Abstract
This bicenter retrospective analysis included 162 patients who had undergone prostate biopsy following prebiopsy MRI, excluding those with PCa identified only in the peripheral zone (PZ). DLR T2WI achieved a 69% reduction in scan time relative to TSE T2WI. The intermethod agreement between the two T2WI sets in terms of the Prostate Imaging Reporting and Data System (PI-RADS) classification and extraprostatic extension (EPE) grade was measured using the intraclass correlation coefficient (ICC) and diagnostic performance was assessed with the area under the receiver operating characteristic curve (AUC). Clinically significant PCa (csPCa) was found in 74 (45.7%) patients. Both T2WI methods showed high intermethod agreement for the overall PI-RADS classification (ICC: 0.907–0.949), EPE assessment (ICC: 0.925–0.957) and lesion size measurement (ICC: 0.980–0.996). DLR T2WI and TSE T2WI showed similar AUCs (0.666–0.814 versus 0.684–0.832) for predicting EPE. The AUCs for detecting csPCa with DLR T2WI (0.834–0.935) and TSE T2WI (0.891–0.935) were comparable in 139 patients with TZ lesions with no significant differences (P > 0.05). The findings suggest that DLR T2WI is an efficient alternative for TZ lesion assessment, offering reduced scan times without compromising diagnostic accuracy.
Keywords: Deep learning-based reconstruction, Acceleration, Prostate cancer, Magnetic resonance imaging, T2-weighted imaging, Staging
Subject terms: Prostate cancer, Cancer imaging
Introduction
Magnetic resonance imaging (MRI) plays an important role in detecting prostate cancer as well as in staging tumors and planning treatment1,2. Additionally, prostate MRI has been used in various clinical settings, such as monitoring prostate cancer in patients undergoing posttreatment follow-up to detect recurrence or those under active surveillance, despite controversy regarding the role of MRI in detecting the progression of PCa3–5. The large number of indications has led to an increase in the number of prostate MRIs6. In situations demanding reduced acquisition times, a biparametric MRI (bpMRI) protocol composed of T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) has been suggested7.
In prostate MRI, DWI plays an important role in detecting prostate cancer in the peripheral zone (PZ), where the majority of prostate cancer occurs. However, T2WI is essential for delineating the anatomic structure of the prostate gland and detecting prostate cancer in the transition zone (TZ), as a variety of benign lesions demonstrate diffusion restriction in the TZ8. Additionally, T2WI is used to evaluate the extraprostatic extension (EPE) of PCa9,10. Even with growing evidence of the efficiency of bpMRI, however, the Prostate Imaging Reporting and Data System (PI-RADS) acknowledges its cautious use under certain circumstances to avoid missing clinically significant PCa (csPCa)8,11–14. As there are conflicting results about the usefulness of dynamic contrast-enhanced MRI in detecting and staging csPCa, there has been great difficulty in adopting bpMRI as a routine protocol15–20.
Deep learning-based reconstruction (DLR) algorithms have been developed for noise reduction in MRI21–23. DLR can enhance the quality of images from accelerated MRI, which are obtained in shorter scan times than conventional MRI22,23. Many studies have shown comparable image quality and diagnostic performance between DL-accelerated T2WI and conventional imaging of the prostate in detecting PCa24–30. However, since T2W images do not play a notable role in detecting PZ cancers, conducting a study with PCa regardless of zonal location would likely not demonstrate the direct impact of DLR T2WI on detecting TZ lesions. Furthermore, while a previous study conducted with patients who underwent prostatectomy for PCa showed minimal impacts of DLR T2WI on EPE evaluation31, , applying DLR to prebiopsy MRI, regardless of the subsequent biopsy outcome, could clarify its impact in current clinical practice.
The purpose of this study was to compare accelerated DLR T2WI with conventional TSE T2WI for detecting TZ PCa and evaluating EPE on prebiopsy MRI.
