Abstract
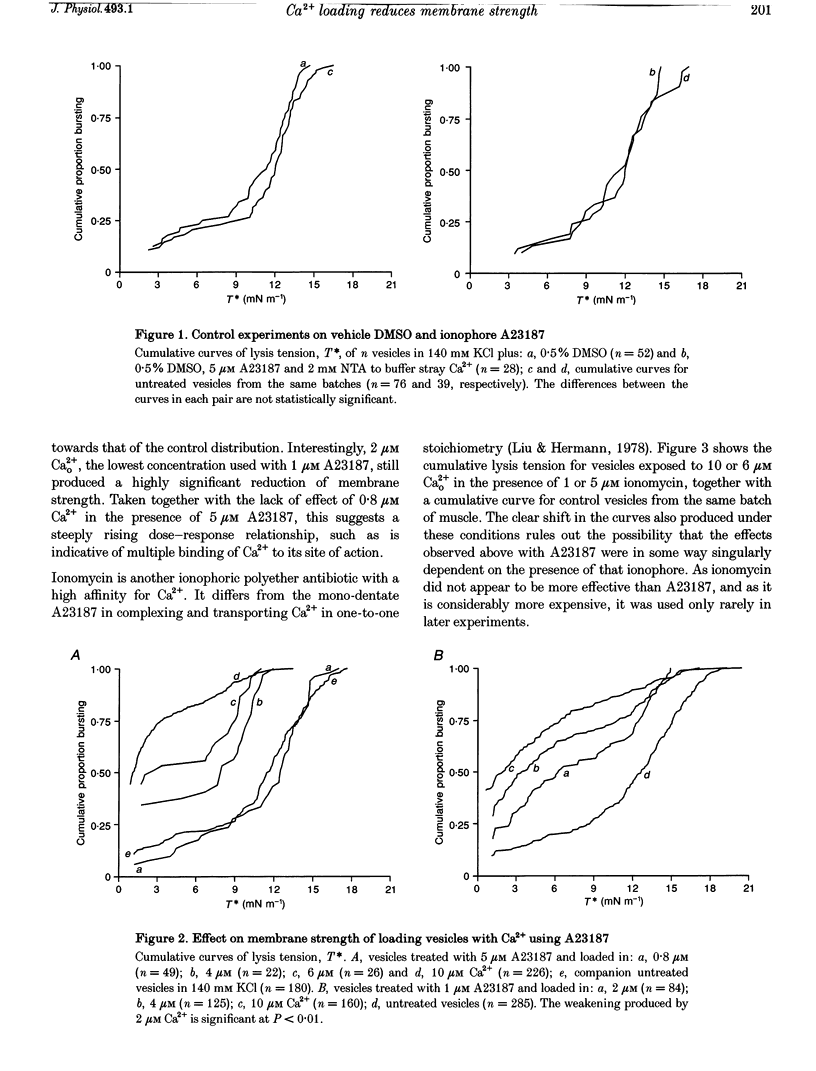
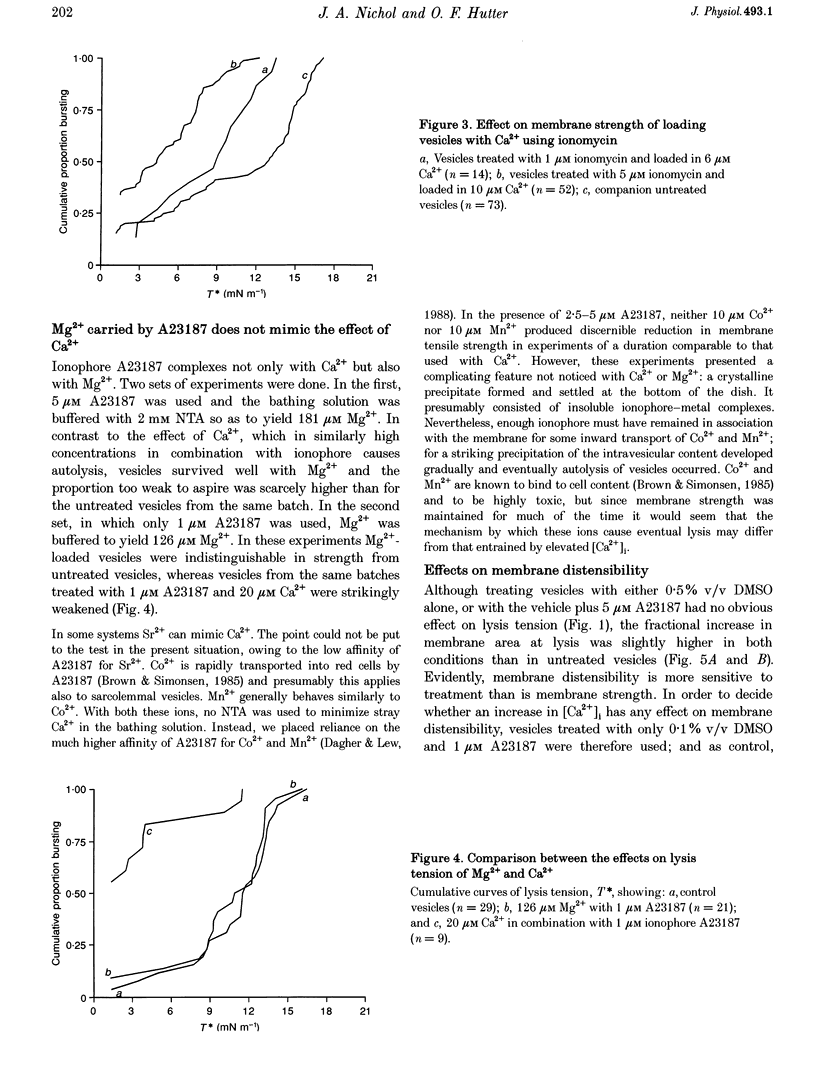
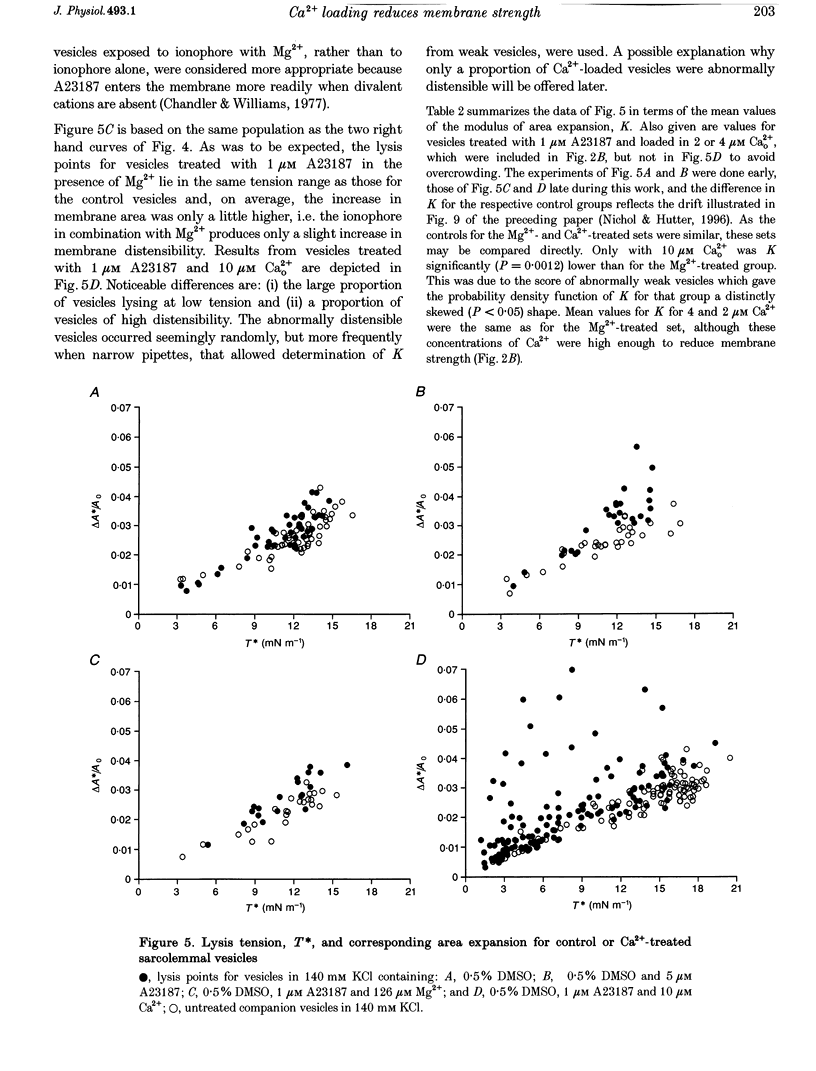
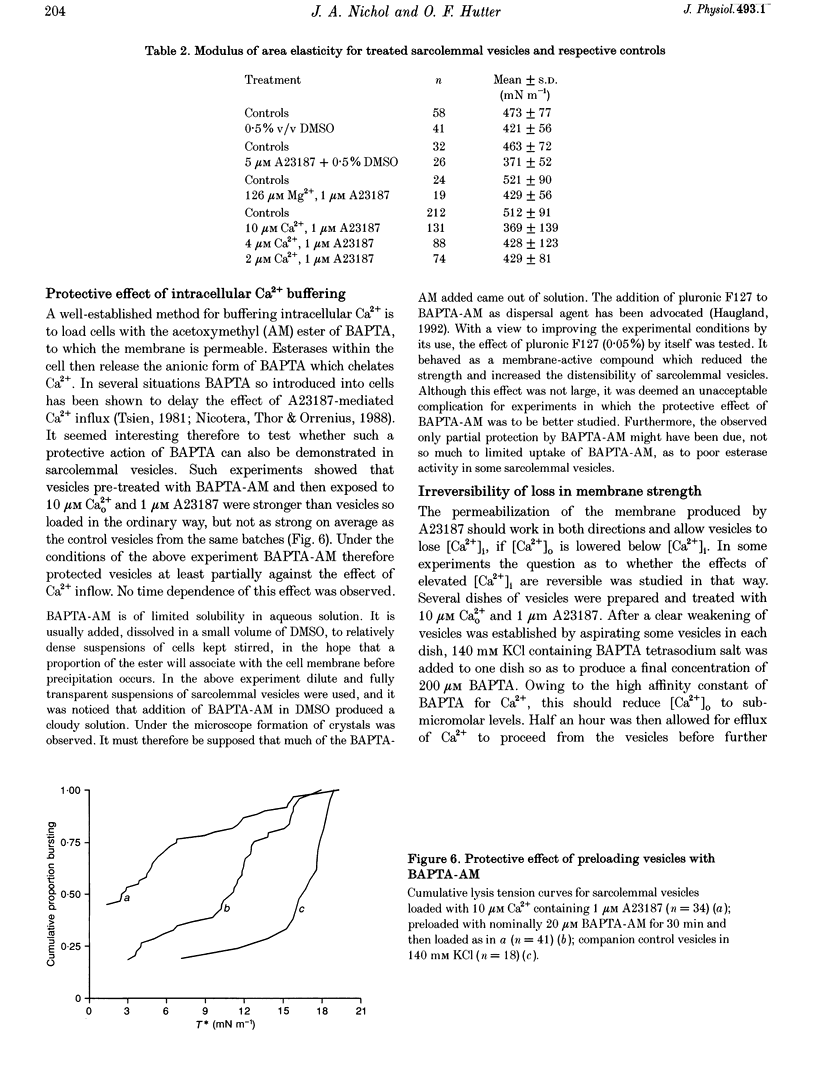
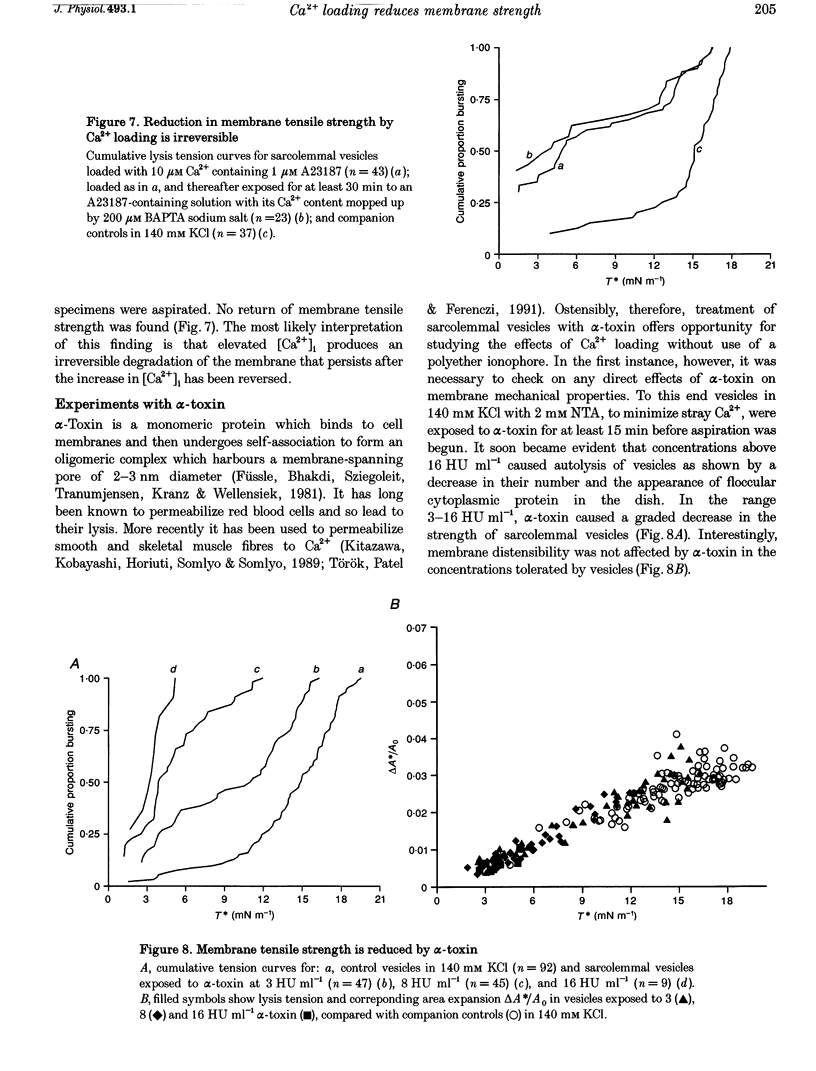
1. Sarcolemmal vesicles shed by rabbit muscle were loaded with Ca2+ by means of A23187 or ionomycin. [Ca2+]0 was buffered between 0.8 and 20 microM. Membrane strength was measured by pipette aspiration. 2. At 20 microM Ca2+ many vesicles underwent autolysis, or were so weak that they burst instantly on aspiration. Between 10 and 2 microM Ca2+ a graded decrease in membrane strength was demonstrable. At 0.8 microM Ca2+ the mechanical properties of the sarcolemma remained unaltered. 3. Mg2+ carried by A23187 does not mimic the effect of Ca2+. The ionophore itself similarly did not cause a decrease in membrane tensile strength. 4. Pre-treatment with BAPTA-AM, so as to buffer internal Ca2+, partly protected vesicles against the decrease in membrane strength produced by Ca2+ loading. 5. Membrane strength was not restored by adding excess BAPTA to the bathing solution, so as to reverse the Ca2+ gradient. An irreversible degradation of the membrane consequent upon raised [Ca2+]1 seems indicated. 