Abstract
OBJECTIVE:
To investigate the mechanism of Bushen Huoxue decoction (补肾活血汤, BSHXD) to treat endometriosis-induced infertility.
MEDHODS:
The main compounds of BSHXD were determined by high performance liquid chromatography-mass spectrometry (HPLC-MS/MS). The effect of BSHXD on Homeobox A10 (HOXA10) and alpha(v)beta(3) (αvβ3) integrin expression of Ishikawa cells, mouse model, and endometriosis-associated infertility women was evaluated by using Western blot analysis, immunohistochemistry and Real-Time quantitative polymerase chain reaction (RT-qPCR). The efficacy of BSHXD on embryo attachment were examined by using the BeWo spheroid and mouse embryo attachment assay. HOXA10 concentration in uterine flushing fluid of endometriosis-associated infertility women treated with BSHXD was measured by Enzyme-Linked immunosorbent assay (ELISA).
RESULTS:
BSHXD improved BeWo spheroid and mice blastocysts attachment to Ishikawa cells and increased embryo implantation rates in mice and pregnancy rates in women with endometriosis-associated infertility. BSHXD enhanced HOXA10 and αvβ3 integrin expression in Ishikawa cell, endometriosis mouse model, and endometriosis-associated infertility women, which potentially improved endometrial receptivity.
CONCLUSIONS:
BSHXD could improve endometrial receptivity of endometriosis-associated infertility in a dose-dependent manner by regulating HOXA10 and αvβ3 integrin expression.
Keywords: infertility, endometriosis, homeobox A10 proteins, integrin alphaVbeta3, Bushen Huoxue decoction
1. INTRODUCTION
Endometriosis is defined as a condition in which functional endometrial glands and stroma appear in ectopic locations.1 About 6%-10% of endometriosis occur at reproductive age and 35%-50% of the patients were accompanied with condition of serious reproductive consequences.2 In the setting of in vitro fertilization (IVF-ET) cycle, even though donor oocytes were obtained from women without endometriosis, the implantation rate and clinical pregnancy rate are significantly lower.3 Endometrial receptivity is a short “window of implantation” period, during which the endometrium is able to support blastocyst implantation. Endometrial receptivity is critical for female reproduction and is affected by some commonly encountered abnormalities including endometriosis.4,5 In addition, a number of key regulators involved in implantation, such as Homeobox A10 (HOXA10), Homeobox A11 (HOXA11), alpha(v)beta(3) (αvβ3) integrin, leukemia inhibitory factor (LIF) and histone deacetylase 3 (HDAC3), are dysregulated in endometriosis.6 Recently, we reported that aberrantly low interleukin 6 (IL-6) and LIF expression in the endometrium and uterine flushing fluid from endometriosis-associated infertility patients resulted in decreased endometrial receptivity, which could be increased by Bushen Huoxue decoction (补肾活血汤, BSHXD).7 In the present study, we used high performance liquid chromatography-mass spectrometry (HPLC-MS/ MS) to identify the main compounds of BSHXD. We also showed that BSHXD upregulated HOXA10 and αvβ3 integrin expression not only in vitro but also in vivo experiments. Our study revealed that an increase in HOXA10 and αvβ3 integrin expression by BSHXD could improve reproduction of endometriosis-associated infertility.
2. MATERIALS AND METHODS
2.1. Formula preparation and composition analysis
BSHXD is composed of eight herbs: Tusizi (Semen Cuscutae) 10 g, Roucongrong (Herba Cistanches Deserticolae) 10 g, Danggui (Radix Angelicae Sinensis) 10 g, Chuanxiong (Rhizoma Chuanxiong) 6 g, Shanyao (Rhizoma Dioscoreae Oppositae) 15 g, Chaihu (Radix Bupleuri Chinensis) 6 g, Baishao (Radix Paeoniae Alba) 15 g, and Dihuang (Radix Rehmanniae) 10 g. All species of medicinal plants used in the formula were provided by Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine. The decoction was made by boiling herbs, and then filtrated. The Traditional Chinese Medicine decoction was commonly heated for 2 h at 100 ℃ to help ingredients dissolved. BSHXD administered to mice was prepared in the same way as for patients.
The BSHXD was mixed with 70% methanol with internal standard extraction solution by a vortex. The sample was centrifuged at 12000 rpm for 15 min at 4 ℃, and the supernatant was taken out and filtered through a 0.22 μm microporous membrane. Then, the supernatant was obtained and subjected to HPLC-MS/MS analyses (UPLC ExionLCTM AD) by Nanjing Changfeng Biotechnology Co., Ltd. (Nanjing, China).
2.2. Medicated serum preparation
Sprague-Dawley (SD) 2-months-old females rats of specific pathogen free (SPF) grade weighing 180-200 g were administered with BSHXD (3 g/mL) and used for preparation of drug-containing serum. The rats were supplied by Comparative Medical Center of Yangzhou University (Certificate for quality No: SYXK[su]2021-0025). All animal experiments were performed using Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine ethics committee approved protocol. The serum was obtained via centrifugation of the SD rats blood at 3000 r/min for 10 min. Then inactivated in 56 ℃ water for 30 min, filtrated with a 0.22 µm cellulose acetate membrane and stored at −80 ℃ for use.
2.3. Patients and sample collection
Endometrial biopsies and uterine flushing fluid for this study were obtained from women with endometriosis-associated infertility, who were admitted to the Center for Reproductive Medicine of Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine. The informed consent was obtained from all patients and this study was approved by the ethics committee. The endometriosis-associated infertility patients were confirmed by combined hysteroscopy and laparoscopy. The primary syndromes were kidney deficiency and blood stasis syndrome.
