Abstract
OBJECTIVE:
To evaluate the effects of Zuyangping (足疡平, ZYP) formula on wound healing in diabetic rats, as well as the molecular mechanisms involved.
METHODS:
The main compounds in ZYP formula were identified by the Liquid chromatography-tandem mass spectrometry. Sprague-Dawley rats, injected with streptozotocin (STZ) to establish diabetes model, then, formed a defective skin trauma in the back, and each group was treated with corresponding drugs once a day. Granulation was taken from each time node for histological analysis. The Western blotting was used to measured protein expression of advanced glycation end products receptor (RAGE) and hypoxia-inducible factor-1α (HIF-1α) axis-related proteins. The relative expression levels of inflammatory cytokines and growth factors were measured by the enzyme-linked immunosorbent assay method.
RESULTS:
The main ingredients were identified in the ZYP formula. Histological analysis showed that the ZYP formula could inhibit the expression of inflammation, promote angiogenesis and collagen deposition. In addition, the ZYP formula could regulate the expression of RAGE and HIF1-α axis-related proteins, thus promoting the wound healing in diabetic rats.
CONCLUSION:
The ZYP formula could accelerate wound healing in diabetic rats.
Keywords: wound healing, angiogenesis, diabetic skin ulcer, Zuyangping formula
1. INTRODUCTION
Diabetes is a global threat to human health, and it can cause many complications, including neuropathy, nephropathy and retinopathy.1 Delayed wound healing in diabetic patients is one of the most common complication. Approximately, 9.1-26.1 million people are estimated to develop this condition annually.2 Under normal circumstances, there are different stages of wound healing, including proliferation and migration of cells to the wound site, appropriate angiogenesis, re-epithelialization and proper biosynthesis. However, in the case of diabetes, hyperglycemia inhibits angiogenesis and reduces wound contraction and closure.3 A large number of studies have shown that the functional impairment of the hypoxia-inducible factor-1α/ vascular endothelial growth factor (HIF-1α/VEGF) signal pathway is the main mechanism underlying diabetic wound-related angiogenesis disorders.4 In hyperglycemia, the combination of advanced glycation end products (AGEs) and advanced glycation end products receptor (RAGE), promotes the secretion of inflammatory cytokines, damages neovascularization and delays healing.5 In fact, persistent inflammation and angiogenic disorders of diabetic wounds eventually lead to difficulty in healing.
In clinical practice, there is still an urgent need to develop new drugs for diabetic wound healing. Over several centuries, various plants have been used to cure disease not only as ingestible medications but also as topical treatments. In Traditional Chinese Medicine, ZYP prescription is a classic prescription for the treatment of chronic ulcers in our hospital in the past 20 years, but the specific mechanisms involved were not clear.6,⇓-8 The main compounds contained in the ZYP formula have been found to have separate anti-inflammatory or angiogenic effects, but the combined effects of these compounds have not been studied.9,10 Therefore, we believe that the effect of ZYP formula on wound healing in diabetic rats may be related to the RAGE/ nuclear factor kappa-B (NF-κB) and HIF1-α/VEGF signal pathway.
2. MATERIALS AND METHODS
2.1. Animals
Fifty healthy 5-week-old male Sprague-Dawley rats (100-200 g) of specific pathogen free grade were purchased from the Experimental Animal Center of the three Gorges University. The production license number of the experimental animal is SCXK (E) 2017-0012, and the license number of the experimental unit is SCXK (E) 2020-0080. The ethics approval number for the study is SY2020-001. The high-fat and high-sugar feed was provided by Wuhan Chunyuhong Experimental Animal Feed Co., Ltd. The rats were raised in a stable feeding environment (25 ℃; 50%-60% humidity). All animal experimental programs were approved by the Animal Research Committee of Wuhan University Tongren Hospital.
2.2. Induction of diabetes in rats
Diabetic rats (n = 40) were intraperitoneally injected with streptozotocin (60 mg/kg body weight, containing 0.1 mmol/L sodium citrate buffer pH = 4.5) (Sigma, St. Louis, MO, USA, Cat. No. 2060675).11,12 After STZ injection, the rats whose random blood glucose level reached 16.7 mmol/L or fasting blood glucose level reached 11.1 mmol/L were included in the diabetic model.10
2.3. Diabetic skin wound model
After 2 weeks of diabetes induction, normal rats and diabetic model rats were anesthetized by intramuscular injection of ketamine (100 mg/kg) (Mackin Biochemical Co., Ltd., Shanghai, China, Cat. No. C923159). Full-thickness skin excision was performed with a diameter of 2 cm on the back of the rat.12,13 At days 0, 7 and 14 post-surgery, the wound was photographed and the wound area was measured by ImageJ software. The wound healing rate was determined by the following equation: wound healing rate (%) = (A0-At)/A0 × 100, where A0 is the wound area immediately after surgery, and represents the wound area at days 7 or 14 after surgery.14
2.4. Drug preparation and ingredient identification
Preparations of the ZYP formula included the following: Huanglian (Rhizoma Coptidis) 20 g; Danggui (Radix Angelicae Sinensis) 10 g; Liujinucao (Herba Artemisiae Anomalae) 5 g; Luganshi (Calamina) 10 g; Duanshigao (Calcined Gypsum Fibrosum) 10 g; Ruxiang (Olibanum) 5 g and Bingpian (Borneolum Syntheticum) 1 g, which were crushed into a fine powder, and subsequently sifted with 100 mesh and finally mixed with Vaseline. The above-mentioned medicinal materials are provided by Tianji Traditional Chinese Medicine Factory (Wuhan, China). For preparing the ZYP extract, place the powder of the above-mentioned herb was decocted with water (1∶10, w/v) for 2 h after 0.5 h of soaking. The extract was filtered and the residues were further decocted (1∶10, w/v) for 1 h. The filtrate was decompressed and concentrated to collect the dried water extract.
