Abstract
Retinal vein occlusion (RVO) is a common retinal vascular disease that causes visual impairment. However, there is limited evidence regarding the relationship between sinusitis and risk of RVO. This study investigated the association between sinusitis and the subsequent risk of RVO disease. A retrospective cohort study was conducted and patients with sinusitis or RVO were selected from the National Health Insurance Research Database (NHIRD) of Taiwan. In this population-based study, we identified a total of 131,532 sinusitis patients, who were matched with 263,064 non-sinusitis individuals based on sex and age. The Cox proportional hazard regression was applied to demonstrate the adjusted hazard ratio (aHR) with 95% confidence interval (CI) of main outcomes. Our results showed that the crude hazard ratio (cHR) for the risk of RVO was 1.35 (95% CI: 1.20–1.52) in the sinusitis cohort compared with the non-sinusitis cohort. After adjustment for demographics and comorbidities in the multivariable cox regression, the adjusted hazard ratio (aHR) was 1.28 (95% CI: 1.13–1.44). Moreover, statistically significant differences were observed in the 14-year cumulative incidence of RVO (p < 0.0001), central RVO (p = 0.0002), and branch RVO (p = 0.0004) between the sinusitis and non-sinusitis cohorts. In conclusion, our study revealed that patients with sinusitis were at higher risk of being diagnosed with RVO.
Keywords: Retinal vein occlusion, Sinusitis, Epidemiology
Subject terms: Diseases, Risk factors
Introduction
Retinal vein occlusion (RVO) is an obstruction of the retinal venous system by thrombus formation and may involve the central, hemi-central or branch retinal vein1. Besides, RVO is a common cause of vision loss in older individuals, and the second most common retinal vascular disease after diabetic retinopathy2.
Known risk factors for RVO include systemic hypertension, diabetes mellitus, dyslipidemia, glaucoma, and hypercoagulability. Furthermore, inflammation contributes to a hypercoagulable state, a phenomenon that can be further described as immunothrombosis3. Neutrophils, endothelial cells, and platelets interact with each other during inflammation. This interaction can trigger a significant coagulation cascade response, leading to thrombotic events. This process involves the fusion of multiple cytokines with the endothelium, including tissue factor pathway inhibitor, thrombomodulin, and protein C4.
Rhinosinusitis and sinusitis, one of the most common diagnoses in outpatient department can lead to serious complications under some circumstances5. For example, a retrospective study which describe septic cavernous sinus thrombosis (SCST) secondary to bacterial sinusitis had been reported6. Other case report mentions about the association between SCST and orbital cellulitis7. Furthermore, a case report indicates a periorbital abscess which cause by acute bacterial rhinosinusitis with combined RVO8. Therefore, eyes, as an adjacent structure of nose, were likely to be affected by the local inflammation caused by sinusitis, which might give rise to concern of developing RVO.
The risk of developing RVO in patients with sinusitis has not been previously investigated by large cohort studies. Therefore, we would like to investigate the association between sinusitis and the subsequent risk of RVO disease by constructing a large cohort study using Taiwan’s National Health Insurance Research Database.
Materials and methods
Data source
This retrospective cohort study employed data from the Longitudinal Health Insurance Research Database 2000 (LHID2000), which is a part of the National Health Insurance Research Dataset (NHIRD). The data covered the period from January 2000 to December 2019. The Health and Welfare Data Science Center, established by Taiwan’s Ministry of Health and Welfare, consolidates the NHIRD with other health databases, enhancing data management and analytical processes. Various analytical methods and policies have been implemented to ensure data privacy and security9. The Institutional Review Board of Chung Shan Medical University Hospital approved this study (IRB approval number: CS1-23034). The necessary of written informed consent was discarded by the above organizations. We confirm that all research was performed in accordance with relevant guidelines/regulations. Written consent from study subjects was not required and waived by the Institutional Review Board of Chung Shan Medical University Hospital, because the NHIRD comprises de-identified data for research purposes.
Study cohorts
The LHID2000 initially comprised 1,998,323 unique individuals, with 1,022,166 males and 976,157 females. Sinusitis-related encounters from January 1, 2000, to December 31, 2019, were identified using ICD-9-CM codes 461 and 473, and ICD-10-CM codes J01 and J32, in both outpatient and inpatient records. A sinusitis patient was defined as having sinusitis if they had three or more recorded visits for sinusitis within six months following their initial sinusitis encounter. A total of 269,468 individuals were identified as sinusitis patients, while the remaining 1,728,855 were categorized as non-sinusitis individuals.
For the sinusitis cohort, the start of the follow-up period (T0) was set as 180 days after the initial diagnosis of sinusitis. To avoid inaccurate estimations of associations, we excluded the following sinusitis patients from the sinusitis group: (1) Patients diagnosed with sinusitis after December 31, 2018 (n = 5,818), (2) Patients diagnosed with sinusitis before January 1, 2002 (n = 97,664), (3) Patients died before T0 (n = 433), (4) Patients had any ophthalmological procedures before T0 (n = 7,682), (5) Patients with conditions such as, hyperhomocyste-inemia, or malignant neoplasms of the eye/adnexa, brain, head, and neck prior to T0 (n = 5,647), (6) Patients who had RVO, glaucoma, dry eye, cataract, keratoconus, uveitis, diabetic retinopathy, central serous chorioretinopathy before T0 were also excluded (n = 25,140), and (7) Patients were not matched with non-sinusitis individuals (n = 3,034). As a result, 131,532 sinusitis patients met the criteria for inclusion in the analysis.