The mid-secretory endometrial and uterine flushing fluid were obtained from twenty endometriosis-associated infertility women before and after BSHXD administration for three menstrual cycles. The follicle stimulating hormone (FSH) of all patients on day 3 of the menstrual cycle were less than 10 mIU/mL.
2.4. Animal studies
To establish BALB/c endometriosis mouse model, 21 6-week-old female BALB/c mice of SPF grade, weighing 18-22 g, were used as donor and the other 42 female BALB/c mice as the recipients. The donor’s uterus was dissected into 1 mm3 pieces and implanted into the abdominal cavity of recipients. After a recovery period of 21 d, 7 recipient mice were chosen randomly to check endometrial implants and the lesions were processed for histological examination. The 35 recipient mice were randomized for treatment by random number table method. The control group (sham surgery group) and the model group received vehicle control. The treatment group received dydrogesterone (3.03 mg·kg-1·d-1), low dose BSHXD (6.218 g·kg-1·d-1), normal dose BSHXD (12.437 g·kg-1·d-1) or high dose BSHXD (24.873 g·kg-1·d-1). The normal dose of dydrogesteroneis and BSHXD for an adult weighing 60 kg is 20 mg/d and 82 g/d respectively. The normal dose of dydrogesteroneis and BSHXD for mice was calculated according to the conventional method as described previously.8 The mice were monitored daily for adverse toxic effects and body weight was measured every week. After 28-day treatment, the mice were mated, with a ratio of female to male at 2 : 1. Pregnant mice were separated from non-pregnant mice. On the 14th day of gestation, the pregnant mice were euthanized, the total number of embryos was calculated, and the endometrium was removed and processed for histological examination, protein and RNA analysis.
2.5. Cell culture and reagents
Ishikawa cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. For the experiments, cells were divided into five groups as follows: Control group, Dydrogesterone group (10 nmol/L), the BSHXD low dose group (treated with 5% BSHXD serum), the BSHXD normal dose group (treated with 10% BSHXD serum), and the BSHXD high dose group (treated with 20% BSHXD serum).
Antibody for αvβ3 integrin was purchased from Santa Cruz Biotechnology (sc-7312, Dallas, TX, USA). HOXA10 antibody was purchased from Abcam (ab191470, Cambridge, UK). GAPHD antibody was purchased from Proteintech (60004-1-Ig, Rosemont, IL, USA). Dydrogesterone was purchased from Abbott Healthcare Products B.V (Olst, Netherlands).
2.6. BeWo spheroid attachment assay
BeWo single-cell suspensions were transferred to an ultra-low attachment multiple well plate (Sigma, St. Louis, MO, USA) to obtain uniform-sized (150-200 μm in diameter) BeWo spheroids. Simultaneously, Ishikawa confluent monolayers were respectively cultured in presence of 10 nmol/l Dydrogesterone, 5% BSHXD serum, 10% BSHXD serum or 20% BSHXD serum in 24-well plate for 12 h. Fifty BeWo spheroids were transferred onto Ishikawa confluent monolayer and incubated together for 1 h. Then the plate was shaken at 180 rpm for 1 min to remove non-attached BeWo spheroids and the attached spheroids were counted. The attachment rate was calculated as a percentage of the total number of BeWo spheroids (% adhesion).9
2.7. Mouse embryo attachment assay
Sexually mature female Institute of Cancer Research mice at 8 weeks of SPF grade, weighing 18-22 g, were injected with 5 IU pregnant mare serum gonadotropin (PMSG, MSD Animal Health Hub, Rahway, NJ, USA) at 1 pm. After 48 h, mice were injected with 10 IU hCG (Livzon Pharmaceutical Group, Zhuhai, China) at 1 pm and one male and two females were housed together for mating at 6 pm. At day 3.5 post-coitum, mice were euthanized, the blastocysts were harvested and transferred onto Ishikawa confluent monolayer treating as same as BeWo spheroid attachment assay and incubated together for 24 h. The embryo attachment stability was measured by a standardized plate movement protocol.
2.8. Western blot analysis and immunohistochemistry
Western blot analysis was performed as described previously.10,11 Briefly, Whole cell and tissue lysates were prepared using RIPA buffer containing protease and phosphatase inhibitors. Total proteins (50 μg) were mixed with 4×sodium dodecyl sulfate (SDS) sample buffer and subjected to SDS-polyacrylamide gel eletrophoresis, and transferred onto nitrocellulose membranes. Blots were incubated with primary antibodies against HOXA10, αvβ3 integrin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), followed by goat anti-rabbit secondary antibody conjugated with HRP. Blots were developed using the electrochemiluminescence kit (Thermo Fisher Scientific, Waltham, MA, USA).
For immunohistochemistry, tissue sections were blocked in 5% normal goat serum followed by incubation with primary antibodies against HOXA10, αvβ3 integrin overnight at 4 ℃ and subsequent secondary antibody incubation for 30 min at room temperature. Immunoreactivity was visualized using diaminobenzidine substrate and counterstained with hematoxylin (Vector Laboratories Inc. Burlingame, CA, USA). Percent of positive cells was calculated in five randomly selected microscopic fields.