6. These findings are discussed in relation to the mechanisms which have been advanced to account for the role of elevated [Ca2+]1 in cell death. 7. An attempt to use staphylococcal alpha-toxin as an alternative means to permeabilize the sarcolemma led to the incidental finding that this pore-forming protein itself greatly weakens the membrane in doses lower than required for effective permeabilization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire A., Piper H. M., Cuthbertson K. S., Cobbold P. H. Cytosolic free Ca2+ in single rat heart cells during anoxia and reoxygenation. Biochem J. 1987 Jun 1;244(2):381–385. doi: 10.1042/bj2440381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton F., Dörstelmann U., Hutter O. F. Single-channel activity in sarcolemmal vesicles from human and other mammalian muscles. Muscle Nerve. 1988 Oct;11(10):1029–1038. doi: 10.1002/mus.880111004. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular uptake and alpha-amylase and lactate dehydrogenase releasing actions of the divalent cation ionophore A23187 in dissociated pancreatic acinar cells. J Membr Biol. 1977 Apr 22;32(3-4):201–230. doi: 10.1007/BF01905220. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Dagher G., Lew V. L. Maximal calcium extrusion capacity and stoichiometry of the human red cell calcium pump. J Physiol. 1988 Dec;407:569–586. doi: 10.1113/jphysiol.1988.sp017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Needham D. Giant vesicle bilayers composed of mixtures of lipids, cholesterol and polypeptides. Thermomechanical and (mutual) adherence properties. Faraday Discuss Chem Soc. 1986;(81):267–280. doi: 10.1039/dc9868100267. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobzik L., Reid M. B., Bredt D. S., Stamler J. S. Nitric oxide in skeletal muscle. Nature. 1994 Dec 8;372(6506):546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Man R. Y., Slater T. L., Pelletier M. P., Choy P. C. Alterations of phospholipids in ischemic canine myocardium during acute arrhythmia. Lipids. 1983 Oct;18(10):677–681. doi: 10.1007/BF02534533. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Nichol J. A., Hutter O. F. Tensile strength and dilatational elasticity of giant sarcolemmal vesicles shed from rabbit muscle. J Physiol. 