Abstract
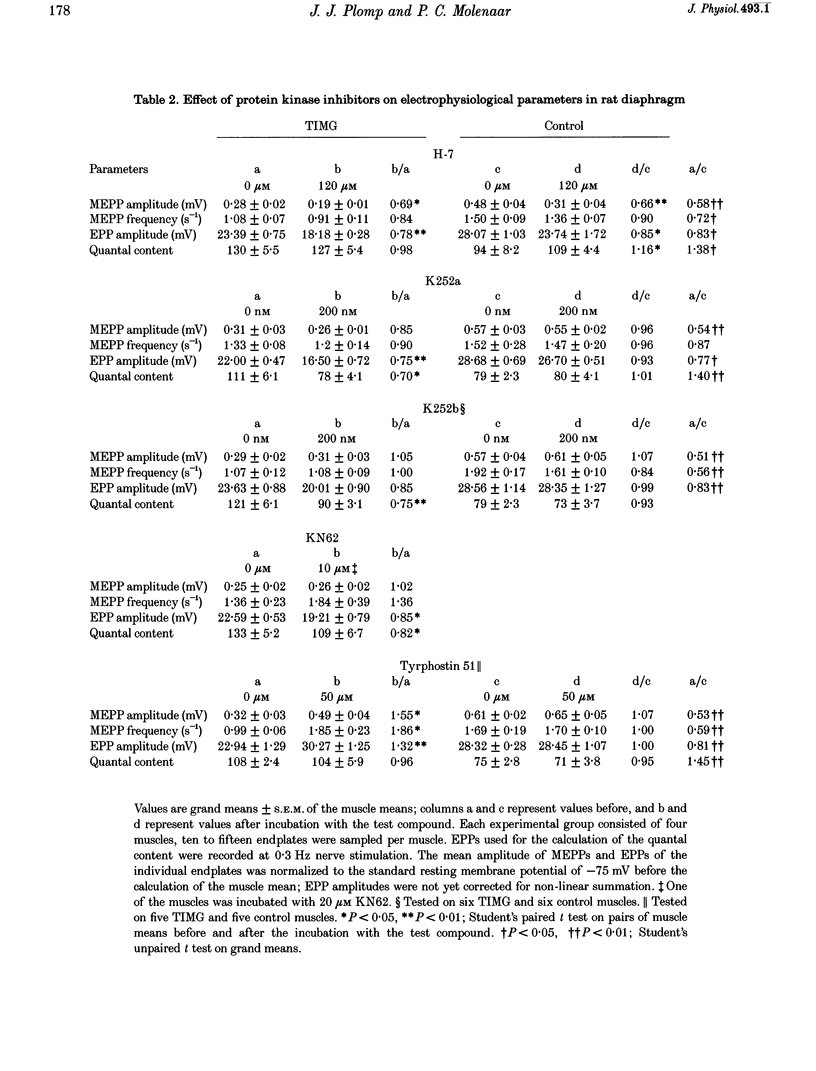
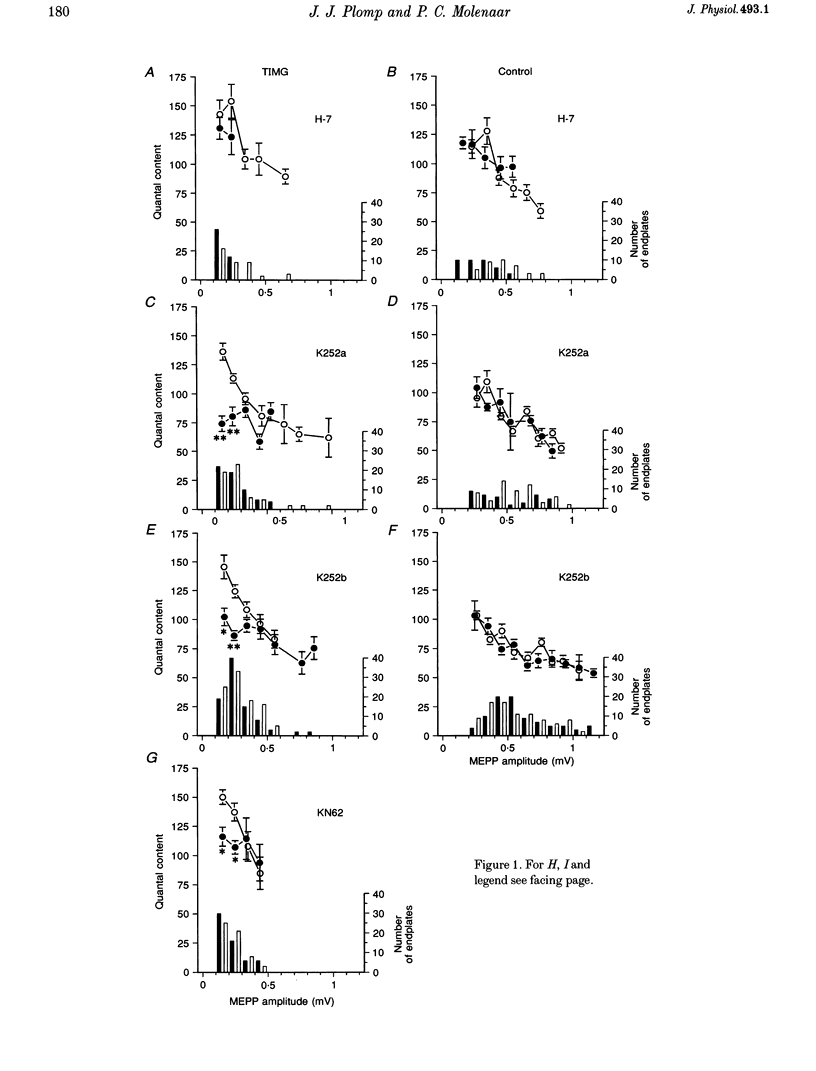
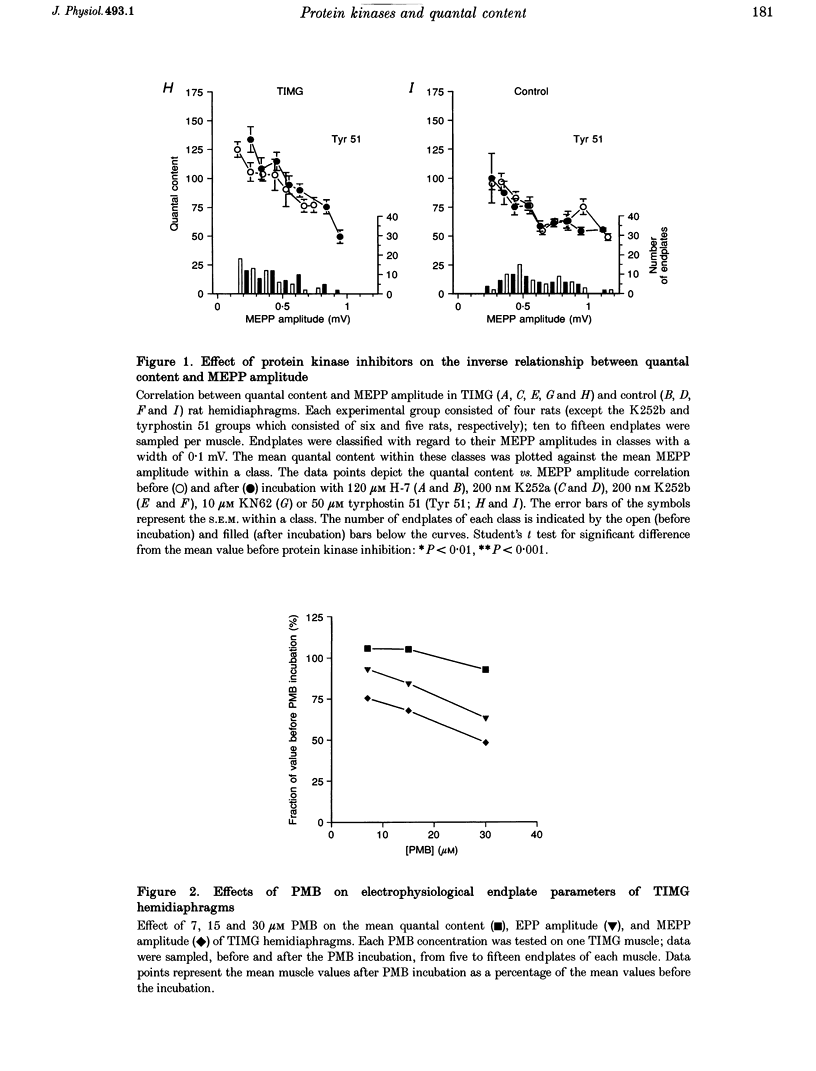
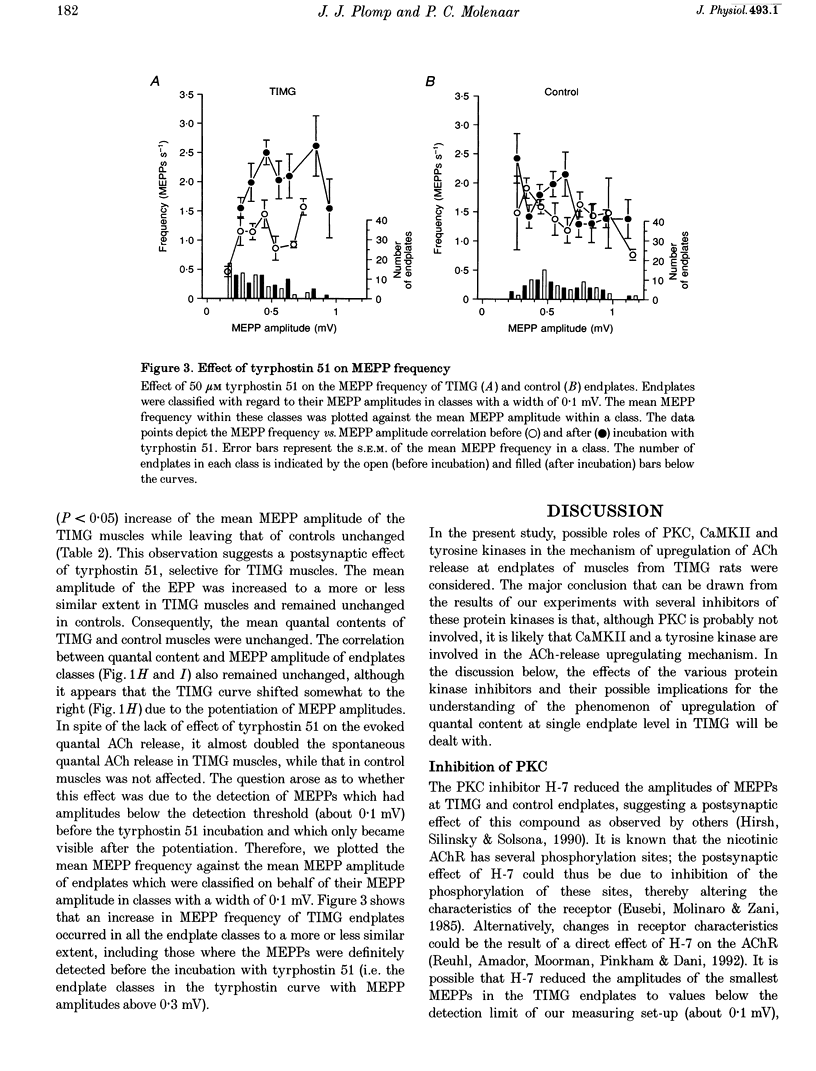
1. ACh release from motor nerve endings in diaphragms of rats treated chronically with alpha-bungarotoxin (alpha-BuTX) is upregulated at the level of the individual endplate. Involvement of protein kinases in this mechanism of synaptic adaptation was investigated. 2. Miniature endplate potentials (MEPPs) and endplate potentials (EPPs) were recorded after mu-conotoxin treatment, which prevents muscle action potentials. The quantal content at endplates was calculated 'directly', i.e. by dividing the EPP amplitude by the MEPP amplitude. 3. Incubation of muscles from control and alpha-BuTX-treated rats with H-7, a protein kinase C (PKC) inhibitor, reduced MEPP amplitudes but had no clear effect on quantal contents. Polymyxin B, another PKC inhibitor, had a similar effect on muscles from alpha-BuTX-treated rats. 4. Incubation of muscles from alpha-BuTX-treated rats with K252a, a broad-spectrum protein kinase inhibitor of, amongst others, PKC, Ca(2+)-calmodulin-dependent protein kinase II (CaMKII) and neurotrophin receptor tyrosine kinases, resulted in a 30% decrease of the quantal content. However, K252a did not change the quantal content of controls. Incubations with the closely related compound K252b, which has an exclusively extracellular action, had a similar effect. 5. KN62, a specific inhibitor of CaMKII, decreased the mean quantal content of muscles from alpha-BuTX-treated rats by 18%. 6. Tyrphostin 51, a selective tyrosine kinase inhibitor, had no effect on quantal contents of muscles from alpha-BuTX-treated and control rats. However, it increased the frequency and amplitude of MEPPs in muscles from alpha-BuTX-treated rats, leaving those of controls unchanged. 