Abstract
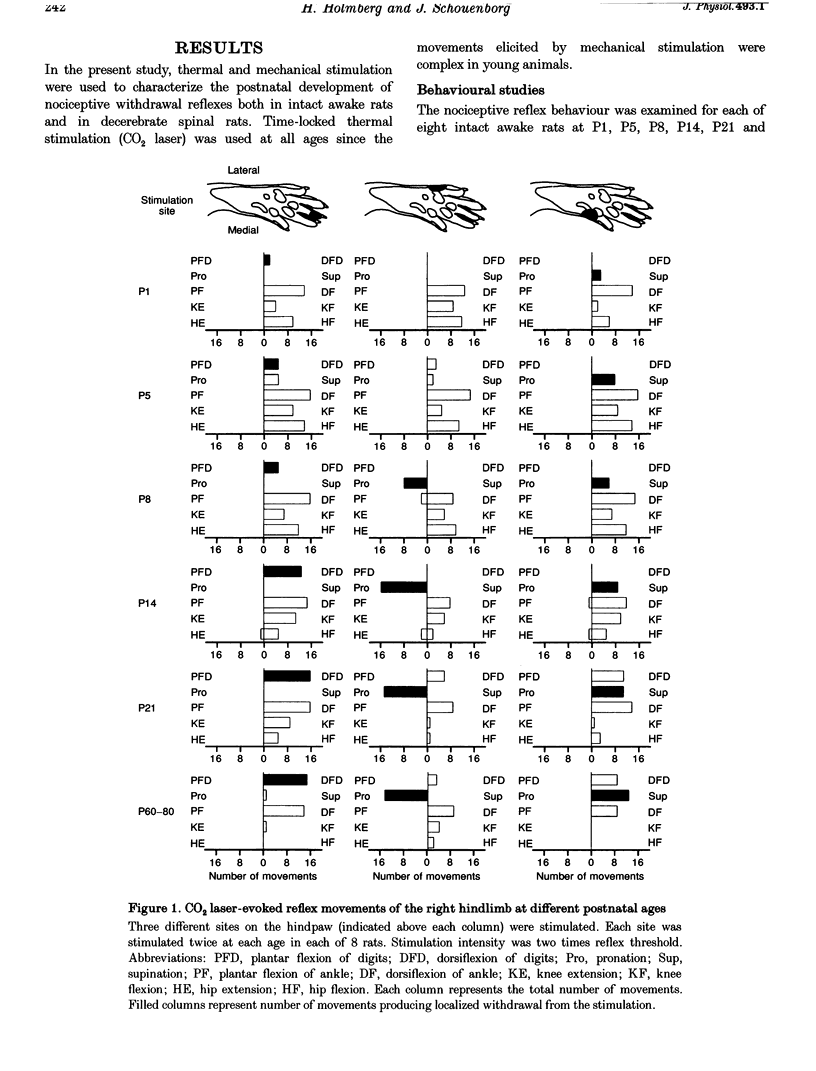
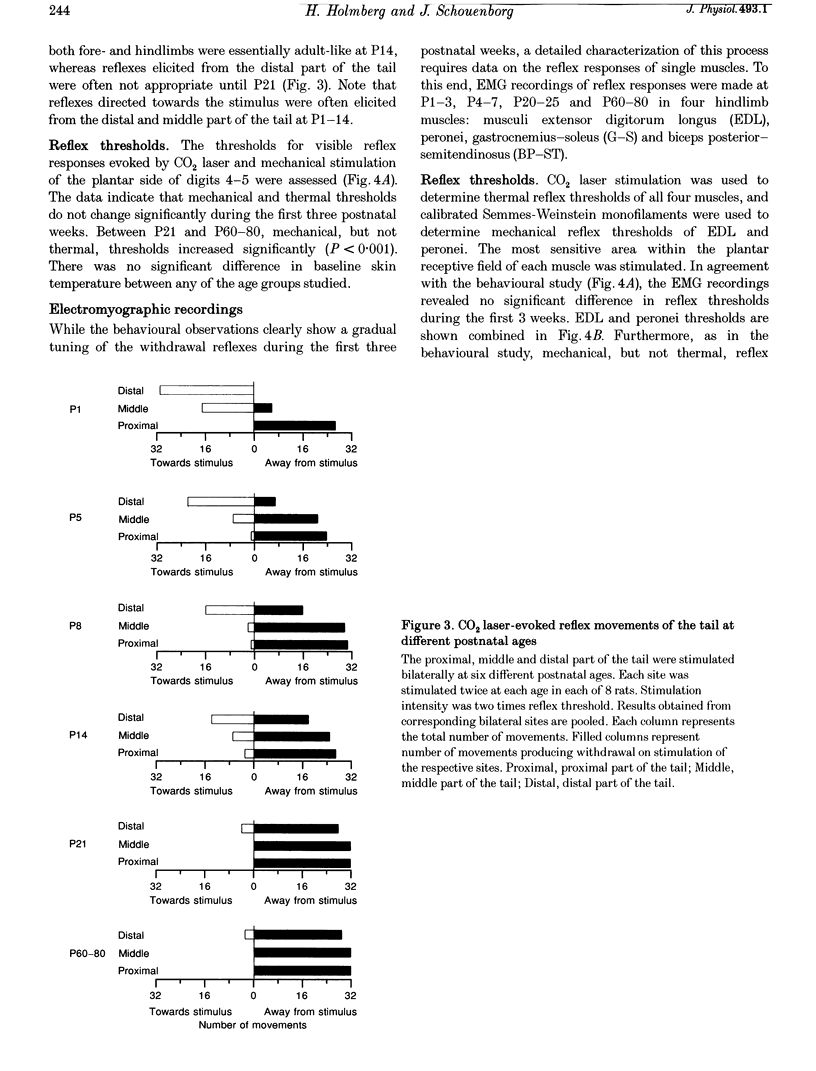
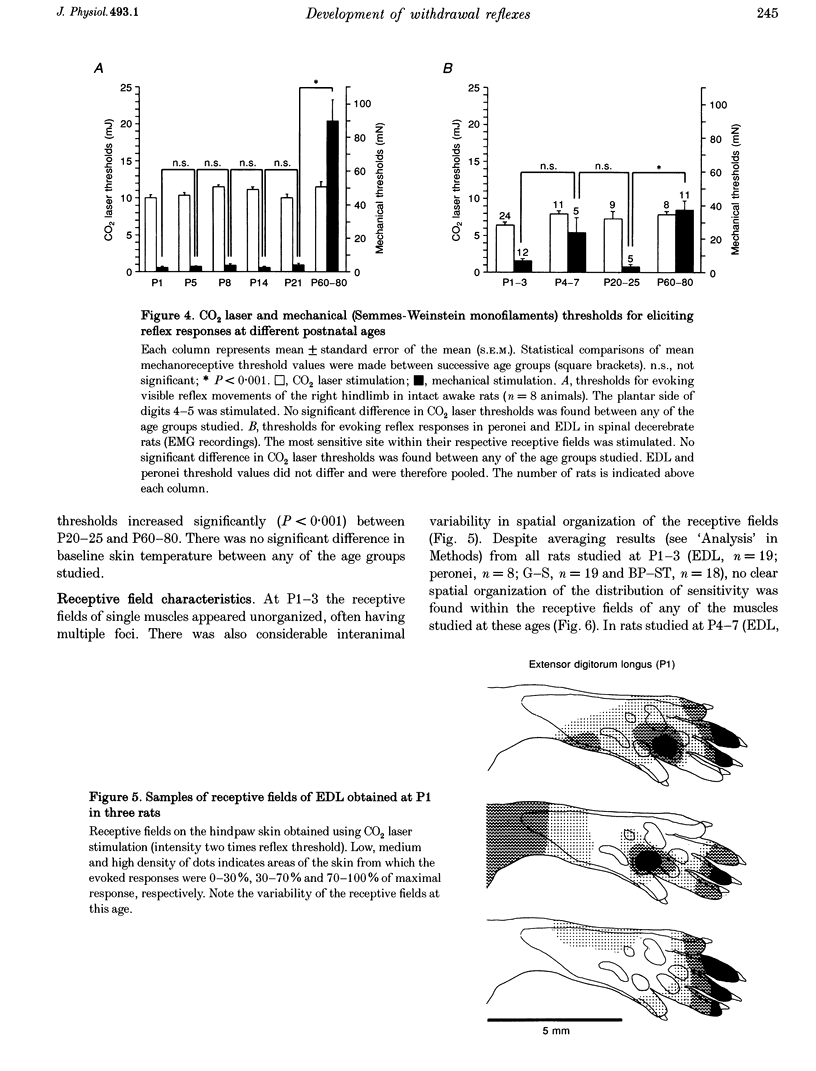
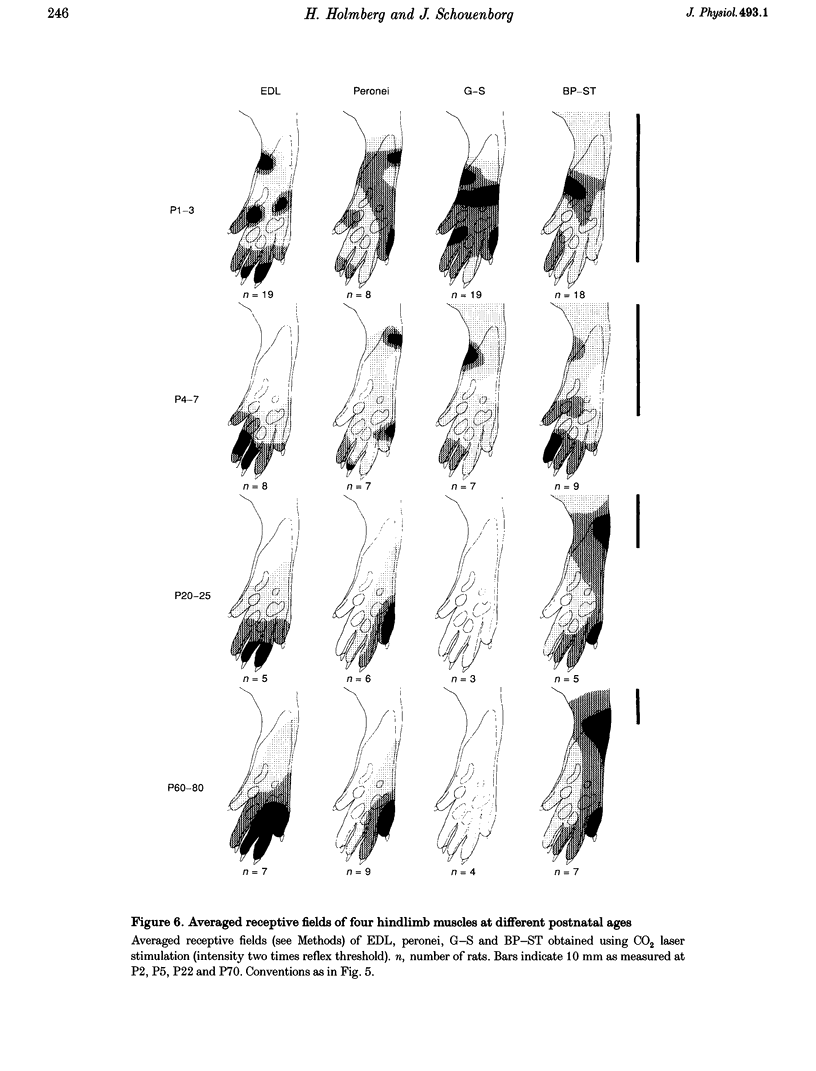
1. The postnatal development of nociceptive withdrawal reflexes was studied. In awake intact rats, forelimb, hindlimb and tail reflexes were recorded on videotape. In decerebrate spinal rats, electromyography (EMG) was used to record nociceptive withdrawal reflexes in musculi extensor digitorum longus (EDL), peronei, gastrocnemius-soleus (G-S) and biceps posterior-semitendinosus (BP-ST). Thermal (short-lasting CO2 laser pulses) and mechanical stimulation were used. 2. In adults, nociceptive withdrawal reflexes were typically well directed and reflex pathways to single hindlimb muscles had functionally adapted receptive fields. By contrast, at postnatal day (P) 1-7, the nociceptive withdrawal reflexes were often inappropriate, sometimes producing movements towards the stimulation, and EMG recordings revealed unadapted variable receptive fields. With increasing age, the nociceptive withdrawal reflexes progressively became well directed, thus producing localized withdrawal. Both withdrawal movements and spatial organization of the receptive fields were adult-like at P20-25. 3. Up to P25, reflex thresholds were more or less constant in both intact awake rats and spinal decerebrate rats, except in G-S in which no nociceptive withdrawal reflexes were evoked from P20 on. After P25, mechanical, but not thermal, thresholds increased dramatically. 4. EMG recordings revealed that during the first three postnatal weeks, the latency of the CO2 laser-evoked nociceptive withdrawal reflexes decreased significantly in peronei and BP-ST, but not in EDL, and thereafter increased significantly in peronei, BP-ST and EDL. The magnitude of the nociceptive withdrawal reflexes in these muscles increased markedly between P7 and P20 and showed little change thereafter. 5. Possible mechanisms underlying the postnatal tuning of the nociceptive withdrawal reflexes are discussed.
