Abstract
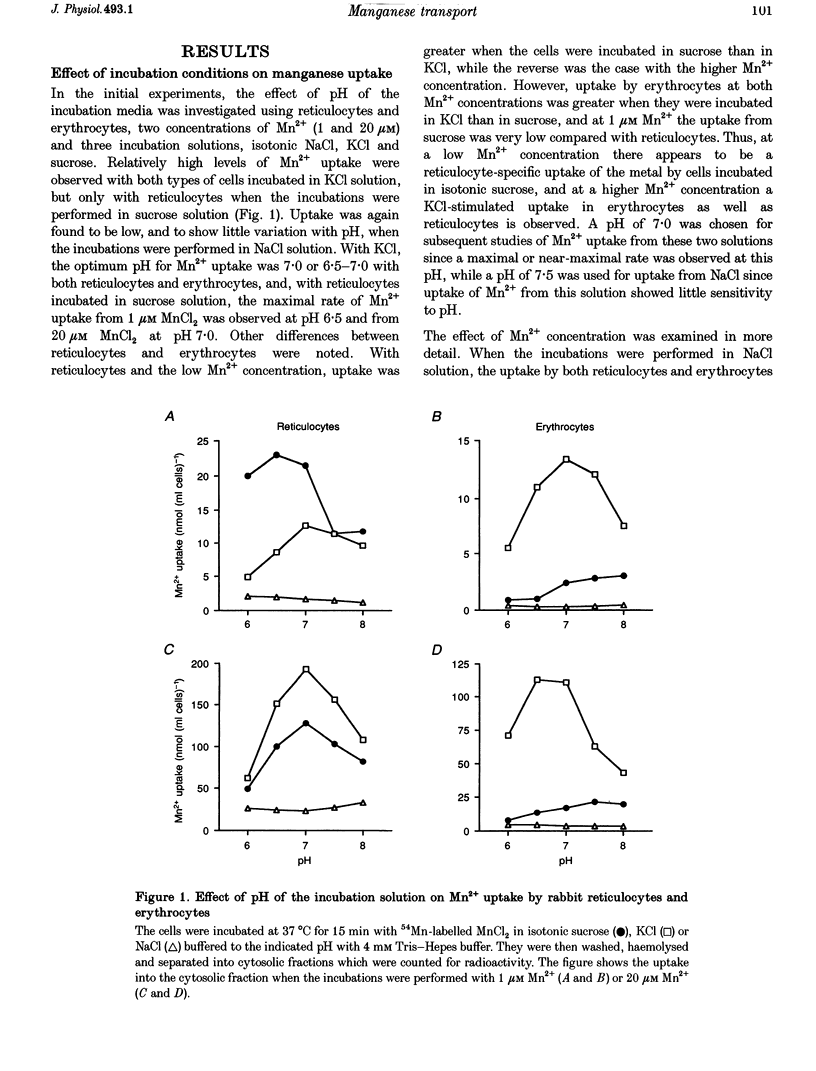
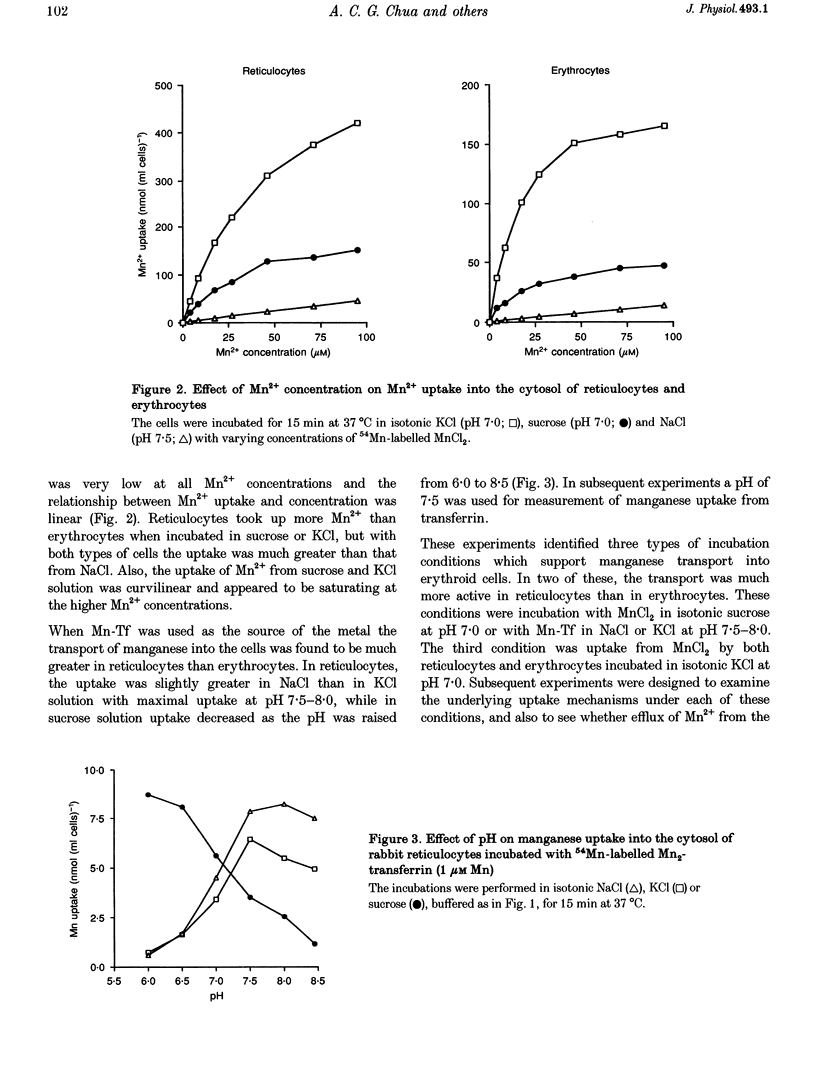
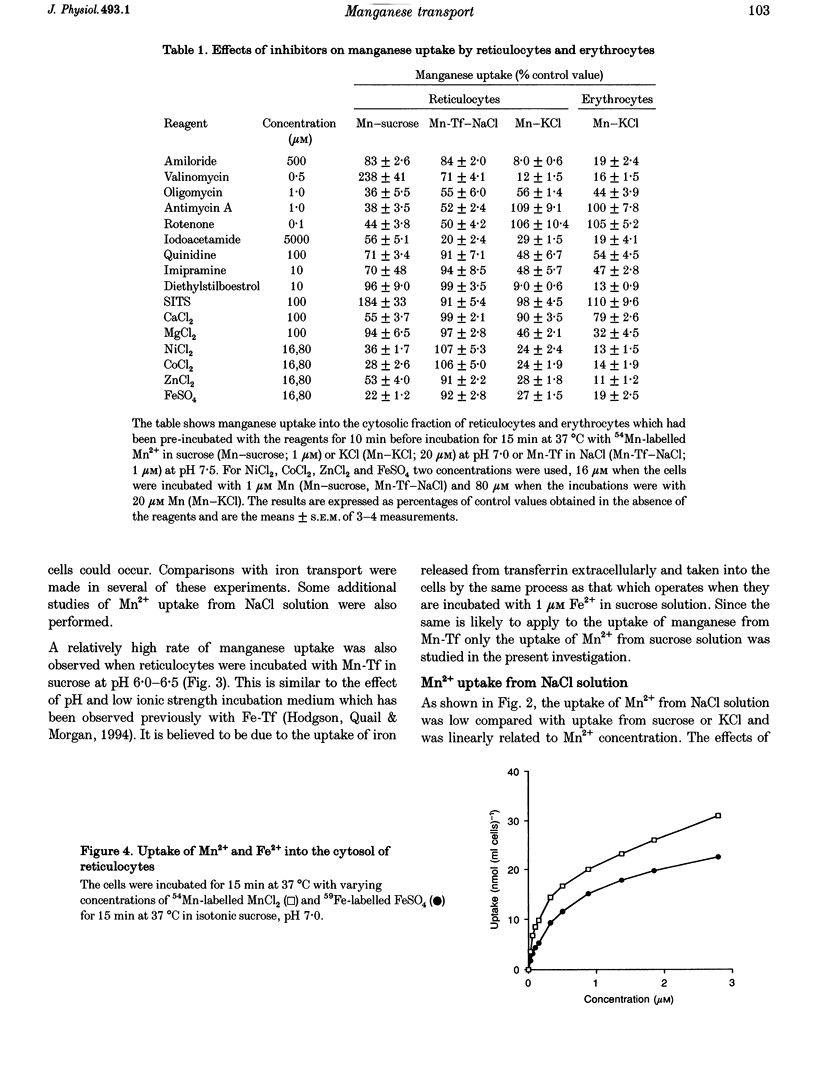
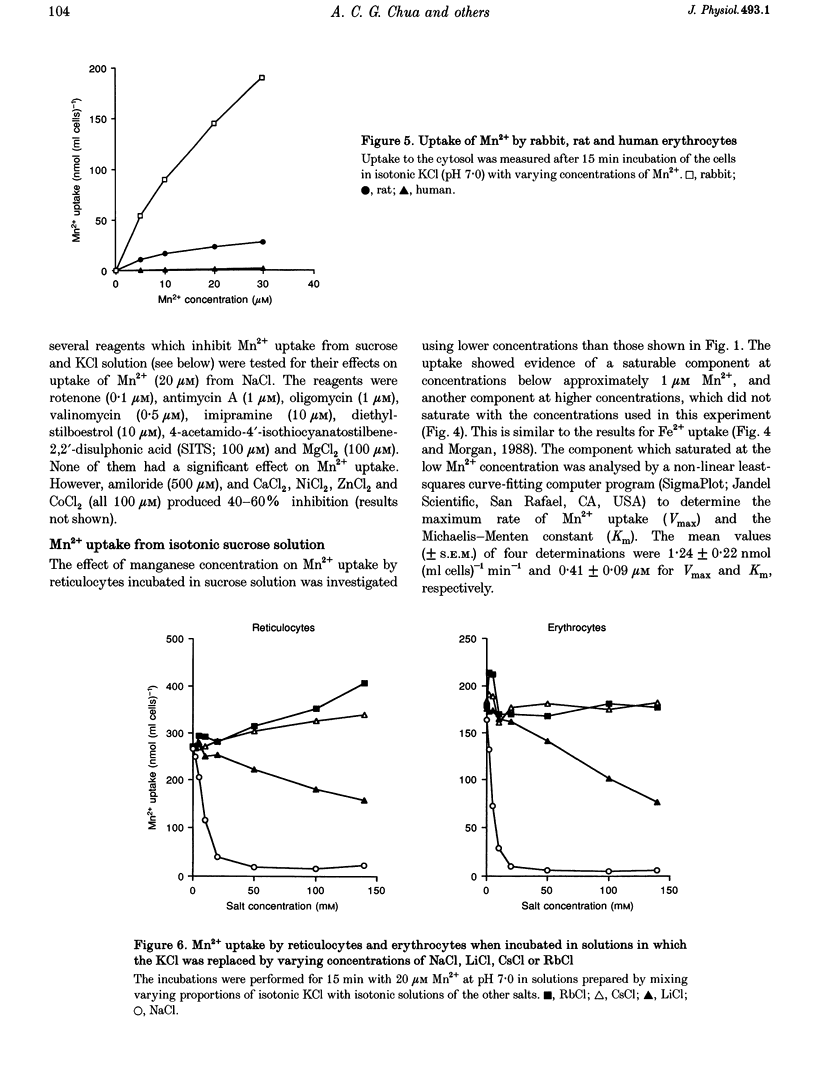
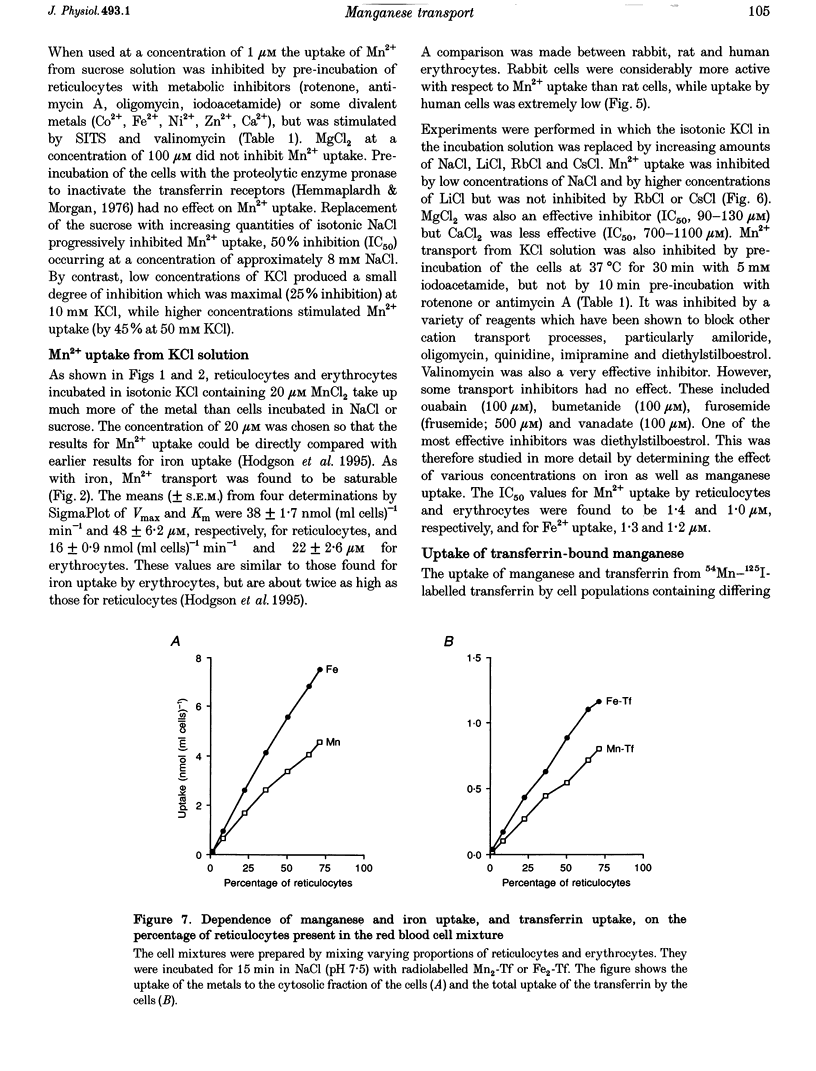
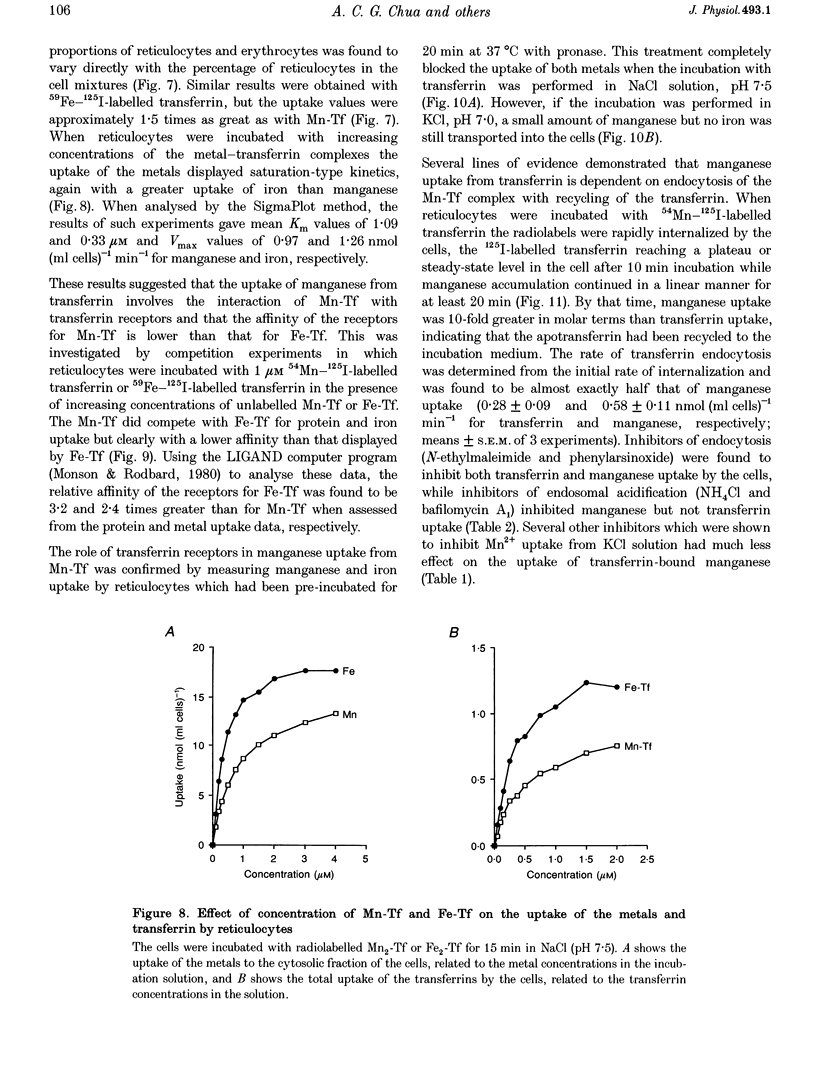
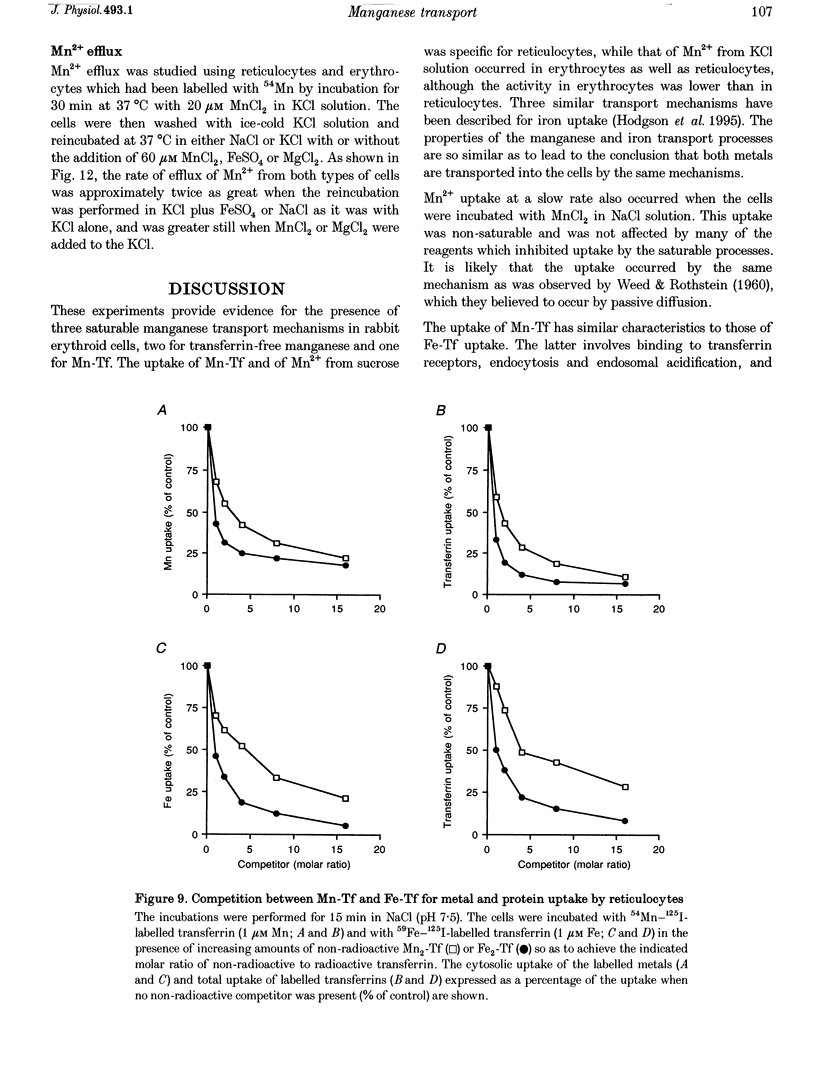
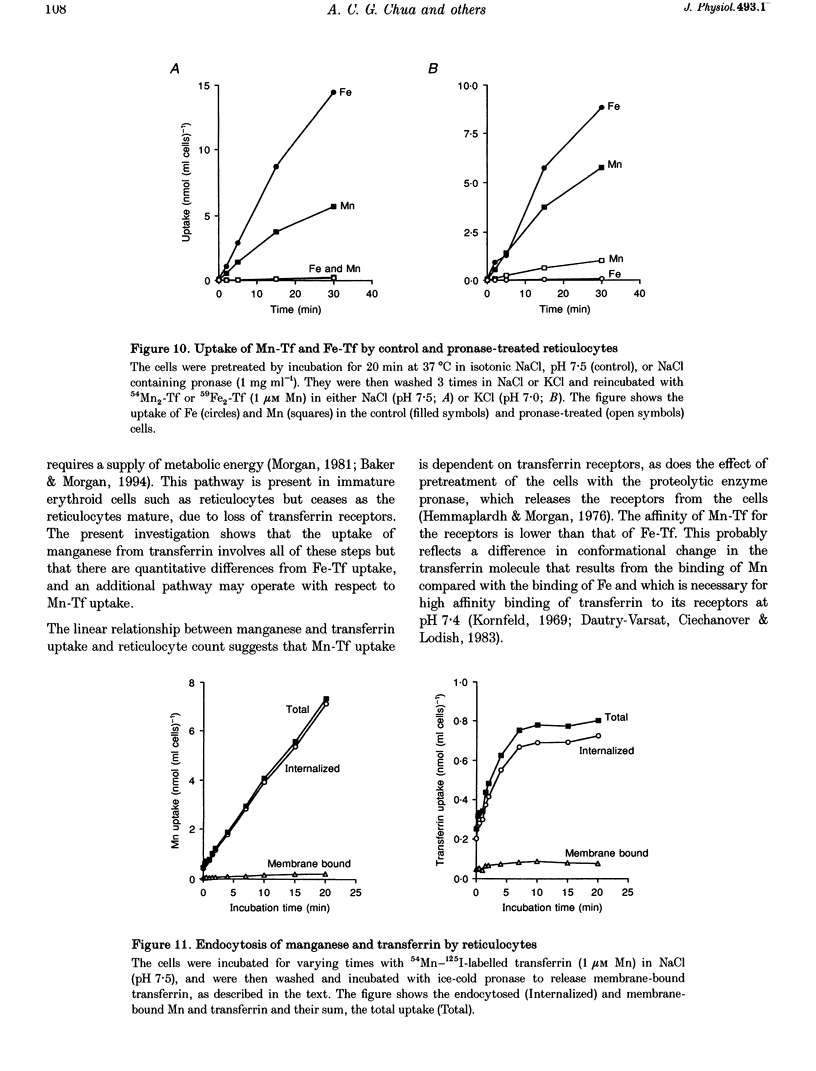
1. The mechanisms of manganese transport into erythroid cells were investigated using rabbit reticulocytes and mature erythrocytes and 54Mn-labelled MnCl2 and Mn2-transferrin. In some experiments iron uptake was also studied. 2. Three saturable manganese transport mechanisms were identified, two for Mn2+ (high and low affinity processes) and one for transferrin-bound manganese (Mn-Tf). 3. High affinity Mn2+ transport occurred in reticulocytes but not erythrocytes, was active only in low ionic strength media such as isotonic sucrose and had a Km of 0.4 microM. It was inhibited by metabolic inhibitors and several metal ions. 4. Low affinity Mn2+ transport occurred in erythrocytes as well as in reticulocytes