2.9. RT-qPCR
Reverse transcription (RT) reactions were performed by using HiScriptⅡ Q RT SuperMix for quantitative polymerase chain reaction (qPCR, vazyme, Nanjing, China), according to the manufacturer’s protocol. Real-time PCR was done using SYBR Green reagents (Takara, Tokyo, Japan) on an Illumina Real-Time PCR system. Primer sequences of the genes used were described previously and listed as below: HOXA10 (F: 5′-GGG TAA GCG GAA TAA ACT-3′ R: 5′-GCA CAG CAG CAA TAC AAT A-3′), ITGB3 (F: 5′-GGT TTC ACT TTC ATC GTC CAG -3′ R: 5′-AAT TAG CCA GGC GTC GTG -3′), GAPDH (F: 5′-GAC CTG ACC TGC CGT CTA-3′ R: 5′-AGG AGT GGG TGT CGC TGT-3′), Hoxa10 (F: 5’-CAG CCC CTT CAG AAA ACA GTA AA-3’ R: 5’-TTC TTC CGG CCG CTC TTT-3’), Itgb3 (F: 5’-AGT GGC CGG GAC AAC TCT G-3’ R: 5’-GGA CTC TCC AAC AAC AAC GC-3’), and Gapdh (F: 5’-GTA TGA CTC CAC TCA CGG CAA A-3’ R: 5’-GGT CTC GCT CCT GGA AGA TG-3’).10
2.10. Enzyme-linked immunosorbent assay (ELISA)
HOXA10 concentration in uterine flushing fluid of endometriosis-associated infertility women was using ELISA (SenBeiJia Bio, Nanjing, China) according to the manufacturer’s protocol.
2.11. Subjects
The study was approved by the Ethics Committee of Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing (No. 2018LWYJ068). Informed consent was obtained from all individual participants included in the study.
2.12. Statistical analysis
All the data represented in plots were shown as mean ± standard deviation. Statistical differences between groups were analyzed by using GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). A P value less than 0.05 was considered statistically significant. All assays in vitro were performed in triplicate and repeated at least three times.
3. RESULTS
3.1. Chemical compositions of BSHXD
The multiple reaction monitoring mode (MRM) metabolite detection multi peak diagram of HPLC showed the substances that can be detected in the BSHXD. The peak area of each chromatographic peak represented the relative content of the corresponding substance. The main components were shown in Table 1.
Table 1.
Main components of Bushen Huoxue decotion identified by high performance liquid chromatography-mass spectrometry (HPLC-MS/MS)
| Num | Compound | RT (min) |
Molecular weight (Da) | Formula | Ionization model | Class I | Class II |
|---|---|---|---|---|---|---|---|
| 1 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | 2.79 | 3.54E+02 | C16H18O9 | [M-H]- | Phenolic acids | Phenolic acids |
| 2 | N-Feruloylagmatine | 2.91 | 3.06E+02 | C15H22N4O3 | [M+H]+ | Alkaloids | Phenolamine |
| 3 | 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | 2.58 | 1.54E+02 | C7H6O4 | [M-H]- | Phenolic acids | Phenolic acids |
| 4 | Protocatechualdehyde | 3.12 | 1.38E+02 | C7H6O3 | [M-H]- | Others | Aldehyde compounds |
| 5 | Luteolin-4'-O-glucoside | 4.06 | 4.48E+02 | C21H20O11 | [M+H]+ | Flavonoids | Flavones |
| 6 | Brevifolin carboxylic acid | 3.05 | 2.92E+02 | C13H8O8 | [M-H]- | Phenolic acids | Phenolic acids |
| 7 | Gallic acid | 1.81 | 1.70E+02 | C7H6O5 | [M-H]- | Phenolic acids | Phenolic acids |
| 8 | Paeonilactone C | 4.34 | 3.18E+02 | C17H18O6 | [M+H]+ | Terpenoids | Monoterpenoids |
| 9 | Quercetin-5-O-β-D-glucoside | 3.82 | 4.64E+02 | C21H20O12 | [M+H]+ | Flavonoids | Flavonols |
| 10 | Quercetin-3-O-glucoside (Isoquercitrin) | 3.82 | 4.64E+02 | C21H20O12 | [M+H]+ | Flavonoids | Flavonols |
| 11 | Cistanoside A | 3.48 | 8.00E+02 | C36H48O20 | [M-H]- | Phenolic acids | Phenolic acids |
| 12 | Luteolin-7-O-glucoside (Cynaroside) | 4.09 | 4.48E+02 | C21H20O11 | [M+H]+ | Flavonoids | Flavones |
| 13 | Kaempferol-4'-O-glucoside | 4.05 | 4.48E+02 | C21H20O11 | [M+H]+ | Flavonoids | Flavonols |
| 14 | Quercetin-3-O-galactoside (Hyperin) | 3.82 | 4.64E+02 | C21H20O12 | [M+H]+ | Flavonoids | Flavonols |
| 15 | Saikosaponin A | 6.37 | 7.80E+02 | C42H68O13 | [M-H]- | Terpenoids | Triterpene Saponin |
| 16 | Isoferulic Acid | 4.03 | 1.94E+02 | C10H10O4 | [M-H]- | Phenolic acids | Phenolic acids |
| 17 | Echinacoside | 3.19 | 7.86E+02 | C35H46O20 | [M-H]- | Phenolic acids | Phenolic acids |
| 18 | Saikogenin Q | 5.54 | 4.88E+02 | C30H48O5 | [M+H]+ | Terpenoids | Triterpene |
| 19 | Calycosin-7-O-glucoside | 3.56 | 4.46E+02 | C22H22O10 | [M+H]+ | Flavonoids | Isoflavones |
| 20 | Catalpol | 1.19 | 3.62E+02 | C15H22O10 | [M-H]- | Terpenoids | Monoterpenoids |
Notes: Num.: number; Compounds: compound name; RT: retention time; Class: classification of compounds; Ionization model: M+H is positive charged, M-H is negative charged.