Full text
PDF



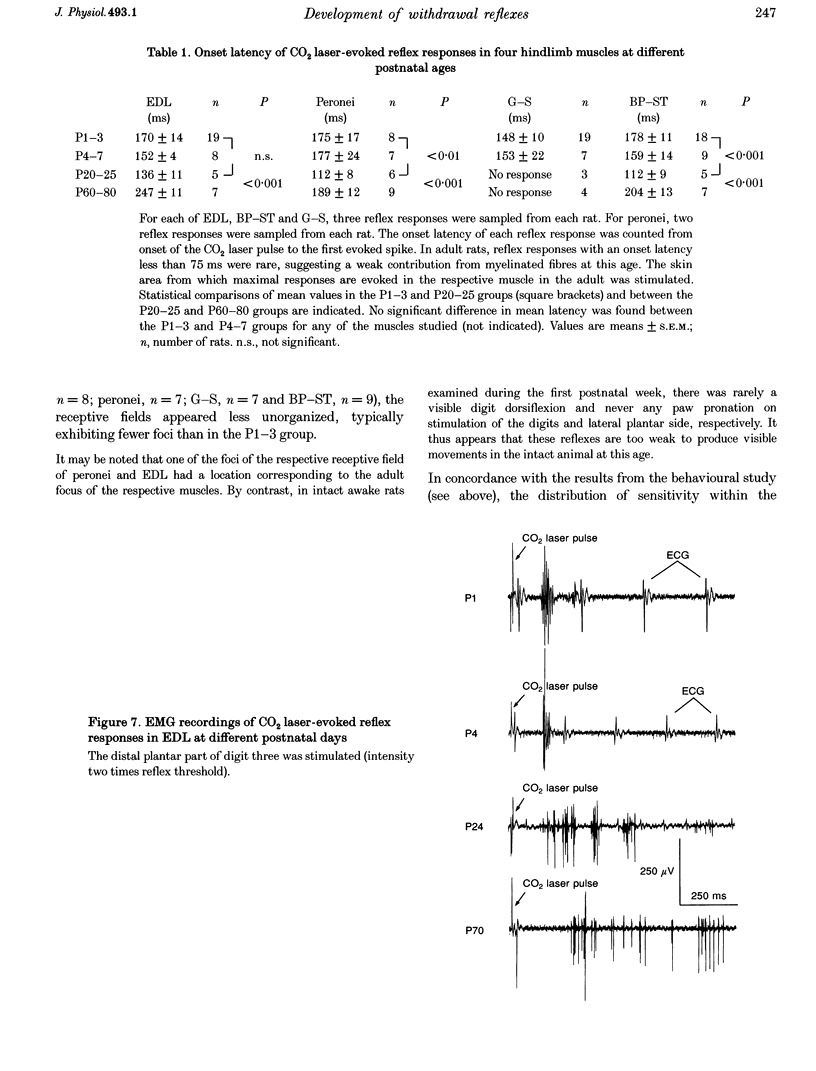
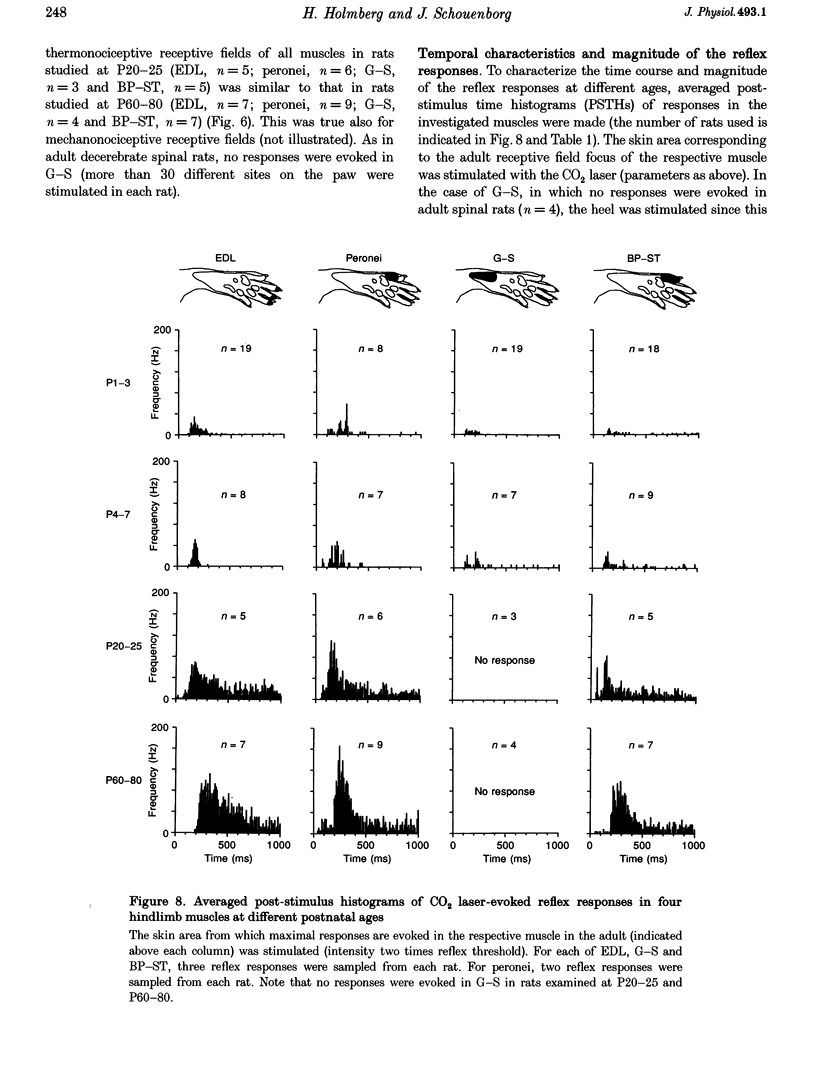




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bicknell H. R., Jr, Beal J. A. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol. 1984 Jul 10;226(4):508–522. doi: 10.1002/cne.902260406. [DOI] [PubMed] [Google Scholar]
- Commissiong J. W. Development of catecholaminergic nerves in the spinal cord of the rat. Brain Res. 1983 Apr 4;264(2):197–208. doi: 10.1016/0006-8993(83)90817-x. [DOI] [PubMed] [Google Scholar]
- Devor M., Carmon A., Frostig R. Primary afferent and spinal sensory neurons that respond to brief pulses of intense infrared laser radiation: a preliminary survey in rats. Exp Neurol. 1982 Jun;76(3):483–494. doi: 10.1016/0014-4886(82)90118-2. [DOI] [PubMed] [Google Scholar]
- Ekholm J. Postnatal changes in cutaneous reflexes and in the discharge pattern of cutaneous and articular sense organs. A morphological and physiological study in the cat. Acta Physiol Scand Suppl. 1967;297:1–130. [PubMed] [Google Scholar]
- Fields R. D., Nelson P. G. Activity-dependent development of the vertebrate nervous system. Int Rev Neurobiol. 1992;34:133–214. doi: 10.1016/s0074-7742(08)60098-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Butcher T., Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. J Comp Neurol. 1994 Oct 8;348(2):225–233. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol. 1987 Feb;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984 Nov;13(3):933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Prenatal growth of fine-diameter primary afferents into the rat spinal cord: a transganglionic tracer study. J Comp Neurol. 1987 Jul 1;261(1):98–104. doi: 10.1002/cne.902610108. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shaw A., MacIntosh N. Postnatal development of the cutaneous flexor reflex: comparative study of preterm infants and newborn rat pups. Dev Med Child Neurol. 