Non-sinusitis individuals were randomly matched to sinusitis case individuals based on year of birth and sex. Each matched non-sinusitis individual was assigned the same T0 (start of follow-up) as their corresponding sinusitis case. These non-sinusitis individuals were then evaluated using the same exclusion criteria applied to sinusitis cases, and those who met any of the criteria were excluded. The matching process continued until each sinusitis case was successfully paired with two non-sinusitis controls. The matching process was conducted without replacement.
Study outcomes and follow-up
The primary outcome of this study was RVO, defined by ICD-9-CM code 362.3 and ICD-10-CM code H34. This classification includes the subcategories of central retinal vein occlusion (CRVO) (ICD-9-CM code 362.35, and ICD-10-CM code H34.819) and branch retinal vein occlusion (BRVO) (ICD-9-CM code 362.36, and ICD-10-CM code H34.831). All study participants were followed from T0 until the earliest occurrence of the primary outcome, death, or December 31, 2019. Taiwan’s National Health Insurance, which covers approximately 99% of the Taiwanese population, ensures accurate capture of all medical encounters related to RVO after T0. Moreover, the comprehensive national death registration data aids in precisely defining right-censored data, thereby enhancing the accuracy of time-to-event risk calculations.
Study covariates
Study covariates were selected based on clinical knowledge and informed by previous randomized controlled trials (RCTs). The baseline covariates were identified within the 180 days preceding T0 and were used to balance the differences between the sinusitis cohort and the non-sinusitis cohort.
In this study, we predefined sociodemographic variables as covariates, including year of index (ranging from 2002 to 2019 in six periods), sex (male, female), age at T0 (categorized as < 40, 40–49, 50–59, 60–69, 70–79, and ≥ 80), urbanization level (ranked from 1 (high) to 7 (low)), and types of insurance coverage (government, labor, farmers’ or fishermen’s association membership, low-income households, non-labor force or unemployed, and others). Additionally, the study covariates encompassed length of hospital stay (categorized as 0 day, 1–6 days, and ≥ 7 days) and comorbidities including hypertension, diabetes mellitus, hyperlipidemia, ischemic heart diseases, peripheral vascular disease, ischemic stroke, hemorrhagic stroke, chronic kidney disease, chronic obstructive pulmonary disease (COPD), alcohol-related disorders, rheumatoid arthritis, and systemic lupus erythematosus. Alcohol-related disorders (defined by ICD-9: 303 and 305.0, or ICD-10: F10) included alcohol abuse and alcohol dependence as recorded in the insurance claim data. Co-medications such as corticosteroids (ATC code: H02), NSAIDs (ATC code: M01A), beta-blockers (ATC code: C07), calcium channel blockers (ATC code: C08), ACE inhibitors (ATC code: C09A and C09B), ARBs (ATC code: C09C and C09D), statins (ATC code: C10AA), diuretics (ATC code: C03), vitamin K antagonists (ATC code: B01AA), and heparin preparations (ATC code: B01AB) were also considered due to their potential effect on the risk of RVO.
Statistical analysis
Baseline characteristics are presented as frequencies and proportions for categorical variables. Absolute standardized differences were calculated to assess balance, with values less than 0.1 indicating good balance10. The incidence density and 95% confidence interval (CI) of study events were calculated using the normal approximation to the Poisson distribution.
To assess the comparative effect of sinusitis on the risk of RVO, stabilized Inverse Probability of Treatment Weighting (IPTW) was used to balance baseline characteristics between the sinusitis-exposed and unexposed groups11. IPTW uses the propensity score to equilibrate patient characteristics at baseline. Each individual is weighted by the inverse of their probability of receiving the exposure they actually received. The weights for individuals are calculated as 1 divided by the propensity score for the exposed group, and 1 divided by (1 minus the propensity score) for the unexposed group. Thus, exposed individuals with a lower likelihood of exposure, and unexposed individuals with a higher likelihood, receive greater weights. This approach amplifies their influence in the comparative analysis. The inclusion of these weights in the analysis ensures that the ‘assignment’ to either the exposed or unexposed groups is not confounded by the variables included in the propensity score model.
In this study, the Kaplan-Meier estimator was used to estimate the cumulative incidence of RVO since T0 among both the sinusitis cohort and the non-sinusitis cohort. The log-rank test was employed to examine whether there were statistically significant differences in the cumulative incidence of RVO between the two cohorts. After assessing the proportional hazards assumption, hazard ratios were estimated using multivariable Cox regression. A hierarchical regression analysis was performed, with aHR1 estimating the risk of RVO in sinusitis patients after adjusting for demographics. The aHR2 model included additional adjustments for comorbidities based on the aHR1 Cox model, and aHR3 further adjusted for co-medications based on the aHR2 Cox model. Finally, the IPTW hazard ratio for sinusitis and the risk of RVO was evaluated. All statistical analyses were conducted using SAS software version 9.4, and a significance level of p < 0.05 was considered statistically significant.