3.2. BSHXD increases pregnancy rate in women with endometriosis-associated infertility
We evaluated the functionality of BSHXD in women with endometriosis-associated infertility. The time for the patients trying to conceive was at least 1 year for infertile women with endometriosis. According to the principle of informed consent, the patients were divided into the treatment group and control group. Early follicular phase FSH level and age were similar between the two groups. The patients in treatment group were administered with BSHXD continuously for three menstrual cycles. BSHXD treatment significantly increased the pregnancy rates [55% (11/20) vs 20% (4/20)] in women with endometriosis-associated infertility during six-month follow-up.
3.3. BSHXD improves embryo attachment in vitro and promoted pregnancy rates in vivo
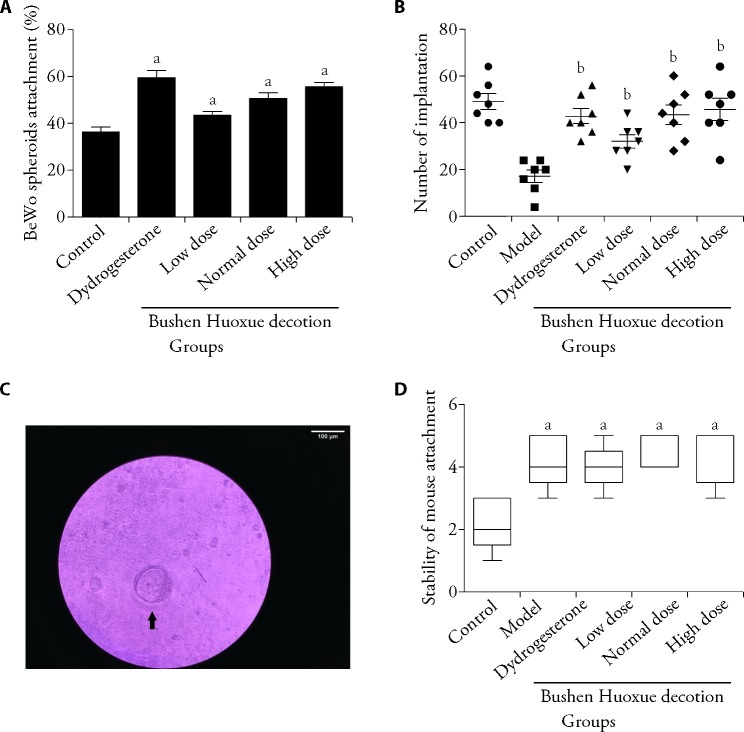
To determine whether BSHXD can improve embryo attachment, monolayer confluent Ishikawa cells were co-cultured with BeWo spheroids. In Figure 1A, it was shown that BSHXD increased BeWo spheroid attachment in a dose-dependent manner. To further confirm the effect of BSHXD on embryo attachment, the mouse embryo attachment assay was used. Embryos transferred onto BSHXD treated Ishikawa cells had higher attachment score (Figures 1C, 1D). These data suggest that BSHXD promote embryo attachment.
Figure 1. BSHXD promotes embryo attachment in vitro and in vivo.
A: adhesion experiments with BeWo spheroids attached to Ishikawa cell monolayers. The attached spheroids were counted and the attachment rate was calculated as a percentage of the total number of BeWo spheroids (% adhesion). Data were presented as mean ± standard deviation (n = 4). B: endometriosis mouse models were treated with dydrogesterone and different dose BSHXD. After 28 d treatment, the mice were mated. The pregnant mice were euthanized on the 14th day of gestation and total number of embryos was calculated. Statistical analysis was carried out using ANOVA tests among these groups. Data were presented as mean ± standard deviation (n = 7) C: Mouse embryo (black arrow) attached to Ishikawa cells (× 100). D: Adhesion experiments of mouse embryos attached to the Ishikawa cell monolayer. The attachment score of each group was described as median with the interquartile range (n = 5). In Figures A and D: Control group: Ishikawa cell cultured in basal medium; Dydrogesterone group: Ishikawa cell cultured in presence of 10 nmol/L dydrogesterone; Low dose BSHXD group: Ishikawa cell cultured in presence of 5% BSHXD serum; Normal dose BSHXD group: Ishikawa cell cultured in presence of 10% BSHXD serum; High dose BSHXD group: Ishikawa cell cultured in presence of 20% BSHXD serum. In Figure B: Control group: sham surgery mice were treated with water; Model group: endometriosis mouse models were treated with water; Dydrogesterone group: endometriosis mouse models were treated with dydrogesterone (3.03 mg·kg-1·d-1); Low dose BSHXD group: endometriosis mouse models were treated with low dose BSHXD (6.218 g·kg-1·d-1); Normal dose BSHXD group: endometriosis mouse models were treated with normal dose BSHXD (12.437 g·kg-1·d-1); High dose BSHXD group: endometriosis mouse models were treated with high dose BSHXD (24.873 g·kg-1·d-1). BSHXD: Bushen Huoxue decotion; ANOVA: analysis of variance. Statistical analysis was carried out using Kruskal-Wallis with Dunn’s multiple comparison tests among these groups. Compared with the Control group, aP < 0.05; compared with the Model group, bP < 0.01.
An in vivo mouse endometriosis pregnancy model showed that BSHXD as well as dydrogesterone significantly increased embryo implantation rates (Figure 1B).
3.4. BSHXD increases HOXA10, αvβ3 integrin expression in Ishikawa cells
As shown in Figure 2A, HOXA10 and ITGB3 mRNA expression were increased significantly in Ishikawa cells after treated with BSHXD as well as dydrogesterone. In addition, the increase of HOXA10 and ITGB3 expression after treated with BSHXD displayed a dose dependent manner. Similarly, as shown in Figures 2B-2D, the protein level of HOXA10 and αvβ3 integrin was also observed to be increased significantly by treatment of BSHXD.
Figure 2. BSHXD induces HOXA10 and αvβ3 integrin expression in Ishikawa cells.