The LC-MS/MS system was composed of a JasperTM liquid chromatography system and a triple quadrupole mass spectrometer equipped with anelectrospray ionization source. The solid phase was WondaSil C18 Superb column (4.6mm × 25 mm, 5 μm) (Shanghai, China). The mobile phase is water (A) containing 0.1% formic acid and acetonitrile (B) containing 0.1% formic acid. The gradient program was as follows: 0-5 min, 40% B; 5-12 min, 40%-80% B; 12-15 min, 80% B; 15-18 min, 80%-25% B. The column temperature was kept at 26 ℃, and the flow rate was 0.8 mL/min. The sample (50 μg/mL) injection was 10 μL. The ionspray voltage was set to 5.5 kV, and the source temperature was maintained at 500 ℃. Qualitative analysis was performed by monitoring [M+H]+ for analytes in selected ion recording mode and the mass range was set at m/z 100-1000, for identification purposes.
2.5. Experimental grouping and treatment
Epidermal growth factor solution (Huashengyuan genetic Engineering Development Co., Ltd, Shenzhen, China, Cat. No. 20200703) and 1% silver sulfadiazine cream (Kun ming Sheng huo Pharmaceytical Co., Ltd., Yunnan, China, Cat. No. H20057720) were used as treatment control groups. After surgery, normal rats represented the normal group (n = 10), whilst diabetic rats were divided into four groups: model group (n = 10), ZYP formula group (n = 10), epidermal growth factor group (n = 10) and 1% sulfadiazine silver cream group (n = 10). 0.59 g/cm2 ZYP formula was applied to the wound surface in the ZYP group. The epidermal growth factor group was treated with 0.4 mL solution. The silver sulfadiazine cream group was locally smeared with 2 mm thick silver sulfadiazine cream. Each group was treated once a day and covered with sterile gauze.
2.6. Preparation of samples
At days 7 and 14 after treatment of the skin wounds, we got the granulation tissue from the skin wound, fixed it with 10% formaldehyde solution, embedded it in paraffin, and observed it under a light microscope. The samples were stored in a freezer at -80 ℃ for further analysis.
2.7. Histology
The paraffin-embedded rat skin granulation tissues were sectioned at 5μm onto glass slides. The tissue sections were stained with hematoxylin and eosin (HE) and Masson's collagen staining. Photomicrographs of the tissue sections were taken using a light microscope (NikonE100, Tokyo, Japan) at a magnification of ×100.
2.8. Immunohistochemistry
Paraformaldehyde was fixed and embedded in paraffin, cut into 3 μm thick slices. After being fixed on the glass sheet, they were rehydrated in an ethanol gradient. 3% H2O2 solution was added to block endogenous catalase, and 0.01 M sodium citrate solution was added to extract antigen. After blocking with 3% bovine serum albumin, the tissue sections were incubated with primary antibody at 4 ℃ overnight. The first antibody included rabbit anti-AGEs polyclonal antibody (PAb) (ABsin Bioscience, Shanghai, China, 1∶1000, Cat. No. abs121210) and anti-CD31 rabbit PAb (Servicebio, Wuhan, China, 1∶1000, Cat. No. GB113151). All tissues were incubated with Goat anti rabbit immunoglobulin G (Hallil)-horse radish peroxidase (HRP) (Servicebio, Wuhan, China, 1∶100, Cat. No. GB23303). Diaminobenzidine and hematoxylin staining 3 min were stained with hematoxylin for 1 h at 37 ℃, and 3 min was dehydrated by graded alcohol. We sealed the film with neutral gum after fixation15. Photomicrographs of the tissue sections were taken using a light microscope (NikonE100, Tokyo, Japan) at a magnification of ×100.
2.9. Enzyme-linked immunosorbent assay (ELISA)
The granulation tissues were weighed and homogenized with 9 times the volume of. After centrifugation, the supernatant was taken, and 50 μL standard working solution or supernatant sample was added to the enzyme plate coated with substrate, and then incubated with antibiotic horseradish peroxidase at 37 ℃ for 60 min. We disposed of the liquid, washed the plate with washing solution 5 times, and subsequently added the chromogenic substrate rationale of 3,3',5,5'-Tetramethylbenzidine and incubate at 37 ℃ for 30 min. Then we added the terminal liquid. The detection absorption wavelength is 450 nm. The kits used were as follows: Rat tumor necrosis factor-α (TNF-α) ELISA kit was purchased from Lun chang shuo Biotechnology Co., Ltd. (Xiamen, China, No. SU-31063). Rat NF-κB p65 ELISA kit was purchased from Lun chang shuo Biotechnology Co., Ltd. (Xiamen, China, No. SU-35152). Rat basic fibroblast growth factor (bFGF) ELISA kit was purchased from Lun Chang Shuo Biotechnology Co., Ltd. (Xiamen, China, No. SU-30506). Rat epidermal growth factor (EGF) ELISA kit was purchased from Sinobestbio Biotechnology Co., Ltd. (Shanghai, China, No. YX-050706R). Rat interleukin-6 (IL-6) ELISA kit was purchased from Sinobestbio Biotechnology Co., Ltd. (Shanghai, China, No. YX-091206R).