Results
We identified a total of 131,532 sinusitis patients, who were matched with 263,064 non-sinusitis individuals based on sex and age at T0 (Fig. 1). Table 1 presents the baseline characteristics, which include the year of index, sex, age at T0, urbanization level, type of insurance coverage, length of hospital stays at baseline, comorbidities, and co-medications. These characteristics were found to be similar (defined as ASD < 0.10) between the sinusitis and non-sinusitis cohorts before IPTW. However, the proportion of individuals with Chronic Obstructive Pulmonary Disease (COPD) was notably higher in the sinusitis group at 2.87%, compared to 0.84% in the non-sinusitis group (ASD = 0.15). Additionally, the use of medications such as systemic corticosteroids (10.37% in the sinusitis group vs. 3.30% in the non-sinusitis group, ASD = 0.28) and NSAIDs (49.17% in the sinusitis group vs. 18.01% in the non-sinusitis group, ASD = 0.70) was unbalanced between the study groups before applying IPTW. Table 2 illustrates the use of the IPTW estimator to balance the baseline characteristics. After applying IPTW, all covariates were balanced (ASD < 0.10) between the sinusitis and non-sinusitis cohorts.
Fig. 1.
Flowchart of study cohort selection. † For each matched non-sinusitis individual, the same T0 (start of follow-up) as their corresponding sinusitis case was assigned. These selected non-sinusitis individuals were then evaluated using the same exclusion criteria and removed from the list if they met any of these criteria. The matching process continued until each sinusitis case was successfully paired with two non-sinusitis controls. The matching process was conducted without replacement.
Table 1.
Baseline characteristics of age and sex matched cohorts before IPTW.
| Non-sinusitis cohort | Sinusitis cohort | ASD | |
|---|---|---|---|
| 263,064 | 131,532 | ||
| Year of index | |||
| 2002–2004 | 123,302 (46.87%) | 61,651 (46.87%) | < 0.01 |
| 2005–2007 | 54,036 (20.54%) | 27,018 (20.54%) | < 0.01 |
| 2008–2010 | 33,116 (12.59%) | 16,558 (12.59%) | < 0.01 |
| 2011–2013 | 27,136 (10.32%) | 13,568 (10.32%) | < 0.01 |
| 2014–2016 | 14,930 (5.68%) | 7465 (5.68%) | < 0.01 |
| 2017–2019 | 10,544 (4.01%) | 5272 (4.01%) | < 0.01 |
| Sex | |||
| Male | 130,106 (49.46%) | 65,053 (49.46%) | < 0.01 |
| Female | 132,958 (50.54%) | 66,479 (50.54%) | < 0.01 |
| Age at T0 | |||
| < 40 | 170,891 (64.96%) | 86,300 (65.61%) | 0.01 |
| 40–49 | 41,808 (15.89%) | 19,815 (15.06%) | 0.02 |
| 50–59 | 31,052 (11.80%) | 15,566 (11.83%) | < 0.01 |
| 60–69 | 13,527 (5.14%) | 6935 (5.27%) | 0.01 |
| 70–79 | 4616 (1.75%) | 2312 (1.76%) | < 0.01 |
| ≥ 80 | 1170 (0.44%) | 604 (0.46%) | < 0.01 |
| Urbanization level | |||
| 1 (high) | 80,612 (30.64%) | 41,470 (31.53%) | 0.02 |
| 2 | 76,778 (29.19%) | 39,804 (30.26%) | 0.02 |
| 3 | 43,570 (16.56%) | 23,177 (17.62%) | 0.03 |
| 4 | 37,196 (14.14%) | 17,061 (12.97%) | 0.03 |
| 5 | 4846 (1.84%) | 1908 (1.45%) | 0.03 |
| 6 | 11,690 (4.44%) | 4502 (3.42%) | 0.05 |
| 7 (low) | 8372 (3.18%) | 3610 (2.74%) | 0.03 |
| Type of insurance coverage | |||
| Government | 17,179 (6.53%) | 9755 (7.42%) | 0.03 |
| Labor | 151,916 (57.75%) | 84,238 (64.04%) | 0.13 |
| Farmers’/fishermen’s membership | 36,091 (13.72%) | 15,643 (11.89%) | 0.05 |
| Low-income households | 1831 (0.70%) | 587 (0.45%) | 0.03 |
| Non-labor force/unemployed | 49,122 (18.67%) | 19,252 (14.64%) | 0.11 |
| Others | 6925 (2.63%) | 2057 (1.56%) | 0.07 |
| Length of hospital stay (within 180 days before T0) | |||
| 0 day | 260,868 (99.17%) | 129,579 (98.52%) | 0.06 |
| 1–6 days | 1264 (0.48%) | 1429 (1.09%) | 0.07 |
| >=7 days | 932 (0.35%) | 524 (0.40%) | 0.01 |
| Co-morbidity (within 180 days before T0) | |||
| Hypertension | 14,694 (5.59%) | 10,390 (7.90%) | 0.09 |
| Diabetes mellitus | 6447 (2.45%) | 3997 (3.04%) | 0.04 |
| Hyperlipidemia | 7174 (2.73%) | 5706 (4.34%) | 0.09 |
| Ischemic heart diseases | 3123 (1.19%) | 2547 (1.94%) | 0.06 |
| Peripheral vascular disease | 327 (0.12%) | 247 (0.19%) | 0.02 |
| Ischemic stroke | 1262 (0.48%) | 739 (0.56%) | 0.01 |
| Hemorrhagic stroke | 404 (0.15%) | 153 (0.12%) | 0.01 |
| Chronic kidney disease | 871 (0.33%) | 448 (0.34%) | < 0.01 |
| COPD | 2211 (0.84%) | 3778 (2.87%) | 0.15 |
| Alcohol-related disorders | 899 (0.34%) | 602 (0.46%) | 0.02 |
| Rheumatoid arthritis | 340 (0.13%) | 295 (0.22%) | 0.02 |
| Systemic lupus erythematosus | 136 (0.05%) | 99 (0.08%) | 0.01 |
| Co-medication (within 180 days before T0) | |||
| Systemic corticosteroids | 8668 (3.30%) | 13,634 (10.37%) | 0.28 |
| NSAIDs | 47,371 (18.01%) | 64,671 (49.17%) | 0.70 |
| Beta- blockers | 7552 (2.87%) | 5700 (4.33%) | 0.08 |
| CCBs | 7981 (3.03%) | 5662 (4.30%) | 0.07 |
| ACEI | 2851 (1.08%) | 1965 (1.49%) | 0.04 |
| ARBs | 4641 (1.76%) | 3201 (2.43%) | 0.05 |
| Statin | 3078 (1.17%) | 2278 (1.73%) | 0.05 |
| Diuretics | 454 (0.17%) | 322 (0.24%) | 0.02 |
| Vitamin K antagonists | 310 (0.12%) | 162 (0.12%) | < 0.01 |
| Heparin preparations | 192 (0.07%) | 126 (0.10%) | 0.01 |
ASD, absolute standardized difference was calculated to assess balance, with values less than 0.1 indicating good balance.