1988 Aug;30(4):520–526. doi: 10.1111/j.1469-8749.1988.tb04779.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985 Jul;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. P., Walton K. Electrophysiological properties of neonatal rat motoneurones studied in vitro. J Physiol. 1986 Jan;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy E. R., Abbott F. V. The behavioral response to formalin in preweanling rats. Pain. 1992 Oct;51(1):81–90. doi: 10.1016/0304-3959(92)90012-Z. [DOI] [PubMed] [Google Scholar]
- HAGBARTH K. E. Excitatory and inhibitory skin areas for flexor and extensor motoneurons. Acta Physiol Scand Suppl. 1952;26(94):1–58. [PubMed] [Google Scholar]
- Kalliomäki J., Schouenborg J., Dickenson A. H. Differential Effects of a Distant Noxious Stimulus on Hindlimb Nociceptive Withdrawal Reflexes in the Rat. Eur J Neurosci. 1992;4(7):648–652. doi: 10.1111/j.1460-9568.1992.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. J Physiol. 1979 Sep;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B. L., Fox K., O'Leary D. D. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993 Aug 12;364(6438):623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Schomburg E. D. Spinal sensorimotor systems and their supraspinal control. Neurosci Res. 1990 Feb;7(4):265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Holmberg H., Weng H. R. Functional organization of the nociceptive withdrawal reflexes. II. Changes of excitability and receptive fields after spinalization in the rat. Exp Brain Res. 1992;90(3):469–478. doi: 10.1007/BF00230929. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Kalliomäki J. Functional organization of the nociceptive withdrawal reflexes. I. Activation of hindlimb muscles in the rat. Exp Brain Res. 1990;83(1):67–78. doi: 10.1007/BF00232194. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Weng H. R., Kalliomäki J., Holmberg H. A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Exp Brain Res. 1995;106(1):19–27. doi: 10.1007/BF00241353. [DOI] [PubMed] [Google Scholar]