and had Km values of approximately 20 and 50 microM for the two types of cells, respectively. The rate of Mn2+ transport was maximal in isotonic KCl, RbCl or CsCl, and was inhibited by NaCl and by amiloride, valinomycin, diethylstilboestrol and other ion transport inhibitors. The direction of Mn2+ transport was reversible, resulting in Mn2+ efflux from the cells. 5. The uptake of transferrin-bound manganese occurred only with reticulocytes and depended on receptor-mediated endocytosis of Mn-Tf. 6. The characteristics of the three saturable manganese transport mechanisms were similar to corresponding mechanisms of iron uptake by erythroid cells, suggesting that the two metals are transported by the same mechanisms. 7. It is proposed that high affinity manganese transport is a surface representation of the process responsible for the transport of manganese across the endosomal membrane after its release from transferrin. Low affinity transport probably occurs by the previously described Na(+)-Mg2+ antiport, and may function in the regulation of intracellular manganese concentration by exporting manganese from the cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Aasa R., Redfield A. G. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969 Sep 10;244(17):4628–4633. [PubMed] [Google Scholar]
- Baker E., Shaw D. C., Morgan E. H. Isolation and characterization of rabbit serum and milk transferrins. Evidence for difference in sialic acid content only. Biochemistry. 1968 Apr;7(4):1371–1378. doi: 10.1021/bi00844a019. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsson L., Lönnerdal B., Sandström B., Kunz C., Keen C. L. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J Nutr. 1989 Oct;119(10):1461–1464. doi: 10.1093/jn/119.10.1461. [DOI] [PubMed] [Google Scholar]
- Davson H. Studies on the permeability of erythrocytes: The effect of reducing the salt content of the medium surrounding the cell. Biochem J. 1939 Mar;33(3):389–401. doi: 10.1042/bj0330389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Smith L. M. Magnesium transport in ferret red cells. J Physiol. 