Abstract
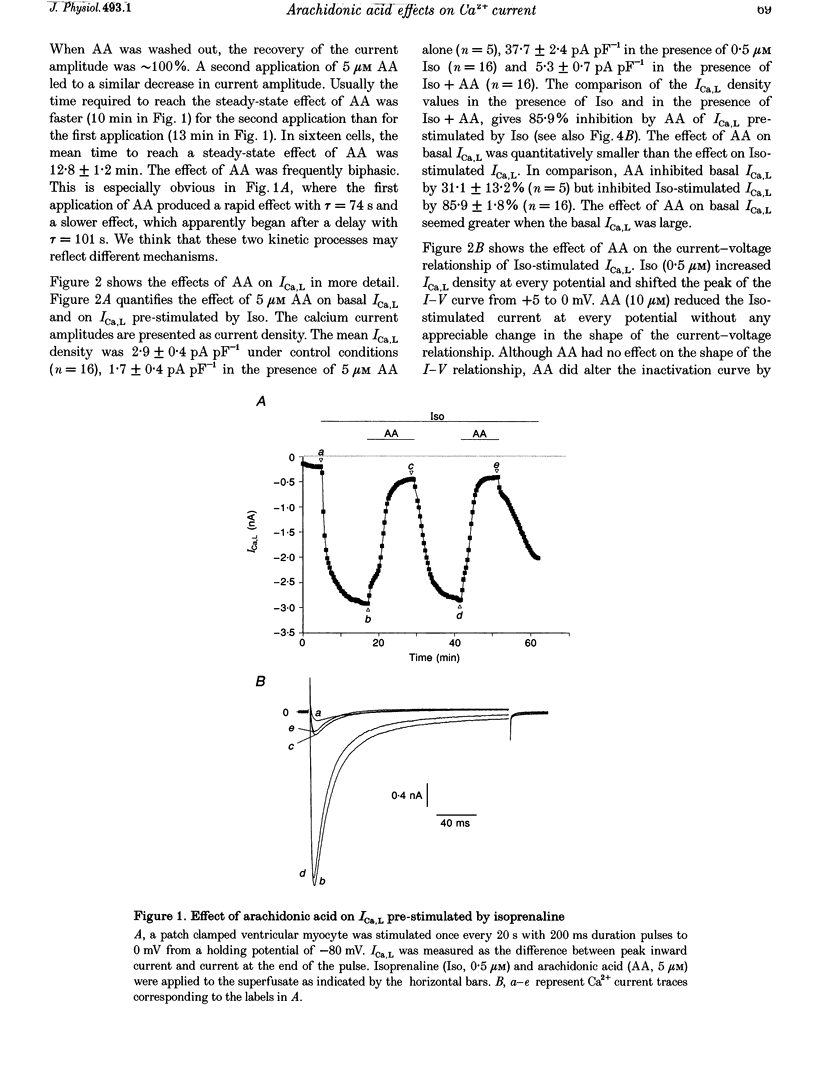
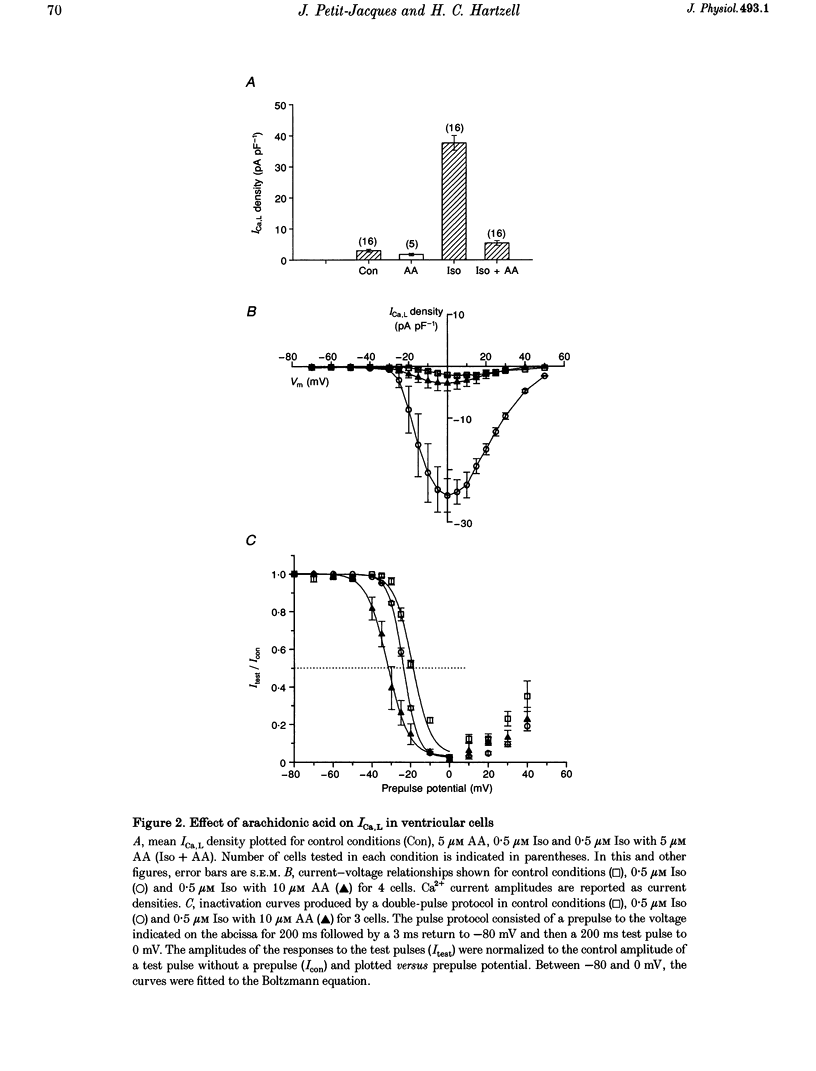
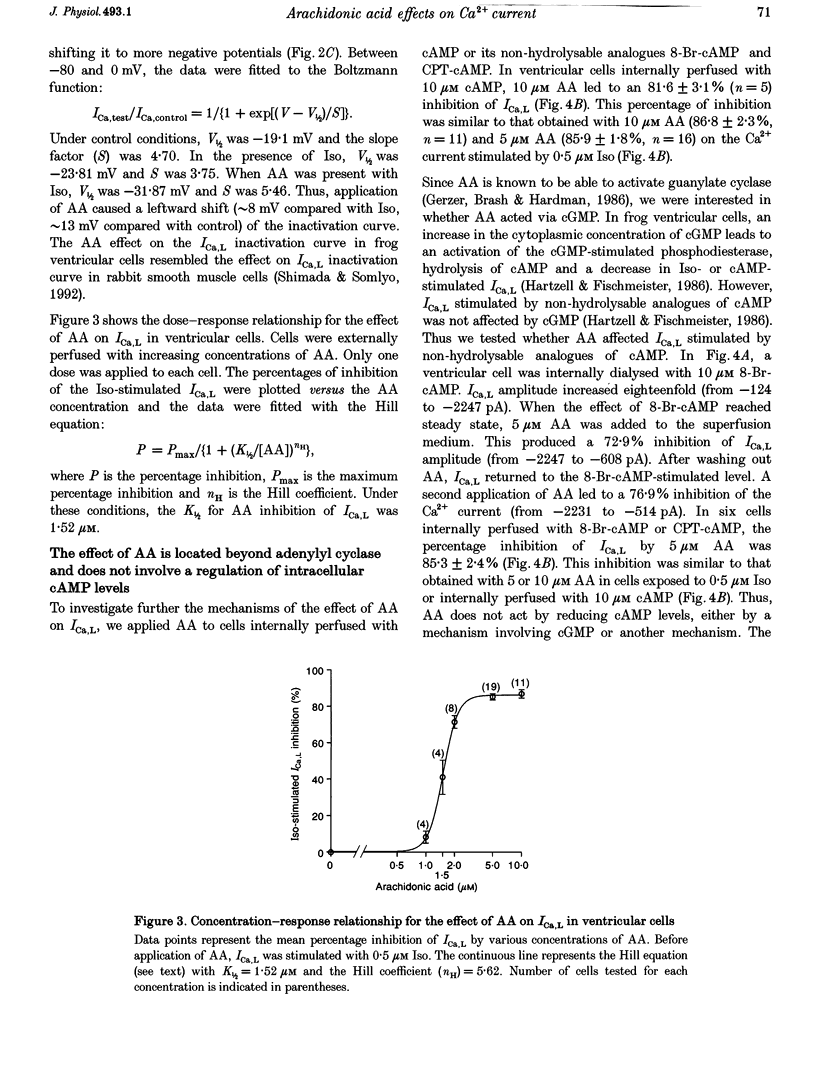
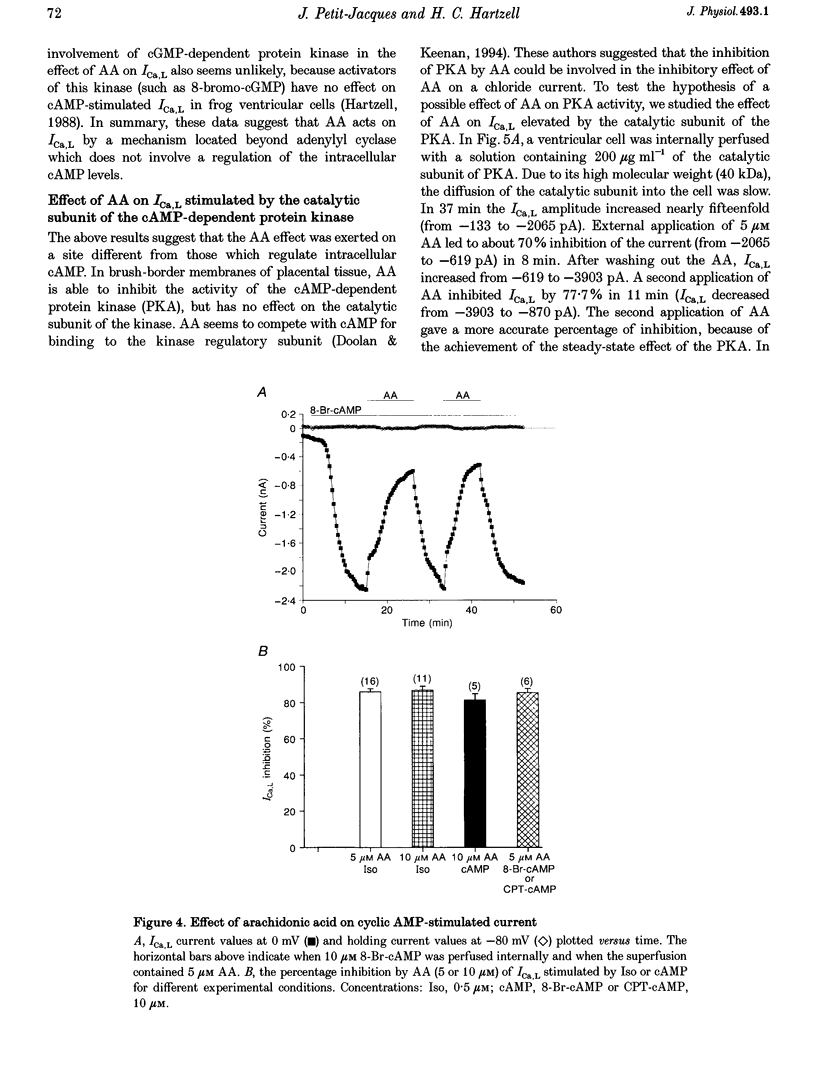
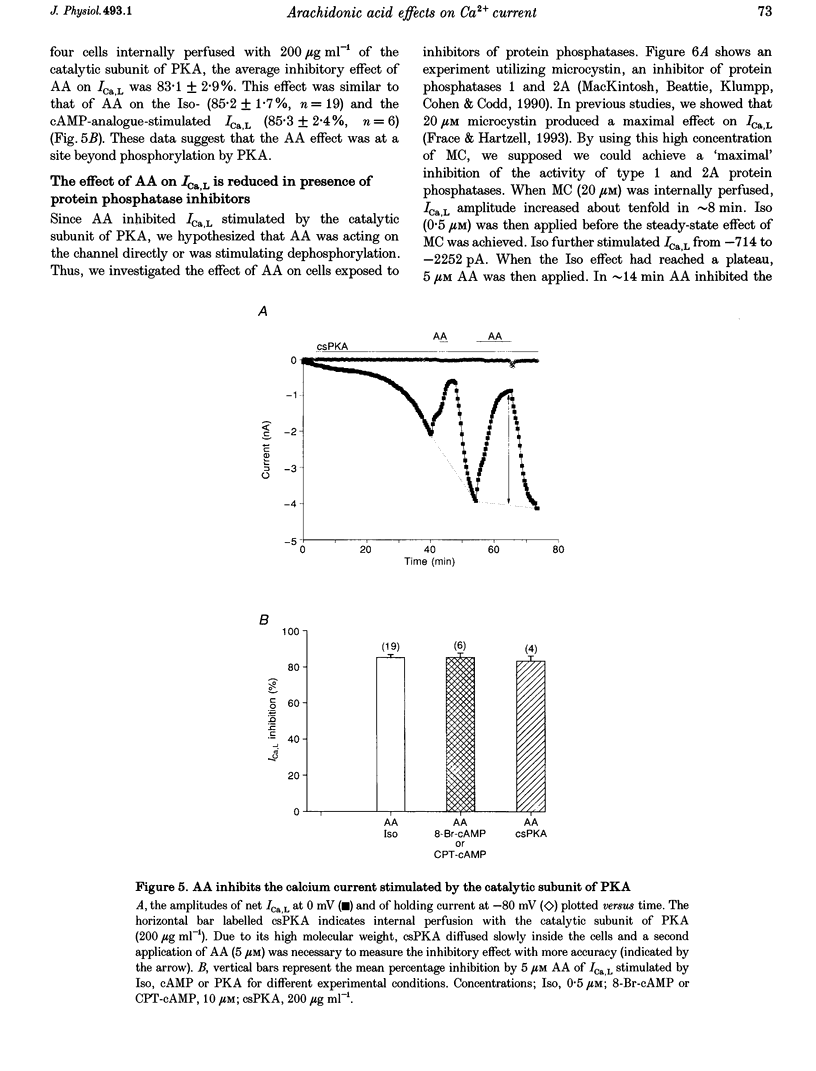
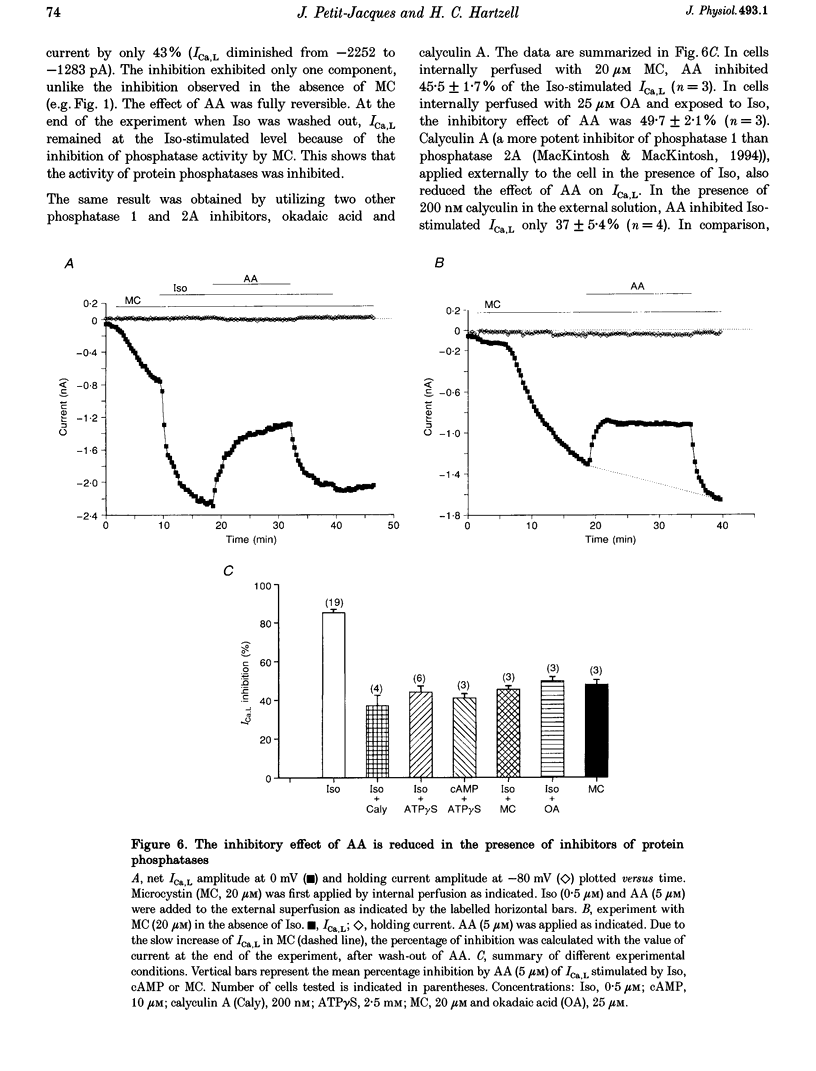
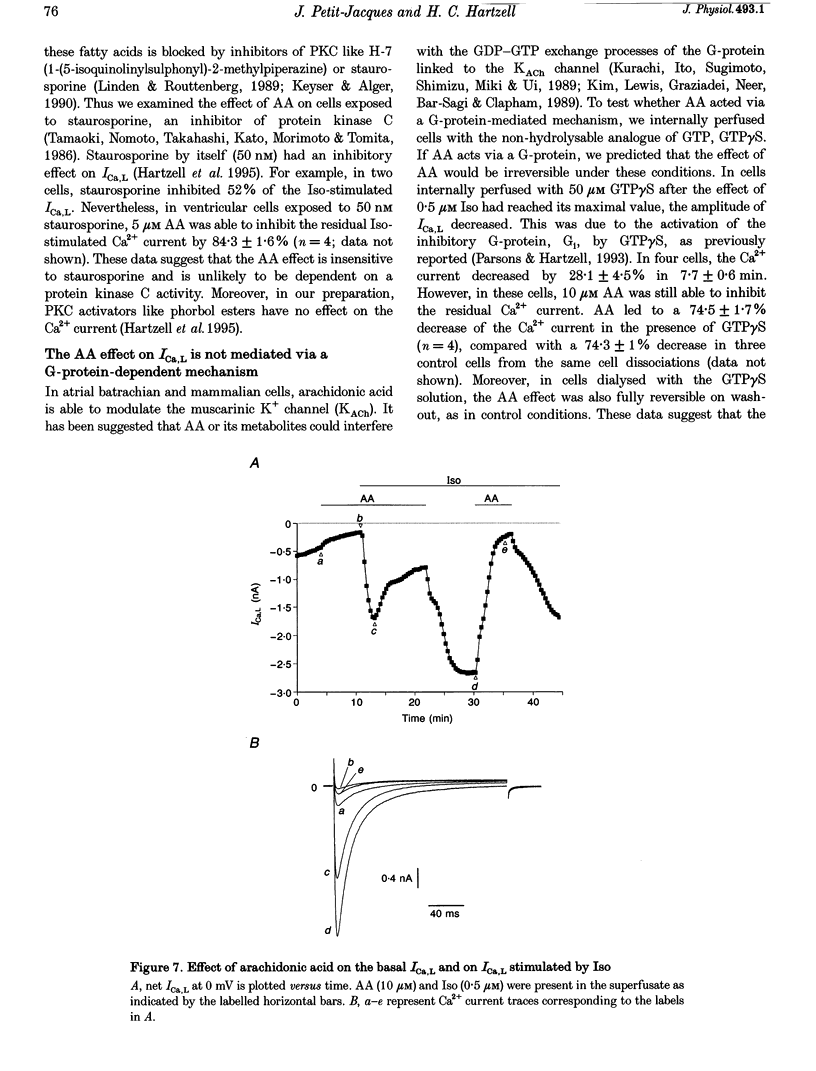
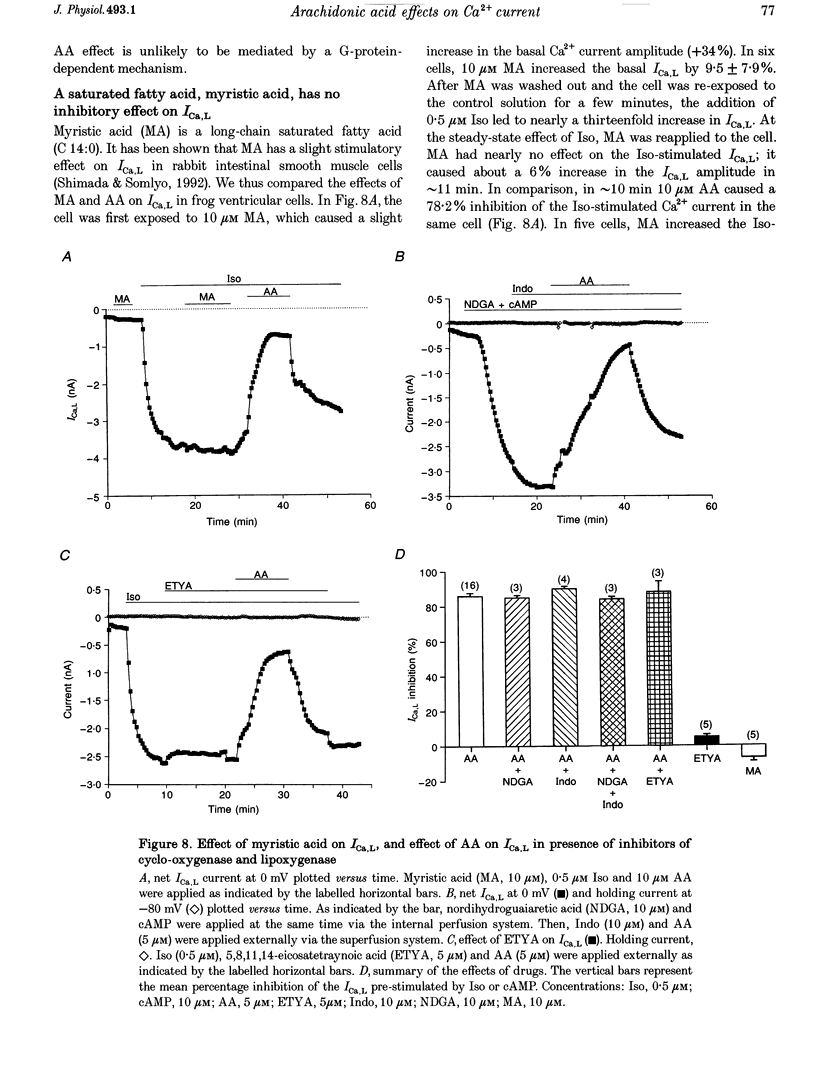
1. External application of the unsaturated fatty acid arachidonic acid (AA) to frog ventricular cells caused a large inhibition (approximately 85%) of the L-type calcium current (ICa,L) previously stimulated by the beta-adrenergic agonist isoprenaline (Iso). The concentration producing half-maximal inhibition (K1/2) was 1.52 microM. The inhibitory effect did not affect the peak current-voltage relationship but produced a negative shift in the inactivation curve. 2. The inhibitory effect of AA also occurred in cells internally perfused with cAMP and non-hydrolysable analogues of cAMP. These data suggest that AA is acting by a mechanism located beyond adenylyl cyclase and does not involve changes in intracellular cAMP levels. 