- Schouenborg J., Weng H. R. Sensorimotor transformation in a spinal motor system. Exp Brain Res. 1994;100(1):170–174. doi: 10.1007/BF00227291. [DOI] [PubMed] [Google Scholar]
- Seebach B. S., Ziskind-Conhaim L. Formation of transient inappropriate sensorimotor synapses in developing rat spinal cords. J Neurosci. 1994 Jul;14(7):4520–4528. doi: 10.1523/JNEUROSCI.14-07-04520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima A. Studies on fibre size in developing sciatic nerve and spinal roots in normal, undernourished, and rehabilitated rats. Acta Physiol Scand Suppl. 1974;406:1–55. [PubMed] [Google Scholar]
- Simon D. K., Prusky G. T., O'Leary D. D., Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983 Oct 10;220(1):29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- Stelzner D. J. The normal postnatal development of synaptic end-feet in the lumbosacral spinal cord and of responses in the hind limbs of the albino rat. Exp Neurol. 1971 Jun;31(3):337–357. doi: 10.1016/0014-4886(71)90237-8. [DOI] [PubMed] [Google Scholar]
- Walton K. D., Navarrete R. Postnatal changes in motoneurone electrotonic coupling studied in the in vitro rat lumbar spinal cord. J Physiol. 1991 Feb;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E. D., Stelzner D. J. Behavioral effects of spinal cord transection in the developing rat. Brain Res. 1977 Apr 15;125(2):241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Physiological and morphological changes in developing peripheral nerves of rat embryos. Brain Res. 1988 Jul 1;470(1):15–28. doi: 10.1016/0165-3806(88)90198-8. [DOI] [PubMed] [Google Scholar]