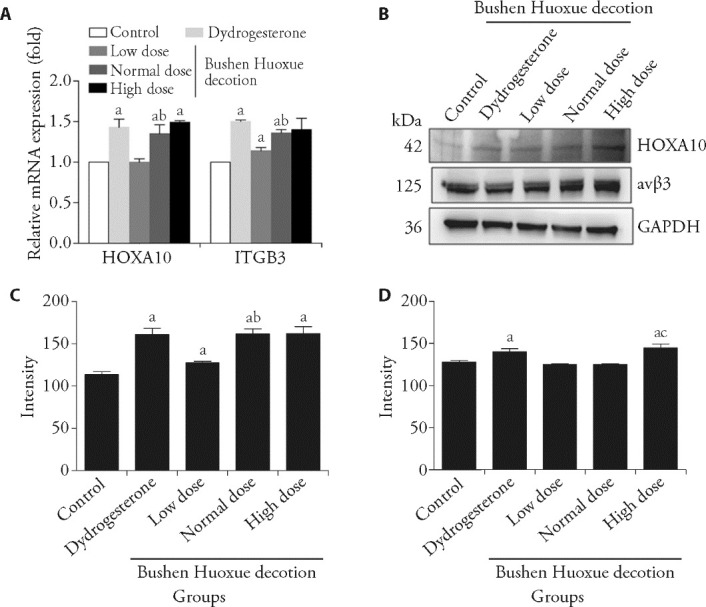
A: Ishikawa cells were treated with Dydrogesterone and different dose BSHXD serum for 24 h and the level of HOXA10 and ITGB3 were detected using RT-qPCR; B: Expression of HOXA10 and αvβ3 integrin were detected by western blotting; C: HOXA10 expression of western blotting was quantitated by using Image J software; D: αvβ3 integrin expression of western blotting was quantitated. Control group: Ishikawa cell cultured in basal medium; Dydrogesterone group: Ishikawa cell cultured in presence of 10 nmol/L dydrogesterone; Low dose BSHXD group: Ishikawa cell cultured in presence of 5% BSHXD serum; Normal dose BSHXD group: Ishikawa cell cultured in presence of 10% BSHXD serum; High dose BSHXD group: Ishikawa cell cultured in presence of 20% BSHXD serum. BSHXD: Bushen Huoxue decotion; HOXA10: Homeobox A10; αvβ3: alpha(v)beta(3) integrin; ITGB3: integrin beta(3); RT-qPCR: real-time quantitative polymerase chain reaction; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ANOVA: analysis of variance. ANOVA tests were used among these groups. Data were presented as mean ± standard deviation (n = 3). Compared with the Control group, aP < 0.05; compared with the Low dose group, bP < 0.05; compared with the Nomal dose dose group, cP < 0.05.
3.5. BSHXD increases HOXA10 and αvβ3 integrin expression in vivo
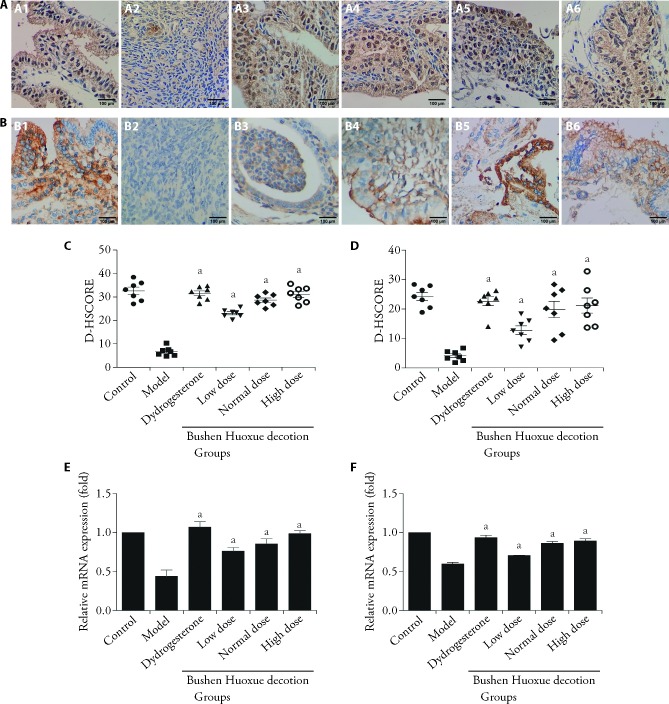
We also analyzed the expression of HOXA10 and αvβ3 integrin in the mouse model’s endometrium. It was shown that BSHXD treatment substantially increased HOXA10 and αvβ3 integrin expression (Figure 3).
Figure 3. BSHXD increases HOXA10 and αvβ3 integrin expression in endometriosis mouse model.