2.10. Western blotting method
The granulation tissues were put into the protein cleavage buffer containing albumin enzyme inhibitors and homogenized by tissue homogenizer to extract protein. The same amount of protein was separated with 8% or 10% Sodium dodecyl sulfate- polyacrylamide gel electrophoresis, electrotransferred to the polyvinylidene fluoride membrane, sealed with 5% milk for 1 h, and then incubated overnight with an antibody at 4 ℃. The antibodies used were as follows: Rabbit anti-RAGE PAb (ABsin Bioscience, Shanghai, China, 1∶2000, Cat. No. abs136138), NF-κB p65 rabbit PAb (Proteintech, Wuhan, China, 1∶2000, Cat. No. 00090164), HIF-1α Ab (Affinity Biosciences, Cincinnati, OH, USA, 1∶2000, Cat. No. AF1009), VEGF rabbit PAb (ABclonal, Wuhan, China, 1∶2000, Cat. No. 3560090114), Beta-actin rabbit PAb (ABclonal, Wuhan, China, 1∶3000, Cat. No. 9100026004). After washing 3 times, the HRP goat anti-rabbit IgG (ABclonal, Wuhan, China, 1∶3000, Cat. No. 9300014001) was incubated with a PVDF membrane and developed with the enhanced chemiluminescence agent.
2.11. Statistical analysis
All data were analyzed using GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). The statistical analysis was performed by a one-way analysis of variance (ANOVA) and Dunnett’s test. Student’s t-test was used to compare differences between the two groups. Values were shown as mean ± standard deviation (). Differences were considered significant if P < 0.05.
3. RESULTS
3.1. Changes in venous blood glucose levels in rats
Diabetic models were generated by injecting STZ. After injecting STZ, the model blood glucose will be higher than 16.7 mmo1/L. Then, we made skin excision, and respectively treating them with each group of drugs. As shown in Table 1, at days 7 and 14 after injection of STZ, the level of venous blood glucose in all diabetic rats was significantly higher than that in the normal control group (P < 0.05). At days 7 and 14 after treatment, the venous blood glucose level of the ZYP group was lower than that of the model group, however, there was significant difference only on the 14th day after treatment (P < 0.05).
Table 1.
Changes in venous blood glucose levels in rats (, mmol/L)
| Time point | Normal group (n = 5) |
Model group (n = 5) |
ZYP group (n = 5) |
rhEGF group (n = 5) |
SSD group (n = 5) |
|---|---|---|---|---|---|
| Before injection of STZ | 6.59±0.35 | 6.78±0.44 | 6.72±0.43 | 6.70±0.48 | 6.64±0.60 |
| Day 7 after STZ injection | 6.17±0.40 | 29.72±3.30a | 30.44±2.66a | 28.20±1.78a | 29.78±3.34a |
| Day 14 after STZ injection | 6.28±0.23 | 31.94±1.38a | 32.90±0.46a | 30.04±2.86a | 29.30±3.87a |
| Day 7 after treatment | 6.21±0.41 | 30.28±3.44a | 27.32±2.70a | 28.40±3.19a | 28.94±3.47a |
| Day 14 after treatment | 6.26±0.31 | 30.02±2.62a | 24.68±2.06ab | 27.20±3.16a | 28.78±3.82a |
Notes: Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. aP < 0.05, compared with the normal control group; bP < 0.05, compared with the model group. All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups.
3.2. ZYP formula accelerated skin wound healing in diabetic rats
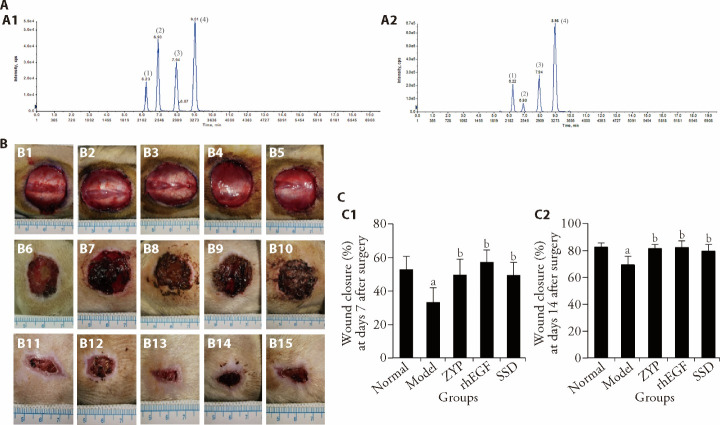
Firstly, its main compositions were identified. The chromatogram of ZYP extract is shown in (Figure 1A2), by comparing the retention time, with the standard chromatogram (Figure 1A1), four main compounds were identified in the ZYP extract. They were respectively: (1) epiberberine, (2) coptisine, (3) bamatine and (4) berberine. As shown in Figure 1B and Figure 1C, our visual inspections showed that at days 7 and 14 post-surgery, compared with the normal group, the wound healing speed of the model group was slower (P < 0.05). At days 7 and 14 post-surgery, the wound healing speed of the ZYP group, rhEGF group and SSD group was faster than the model group (P < 0.05). As shown in supplementary Figures 1A and 1B, the images of the HE staining and Masson's trichrome staining showed that at day 7 post-surgery, compared with the model group, the number of capillaries in the granulation tissue increased in the normal group, ZYP group, rhEGF group and SSD group; there were a greater abundance of fibroblasts, and collagen fibers were arranged densely. At day 14 post-surgery, compared with the model group, the inflammatory infiltration was reduced, the collagen fibers were neatly arranged, a large number of collagen fibers were deposited in the normal group and all treatment groups, indicating that the repair was better.