1990 Dec;431:11–25. doi: 10.1113/jphysiol.1990.sp018318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féray J. C., Garay R. A one-to-one Mg2+:Mn2+ exchange in rat erythrocytes. J Biol Chem. 1987 Apr 25;262(12):5763–5768. [PubMed] [Google Scholar]
- Günther T. Mechanisms and regulation of Mg2+ efflux and Mg2+ influx. Miner Electrolyte Metab. 1993;19(4-5):259–265. [PubMed] [Google Scholar]
- Günther T., Vormann J., Cragoe E. J., Jr Species-specific Mn2+/Mg2+ antiport from Mg2(+)-loaded erythrocytes. FEBS Lett. 1990 Feb 12;261(1):47–51. doi: 10.1016/0014-5793(90)80633-t. [DOI] [PubMed] [Google Scholar]
- Günther T., Vormann J. Reversibility of Na+/Mg2+ antiport in rat erythrocytes. Biochim Biophys Acta. 1995 Mar 8;1234(1):105–110. doi: 10.1016/0005-2736(94)00267-s. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The mechanism of iron exchange between synthetic iron chelators and rabbit reticulocytes. Biochim Biophys Acta. 1974 Nov 27;373(1):84–99. doi: 10.1016/0005-2736(74)90108-4. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. Transferrin uptake and release by reticulocytes treated with proteolytic enzymes and neuraminidase. Biochim Biophys Acta. 1976 Mar 19;426(3):385–398. doi: 10.1016/0005-2736(76)90384-9. [DOI] [PubMed] [Google Scholar]
- Hodgson L. L., Quail E. A., Morgan E. H. Iron transport mechanisms in reticulocytes and mature erythrocytes. J Cell Physiol. 1995 Feb;162(2):181–190. doi: 10.1002/jcp.1041620204. [DOI] [PubMed] [Google Scholar]
- Hodgson L. L., Quail E. A., Morgan E. H. Receptor-independent uptake of transferrin-bound iron by reticulocytes. Arch Biochem Biophys. 1994 Jan;308(1):318–326. doi: 10.1006/abbi.1994.1045. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Morgan E. H. The kinetics of transferrin endocytosis and iron uptake from transferrin in rabbit reticulocytes. J Biol Chem. 1983 Aug 10;258(15):9108–9115. [PubMed] [Google Scholar]
- Jan K. M., Chien S. Influence of the ionic composition of fluid medium on red cell aggregation. J Gen Physiol. 1973 May;61(5):655–668. doi: 10.1085/jgp.61.