Abstract
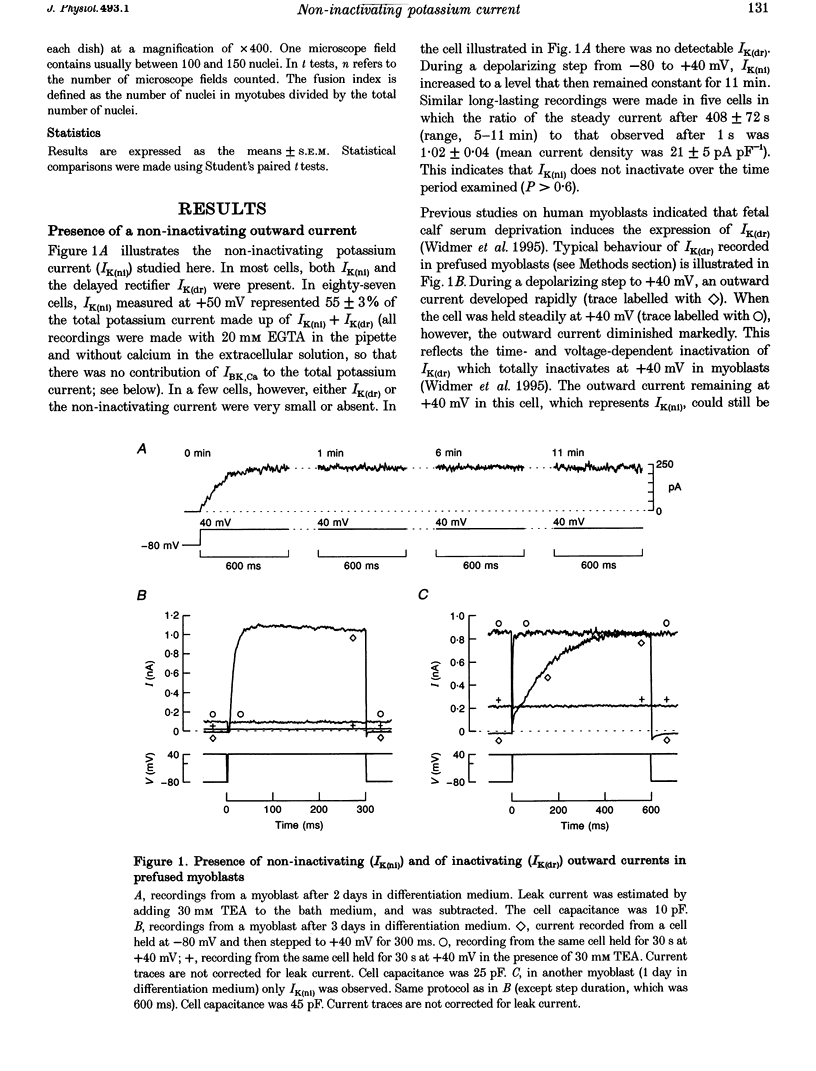
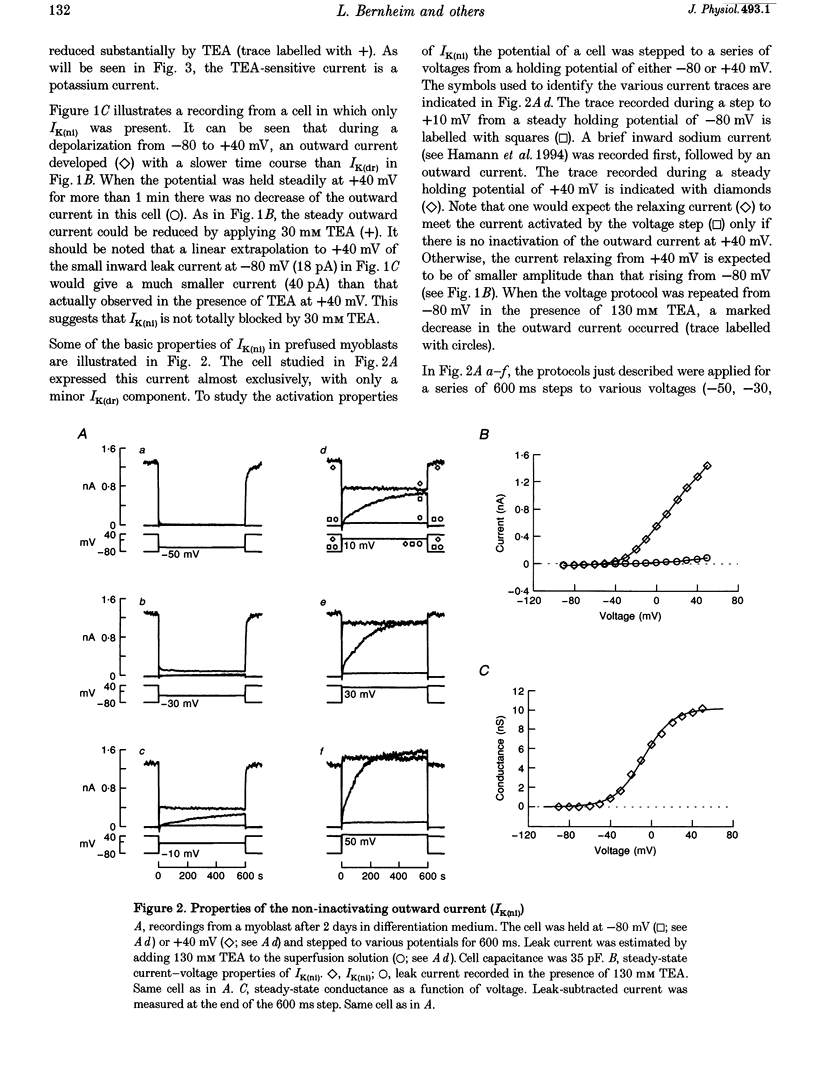
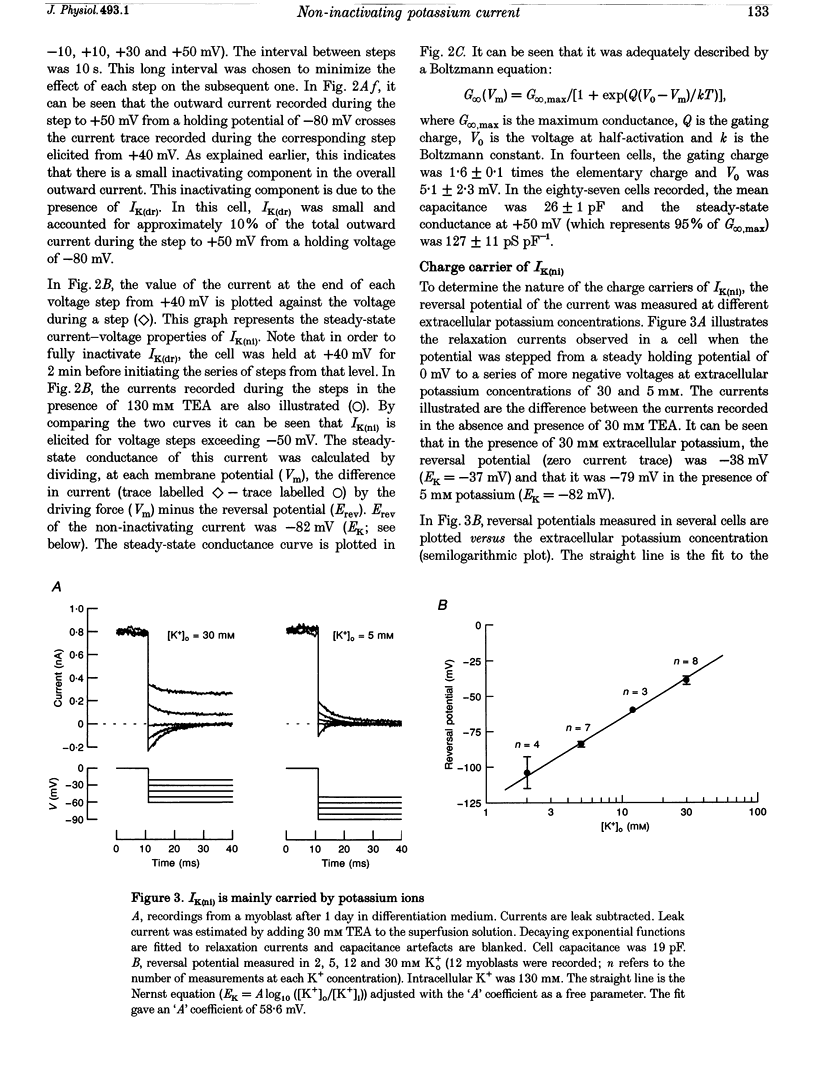
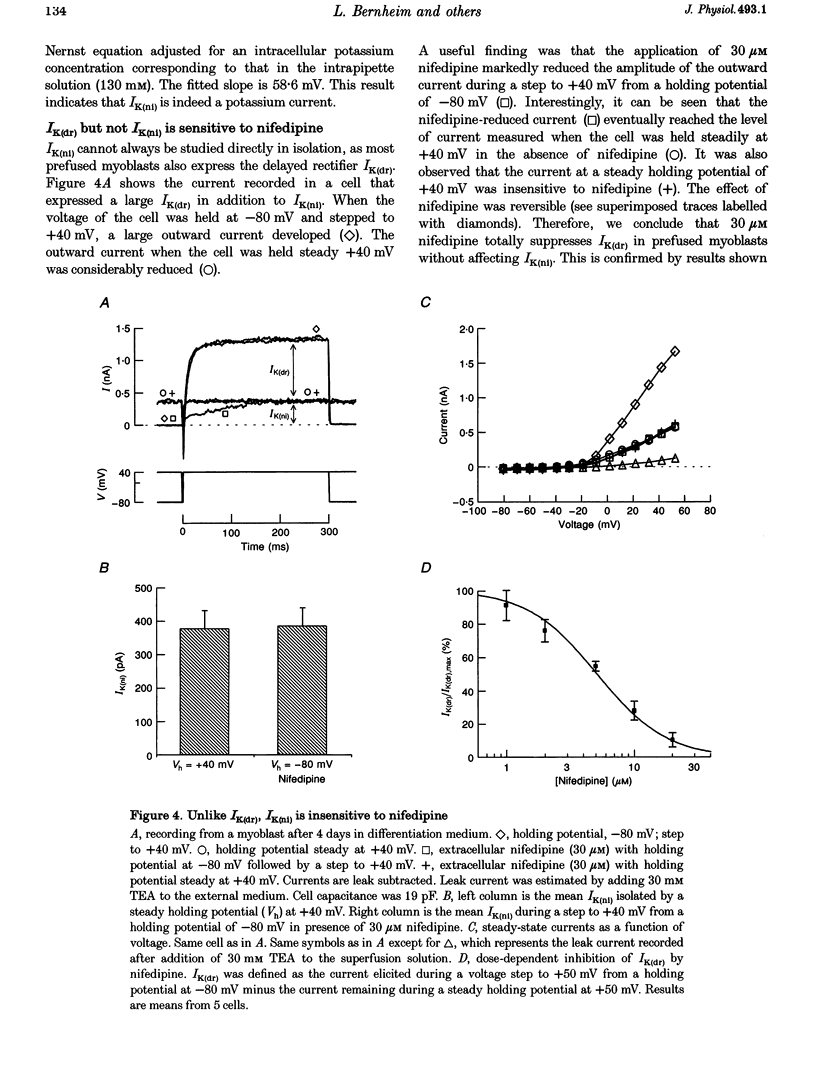
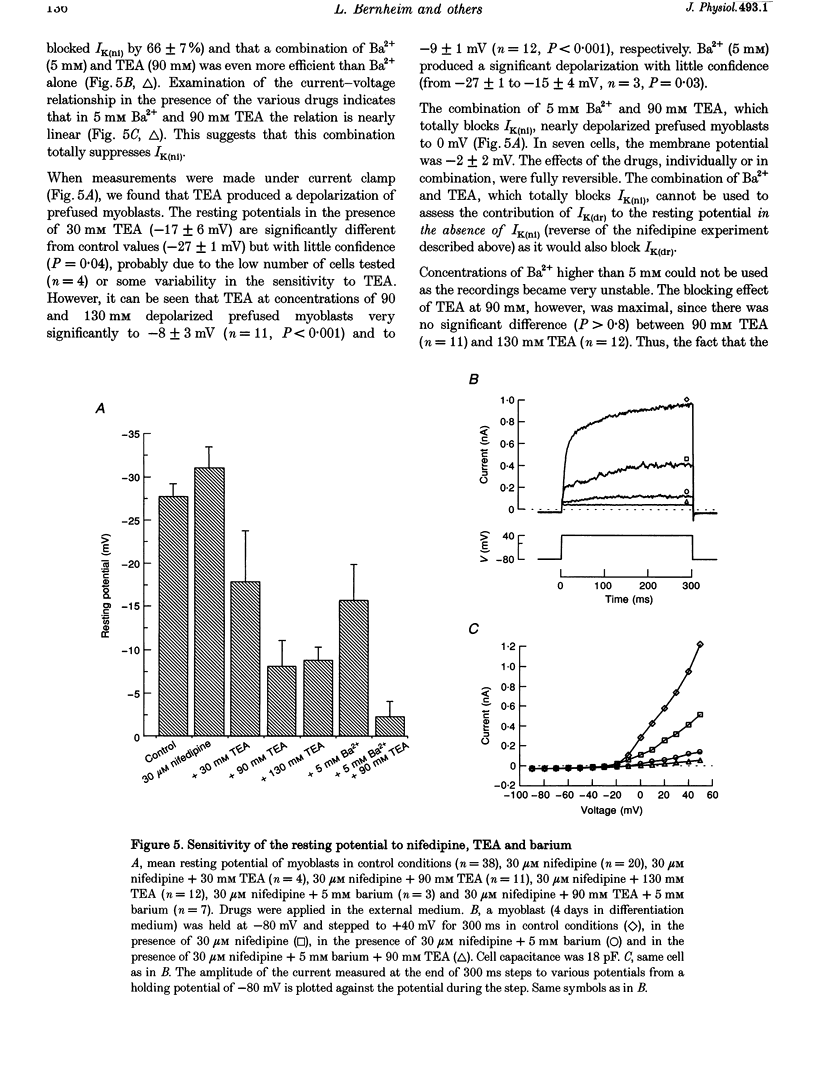
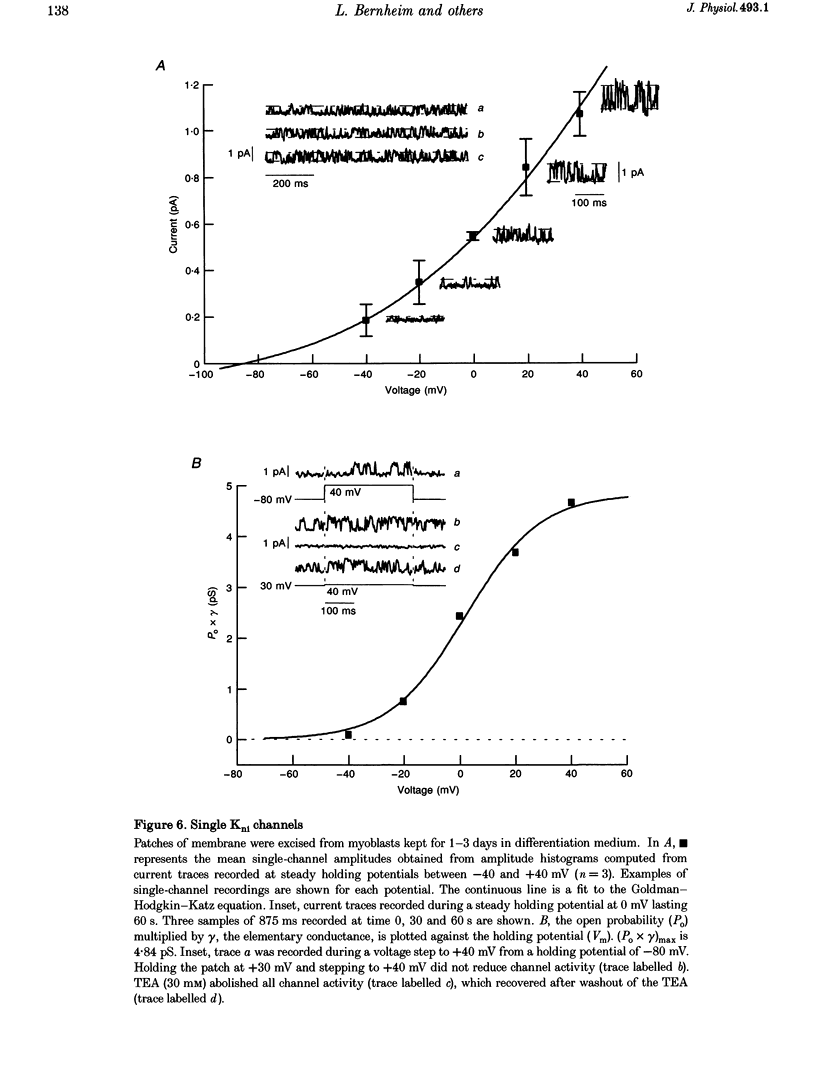
1. Using the patch-clamp technique, a new non-inactivating voltage-gated potassium current, IK(ni), was studied in cultured fusion-competent human myoblasts. 2. IK(ni) is activated at voltages above -50 mV and its conductance reaches its maximum around +50 mV. Once activated, the current remains at a steady level for minutes. 3. Reversal potential measurements at various extracellular potassium concentrations indicate that potassium ions are the major charge carriers of IK(ni). 4. IK(ni) is insensitive to potassium channel blockers such as charybdotoxin, dendrotoxins, mast cell degranulating (MCD) peptide, 4-aminopyridine (4-AP), 3,4-diaminopyridine (3,4-DAP) and apamin, but can be blocked by high concentrations of TEA and by Ba2+. 