Figure 1. Morphological effects on rat wounds.
A: chromatograms of standard and ZYP extracts; A1: chromatograms of standard; A2: chromatograms of ZYP extracts. B1-B5: representative images of skin wounds at days 0 after surgery from all groups. B6-B10: representative images of skin wounds at days 7 after surgery from all groups. B11-B15: representative images of skin wounds at days 14 after surgery from all groups. B1, B6, B11: Normal group; B2, B7, B12: Model group; B3, B8, B13: ZYP group; B4, B9, B14: rhEGF group; B5, B10, B15: SSD group. C1-C2: wound closure rate at days 7 and 14 post surgery in each group. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 5). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group, bP < 0.05, compared with the model group.
3.3. ZYP formula reduced the expression of AGEs and promoted the expression of CD31 in wound healing of diabetic rats.
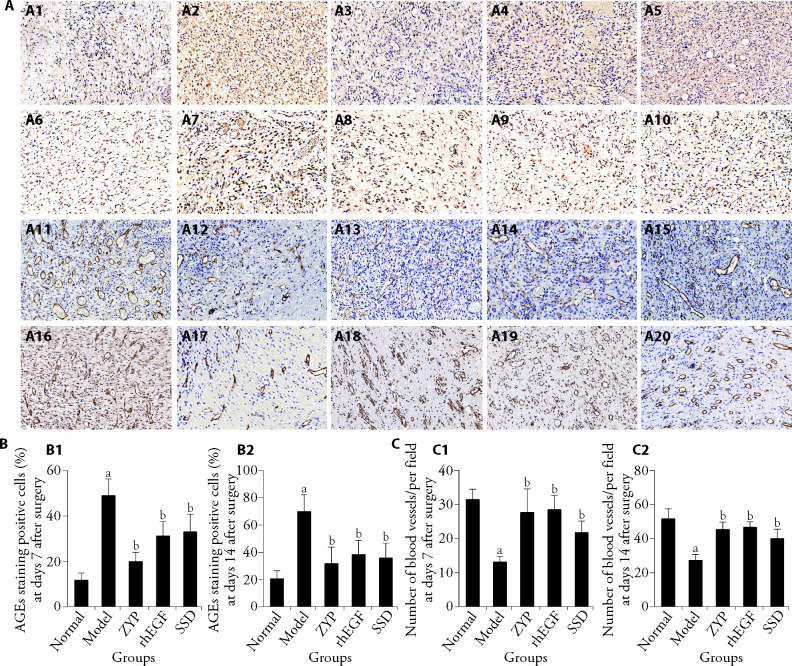
In this study, immunohistochemical method was used to detect the expression level of AGEs and CD31 in granulation tissue of rats (Figure 2A). The results showed that the number of AGEs-positive cells (Figure 2B) in the model group was significantly higher than that in the normal group on days 7 and 14 post-surgery (P < 0.05). At days 7 and 14, the number of AGEs-positive cells (Figure 2B) in all treatment groups was lower than that in the model group (P < 0.05). As shown in Figure 2C, the number of capillary vessels in the normal control group was significantly higher than that in the model group at days 7 and 14 after surgery (P < 0.05). Compared with the model group, the number of capillary vessels in all treatment groups (Figure 2C) increased in varying degrees at days 7 and 14 after surgery (P < 0.05). It was observed that the ZYP group could promote the expression of CD31 in wound healing.
Figure 2. Representative immunohistochemical staining of AGEs and CD31 in the granulation tissue of rats (×100).
A1-A5: representative images of immunohistochemistry staining of AGEs at days 7 after surgery from all groups; A6-A10: Representative images of immunohistochemistry staining of AGEs at days 14 after surgery from all groups; A11-A15: representative images of immunohistochemistry staining of CD31 at days 7 after surgery from all groups; A16-A20: representative images of immunohistochemistry staining of CD31 at days 14 after surgery from all groups. A1, A6, A11, A16: Normal group; A2, A7, A12, A17: Model group; A3, A8, A13, A18: ZYP group; A4, A9, A14, A19: rhEGF group; A5, A10, A15, A20: SSD group. B1-B2: statistical analysis of AGEs staining positive cells at days 7 and 14 post surgery, cells were calculated by IPP software. C1-C2: statistical analysis of the number of capillary vessels at days 7 and 14 post surgery, capillary vessels per field were calculated by IPP software. Normal group: normal rats with skin ulcer; Model group: Non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. AGEs: advanced glycation end products; CD31: platelet endothelial cell adhesion molecule-1; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 3). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.