Table 2.
Baseline characteristics of age and sex matched cohorts after IPTW.
| IPTW % | ASD | ||
|---|---|---|---|
| Non-sinusitis cohort | Sinusitis cohort | ||
| Year of index | |||
| 2002–2004 | 47.00 | 47.09 | < 0.01 |
| 2005–2007 | 20.45 | 20.36 | < 0.01 |
| 2008–2010 | 12.53 | 12.44 | < 0.01 |
| 2011–2013 | 10.31 | 10.30 | < 0.01 |
| 2014–2016 | 5.69 | 5.73 | < 0.01 |
| 2017–2019 | 4.02 | 4.08 | < 0.01 |
| Sex | |||
| Male | 49.43 | 49.27 | < 0.01 |
| Female | 50.57 | 50.73 | < 0.01 |
| Age at T0 | |||
| < 40 | 64.73 | 64.61 | < 0.01 |
| 40–49 | 15.58 | 15.49 | < 0.01 |
| 50–59 | 11.97 | 11.95 | < 0.01 |
| 60–69 | 5.40 | 5.54 | 0.01 |
| 70–79 | 1.84 | 1.91 | 0.01 |
| ≥ 80 | 0.48 | 0.50 | < 0.01 |
| Urbanization level | |||
| 1 (high) | 30.73 | 30.34 | 0.01 |
| 2 | 29.63 | 29.75 | < 0.01 |
| 3 | 16.89 | 16.93 | < 0.01 |
| 4 | 13.84 | 13.98 | < 0.01 |
| 5 | 1.72 | 1.74 | < 0.01 |
| 6 | 4.11 | 4.16 | < 0.01 |
| 7 (low) | 3.07 | 3.10 | < 0.01 |
| Type of insurance coverage | |||
| Government | 6.87 | 6.93 | < 0.01 |
| Labor | 59.94 | 60.23 | 0.01 |
| Farmers’/fishermen’s membership | 13.27 | 13.59 | 0.01 |
| Low-income households | 0.61 | 0.60 | < 0.01 |
| Non-labor force/unemployed | 17.08 | 16.54 | 0.01 |
| Others | 2.23 | 2.10 | 0.01 |
| Length of hospital stay (within 180 days before T0) | |||
| 0 day | 98.86 | 98.79 | 0.01 |
| 1–6 days | 0.76 | 0.77 | < 0.01 |
| >=7 days | 0.39 | 0.44 | 0.01 |
| Co-morbidity (within 180 days before T0) | |||
| Hypertension | 6.73 | 6.95 | 0.01 |
| Diabetes mellitus | 2.76 | 2.86 | 0.01 |
| Hyperlipidemia | 3.43 | 3.48 | < 0.01 |
| Ischemic heart diseases | 1.58 | 1.59 | < 0.01 |
| Peripheral vascular disease | 0.15 | 0.16 | < 0.01 |
| Ischemic stroke | 0.15 | 0.16 | < 0.01 |
| Hemorrhagic stroke | 0.15 | 0.17 | 0.01 |
| Chronic kidney disease | 0.35 | 0.37 | < 0.01 |
| COPD | 1.79 | 1.60 | 0.01 |
| Alcohol-related disorders | 0.40 | 0.41 | < 0.01 |
| Rheumatoid arthritis | 0.18 | 0.18 | 0.01 |
| Systemic lupus erythematosus | 0.06 | 0.07 | 0.01 |
| Co-medication (within 180 days before T0) | |||
| Systemic corticosteroids | 6.30 | 5.96 | 0.01 |
| NSAIDs | 28.89 | 28.69 | < 0.01 |
| Beta- blockers | 3.52 | 3.61 | < 0.01 |
| CCBs | 3.70 | 3.82 | 0.01 |
| ACEI | 1.32 | 1.37 | < 0.01 |
| ARBs | 2.11 | 2.18 | < 0.01 |
| Statin | 1.44 | 1.47 | < 0.01 |
| Diuretics | 0.21 | 0.22 | < 0.01 |
| Vitamin K antagonists | 0.13 | 0.14 | < 0.01 |
| Heparin preparations | 0.09 | 0.09 | < 0.01 |
ASD, absolute standardized difference was calculated to assess balance, with values less than 0.1 indicating good balance.