5. A potassium channel of small conductance (8.4 pS at +40 mV) with potential dependence and pharmacological properties corresponding to those of IK(ni) in whole-cell recording is described. 6. IK(ni) participates in the control of the resting potential of fusion-competent myoblasts, suggesting that it may play a key role in the process of myoblast fusion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroffio A., Aubry J. P., Kaelin A., Krause R. M., Hamann M., Bader C. R. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993 May;16(5):498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Cognard C., Constantin B., Rivet-Bastide M., Raymond G. Intracellular calcium transients induced by different kinds of stimulus during myogenesis of rat skeletal muscle cells studied by laser cytofluorimetry with Indo-1. Cell Calcium. 1993 Apr;14(4):333–348. doi: 10.1016/0143-4160(93)90054-a. [DOI] [PubMed] [Google Scholar]
- Entwistle A., Zalin R. J., Bevan S., Warner A. E. The control of chick myoblast fusion by ion channels operated by prostaglandins and acetylcholine. J Cell Biol. 1988 May;106(5):1693–1702. doi: 10.1083/jcb.106.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Nameroff M., Nelson P. G. Electrical properties of chick skeletal muscle fibers developing in cell culture. J Cell Physiol. 1971 Oct;78(2):289–299. doi: 10.1002/jcp.1040780218. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Washabau R. J., Kotlikoff M. I. Control of resting membrane potential by delayed rectifier potassium currents in ferret airway smooth muscle cells. J Physiol. 1993 Sep;469:625–638. doi: 10.1113/jphysiol.1993.sp019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M., Widmer H., Baroffio A., Aubry J. P., Krause R. M., Kaelin A., Bader C. R. Sodium and potassium currents in freshly isolated and in proliferating human muscle satellite cells. J Physiol. 1994 Mar 1;475(2):305–317. doi: 10.1113/jphysiol.1994.sp020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Dobi E. T., Woodford E. J., Petrides S., Ernst M., Nichols E. B. Prostaglandin binding activity and myoblast fusion in aggregates of avian myoblasts. Dev Biol. 1986 Jan;113(1):40–48. doi: 10.1016/0012-1606(86)90106-5. [DOI] [PubMed] [Google Scholar]