Results
Table 1 presents the baseline characteristics of the 162 patients included in this study. The median age was 69 years, with an IQR of 65.0 to 75.3, and there was no significant difference between the two hospitals. The median PSA level was 8.0 ng/mL, with patients from Hospital B exhibiting a significantly higher median PSA level of 8.4 ng/mL. Over 90% of patients underwent MRI using the multiparametric MRI (mpMRI) protocol, and no MRI scans in Hospital B were performed using the bpMRI protocol. During the biopsy, 32 patients had multifocal target lesions. The number of biopsy core was significantly different between two hospitals (12 versus 15, P < 0.001). PCa and csPCa were diagnosed in 94 and 74 patients, respectively. While the frequency of PCa was significantly higher in Hospital B (P = 0.001), the frequency of csPCa did not differ significantly between the hospitals (38.4% versus 53.9%, P = 0.058). The distribution of Gleason grade group was comparable across the two hospitals (P = 0.253). Prostatectomy was performed in 32 patients, with EPE was confirmed in 10 of them (31.2%).
Table 1.
Baseline patient characteristics.
| Total (n = 162) |
Hospital A (n = 86) |
Hospital B (n = 76) |
P value | |
|---|---|---|---|---|
| Age (years) | 69.0 (65.0–75.3) | 69.0 (65.8–75.3) | 69.5 (64.3–77.3) | 0.971 |
| PSA (ng/mL) | 8.0 (5.2–15.2) | 6.8 (5.1–12.1) | 8.4 (5.8–20.3) | 0.033 |
| MRI protocol | < 0.001 | |||
| Multiparametric MRI | 147 (90.7) | 71 (82.6) | 76 (100) | |
| Biparametric MRI | 15 (9.3) | 15 (17.4) | 0 (0) | |
| MRI-biopsy interval (days) | 16 (9–24) | 17 (7–26) | 16 (11–21) | < 0.001 |
| Number of biopsy cores | 12 (12–15) | 15 (15–15) | 12 (12–12) | < 0.001 |
| Biopsy-confirmed prostate cancer | 94 (58.0) | 39 (45.3) | 55 (72.4) | 0.001 |
| Biopsy-confirmed clinically significant prostate cancer | 74 (45.7) | 33 (38.4) | 41 (53.9) | 0.058 |
| Highest Gleason grade group* | 0.253 | |||
| 1 | 20 (21.3) | 6 (15.4) | 14 (25.5) | |
| 2 | 29 (30.9) | 11 (28.2) | 18 (32.7) | |
| 3 | 24 (25.5) | 13 (33.3) | 11 (20.0) | |
| 4 | 13 (13.8) | 5 (12.8) | 8 (14.5) | |
| 5 | 8 (8.5) | 4 (10.3) | 4 (7.3) | |
| Radical prostatectomy | 32 (19.8) | 10 (11.6) | 22 (28.9) | 0.006 |
| Positive EPE† | 10 (31.2) | 3 (30.0) | 7 (31.8) | 0.919 |
PSA, prostate-specific antigen; PI-RADS, Prostate Imaging Reporting and Data System; EPE, extraprostatic extension.
The data are presented as medians with interquartile ranges in parentheses or as frequencies (proportions).
* Proportion among patients with biopsy-confirmed prostate cancer.
† Proportion among patients who underwent prostatectomy.
Table 2 summarizes the image review results from the two radiologists and the intermethod agreement between the two different T2WI sets. PZ lesions were detected in 23 and 20 patients in the TSE T2WI set, and in 23 and 21 patients in the DLR T2WI set by readers 1 and 2, respectively. A PI-RADS 4 or higher was assigned to 87 and 86 patients based on TSE T2WI and to 92 and 86 patients based on DLR T2WI by reader 1 and reader 2, respectively. Comparisons between the TSE T2WI and DLR T2WI for overall PI-RADS classification and T2WI PI-RADS classification revealed good to excellent intermethod agreement for both readers, with ICCs ≥ 0.899. Both readers demonstrated excellent intermethod agreement for EPE grading with ICC values of 0.957 and 0.925.