3.4. ZYP formula could inhibit the expression of RAGE/NF-κB p65 and promote the expression of HIF-1α/VEGF in wound healing of diabetic rats
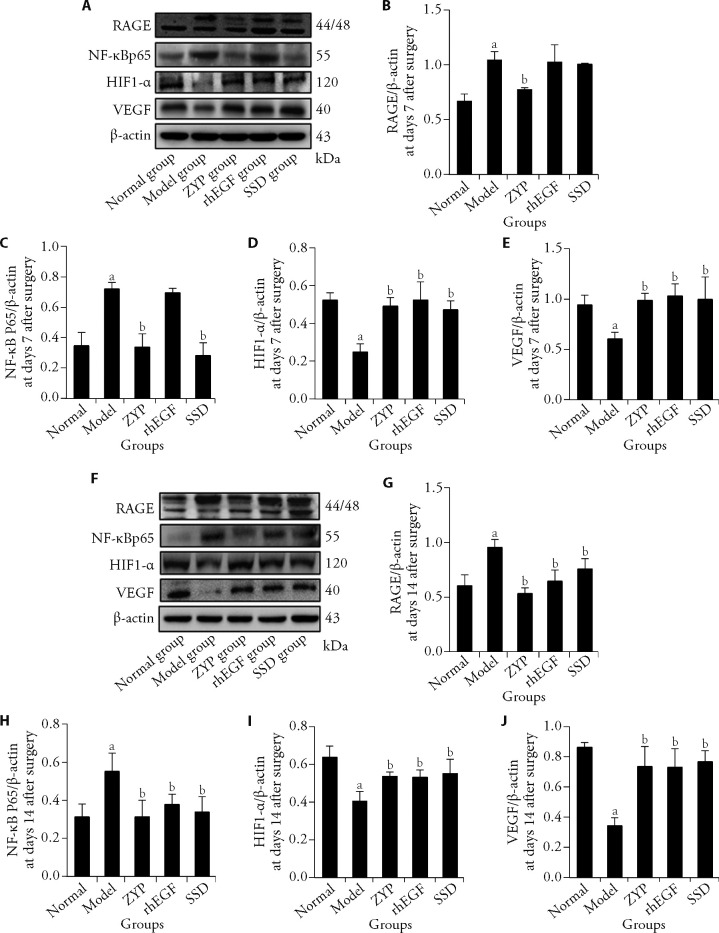
The Western blotting was used to detect the expression of RAGE and NF-κB p65 (Figures 3A, 3F). The results showed that the expression of RAGE and NF-κB p65 (Figures 3B, 3C) in the model group was significantly higher than that in the normal group at day 7 after surgery (P < 0.05). Compared with the model group, the expression of NF- κ B p65 (Figure 3C) in the ZYP group and SSD group decreased significantly (P < 0.05). At day 14 after surgery, the expression of RAGE and NF-κB p65 (Figure 3G, 3H) in the ZYP group, rhEGF group and SSD group was significantly lower than that of the model group (P < 0.05). Compared with the model group, the ZYP group showed significant inhibition of the expression of RAGE and NF-κB p65 (Figures 3B, 3C) at day 7 after surgery (P < 0.05), thus the effect of the ZYP group was stronger in the early stages of wound healing.
Figure 3. Expression of angiogenesis-associated molecules in granulation tissue of diabetic rats at days 7 and 14 post-surgery and the effect of ZYP formula.
A: Western blotting images at day 7 post surgery; B: after 7 d of surgery, statistical analysis of RAGE expression; C: after 7 d of surgery, statistical analysis of NF-κB p65 expression; D: after 7 d of surgery, statistical analysis of HIF-1α expression; D: after 7 d of surgery, statistical analysis of VEGF expression; F: Western blotting images at day 14 post surgery; G: after 14 d of surgery, statistical analysis of RAGE expression; H: after 14 d of surgery, statistical analysis of NF-κB p65 expression; I: after 14 d of surgery, statistical analysis of HIF-1α expression; J: after 7 d of surgery, statistical analysis of VEGF expression. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. β-actin: beta-actin; RAGE: advanced glycation end products receptor; NF-κB p65: nuclear factor-κB p65; HIF-1α: hypoxia-inducible factor-1α; VEGF: vascular endothelial growth factor; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 3). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.
Angiogenesis-associated molecules HIF1-α/VEGF were also studied by Western blotting (Figures 3A, 3F). The findings showed that the expression of HIF-1α/VEGF in the model group was significantly lower than that in the normal group at day 7 after surgery (Figures 3D, 3E, P < 0.05). Meanwhile the expression in the ZYP group, rhEGF and SSD group was significantly higher than that of the model group (Figures 3D, 3E, P < 0.05). At day 14 after surgery, the expression of HIF-1α/VEGF in the model group was significantly lower than that in the normal group (Figures 3I, 3J, P < 0.05). At this time, the expression of HIF-1α/VEGF in the ZYP group, rhEGF group and SSD group was significantly higher than that in the model group (Figures 3I, 3J, P < 0.05).
3.5. Effect of the ZYP formula on the expression levels of inflammatory cytokines and growth factors in wound healing of diabetic rats
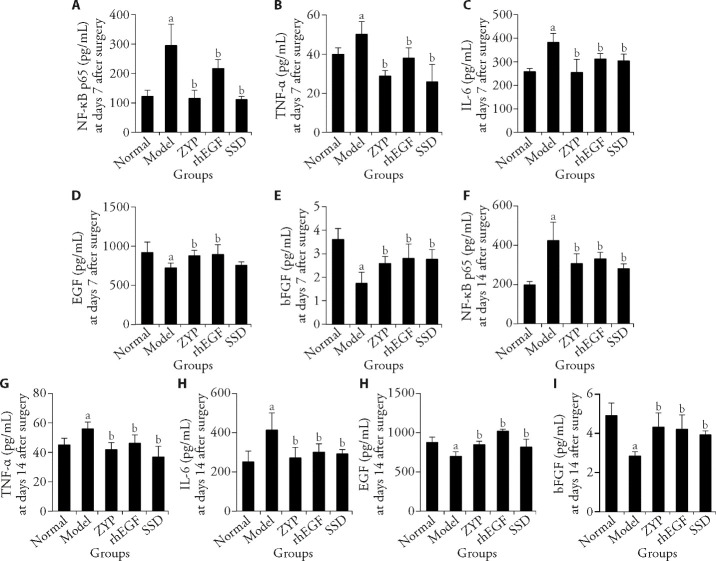
The ELISA assay showed that compared with the normal group, the expression levels of NF-κB p65, TNF-α, and IL-6 (Figures 4A-4C and Figures 4F-4H) in the model group were significantly increased on days 7 and 14 post-surgery (P < 0.05), while the expression levels of EGF and bFGF (Figures 4D, 4E and Figures 4I, 4J) were significantly decreased in the model group (P < 0.05). Compared with the model group, the expression levels of NF-κB p65, TNF-α and IL-6 (Figures 4A-4C and Figures 4F-4H) in the ZYP group, decreased significantly on days 7 and 14 post-surgery (P < 0.05). Compared with the model group, the expression of EGF and bFGF (Figure 4D, 4E and 4I, 4J) in the ZYP group and rhEGF group increased significantly on days 7 and 14 post-surgery (P < 0.05).