Figure 2A–C provides the 14-year cumulative incidence of RVO, CRVO, and BRVO, respectively. Figure 2A shows the 5-year, 10-year, and 14-year cumulative incidence (95% CI) of RVO in the sinusitis cohort as 0.113 (0.096–0.133), 0.282 (0.253–0.314), and 0.404 (0.367–0.445), respectively. In the non-sinusitis cohort, these probabilities were 0.099 (0.087–0.112), 0.201 (0.183–0.220), and 0.291 (0.269–0.315), respectively. The 14-year cumulative incidence of RVO showed statistically significant differences, with a log-rank p-value of < 0.0001. The 14-year cumulative incidence of CRVO (Fig. 2B) and BRVO (Fig. 2C) also showed statistically significant differences, with log-rank p-values of 0.0002 and 0.0004, respectively.
Fig. 2.
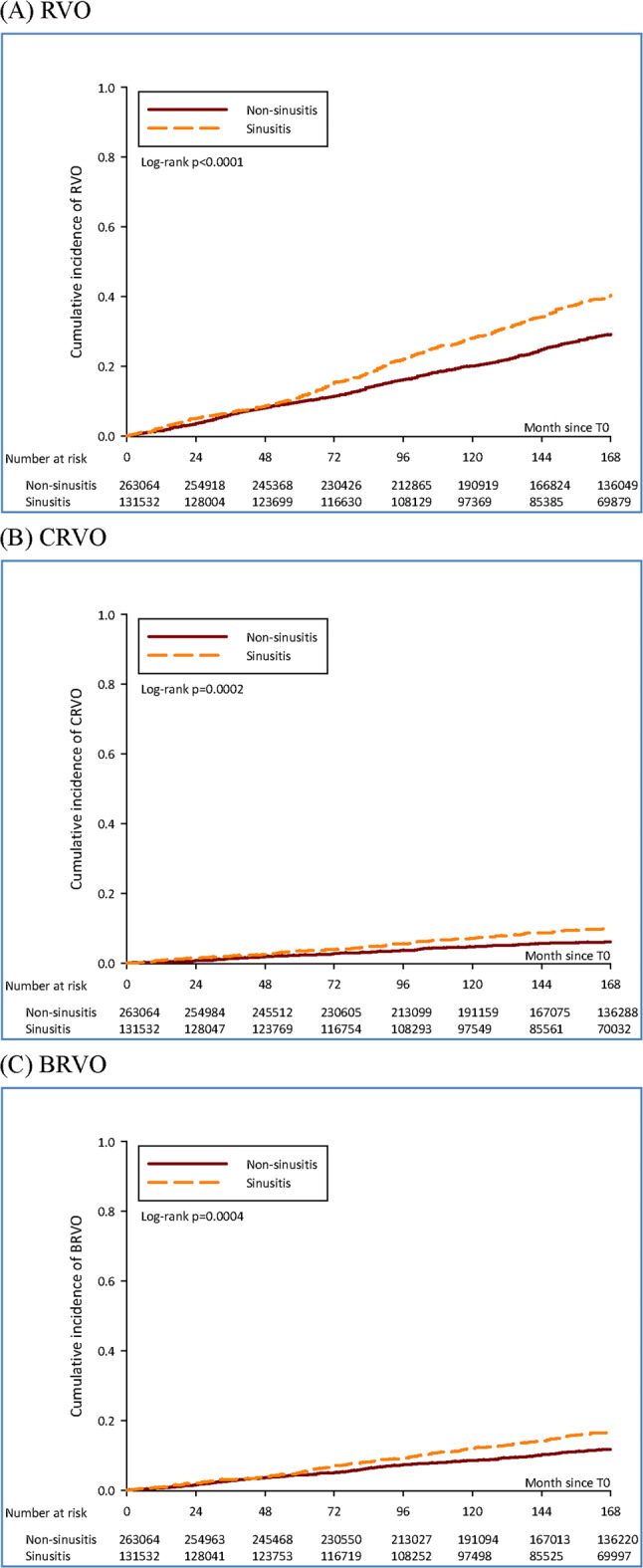
Cumulative incidence of RVO, CRVO and BRVO in study cohorts. (A) Cumulative incidence of RVO in study cohorts. (B) Cumulative incidence of CRVO in study cohorts. (C) Cumulative incidence of BRVO in study cohorts.
Table 3 shows the comparative risk of RVO between sinusitis and non-sinusitis cohorts. The incidence density, expressed as cases per 100,000 person-months (95% CI), was 1.76 (1.63–1.90) in the non-sinusitis cohort and 2.38 (2.17–2.60) in the sinusitis cohort. The crude hazard ratio (cHR) for the risk of RVO in the sinusitis cohort compared to the non-sinusitis cohort was 1.35 (95% CI: 1.20–1.52). After adjusting for demographics, comorbidities, and co-medications in the multivariable Cox regression model, the adjusted hazard ratio (aHR) was 1.28 (95% CI: 1.13–1.44). Additionally, the hazard ratio using IPTW HR was calculated as 1.32 (95% CI: 1.22–1.43). For the risk of CRVO, the incidence density was 0.35 (0.30–0.42) in the non-sinusitis cohort and 0.56 (0.47–0.68) in the sinusitis cohort, with an IPTW HR of 1.58 (95% CI: 1.33–1.88). As for BRVO, the incidence density was 0.68 (0.60–0.77) in the non-sinusitis cohort and 0.95 (0.82–1.09) in the sinusitis cohort, with an IPTW HR of 1.41 (95% CI: 1.25–1.60).