A: endometriosis mouse models were treated with dydrogesterone and different dose BSHXD, after 28 d treatment, the mice were mated. The pregnant mice were euthanized on the 14th day of gestation and the endometrium was subjected to immunohistochemical staining using HOXA10 antibody (× 100). A1: Control group; A2: Model group; A3: Dydrogesterone group; A4: Low dose BSHXD group; A5: Normal dose BSHXD group; A6: High dose BSHXD group. B: The endometrium was subjected to immunohistochemical staining using αvβ3 integrin antibody (× 100). B1: Control group; B2: Model group; B3: Dydrogesterone group; B4: Low dose BSHXD group; B5: Normal dose BSHXD group; B6: High dose BSHXD group. C: the intensity of HOXA10 staining was quantitated by using Image J software. D: the intensity of αvβ3 integrin staining was quantitated. E: the expression level of Hoxa10 was determined using RT-qPCR. F: the expression level of Itgb3 was determined by RT-qPCR. Control group: sham surgery mice were treated with water; Model group: endometriosis mouse models were treated with water; Dydrogesterone group: endometriosis mouse models were treated with dydrogesterone (3.03 mg·kg-1·d-1); Low dose BSHXD group: endometriosis mouse models were treated with low dose BSHXD (6.218 g·kg-1·d-1); Normal dose BSHXD group: endometriosis mouse models were treated with normal dose BSHXD (12.437 g·kg-1·d-1); High dose BSHXD group: endometriosis mouse models were treated with high dose BSHXD (24.873 g·kg-1·d-1). BSHXD: Bushen Huoxue decotion; HOXA10: Homeobox A10; αvβ3: alpha(v)beta(3) integrin; Itgb3: Integrin beta(3); RT-qPCR: real-time quantitative polymerase chain reaction; D-HSCORE: digital histological score; mRNA: messenger ribonucleic acid; ANOVA: analysis of variance. Statistical analysis was carried out using ANOVA tests among these groups. Data were presented as mean ± standard deviation (n = 7). Compared with the model group, aP < 0.05.
3.6. BSHXD induces HOXA10 expression in endometrium and uterine flushing fluid of endometriosis-associated infertility women
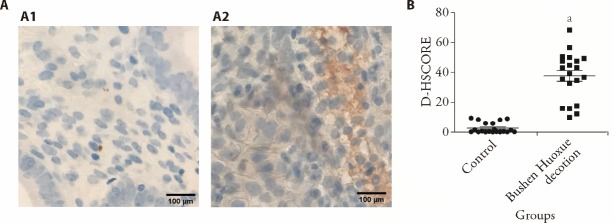
We tested the effect of BSHXD on HOXA10 expression in the endometrium and uterine flushing fluid obtained from the patients with endometriosis-associated infertility. Immunohistochemistry assays showed that the expression of HOXA10 was significantly increased in BSHXD-treated women compared with control (Figure 4). Using ELISA, we analyzed the concentration of HOXA10 in uterine flushing fluid of endometriosis-associated infertility women. Our results demonstrated that BSHXD increased the concentration of HOXA10 in uterine flushing fluid [(142 ± 16) vs (158 ± 18) pg/mL]. The ELISA analysis confirmed a substantially activation of HOXA10 in BSHXD-treated endometriosis-associated infertility women.
Figure 4. BSHXD induces HOXA10 and αvβ3 integrin expression in endometriosis-associated infertility women.
A: The mid-secretory endometrial biopsy specimens were obtained from twenty endometriosis-associated infertility women before and after BSHXD treatment for three menstrual cycles and were subjected to immunohistochemical staining by using HOXA10 antibody (×100). A1: Control group; A2: BSHXD group. B: The intensity of HOXA10 staining was quantitated by using Image J software. Control group: patients not treated with BSHXD; BSHXD group: patients treated with BSHXD (82 g/d) for three menstrual cycles. BSHXD: Bushen Huoxue decotion; ELISA: enzyme-linked immunosorbent assay; D-HSCORE: digital histological score. Student’s t-tests were used for comparisons of two groups. Data were presented as mean ± standard deviation (n = 20). Compared with the control group, aP < 0.05.
4. DISCUSSION
Among the 20 compounds of BSHXD, many have been proved to have activities in reproductive dysfunction. For instance, hyperin can increase proliferation, progesterone receptor levels, and estradiol levels in premature ovarian insufficiency.12 Isoquercitrin has a anti-apoptotic and antioxidative effect on repairing bone injury.13 Cynaroside is a flavonoid and exerts antibacterial, antifungal, antileishmanial, antioxidant, hepatoprotective, antidiabetic, anti-inflammatory, and anticancer effects.14 Paeoniflorin can promote estradiol synthesis in ovariectomized mice.15 In addition, we also found multiple compounds in BSHXD, such as feruloylagmatine, cistanoside, isoferulic acid, echinacoside and catalpol. Many components of BSHXD have extensive pharmacological actions, further proving the complexity of Traditional Chinese Medicine prescriptions. And these results also provide valuable clues for future study of the therapeutic mechanism of BSHXD.
Endometrial receptivity is a complex process of endometrial maturation that the trophectoderm of blastocyst can attach to the endometrial epithelial cells, adhere and invade into the endometrial vasculature and stroma.16 The altered endometrial receptivity is one of the major factors to infertility, and almost one-half of infertile women have the evidence of endometriosis.2,17 At the same time, endometriosis can also impair endometrial receptivity. Previous studies have shown that eutopic endometrium from women with endometriosis exhibits irregular molecular changes, such as dysregulation of integrins and HOXA10.18 However, rationally-designed novel therapies to target these interactions were limited. In this study, by using Western blot analysis, immun-ohistochemistry, RT-qPCR and ELISA, we demonstrated that BSHXD improved reproduction of endometriosis-associated infertility through upregulation of HOXA10 and αvβ3 integrin expression.
Recent study showed that Traditional Chinese Medicine can improve live birth rates of in-vitro fertilization.19 According to Traditional Chinese Medicine theories, BSHXD which is made upon the treatment principles of nourishing the kidney and activating blood circulation is effective and safe for endometriosis-induced infertility.8,20,21 For instance, BSHXD can improve oocytes quality by increasing intestinal barrier function via hsp-16.2-mediated heat-shock signaling pathway in C.elegans.22 Further, in polystic ovary syndrome, BSHXD improve the inflammatory state and intervenes in the endometrial epithelial-mesenchymal transition process through the transforming growth factor beta/nuclear factor kappa-light-chain-enhancer of activated B cells pathway to improve pregnancy outcome.23 And in recurrent implantation failure patients, BSHXD combined with acupuncture can down-regulate serum p38 mitogen-activated protein kinases and janus kinase/signal transducer and activator of transcription protein expression to improve embryo implantation rate.24 In line with previous research, we discovered that BSHXD can improve endometrial classification distribution and subendometrial blood flow, increase endometrial thickness and also enhance IL-6, LIF expression, which is an effective Chinese herbal medicine for improving endometrial receptivity and increasing pregnancy rate of patients with endometriosis-induced infertility.7 Collectively, these findings implicated that BSHXD has potentials to improve endometrial receptivity for treating endometriosis-induced infertility.