Figure 4. ELISA method was used to detect the expression level of related cytokines in granulation tissue of diabetic rats at days 7 and 14 post-surgery and the effect of ZYP formula.
A: NF-κB p65 expression level at day 7 post-surgery; B: TNF-α expression level at days 7 post-surgery; C: IL-6 expression level at day 7 post-surgery, D: EGF expression level at day 7 post-surgery; E: bFGF expression level at day 7 post-surgery; F: NF-κB p65 expression level at day 14 post-surgery; G: TNF-α expression level at day 14 post-surgery; H: IL-6 expression level at days 14 post-surgery; I: EGF expression level at days 14 post-surgery; J: bFGF expression level at day 14 post-surgery. Normal group: normal rats with skin ulcer; Model group: non-intervention group after the establishment of diabetic ulcer model; ZYP group: after modelling diabetic ulcer, treatment with ZYP formula; rhEGF group: after modelling diabetic ulcer, treatment with epidermal growth factor; SSD group: after modelling diabetic ulcer, treatment with 1% sulfadiazine silver cream. ELISA: enzyme-linked immunosorbent assay; NF-κB p65: nuclear factor-κB p65; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor; ZYP: Zuyangping; rhEGF: epidermal growth factor; SSD: 1% sulfadiazine silver cream. Values are shown as mean ± standard deviation (n = 5). All the treated groups were compared with the model group using the Dunnett’s test. Student’s t-test was used to compare differences between the normal and model groups. aP < 0.05, compared with the normal group; bP < 0.05, compared with the model group.
4. DISCUSSION
The number of patients suffering with diabetes are expected to reach 400 million by 2030. Complications of wound healing, including foot ulcers or even amputation, are major factors contributing to its mortality.16,17 At present, it is necessary to research appropriate drugs to promote the wound healing in diabetes.
Our study found that ZYP formula has a good effect in promoting the wound healing in diabetic rats. But ZYP formulas contain mineral components, we detected four components (epiberberine, coptisine, palmatine, and berberine) for the quality control by LC-MS/MS. All belonged to isoquinoline alkaloids and showed bacteriostatic and anti-inflammatory effects.18 Considerable evidence have shown that berberine alkaloid in Huanglian (Rhizoma Coptidis) could inhibit inflammatory factors such as TNF-α, IL-6, IL-1β caused by the RAGE/NF-κB signalling pathway. Furthermore, this alkaloid could up-regulate the expression of VEGF and CD31, and thus reduced the inflammation-related injury of vascular endothelial cells and promoted angiogenesis.19,20 Studies have found that polysaccharides and ferulic acid in Danggui (Radix Angelicae Sinensis) could up-regulate the expression of CD31, VEGF and HIF-1α, thus increasing angiogenesis.21,⇓-23 Medicinal properties of Ruxiang (Olibanum) were also widely recognized for its antimicrobial activity and use in inflammatory conditions. Bingpian (Borneolum Syntheticum) was a natural transdermal absorption enhancer.24,25 Duanshigao (Calcined Gypsum Fibrosum) and Luganshi (Calamina) have been used as mineral drugs that had good effects in antibacterial, anti-inflammatory.26,27 Different components such as polysaccharides, ferulic acid and berberine alkaloids in ZYP formula could directly or indirectly promote the expression of CD31 and induce neovas-cularization at days 7 and 14 after surgery, which is consistent with the results of Figure 2. However, although rhEGF and SSD could also promote the expression of CD31, they both had more or less the same effects. The rhEGF solution had no direct bacteriostatic effect, but it could promote the expression of endogenous EGF and form a protective film on the wound surface. It also promoted cell proliferation and angiogenesis, and improved diabetic wound healing.28,⇓-30 Studies have shown that silver sulfadiazine modulates oxidative stress and inflammatory responses and improved the wound microenvironment, thereby promoted wound healing.31,⇓-33 But silver-based products should be avoided because of its respective side effects. In supplementary Figures 1A, 1B and Figure 2C, ZYP group promoted the expression of CD31 and VEGF, with more capillaries increasing at day 7 after surgery, and collagen deposition and more compact arrangement at day 14 after surgery, indicating that wound healing speed was faster, similar to the results of other studies.34,35
The reason underlying difficult wound healing of diabetes was related to the AGEs/RAGE/NF-κB p65 signal pathway. Patients with diabetes were prone to produce AGEs due to long-term hyperglycemia, which became a risk factor for injury and chronic ulcers.36,37 RAGE, the receptor of AGEs, was one of the crucial ways to mediate the pathological effects of glycosylated products in ulcers. When AGEs bound to RAGE, it could trigger intracellular oxidative stress and inflammation, and thus activate NF-κB. Among them, the increased expression of NF-κB p65 could promote RAGE to increase the activity of pro-inflammatory cytokines, such as TNF-α, IL-6, resulting in cell dysfunction and tissue repair difficulty.38 As shown in Figure 2B and Figures 3B-3C, compared with model group during the first 7 d, ZYP formula could significantly reduce the level of AGEs, further inhibit the binding to RAGE, and reduce the expression of NF-κB39. This inhibited the secretion of inflammatory cytokines TNF-α and IL-6 (Figures 4G-4H) during 14 d of treatment, and reduced the injury of inflammatory response.40
