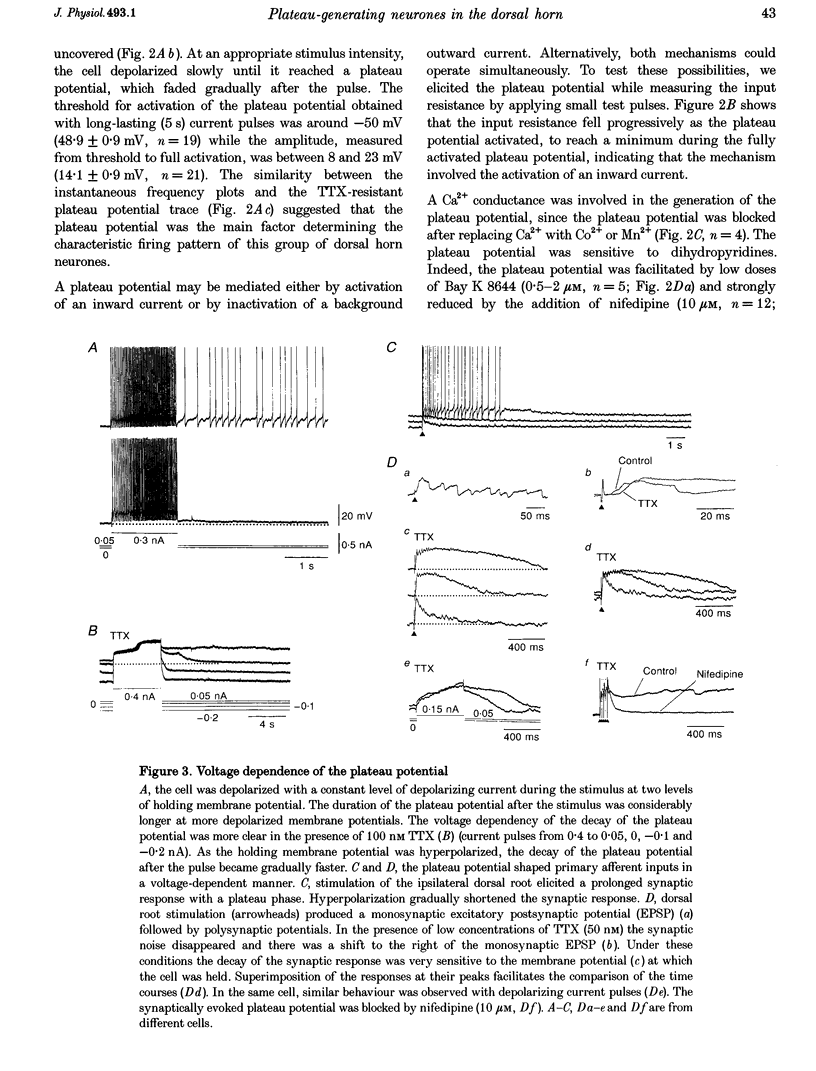
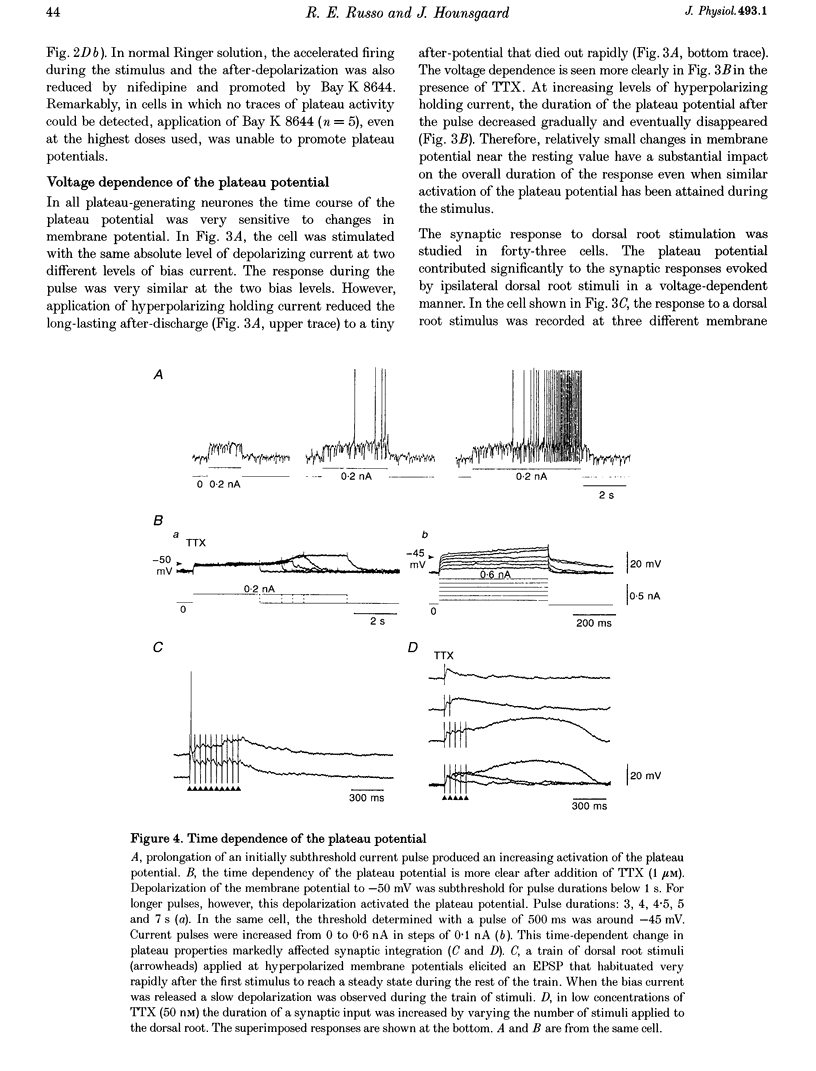
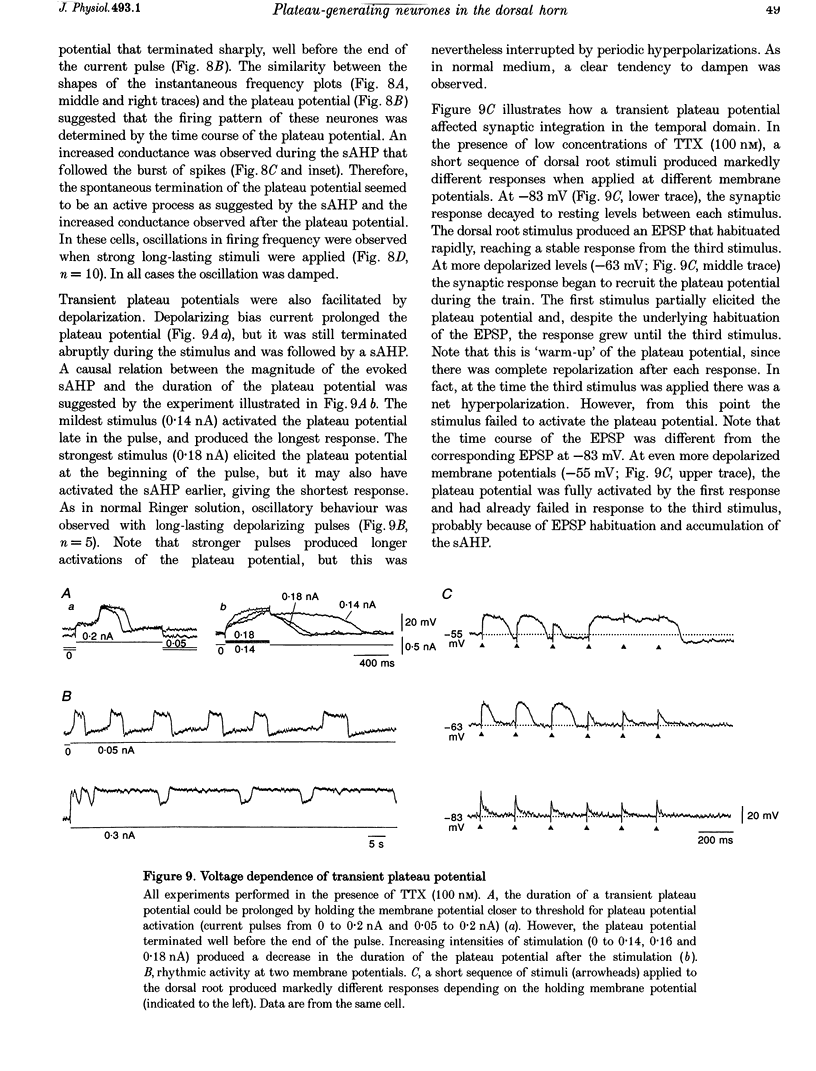
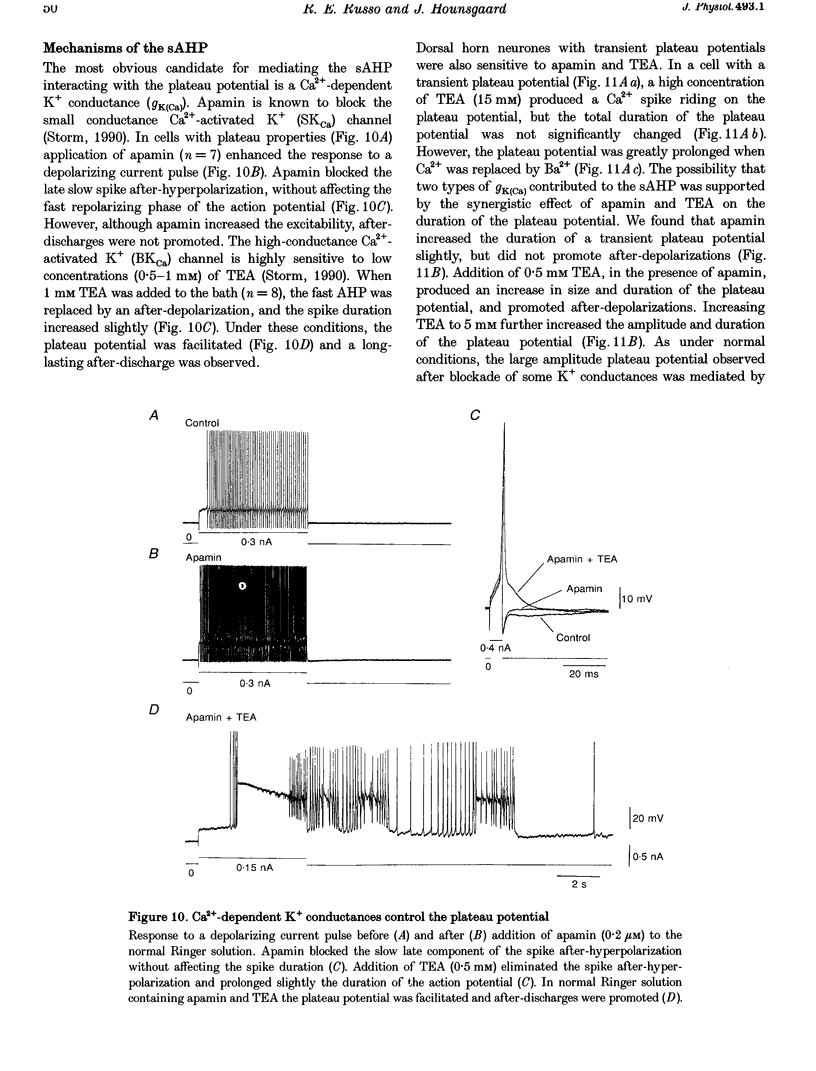
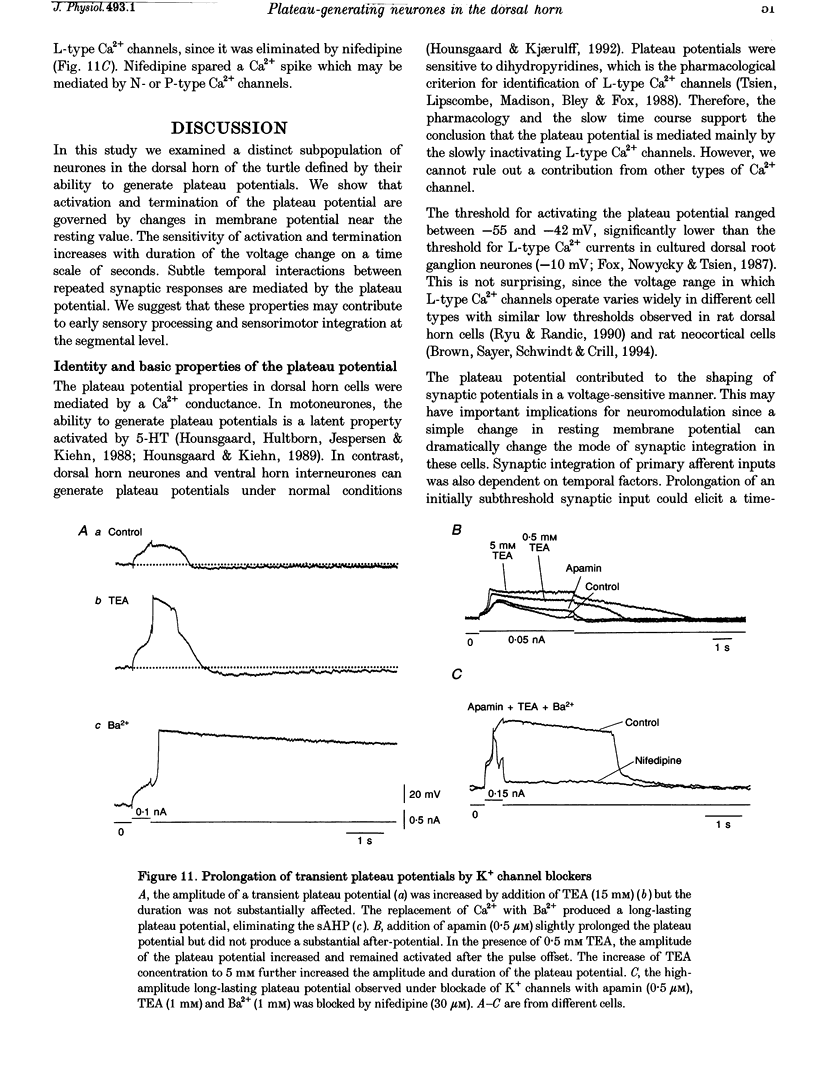
1. In transverse slices of the spinal cord of the turtle, intracellular recordings were used to characterize and analyse the responses to injected current and activation of primary afferents in dorsal horn neurones. 2. A subpopulation of neurones, with cell bodies located laterally in the deep