- Krause R. M., Hamann M., Bader C. R., Liu J. H., Baroffio A., Bernheim L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. J Physiol. 1995 Dec 15;489(Pt 3):779–790. doi: 10.1113/jphysiol.1995.sp021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche H., Fahlke C., Iaizzo P. A., Lehmann-Horn F. Characterization of the high-conductance Ca(2+)-activated K+ channel in adult human skeletal muscle. Pflugers Arch. 1995 Mar;429(5):738–747. doi: 10.1007/BF00373997. [DOI] [PubMed] [Google Scholar]
- Mlinar B., Enyeart J. J. Identical inhibitory modulation of A-type potassium currents by dihydropyridine calcium channel agonists and antagonists. Mol Pharmacol. 1994 Oct;46(4):743–749. [PubMed] [Google Scholar]
- Nerbonne J. M., Gurney A. M. Blockade of Ca2+ and K+ currents in bag cell neurons of Aplysia californica by dihydropyridine Ca2+ antagonists. J Neurosci. 1987 Mar;7(3):882–893. doi: 10.1523/JNEUROSCI.07-03-00882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabia V., Klip A. Regulation of cytosolic Ca2+ in clonal human muscle cell cultures. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1130–1137. doi: 10.1016/0006-291x(89)92720-4. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Spector I., Prives J. M. Development of electrophysiological and biochemical membrane properties during differentiation of embryonic skeletal muscle in culture. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5166–5170. doi: 10.1073/pnas.74.11.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair J. A., Meyer-Demarest S. D., Ham R. G. Improved medium with EGF and BSA for differentiated human skeletal muscle cells. Muscle Nerve. 1992 Jul;15(7):774–779. doi: 10.1002/mus.880150705. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Delaporte C., Marty A. Voltage-dependent channels of human muscle cultures. Pflugers Arch. 1986 Feb;406(2):163–172. doi: 10.1007/BF00586678. [DOI] [PubMed] [Google Scholar]
- Valmier J., Richard S., Devic E., Nargeot J., Simonneau M., Baldy-Moulinier M. Dihydropyridines interact with calcium-independent potassium currents in embryonic mammalian sensory neurons. Pflugers Arch. 1991 Oct;419(3-4):281–287. doi: 10.1007/BF00371108. [DOI] [PubMed] [Google Scholar]
- Wei A., Covarrubias M., Butler A., Baker K., Pak M., Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990 May 4;248(4955):599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- Widmer H., Hamann M., Baroffio A., Bijlenga P., Bader C. R. Expression of a voltage-dependent potassium current precedes fusion of human muscle satellite cells (myoblasts). J Cell Physiol. 1995 Jan;162(1):52–63. doi: 10.1002/jcp.1041620108. [DOI] [PubMed] [Google Scholar]
- Zhao B., Rassendren F., Kaang B. K., Furukawa Y., Kubo T., Kandel E. R. A new class of noninactivating K+ channels from aplysia capable of contributing to the resting potential and firing patterns of neurons. Neuron. 1994 Nov;13(5):1205–1213. doi: 10.1016/0896-6273(94)90058-2. [DOI] [PubMed] [Google Scholar]