In addition, the delayed healing property of diabetic wounds was the result of reduced neovascularization during hypoxia. Thangarajah et al 41,42 have shown that the decreased expression of vascular endothelial growth factor, induced by hypoxia, was due to the potential mechanism of hypoxia-induced impaired angiogenesis, which was due to the deficiency of hypoxia-induced transactivation of HIF-1α. Botusan et al 43 demonstrated that hyperglycaemia interferes with the stability of HIF-1α through a von Hippel-Lindau tumor suppressor protein-dependent mechanism, resulting in the destabilisation of HIF-1α and the suppression of its downstream genes (especially VEGF), which was a central pathogenic mechanism that delays wound healing in diabetes mellitus.43 Therefore, the impairment of the HIF-1α/VEGF axis function was acknowledged to be the main cause of impaired angiogenesis and delayed wound healing in diabetic patients. Existing studies have shown that different means of activating HIF-1α and its downstream VEGF could promote angiogenesis and thus accelerate ulcer healing.44,45 In Figures 3D-3E and Figures 3I-3J, we found that the ZYP formula could activate HIF-1α/VEGF signal transduction to promote the expression of CD31, thus promoting angiogenesis at different time points.
In conclusion, this study demonstrated that the ZYP formula is a pharmacologically active formulation that can promote wound healing. ZYP could promote wound healing by reducing inflammatory responses and accelerating angiogenesis. Due to conditional limitations, the quality control components have only been preliminarily studied. Because of this, its effective components and other action mechanisms will be further explored in the future.
5. SUPPORTING INFORMATION
Supporting data to this article can be found online at http://journaltcm.cn.
Funding Statement
Supported by Hubei Provincial Central Guidance Local Science and Technology Development Project: Wuhan Precision Diagnosis and Treatment Clinical Medical Research Center for Severe Infections (No. 2020ZYYD026), Wuhan Municipal Health Commission Scientific Research Project: Study on the effect of Zuyangping formula on Diabetes Wound Healing through Advanced Glycation End Products Receptor/ Nuclear Factor Kappa-B p65/Nucleotide-binding Oligomerization Domain, Leucine-Rich Repeat and Pyrin Domain-Containing 3 Mediated Inflammatory Reaction (No. WZ22A01), Wuhan Applied Basic Frontier Project: Development of Scar Eliminating Cream for Burn Scar Clinical Agreement (No. 2020020601012301), and Wuhan Clinical Medical Research Project: Preclinical Study of Mitogen-activated Protein Kinase 12-based Pituitary Prolactinoma and Adrenocorticotropin Adenoma in the Treatment of New Drug Barley Maltamine (No. WX20M02)
Contributor Information
Hong ZHANG, Email: 390479135@qq.com.
Yonggang CHEN, Email: cyg508@163.com.
REFERENCES
- 1. Rosenson RS, Fioretto P, Dodson PM. . Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 2011; 218: 13-8. [DOI] [PubMed] [Google Scholar]
- 2. Jeffcoate WJ, Vileikyte L, Boyko EJ, et al. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018; 41: 645-52. [DOI] [PubMed] [Google Scholar]
- 3. Daemi A, Lotfi M, Farahpour MR, et al. Topical application of cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol 2019; 57: 799-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong WX, Hu MS, Esquivel M, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle) 2014; 3: 390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han Y, Sun T, Tao R, et al. Clinical application prospect of umbilical cord-derived mesenchymal stem cells on clearance of advanced glycation end products through autophagy on diabetic wound. Eur J Med Res 2017; 22: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou XZ, Luo M, Wu J, et al. Antibacterial effect of foot ulcer cure ointment on ulceration of diabetic rat: an experimental study. Chin J Nosocomiol 2006: 1207-9. [Google Scholar]
- 7. Zhang H, Zhang Y, Cao YL, et al. Effect of Fufang Zuyangping on wound healing of skin ulcer and expression of RAGE/NF-κBP65/VEGF in diabetic rats. Zhong Guo Yi Yuan Yao Xue Za Zhi 2021; 41: 1405-9. [Google Scholar]
- 8. Cai JY, Zhou XZ, Wu J, et al. Clinical study of Zuyangping ointment in the treatment of diabetic foot ulcer. Zhong Guo Yi Shi Za Zhi 2008: 979-80. [Google Scholar]
- 9. Qiu YY, Tang LQ, Wei W. . Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol Cell Endocrinol 2017; 443: 89-105. [DOI] [PubMed] [Google Scholar]
- 10. Zhang XN, Ma ZJ, Wang Y, et al. Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS One 2017; 12: e0177862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarandy MM, Novaes RD, Xavier AA, et al. Hydroethanolic extract of strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type I diabetes. Biomed Res Int 2017; 2017: 9538351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kant V, Gopal A, Kumar D, et al. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res 2015; 193: 978-88. [DOI] [PubMed] [Google Scholar]
- 13. Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther 2020; 11: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li G, Ko CN, Li D, et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat Commun 2021; 12: 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonab FS, Farahpour MR. . Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Path 2017; 26: 885-92. [Google Scholar]
- 16. Tam JC, Lau KM, Liu CL, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol 2011; 134: 831-8. [DOI] [PubMed] [Google Scholar]