Table 3.
The comparative risk of RVO between sinusitis and non-sinusitis cohorts.
| Event: Retinal vein occlusion | Event: Central retinal vein occlusion | Event: Branch retinal vein occlusion | ||||
|---|---|---|---|---|---|---|
| Non-sinusitis cohort | Sinusitis cohort | Non-sinusitis cohort | Sinusitis cohort | Non-sinusitis cohort | Sinusitis cohort | |
| Follow-up person-month | 39,821,930 | 20,197,467 | 39,858,965 | 20,221,827 | 39,848,598 | 20,216,180 |
| Event | 701 | 481 | 141 | 114 | 272 | 192 |
| Incidence density (95% CI) |
1.76 (1.63–1.90) |
2.38 (2.17–2.60) |
0.35 (0.30–0.42) |
0.56 (0.47–0.68) |
0.68 (0.60–0.77) |
0.95 (0.82–1.09) |
| Crude HR | Reference | 1.35 (1.20–1.52) | Reference | 1.60 (1.25–2.04) | Reference | 1.39 (1.16–1.68) |
| aHR1 | Reference | 1.33 (1.18–1.49) | Reference | 1.56 (1.22-2.00) | Reference | 1.37 (1.14–1.65) |
| aHR2 | Reference | 1.27 (1.13–1.43) | Reference | 1.50 (1.16–1.92) | Reference | 1.33 (1.10–1.60) |
| aHR3 | Reference | 1.28 (1.13–1.44) | Reference | 1.51 (1.16–1.96) | Reference | 1.36 (1.12–1.65) |
| IPTW HR | Reference | 1.32 (1.22–1.43) | Reference | 1.58 (1.33–1.88) | Reference | 1.41 (1.25–1.60) |
Incidence density, defined as event cases per 100,000 person-months, along with the 95% confidence interval (CI), was calculated using the normal approximation to the Poisson distribution.
aHR1, adjusted for demographics.
aHR2, adjusted for demographics and co-morbidities.
aHR3, adjusted for demographics, co-morbidities, and co-medications.
IPTW HR, inverse probability of treatment weighted hazard ratio.
Table 4 presents the associations between baseline characteristics and the risk of RVO as identified in the multivariable Cox regression analysis. Significant factors associated with an increased risk of RVO include sex, age, and comorbidities such as hypertension, diabetes mellitus, and chronic kidney disease.
Table 4.
The aHRs (95% CI) for retinal vein occlusion by multivariable Cox regression.
| Variable | aHR (95% CI) | ||
|---|---|---|---|
| For RVO risk | For CRVO risk | For BRVO risk | |
| Cohort | |||
| Non-sinusitis | Reference | Reference | Reference |
| Sinusitis | 1.28 (1.13–1.44) | 1.51 (1.16–1.96) | 1.36 (1.12–1.65) |
| Year of index | |||
| 2002–2004 | Reference | Reference | Reference |
| 2005–2007 | 1.08 (0.94–1.25) | 1.02 (0.76–1.37) | 1.01 (0.81–1.26) |
| 2008–2010 | 0.93 (0.76–1.13) | 0.52 (0.33–0.83) | 0.76 (0.56–1.03) |
| 2011–2013 | 0.87 (0.68–1.11) | 0.78 (0.48–1.27) | 0.52 (0.34–0.78) |
| 2014–2016 | 0.83 (0.57–1.22) | 0.29 (0.09–0.93) | 0.41 (0.20–0.84) |
| 2017–2019 | 0.75 (0.36–1.53) | 0.71 (0.17-3.00) | Not estimated |
| Sex | |||
| Male | 1.17 (1.04–1.31) | 1.12 (0.87–1.43) | 1.12 (0.94–1.35) |
| Female | Reference | Reference | Reference |
| Age at T0 | |||
| < 40 | 0.13 (0.06–0.27) | 0.08 (0.02–0.26) | 0.09 (0.03–0.29) |
| 40–49 | 0.61 (0.29–1.31) | 0.29 (0.09–0.97) | 0.66 (0.21–2.10) |
| 50–59 | Reference | Reference | Reference |
| 60–69 | 1.25 (0.59–2.65) | 0.77 (0.24–2.49) | 1.39 (0.44–4.40) |
| 70–79 | 1.51 (0.71–3.23) | 0.76 (0.23–2.48) | 1.59 (0.50–5.06) |
| ≥ 80 | 1.61 (0.74–3.50) | 0.94 (0.27–3.21) | 1.42 (0.43–4.71) |
| Urbanization level | |||
| 1 (high) | Reference | Reference | Reference |
| 2 | 1.03 (0.89–1.19) | 0.86 (0.63–1.17) | 0.94 (0.74–1.19) |
| 3 | 0.98 (0.82–1.17) | 0.89 (0.61–1.30) | 1.13 (0.85–1.48) |
| 4 | 1.07 (0.88–1.29) | 0.79 (0.51–1.23) | 0.92 (0.66–1.27) |
| 5 | 0.78 (0.47–1.29) | 1.01 (0.39–2.60) | 0.90 (0.41–1.97) |
| 6 | 1.10 (0.81–1.50) | 1.02 (0.52–1.99) | 1.39 (0.87–2.22) |
| 7 (low) | 0.87 (0.60–1.27) | 0.69 (0.29–1.65) | 1.23 (0.72–2.12) |
| Type of insurance coverage | |||