HOXA10, one of the most important HOX transcription factors, is located in a cluster of chromosome 7 and playing critical roles in fertility, embryo viability, hematopoietic, tumorgenesis.25,26 Enough HOXA10 expression in the implantation window is essential for endometrial cell differentiation and receptivity which is necessary for embryo implantation.25 Hoxa10-mutated female homozygous mice ovulate normally, but about 80% embryos died of defective endometrial receptivity.27 Equally, downregulation of HOXA10 expression in endometriosis contributes to their repressed endometrial receptivity.28 HOXA10 improves endometrial receptivity by activation of downstream genes including αvβ3 integrin which is specific to the implantation window.29 Our data showed that BSHXD significantly increased the pregnancy rates in endometriosis-associated infertility women and endometriosis mouse model. Using BeWo spheroid and mouse embryo attachment assay, we showed that BSHXD improved embryo attachment. Furthermore, we showed that BSHXD treatment could activate HOXA10 signaling in Ishikawa cells. We also provided evidence that increase of αvβ3 integrin and HOXA10 expression by using BSHXD in endometriosis mouse model. Finally, our study demonstrated that BSHXD enhanced HOXA10 expression in the endo-metrium of endometriosis-associated infertility women.
Detecting molecular mark expression in endometrial biopsies during implantation window is an important method used frequently to evaluate uterine receptivity. However, this is an invasive technique which brings pain and lesion to the patient. Recent studies suggest that measuring endometrial marker concentration, such as LIF and αVβ3 integrin, in uterine flushing fluid is a non-invasive, reproducible and reliable technique which enabled us to predict endometrial receptivity.30,⇓-32 In this study, we firstly presented and measured HOXA10 concentrations in uterine flushing fluid of endometriosis-associated infertility women during the implantation window by EILISA. Our results demonstrated that BSHXD increased the concentration of HOXA10 in uterine flushing fluid and improved the pregnancy rate of endometriosis-associated infertility women during six-month follow-up. This suggests that HOXA10 is secreted into the human uterine cavity from eutopic endometrium and its concentration in uterine flushing fluid would be regarded as to be a marker to evaluate the efficacy of BSHXD on endometriosis-associated infertility.
In summary, our data demonstrated that BSHXD could improve endometrial receptivity of endometriosis-associated infertility women in a dose-dependent manner by regulating HOXA10 and αvβ3 integrin expression. These provides an evidence for this herbal prescription as a novel therapy in treating endometriosis-associated infertility.
Funding Statement
Supported by National Natural Science Foundation-funded Project: the Mechanism of Effect of Bushen Huoxue Decotion on Endometrial Receptivity in Endometriosis (No. 81603372) and National Natural Science Foundation-funded Project: to Investigate the Mechanism of Bushen Huoxue Decoction in Improving Endometrial Receptivity Based on SUMOylation of Homeobox A10 (No. 82274190) and Jiangsu Commission of Health Project: to Investigate the Mechanism of Bushen Huoxue Decoction in Improving Endometrial Receptivity Based on Leukemia Inhibitory Factor Signaling (No. M2022079)
REFERENCES
- 1. Ye L, Whitaker LHR, Mawson RL, Hickey M. . Endometriosis. BMJ 2022; 379: e068950. [DOI] [PubMed] [Google Scholar]
- 2. Pirtea P, Vulliemoz N, De Ziegler D, Ayoubi JM. . Infertility workup: identifying endometriosis. Fertil Steril 2022; 118: 29-33. [DOI] [PubMed] [Google Scholar]
- 3. Qu H, Du Y, Yu Y, et al. The effect of endometriosis on Ivf/Icsi and perinatal outcome: a systematic review and Meta-analysis. J Gynecol Obstet Hum Reprod 2022; 51: 102446. [DOI] [PubMed] [Google Scholar]
- 4. Munro MG. . Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril 2019; 111: 629-40. [DOI] [PubMed] [Google Scholar]
- 5. Guo SW. . Genesis, genes and epigenetics of endometriosis-associated infertility. Nat Rev Endocrinol 2019; 15: 259-60. [DOI] [PubMed] [Google Scholar]
- 6. Naqvi H, Mamillapalli R, Krikun G, Taylor HS. . Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci 2016; 23: 186-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang WW, Zhu L, Huang MH, Liu KL, Wan GP. . Influence of bushen huoxue decoction on Il-6 and Lif expression in uterine flushing fluid of endometriosis. Jilin Zhong Yi Yao 2018; 38:1285-7. [Google Scholar]
- 8. Song Y, Zhou F, Tan X, et al. Bushen huoxue recipe attenuates early pregnancy loss via activating endometrial cox2-pge 2 angiogenic signaling in mice. BMC Complement Med Ther 2021; 21: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xue P, Zhou W, Fan W, et al. Increased mettl3-mediated m(6)a methylation inhibits embryo implantation by repressing Hoxa10 expression in recurrent implantation failure. Reprod Biol Endocrinol 2021; 19: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang W, Jiang Y, Mu X, et al. Mir-135a functions as a tumor suppressor in epithelial ovarian cancer and regulates Hoxa10 expression. Cell Signal 2014; 26: 1420-6. [DOI] [PubMed] [Google Scholar]