- 17. Levin ME. . Management of the diabetic foot: preventing amputation. South Med J 2002; 95: 10-20. [PubMed] [Google Scholar]
- 18. Shang XF, Yang CJ, Morris-Natschke SL, et al. Biologically active isoquinoline alkaloids covering 2014-2018. Med Res Rev 2020; 40: 2212-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Q, Mo R, Wu N, et al. Berberine ameliorates diabetes-associated cognitive decline through modulation of aberrant inflammation response and insulin signaling pathway in DM rats. Front Pharmacol 2017; 8: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, He L, Zhang J, et al. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-kappaB pathway. Colloids Surf B Biointerfaces 2020; 187: 110647. [DOI] [PubMed] [Google Scholar]
- 21. Lin CM, Chiu JH, Wu IH, et al. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1 alpha. J Nutr Biochem 2010; 21: 627-33. [DOI] [PubMed] [Google Scholar]
- 22. Lam HW, Lin HC, Lao SC, et al. The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo. J Cell Biochem 2008; 103: 195-211. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Chin A, Zhang L, et al. The role of Traditional Chinese Medicines in osteogenesis and angiogenesis. Phytother Res 2014; 28: 1-8. [DOI] [PubMed] [Google Scholar]
- 24. Siddiqui MZ. . Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci 2011; 73: 255-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai X, Wang R, Wu Z, et al. Permeation-enhancing effects and mechanisms of borneol and menthol on ligustrazine: a multiscale study using in vitro and coarse-grained molecular dynamics simulation methods. Chem Biol Drug Des 2018; 92: 1830-7. [DOI] [PubMed] [Google Scholar]
- 26. Liu K, Han S, Gao W, et al. Changes of mineralogical properties and biological activities of gypsum and its calcined products with different phase structures. Evid Based Complement Alternat Med 2021; 2021: 6676797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta M, Mahajan VK, Mehta KS, et al. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014: 709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mimura Y, Ihn H, Jinnin M, et al. Epidermal growth factor induces fibronectin expression in human dermal fibroblasts via protein kinase C delta signaling pathway. J Invest Dermatol 2004; 122: 1390-8. [DOI] [PubMed] [Google Scholar]
- 29. Garcia-Honduvilla N, Cifuentes A, Ortega MA, et al. Immuno-modulatory effect of local rhEGF treatment during tissue repair in diabetic ulcers. Endocr Connect 2018; 7: 584-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen 2014; 22: 497-503. [DOI] [PubMed] [Google Scholar]
- 31. Muhammad AA, Arulselvan P, Cheah PS, et al. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Devel Ther 2016; 10: 1715-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamdi SP, Raval A, Nakhate KT. . Effect of apple peel extract on diabetes-induced peripheral neuropathy and wound injury. J Diabetes Metab Disord 2021; 20: 119-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang JY, Chen L, Zhou ZZ, et al. Effect of Jiedu Shengji ointment on local IL-6 and TNF-α expression in diabetic ulcer rats. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 2021; 27: 1567-71. [Google Scholar]
- 34. He T, Sun P, Liu B, et al. Puffball spores improve wound healing in a diabetic rat model. Front Endocrinol (Lausanne) 2022; 13: 942549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gan D, Su Q, Su H, et al. Burn ointment promotes cutaneous wound healing by modulating the PI3K/AKT/mTOR signaling pathway. Front Pharmacol 2021; 12: 631102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gugliucci A. . Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr 2017; 8: 54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richards JE, Hutchinson J, Mukherjee K, et al. Stress hyperglycemia and surgical site infection in stable nondiabetic adults with orthopedic injuries. J Trauma Acute Care Surg 2014; 76: 1070-5. [DOI] [PubMed] [Google Scholar]
- 38. Ghosh G, Wang VY, Huang DB, et al. NF-kappa B regulation: lessons from structures. Immunol Rev 2012; 246: 36-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fei J, Ling YM, Zeng MJ, et al. Shixiang plaster, a Traditional Chinese Medicine, promotes healing in a rat model of diabetic ulcer through the receptor for advanced glycation end products (RAGE)/nuclear factor kappa B (NF-kappa B) and vascular endothelial growth factor (VEGF)/vascular cell adhesion molecule-1 (VCAM-1)/endothelial nitric oxide synthase (eNOS) signaling pathways. Med Sci Monit 2019; 25: 9446-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parmar KM, Shende PR, Katare N, et al. Wound healing potential of Solanum xanthocarpum in streptozotocin-induced diabetic rats. J Pharm Pharmacol 2018; 70: 1389-400. [DOI] [PubMed] [Google Scholar]
- 41. Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle 2010; 9: 75-9. [DOI] [PubMed] [Google Scholar]
- 42. Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009; 106: 13505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 2008; 105: 19426-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sunkari VG, Lind F, Botusan IR, et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen 2015; 23: 98-103. [DOI] [PubMed] [Google Scholar]
- 45. Chen H, Jia P, Kang H, et al. Upregulating Hif-1alpha by hydrogel nanofibrous scaffolds for rapidly recruiting angiogenesis relative cells in diabetic wound. Adv Healthc Mater 2016; 5: 907-18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting data to this article can be found online at http://journaltcm.cn.