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfakakou V., Simons T. J. Anionic mechanisms of zinc uptake across the human red cell membrane. J Physiol. 1990 Feb;421:485–497. doi: 10.1113/jphysiol.1990.sp017957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer R. C., Barak A. J., Boyett J. D. Binding of manganese and transferrin in rat serum. Biochim Biophys Acta. 1970 Nov 17;221(2):390–393. doi: 10.1016/0005-2795(70)90284-9. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. The effect of metal attachment to human apotransferrin on its binding to reticulocytes. Biochim Biophys Acta. 1969 Nov 11;194(1):25–33. doi: 10.1016/0005-2795(69)90175-5. [DOI] [PubMed] [Google Scholar]
- Morgan E. H. Membrane transport of non-transferrin-bound iron by reticulocytes. Biochim Biophys Acta. 1988 Sep 1;943(3):428–439. doi: 10.1016/0005-2736(88)90374-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Qian Z. M., Morgan E. H. Changes in the uptake of transferrin-free and transferrin-bound iron during reticulocyte maturation in vivo and in vitro. Biochim Biophys Acta. 1992 Apr 30;1135(1):35–43. doi: 10.1016/0167-4889(92)90163-6. [DOI] [PubMed] [Google Scholar]
- Quail E. A., Morgan E. H. Role of membrane surface potential and other factors in the uptake of non-transferrin-bound iron by reticulocytes. J Cell Physiol. 1994 May;159(2):238–244. doi: 10.1002/jcp.1041590207. [DOI] [PubMed] [Google Scholar]
- Rossander-Hultén L., Brune M., Sandström B., Lönnerdal B., Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991 Jul;54(1):152–156. doi: 10.1093/ajcn/54.1.152. [DOI] [PubMed] [Google Scholar]
- Scheuhammer A. M., Cherian M. G. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985 Jun 18;840(2):163–169. doi: 10.1016/0304-4165(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Singh A. K., Kasinath B. S., Lewis E. J. Interaction of polycations with cell-surface negative charges of epithelial cells. Biochim Biophys Acta. 1992 Apr 17;1120(3):337–342. doi: 10.1016/0167-4838(92)90257-e. [DOI] [PubMed] [Google Scholar]