dorsal horn and dendrites radiating towards the pial surface, was distinguished by the ability to generate plateau potentials. Activation of the plateau potential by a suprathreshold depolarizing current pulse produced an increasing firing frequency during the first few seconds and a sustained after-discharge. 3. The plateau potential was assumed to be mediated by L-type Ca2+ channels since it was blocked by Co2+ (3 mM) and nifedipine (10 microM) and enhanced by Bay K 8644 (0.5-2 microM). 4. The threshold for activating the plateau potential declined during the first few seconds of depolarization. The decline in threshold gradually subsided over 3-10 s after repolarization. 5. Frequency potentiation of the plateau potential contributed to wind-up of the response to depolarizing current pulses and primary afferent stimuli repeated at frequencies higher than 0.1-0.3 Hz. 6. The sustained after-discharge mediated by the plateau potential was curtailed by a slow after-hyperpolarization (sAHP) evoked by strong depolarizations. The relative strength of the plateau potential and sAHP varied among cells. In some cells the plateau potential and sAHP interacted to produce damped oscillations upon depolarization. The sAHP was mediated by both apamin and tetraethylammonium (TEA)-sensitive K+ channels. 7. Our findings suggest that basic properties of sensory integration may reside with the specialized intrinsic response properties of particular subtypes of neurones in the dorsal horn.