| Government | 0.84 (0.67–1.07) | 0.86 (0.52–1.43) | 0.88 (0.61–1.28) |
| Labor | Reference | Reference | Reference |
| Farmers’ /Fishermen’s membership | 1.07 (0.89–1.30) | 0.93 (0.60–1.44) | 0.95 (0.70–1.30) |
| Low-income households | 0.73 (0.27–1.96) | 0.82 (0.11–5.86) | 0.46 (0.06–3.27) |
| Non-labor force/unemployed | 0.92 (0.77–1.09) | 0.93 (0.65–1.34) | 0.90 (0.68–1.18) |
| Others | 0.75 (0.48–1.18) | 0.84 (0.34–2.07) | 1.04 (0.57–1.91) |
| Length of hospital stay (within 180 days before T0) | |||
| 0 day | Reference | Reference | Reference |
| 1–6 days | 1.07 (0.53–2.18) | 1.41 (0.34–5.87) | 0.79 (0.19–3.25) |
| >=7 days | 0.84 (0.31–2.28) | Not estimated | 0.59 (0.08–4.33) |
| Co-morbidity (within 180 days before T0) | |||
| Hypertension | 1.62 (1.31-2.00) | 1.90 (1.22–2.98) | 1.26 (0.89–1.79) |
| Diabetes mellitus | 1.49 (1.20–1.85) | 1.62 (1.03–2.55) | 1.46 (1.04–2.05) |
| Hyperlipidemia | 0.93 (0.73–1.19) | 0.75 (0.43–1.31) | 0.80 (0.54–1.20) |
| Ischemic heart diseases | 1.08 (0.82–1.42) | 1.79 (1.08–2.98) | 1.17 (0.77–1.77) |
| Peripheral vascular disease | 0.44 (0.11–1.76) | Not estimated | 0.52 (0.07–3.72) |
| Ischemic stroke | 0.99 (0.61–1.59) | 0.73 (0.23–2.32) | 1.18 (0.60–2.34) |
| Hemorrhagic stroke | 1.64 (0.73–3.68) | 2.82 (0.68–11.60) | 1.25 (0.31–5.10) |
| Chronic kidney disease | 4.29 (2.94–6.28) | 5.10 (2.36–11.01) | 4.66 (2.63–8.25) |
| COPD | 1.18 (0.88–1.58) | 1.07 (0.57–1.99) | 1.07 (0.66–1.71) |
| Alcohol-related disorders | 0.89 (0.37–2.16) | 1.76 (0.44–7.14) | Not estimated |
| Rheumatoid arthritis | 0.96 (0.36–2.56) | 1.06 (0.15–7.65) | 0.58 (0.08–4.14) |
| Systemic lupus erythematosus | Not estimated | Not estimated | Not estimated |
| Co-medication (within 180 days before T0) | |||
| Systemic corticosteroids | 0.97 (0.77–1.21) | 1.00 (0.62–1.59) | 1.12 (0.80–1.58) |
| NSAIDs | 1.00 (0.88–1.13) | 0.97 (0.74–1.29) | 0.89 (0.72–1.09) |
| Beta-blockers | 1.17 (0.95–1.44) | 0.92 (0.58–1.46) | 1.23 (0.89–1.72) |
| CCBs | 1.00 (0.80–1.25) | 1.11 (0.70–1.77) | 1.49 (1.05–2.13) |
| ACEI | 0.99 (0.74–1.32) | 0.89 (0.48–1.64) | 0.89 (0.55–1.42) |
| ARBs | 1.05 (0.80–1.38) | 0.81 (0.43–1.51) | 1.10 (0.71–1.70) |
| Statin | 0.88 (0.62–1.26) | 0.51 (0.19–1.33) | 0.70 (0.37–1.29) |
| Diuretics | 0.91 (0.47–1.78) | 0.80 (0.19–3.26) | 0.51 (0.13–2.06) |
| Vitamin K antagonists | 0.58 (0.14–2.33) | Not estimated | 1.31 (0.32–5.38) |
| Heparin preparations | 0.85 (0.21–3.47) | Not estimated | 1.86 (0.44–7.79) |
Discussion
Our study revealed increased incidence of RVO in patient with sinusitis. To the best of our knowledge, this is the largest-ever cohort study to show a positive correlation between RVO and sinusitis. There were a few case reports of patient with sinusitis induced cavernous sinus syndrome which led to RVO6. Since there are no valves in the dural sinuses and the cerebral and emissary veins, blood was able to flow in either direction according to pressure gradients, which makes cavernous sinuses vulnerable to septic thrombosis resulting from infection at multiple sites12. Clinical presentation of septic cavernous sinus syndrome can be extremely varied, and symptoms can evolve over hours to a few weeks, which makes it difficult to diagnose13. In a Dutch-European study, the most frequent symptoms and signs were headache14. The diagnosis of septic cavernous sinus syndrome is best made on clinical grounds and confirmed by appropriate radiographic studies such as Contrast enhanced computed tomography (CT) scan and magnetic resonance imaging15. However, due to its varied and unspecific symptoms, misdiagnosing, or failure to diagnose were often found in patient with septic cavernous sinus syndrome16.