3. AA also inhibited the calcium current stimulated by internal perfusion with the catalytic subunit of protein kinase A (PKA), suggesting that AA acts downstream of channel phosphorylation. 4. The inhibitory effect of AA on the isoprenaline- or cAMP-stimulated ICa,L is largely reduced in cells internally perfused with the thiophosphate donor analogue of ATP, ATP gamma S, or protein phosphatase 1 and 2A inhibitors like microcystin (MC) or okadaic acid (OA). External application of the phosphatase inhibitor calyculin (Caly) also reduced the AA effect. These data suggested that the AA effect on ICa,L involves activation of protein phosphatase activity. 5. The effect of AA on ICa,L was not affected by staurosporine, an inhibitor of protein kinases. It was also unaffected in cells internally perfused with GTP gamma S. These results suggest that neither a PKC- nor a G-protein-mediated mechanism are likely to be involved in the effect of AA on ICa,L. 6. A saturated fatty acid, myristic acid (MA), had no inhibitory effect on the isoprenaline-stimulated Ca2+ current, whereas, in the same cells arachidonic acid produced approximately 85% inhibition of ICa,L. 7. The inhibitory effect of AA was not affected by exposing the cells to indomethacin (Indo), an inhibitor of the metabolism of AA by cyclo-oxygenase, nor nordihydroguaiaretic acid (NDGA), an inhibitor of the lipoxygenase pathway. However, the non-metabolizable analogue of AA, 5,8,11,14-eicosatetraynoic acid (ETYA), was without effect on the isoprenaline-stimulated ICa,L. 8. These results suggest that AA inhibits ICa,L via a mechanism which involves, in part, stimulation of protein phosphatase activity. This process could provide a new mechanism in the modulation of calcium channel activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Welsh M. J. Fatty acids inhibit apical membrane chloride channels in airway epithelia. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7334–7338. doi: 10.1073/pnas.87.18.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan C. M., Keenan A. K. Inhibition by fatty acids of cyclic AMP-dependent protein kinase activity in brush border membranes isolated from human placental vesicles. Br J Pharmacol. 1994 Feb;111(2):509–514. doi: 10.1111/j.1476-5381.1994.tb14766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M., Wang J., Foster C. D., Adelman J. P., North R. A., Levitan I. B. Cloned Ca(2+)-dependent K+ channel modulated by a functionally associated protein kinase. Nature. 1994 Jun 16;369(6481):563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frace A. M., Hartzell H. C. Opposite effects of phosphatase inhibitors on L-type calcium and delayed rectifier currents in frog cardiac myocytes. J Physiol. 1993 Dec;472:305–326. doi: 10.1113/jphysiol.1993.sp019948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Brash A. R., Hardman J. G. Activation of soluble guanylate cyclase by arachidonic acid and 15-lipoxygenase products. Biochim Biophys Acta. 1986 May 29;886(3):383–389. doi: 10.1016/0167-4889(86)90173-4. [DOI] [PubMed] [Google Scholar]
- Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992 Oct 25;267(30):21492–21498. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Hirayama Y., Petit-Jacques J. Effects of protein phosphatase and kinase inhibitors on the cardiac L-type Ca current suggest two sites are phosphorylated by protein kinase A and another protein kinase. J Gen Physiol. 1995 Sep;106(3):393–414. doi: 10.1085/jgp.106.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W., Mieskes G., Söling H. D. Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem. 1987 Jun 1;165(2):261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- Huang J. M., Xian H., Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser D. O., Alger B. E. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990 Oct;5(4):545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Khurana G., Bennett M. R. Nitric oxide and arachidonic acid modulation of calcium currents in postganglionic neurones of avian cultured ciliary ganglia. Br J Pharmacol. 1993 Jun;109(2):480–485. doi: 10.1111/j.1476-5381.1993.tb13594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol. 1990 Oct;38(4):524–530. [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., MacKintosh R. W. Inhibitors of protein kinases and phosphatases. Trends Biochem Sci. 1994 Nov;19(11):444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Massey K. D., Minnich B. N., Burt J. M. Arachidonic acid and lipoxygenase metabolites uncouple neonatal rat cardiac myocyte pairs. Am J Physiol. 1992 Aug;263(2 Pt 1):C494–C501. doi: 10.1152/ajpcell.1992.263.2.C494. [DOI] [PubMed] [Google Scholar]
- Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994 Jun;43(2):175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Two phosphatase sites on the Ca2+ channel affecting different kinetic functions. J Physiol. 1993 Oct;470:73–84. doi: 10.1113/jphysiol.1993.sp019848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Hartzell H. C. Regulation of Ca2+ current in frog ventricular cardiomyocytes by guanosine 5'-triphosphate analogues and isoproterenol. J Gen Physiol. 1993 Sep;102(3):525–549. doi: 10.1085/jgp.102.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Wiechen K., Yue D. T., Herzig S. Two distinct functional effects of protein phosphatase inhibitors on guinea-pig cardiac L-type Ca2+ channels. J Physiol. 1995 May 1;484(Pt 3):583–592. doi: 10.1113/jphysiol.1995.sp020688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Terzic A., Kurachi Y. Regulation of potassium channels by G-protein subunits and arachidonic acid metabolites. Methods Enzymol. 1994;238:394–422. doi: 10.1016/0076-6879(94)38036-8. [DOI] [PubMed] [Google Scholar]