Full text
PDF

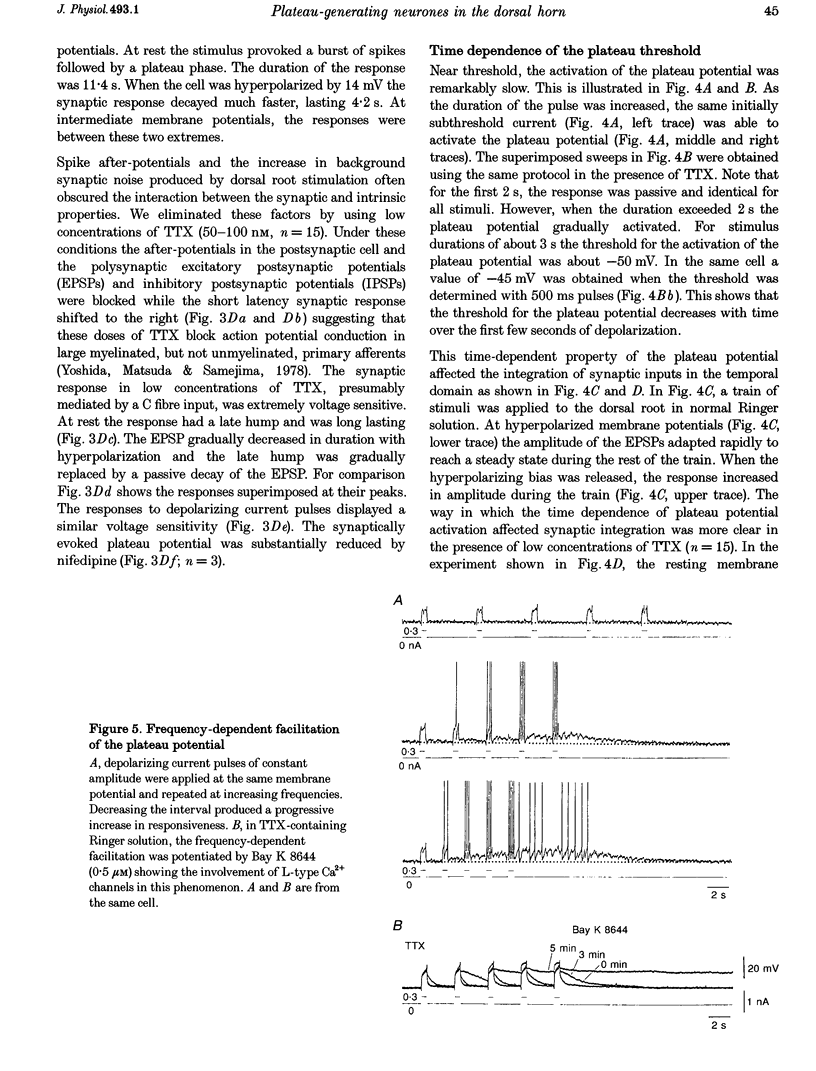
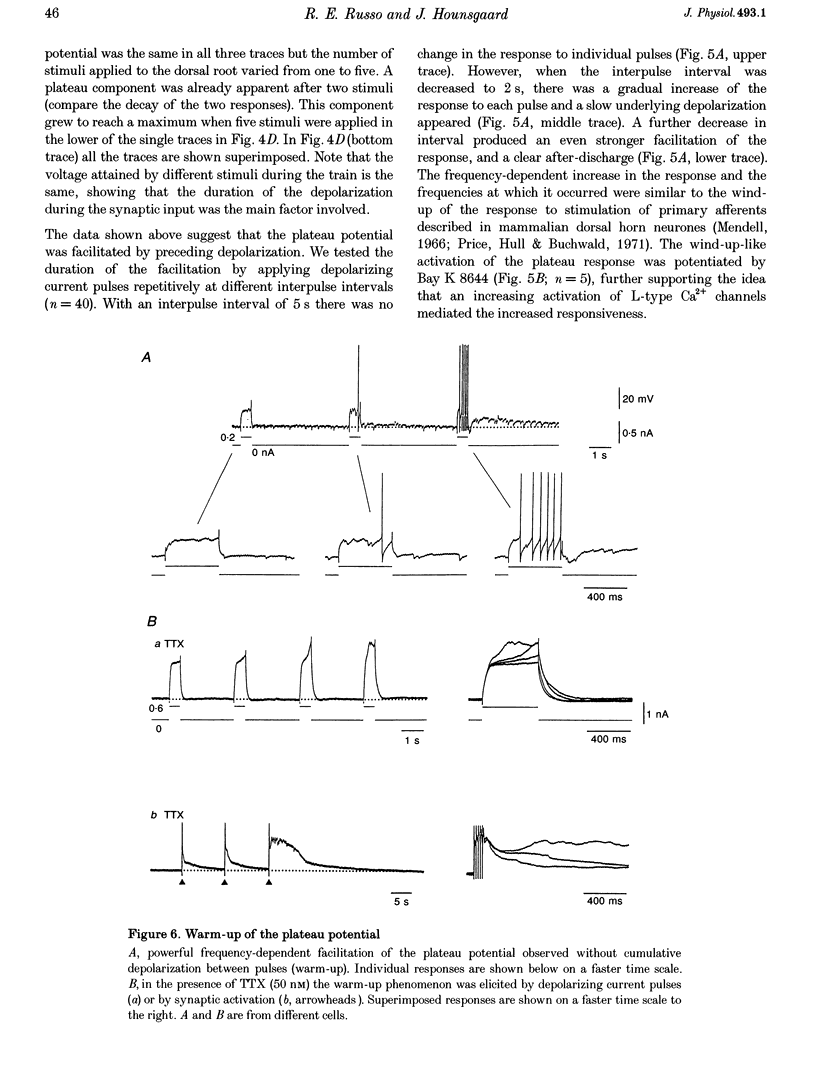
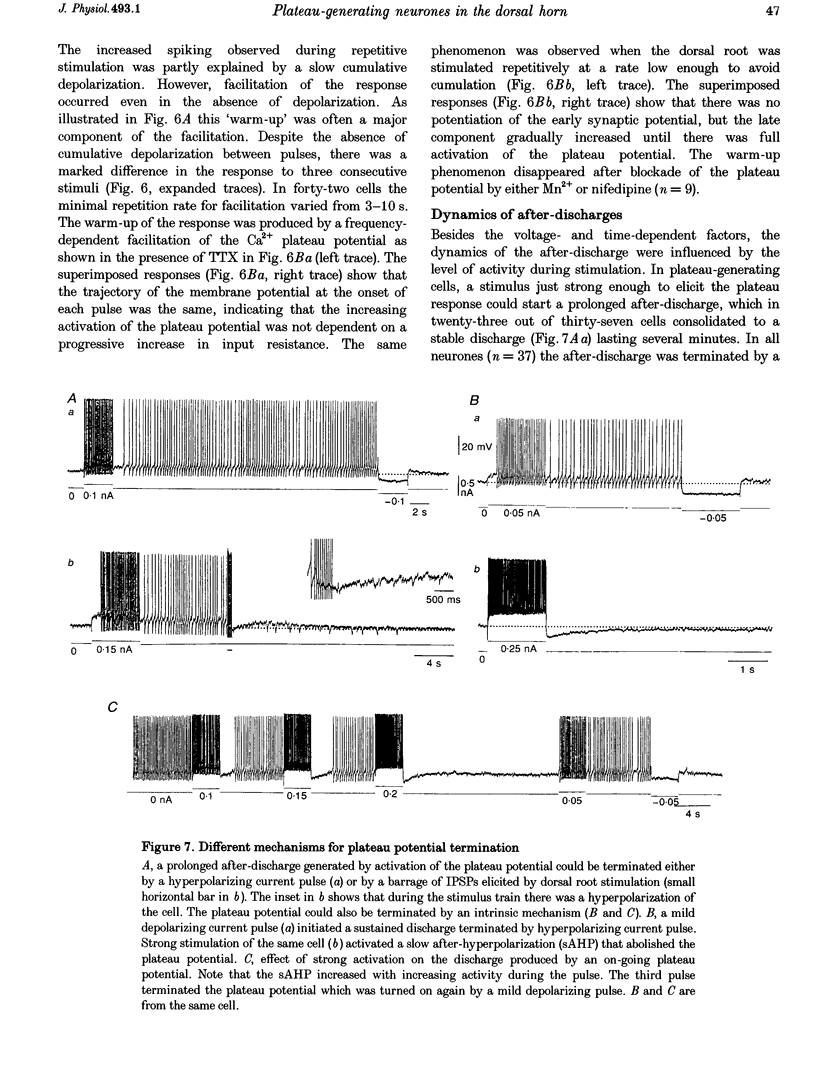
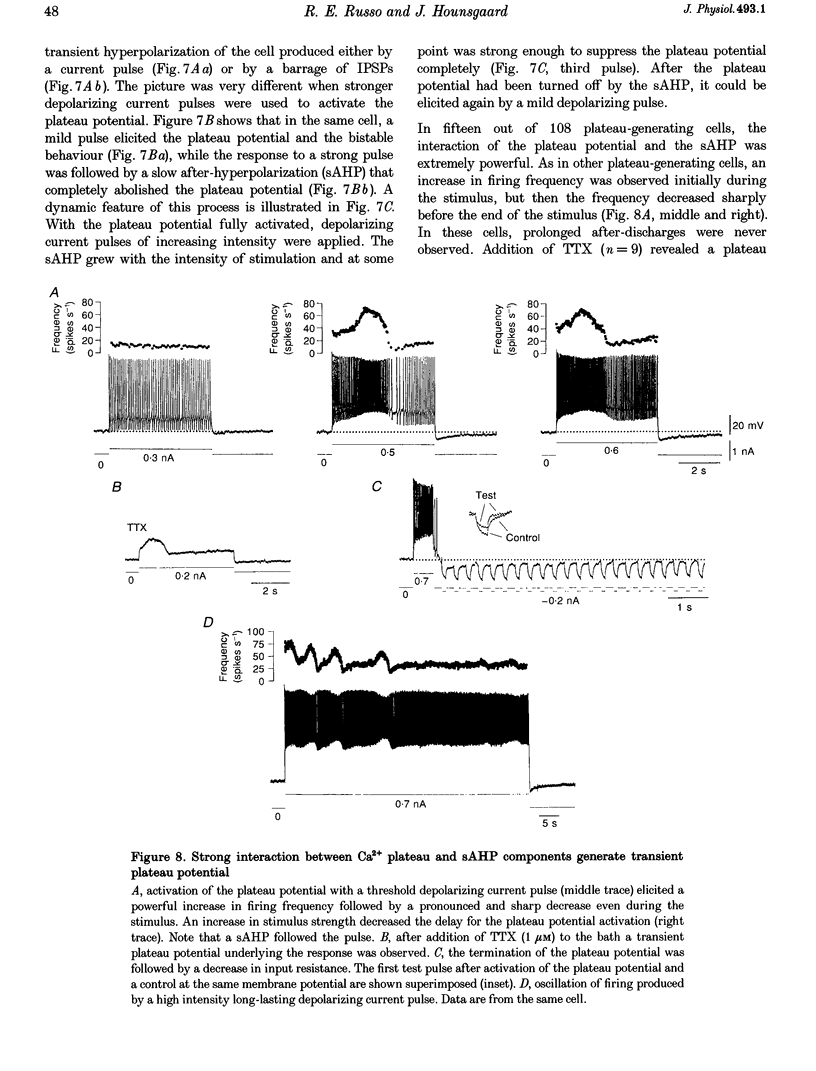



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Sayer R. J., Schwindt P. C., Crill W. E. P-type calcium channels in rat neocortical neurones. J Physiol. 1994 Mar 1;475(2):197–205. doi: 10.1113/jphysiol.1994.sp020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992 Sep;12(9):3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie S. N., Stein P. S. Cutaneous stimulation evokes long-lasting excitation of spinal interneurons in the turtle. J Neurophysiol. 1990 Oct;64(4):1134–1148. doi: 10.1152/jn.1990.64.4.1134. [DOI] [PubMed] [Google Scholar]
- Del Pozo E., Caro G., Baeyens J. M. Analgesic effects of several calcium channel blockers in mice. Eur J Pharmacol. 1987 Jun 4;137(2-3):155–160. doi: 10.1016/0014-2999(87)90216-0. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Avila-Sakar A. J., Stefani E. Repetitive stimulation increases the activation rate of skeletal muscle Ca2+ currents. Pflugers Arch. 1990 Apr;416(1-2):210–212. doi: 10.1007/BF00370245. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988 Nov;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O., Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988 Apr;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J., Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989 Jul;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y. Calcium channels in isolated rat dorsal horn neurones, including labelled spinothalamic and trigeminothalamic cells. J Physiol. 1989 Apr;411:161–177. doi: 10.1113/jphysiol.1989.sp017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A., Alvarez O., Latorre R. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J Gen Physiol. 1988 Jul;92(1):67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. D., Hayes R. L., Ruda M., Dubner R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol. 1978 Jul;41(4):933–947. doi: 10.1152/jn.1978.41.4.933. [DOI] [PubMed] [Google Scholar]
- Price D. D., Hull C. D., Buchwald N. A. Intracellular responses of dorsal horn cells to cutaneous and sural nerve A and C fiber stimuli. Exp Neurol. 1971 Nov;33(2):291–309. doi: 10.1016/0014-4886(71)90022-7. [DOI] [PubMed] [Google Scholar]
- Russell D. F., Hartline D. K. Bursting neural networks: a reexamination. Science. 1978 Apr 28;200(4340):453–456. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- Russo R. E., Hounsgaard J. Burst-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996 May 15;493(Pt 1):55–66. doi: 10.1113/jphysiol.1996.sp021364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R. E., Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994 Jul;61(2):191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Ryu P. D., Randic M. Low- and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J Neurophysiol. 1990 Feb;63(2):273–285. doi: 10.1152/jn.1990.63.2.273. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Thomson Alex M., West David C., Headley P. Max. Membrane Characteristics and Synaptic Responsiveness of Superficial Dorsal Horn Neurons in a Slice Preparation of Adult Rat Spinal Cord. Eur J Neurosci. 1989 Sep;1(5):479–488. doi: 10.1111/j.1460-9568.1989.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Urban L., Thompson S. W., Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends Neurosci. 1994 Oct;17(10):432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. C-primary afferent fibre mediated inhibitions in the dorsal horn of the decerebrate-spinal rat. Exp Brain Res. 1983;51(2):283–290. doi: 10.1007/BF00237204. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., King A. E. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. J Neurophysiol. 1987 Sep;58(3):460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]