In meta-analysis showed that comorbidities such as hypertension, diabetes mellitus, and chronic kidney disease also increase the risk of developing RVO17. However, in our study, there was no significant association of hyperlipidemia and RVO. Another meta-analysis study showed chronic kidney disease was related to RVO18. The condition may be due to a combination of three systemic changes known as Virchow’s triad: hemodynamic changes (venous stasis), degenerative changes of the vessel wall and blood hypercoagulability19. However, the pathogenesis is still not completely understood and needs to be further investigated in the future.
This study has inherent limitations that warrant acknowledgment. As a retrospective population study, the possibility of unmeasured confounding variables between the two cohorts introduces potential bias. In other words, the National Health Insurance Research Database (NHIRD) lacks detailed personal data on factors such as smoking history, alcohol consumption, body mass index, physical activity level, and dietary habits, which were consequently not adjusted for in this analysis, posing potential sources of bias. In our study, chronic sinusitis and acute sinusitis are not clearly distinguished, which may have resulted in discrepancies due to the differences in treatments for the two conditions. Patients who had myeloproliferative disorders, inherited hypercoagulable states, inflammatory disease associated with occlusive periphlebitis were not excluded in the study. The absence of imaging data, such as contrast-enhanced CT and magnetic resonance imaging (MRI), in the NHIRD limits our ability to determine the causal relationship between sinusitis and the development of RVO. Additionally, the control group may have included patients with undiagnosed BRVO, as it can be associated with only a slight decrease in visual acuity or a visual field defect, making detection less likely. Therefore, a more in-depth prospective cohort study that considers these factors is essential for a comprehensive understanding of the association between sinusitis and RVO.
In conclusion, in this large retrospective cohort study, patients with rhinosinusitis were at higher risk of being diagnosed with RVO. It is therefore important to be cognizant of the possibility of RVO in patients with a history of sinusitis, especially in patient with hypertension, diabetes mellitus, and chronic kidney disease. Further investigations involving prospective cohorts are necessary for clinical knowledge.
Author contributions
TYT, JYH, SCC, SFY, and HYL designed and conceptualized the study. JYH and SFY are responsible for the statistical analyses. TYT, JYH, SCC, SMH, SFY, and HYL are responsible for the manuscript draft. All authors read and approved the final manuscript.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethical approval
This study was approved by both the National Health Insurance Administration of Taiwan and Institutional Review Board of Chung Shan Medical University Hospital (Project code: CS1-23034).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tsan-Yu Tsai and Jing-Yang Huang contributed equally to this work.
References
- 1.Song, P., Xu, Y., Zha, M., Zhang, Y. & Rudan, I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J. Glob Health. 9, 010427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cugati, S. et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology. 114, 520–524 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Delvaeye, M. & Conway, E. M. Coagulation and innate immune responses: can we view them separately? Blood. 114, 2367–2374 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Galli, E., Maggio, E. & Pomero, F. Venous thromboembolism in Sepsis: from bench to Bedside. Biomedicines. 10, (2022). [DOI] [PMC free article] [PubMed]
- 5.Fieux, M., Rumeau, C., De Bonnecaze, G., Papon, J. F. & Mortuaire, G. Surgery for chronic rhinosinusitis with nasal polyps: an update. Eur. Ann. Otorhinolaryngol. Head Neck Dis.140, 297–304 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Lize, F. et al. Septic cavernous sinus thrombosis secondary to acute bacterial sinusitis: a retrospective study of seven cases. Am. J. Rhinol Allergy. 29, e7–12 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Branson, S. V., McClintic, E. & Yeatts, R. P. Septic cavernous sinus thrombosis associated with orbital cellulitis: a report of 6 cases and review of literature. Ophthalmic Plast. Reconstr. Surg.35, 272–280 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Pedrosa, R. et al. A periorbital abscess with combined retinal artery occlusion and retinal vein occlusion: a case report. Am. J. Case Rep.22, e930808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh, C. Y. et al. Taiwan’s National Health Insurance Research Database: past and future. Clin. Epidemiol.11, 349–358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin, P. C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med.28, 3083–3107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesnaye, N. C. et al. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J.15, 14–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebright, J. R., Pace, M. T. & Niazi, A. F. Septic thrombosis of the cavernous sinuses. Arch. Intern. Med.161, 2671–2676 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Kimber, J. Cerebral venous sinus thrombosis. QJM. 95, 137–142 (2002). [DOI] [PubMed] [Google Scholar]
- 14.de Bruijn, S. F., de Haan, R. J. & Stam, J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For the cerebral venous sinus thrombosis Study Group. J. Neurol. Neurosurg. Psychiatry. 70, 105–108 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon, M. L. et al. Cavernous sinus thrombosis complicating sinusitis. Pediatr. Crit. Care Med.5, 86–88 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Sasidharan, P. K. Cerebral vein thrombosis misdiagnosed and mismanaged. Thrombosis. 2012, 210676 (2012). [DOI] [PMC free article] [PubMed]
- 17.Kolar, P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J. Ophthalmol.2014, 724780 (2014). [DOI] [PMC free article] [PubMed]
- 18.Fang, L. J., Dong, L., Li, Y. F. & Wei, W. B. Retinal vein occlusion and chronic kidney disease: a meta-analysis. Eur. J. Ophthalmol.31, 1945–1952 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Rehak, M. & Wiedemann, P. Retinal vein thrombosis: pathogenesis and management. J. Thromb. Haemost. 8, 1886–1894 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.