1996 May 15;493(Pt 1):187–198. doi: 10.1113/jphysiol.1996.sp021374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Radi R., Beckman J. S., Bush K. M., Freeman B. A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991 Aug 1;288(2):481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- Saberwal G., Nagaraj R. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim Biophys Acta. 1994 Jun 29;1197(2):109–131. doi: 10.1016/0304-4157(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Salamino F., Sparatore B., Melloni E., Michetti M., Viotti P. L., Pontremoli S., Carafoli E. The plasma membrane calcium pump is the preferred calpain substrate within the erythrocyte. Cell Calcium. 1994 Jan;15(1):28–35. doi: 10.1016/0143-4160(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Verhoven B., Schlegel R. A., Williamson P. Rapid loss and restoration of lipid asymmetry by different pathways in resealed erythrocyte ghosts. Biochim Biophys Acta. 1992 Feb 17;1104(1):15–23. doi: 10.1016/0005-2736(92)90126-7. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Post J. A. Physico-chemical properties and organization of lipids in membranes: their possible role in myocardial injury. Basic Res Cardiol. 1987;82 (Suppl 1):85–91. doi: 10.1007/978-3-662-08390-1_10. [DOI] [PubMed] [Google Scholar]
- Williamson P., Algarin L., Bateman J., Choe H. R., Schlegel R. A. Phospholipid asymmetry in human erythrocyte ghosts. J Cell Physiol. 1985 May;123(2):209–214. doi: 10.1002/jcp.1041230209. [DOI] [PubMed] [Google Scholar]
- Williamson P., Kulick A., Zachowski A., Schlegel R. A., Devaux P. F. Ca2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry. 1992 Jul 14;31(27):6355–6360. doi: 10.1021/bi00142a027. [DOI] [PubMed] [Google Scholar]
- Zachowski A., Favre E., Cribier S., Hervé P., Devaux P. F. Outside-inside translocation of aminophospholipids in the human erythrocyte membrane is mediated by a specific enzyme. Biochemistry. 1986 May 6;25(9):2585–2590. doi: 10.1021/bi00357a046. [DOI] [PubMed] [Google Scholar]
- Zaidi S. I., Narahara H. T. Degradation of skeletal muscle plasma membrane proteins by calpain. J Membr Biol. 1989 Sep;110(3):209–216. doi: 10.1007/BF01869151. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Hutter O. F., Karpati G., Klamut H. J., Bulman D. E., Hodges R. S., Worton R. G., Ray P. N. Dystrophin is tightly associated with the sarcolemma of mammalian skeletal muscle fibers. Exp Cell Res. 1991 Jan;192(1):278–288. doi: 10.1016/0014-4827(91)90187-y. [DOI] [PubMed] [Google Scholar]