7. The extent of reduction of quantal content, caused by K252a, K252b and KN62, varied between endplates of individual muscles from alpha-BuTX-treated rats; quantal contents at endplates with small MEPPs were more sensitive than those at endplates with large MEPPs. 8. It is concluded that PKC does not play a role in the mechanism of upregulation of ACh release at endplates of alpha-BuTX-treated rats. Instead, CaMKII and some tyrosine kinases in the presynaptic membrane, as well as in the cytoplasm, might be involved.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castrén E., Pitkänen M., Sirviö J., Parsadanian A., Lindholm D., Thoenen H., Riekkinen P. J. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993 Jul;4(7):895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Cheng B., Barger S. W., Mattson M. P. Staurosporine, K-252a, and K-252b stabilize calcium homeostasis and promote survival of CNS neurons in the absence of glucose. J Neurochem. 1994 Apr;62(4):1319–1329. doi: 10.1046/j.1471-4159.1994.62041319.x. [DOI] [PubMed] [Google Scholar]
- Di Gregorio F., Fesce R., Cereser S., Favaro G., Fiori M. G. Spontaneous and nerve-evoked quantal transmission in regenerated motor terminals. Cell Biol Int Rep. 1989 Dec;13(12):1119–1126. doi: 10.1016/0309-1651(89)90025-8. [DOI] [PubMed] [Google Scholar]
- Durant N. N., Lambert J. J. The action of polymyxin B at the frog neuromuscular junction. Br J Pharmacol. 1981 Jan;72(1):41–47. doi: 10.1111/j.1476-5381.1981.tb09102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H., Belluardo N., Arenas E., Yamamoto Y., Casabona A., Persson H., Ibáez C. F. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995 Jun 9;268(5216):1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Nakayama T., Teramoto T., Kato H., Watanabe T., Kinoshita M., Tsukamoto K., Tokunaga K., Kurokawa K., Nakanishi S. Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative, KT5926. Biochem Biophys Res Commun. 1991 Nov 27;181(1):423–429. doi: 10.1016/s0006-291x(05)81436-6. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hirsh J. K., Silinsky E. M., Solsona C. S. The role of cyclic AMP and its protein kinase in mediating acetylcholine release and the action of adenosine at frog motor nerve endings. Br J Pharmacol. 1990 Oct;101(2):311–318. doi: 10.1111/j.1476-5381.1990.tb12707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn A., Leibrock J., Bailey K., Barde Y. A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990 Mar 22;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989 Jul;97(3):934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Schuman E. M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995 Mar 17;267(5204):1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Knipper M., da Penha Berzaghi M., Blöchl A., Breer H., Thoenen H., Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994 Apr 1;6(4):668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992 Dec;59(6):1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995 Mar 24;267(5205):1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof A. M., Ip N. Y., Poo M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Mazzei G. J., Katoh N., Kuo J. F. Polymyxin B is a more selective inhibitor for phospholipid-sensitive Ca2+-dependent protein kinase than for calmodulin-sensitive Ca2+-dependent protein kinase. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1129–1133. doi: 10.1016/0006-291x(82)91894-0. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Nakanishi S., Matsuda Y. Inhibition of nerve growth factor-induced neurite outgrowth of PC12 cells by a protein kinase inhibitor which does not permeate the cell membrane. FEBS Lett. 1991 Nov 18;293(1-2):119–123. doi: 10.1016/0014-5793(91)81165-5. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Yin Q. W., Prevette D., Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992 Dec 24;360(6406):755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- Pettit D. L., Perlman S., Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994 Dec 16;266(5192):1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- Plomp J. J., Van Kempen G. T., De Baets M. B., Graus Y. M., Kuks J. B., Molenaar P. C. Acetylcholine release in myasthenia gravis: regulation at single end-plate level. Ann Neurol. 1995 May;37(5):627–636. doi: 10.1002/ana.410370513. [DOI] [PubMed] [Google Scholar]
- Plomp J. J., van Kempen G. T., Molenaar P. C. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992 Dec;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp J. J., van Kempen G. T., Molenaar P. C. The upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats: its dependency on calcium. J Physiol. 1994 Jul 1;478(Pt 1):125–136. doi: 10.1113/jphysiol.1994.sp020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuhl T. O., Amador M., Moorman J. R., Pinkham J., Dani J. A. Nicotinic acetylcholine receptors are directly affected by agents used to study protein phosphorylation. J Neurophysiol. 1992 Aug;68(2):407–416. doi: 10.1152/jn.1992.68.2.407. [DOI] [PubMed] [Google Scholar]
- Rosahl T. W., Spillane D., Missler M., Herz J., Selig D. K., Wolff J. R., Hammer R. E., Malenka R. C., Südhof T. C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995 Jun 8;375(6531):488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Tapley P., Lamballe F., Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992 Feb;7(2):371–381. [PubMed] [Google Scholar]
- Thoenen H., Hughes R. A., Sendtner M. Trophic support of motoneurons: physiological, pathophysiological, and therapeutic implications. Exp Neurol. 1993 Nov;124(1):47–55. doi: 10.1006/exnr.1993.1173. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Wong V., Arriaga R., Ip N. Y., Lindsay R. M. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur J Neurosci. 1993 May 1;5(5):466–474. doi: 10.1111/j.1460-9568.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]