- Suárez N., Eriksson H. Receptor-mediated endocytosis of a manganese complex of transferrin into neuroblastoma (SHSY5Y) cells in culture. J Neurochem. 1993 Jul;61(1):127–131. doi: 10.1111/j.1471-4159.1993.tb03546.x. [DOI] [PubMed] [Google Scholar]
- Tan A. T., Woodworth R. C. Ultraviolet difference spectrl studies of conalbumin complexes with transition metal ions. Biochemistry. 1969 Sep;8(9):3711–3716. doi: 10.1021/bi00837a033. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Olatunbosun D., Valverg L. S. Interrelation of intestinal transport system for manganese and iron. J Lab Clin Med. 1971 Oct;78(4):642–655. [PubMed] [Google Scholar]
- Torrubia J. O., Garay R. Evidence for a major route for zinc uptake in human red blood cells: [Zn(HCO3)2Cl]- influx through the [Cl-/HCO3-] anion exchanger. J Cell Physiol. 1989 Feb;138(2):316–322. doi: 10.1002/jcp.1041380214. [DOI] [PubMed] [Google Scholar]
- WEED R. I., ROTHSTEIN A. The uptake of divalent manganese ion by mature normal human red blood cells. J Gen Physiol. 1960 Nov;44:301–314. doi: 10.1085/jgp.44.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