- 11. Tang W, Ramasamy K, Pillai SMA, et al. Lif/lifr oncogenic signaling is a novel therapeutic target in endometrial cancer. Cell Death Discov 2021; 7: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. You F, Cao J, Cheng L, Liu X, Zeng L. . Hyperin alleviates triptolide-induced ovarian granulosa cell injury by regulating Akt/Tsc1/Mtorc1 signaling. Evid Based Complement Alternat Med 2021; 2021: 9399261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Zhou D, Yang D, et al. Isoquercitrin attenuates osteogenic injury in Mc3t 3 osteoblastic cells and the zebrafish model via the Keap1-Nrf2-Are pathway. Molecules 2022; 27: 3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouyahya A, Taha D, Benali T, et al. Natural sources, biological effects, and pharmacological properties of cynaroside. Biomed Pharmacother 2023; 161: 114337. [DOI] [PubMed] [Google Scholar]
- 15. Park KS, Kim H, Kim HJ, et al. Paeoniflorin alleviates skeletal muscle atrophy in ovariectomized mice through the Erα/Nrf 1 mitochondrial biogenesis pathway. Pharmaceuticals (Basel) 2022; 15: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haas J, Casper RF. . Observations on clinical assessment of endometrial receptivity. Fertil Steril 2022; 118: 828-31. [DOI] [PubMed] [Google Scholar]
- 17. Alecsandru D, Garcia Velasco JA. . The excessive presence (percentage or number) of endometrial immune cells in patients with chronic endometritis cannot be associated with reduced endometrial receptivity or recurrent pregnancy failure. Fertil Steril 2020; 113: 85-6. [DOI] [PubMed] [Google Scholar]
- 18. Esfandiari F, Chitsazian F, Jahromi MG, et al. Hox cluster and their cofactors showed an altered expression pattern in eutopic and ectopic endometriosis tissues. Reprod Biol Endocrinol 2021; 19: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casale M. . Improving the health and treatment success rates of in vitro fertilization patients with Traditional Chinese Medicine: need for more robust evidence and innovative approaches. J Integr Med 2022; 20: 187-92. [DOI] [PubMed] [Google Scholar]
- 20. Cao Y, Chen Y, Wang P, et al. Network pharmacology and experimental validation to explore the molecular mechanisms of Bushen Huoxue for the treatment of premature ovarian insufficiency. Bioengineered 2021; 12: 10345-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma K, Yuan Y, Chen YX, et al. Efficacy of Bushen Culuan decoction on ovarian follicle and follicular granulosa cells in mice with premature ovarian insufficiency induced by tripterygium wilfordii polyglycoside. J Tradit Chin Med 2022; 42: 23-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu K, Zhao X, Xiao X, et al. Bushen huoxue decoction improves fertility through intestinal hsp-16.2-mediated heat-shock signaling pathway in caenorhabditis elegans. Front Pharmacol 2023; 14: 1210701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu H, Wu P, Zhu Y, et al. Bushen huoxue recipe inhibits endometrial epithelial-mesenchymal transition through the transforming growth factor-Β/nuclear factor kappa-B pathway to improve polycystic ovary syndrome-mediated infertility. Gynecol Endocrinol 2024; 40: 2325000. [DOI] [PubMed] [Google Scholar]
- 24. You XM, Xu JB, Yang J, Liao J. . Clinical efficacy of acupuncture combined with chinese herbal medication for recurrent implantation failure infertility of kidney deficiency and blood stasis and its effects on serum P38mapk and Jak/stat protein expression. Zhong Guo Zhen Jiu 2023; 43: 1399-404. [DOI] [PubMed] [Google Scholar]
- 25. Du H, Taylor HS. . The Role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med 2015; 6: a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang M, Hao C, Huang X, et al. Aberrant expression of lncrna ( Hoxa11-As1) and Homeobox a ( Hoxa9, Hoxa10, Hoxa11, and Hoxa13) genes in infertile women with endometriosis. Reprod Sci 2018; 25: 654-61. [DOI] [PubMed] [Google Scholar]
- 27. Ekanayake DL, Małopolska MM, Schwarz T, Tuz R, Bartlewski PM. . The roles and expression of Hoxa/Hoxa10 gene: a prospective marker of mammalian female fertility? Reprod Biol 2022; 22: 100647. [DOI] [PubMed] [Google Scholar]
- 28. Cheng J, Li C, Ying Y, et al. Metformin alleviates endometriosis and potentiates endometrial receptivity via decreasing vegf and mmp9 and increasing leukemia inhibitor factor and Hoxa10. Front Pharmacol 2022; 13: 750208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan Q, Huang C, Jiang Y, et al. Calpain7 impairs embryo implantation by downregulating Β3-integrin expression via degradation of Hoxa10. Cell Death Dis 2018; 9: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukui Y, Hirota Y, Aikawa S, et al. Uterine receptivity is reflected by Lif expression in the cervix. Reprod Sci 2022; 29: 1457-62. [DOI] [PubMed] [Google Scholar]
- 31. Marron K, Harrity C, Dunne H, Shkrobot L, Kennedy J. . Cytometric assessment of uterine receptivity via epithelial Β3 integrin expression. Reprod Biomed Online 2019; 39: 294-303. [DOI] [PubMed] [Google Scholar]
- 32. Dokuzeylül Güngör N, Önal M, Madenli AA, Ağar M. . Surgical removal of Figo type 0 and 1 fibroids ameliorates the expression of endometrial proinflammatory transcription factors and receptivity modulators. Fertil Steril 2023; 119: 504-13. [DOI] [PubMed] [Google Scholar]