Table 2.
Comparison of image review results between the two sets of T2-wieghted imaging sets.
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
| TSE T2WI | DLR T2WI | Intermethod agreement (95% CI) |
TSE T2WI | DLR T2WI | Intermethod agreement (95% CI) |
|
| Lesion location | ||||||
| No lesion | 36 (22.2) | 36 (22.2) |
1.000e (1.000–1.000) |
36 (22.2) | 36 (22.2) |
0.977* (0.951-1.000) |
| PZ | 23 (14.2) | 23 (14.2) | 20 (12.3) | 21 (13.0) | ||
| TZ | 103 (63.6) | 103 (63.6) | 106 (65.4) | 105 (64.8) | ||
| Overall PI-RADS score | ||||||
| ≤ 2 | 48 (29.6) | 47 (29.0) |
0.949† (0.931–0.963) |
73 (45.1) | 69 (42.6) |
0.907† (0.873–0.932) |
| 3 | 27 (16.7) | 23 (14.2) | 4 (2.5) | 7 (4.3) | ||
| 4 | 31 (19.1) | 29 (17.9) | 23 (14.2) | 21 (13.0) | ||
| 5 | 56 (34.6) | 63 (38.9) | 62 (38.3) | 65 (40.1) | ||
| T2WI PI-RADS score | ||||||
| ≤ 2 | 63 (38.9) | 63 (38.9) |
0.943† (0.922–0.958) |
74 (45.7) | 74 (45.7) |
0.899† (0.863–0.926) |
| 3 | 16 (9.9) | 12 (7.4) | 4 (2.5) | 4 (2.5) | ||
| 4 | 28 (17.3) | 26 (16.0) | 22 (13.6) | 20 (12.3) | ||
| 5 | 55 (34.0) | 61 (37.7) | 62 (38.3) | 64 (39.5) | ||
| MRI EPE grade | ||||||
| 0 | 112 (69.1) | 105 (64.8) |
0.957† (0.942–0.969) |
115 (71.0) | 118 (72.8) |
0.925† (0.897-945) |
| 1 | 19 (11.7) | 21 (13.0) | 17 (10.5) | 16 (9.9) | ||
| 2 | 13 (8.0) | 15 (9.3) | 8 (4.9) | 4 (2.5) | ||
| 3 | 18 (11.1) | 21 (13.0) | 22 (13.6) | 24 (14.8) | ||
| Size (cm)‡ | 2.09 ± 1.52 | 2.03 ± 1.50 |
0.996† (0.994–0.997) |
2.36 ± 1.58 | 2.46 ± 1.56 |
0.980† (0.969–0.987) |
Unless otherwise specified, the data are presented as frequencies (percentages).
*Kappa value.
†Intraclass correlation coefficient.
‡Data are presented as the mean ± standard deviation.
Interreader agreements for PI-RADS classification and EPE evaluation were good for TSE T2WI, with ICCs of 0.860 and 0.898, respectively, as shown in Table 3. For DLR T2WI, the agreement was excellent, with ICCs of 0.996 and 0.915, respectively. The measurement of tumor size demonstrated excellent interreader and intermethod agreement, as detailed in Tables 2 and 3.
Table 3.
Interreader agreement per index lesion.
| ICC | |
|---|---|
| Interreader agreement | |
| PI-RADS classification | |
| Turbo spin echo | 0.860 (0.809–0.897) |
| Deep learning-based reconstruction | 0.996 (0.994–0.997) |
| Extraprostatic extension | |
| Turbo spin echo | 0.898 (0.842–0.934) |
| Deep learning-based reconstruction | 0.915 (0.870–0.944) |
| Size | |
| Turbo spin echo | 0.988 (0.982–0.992) |
| Deep learning-based reconstruction | 0.980 (0.969–0.987) |
PI-RADS, Prostate Imaging Reporting and Data System.
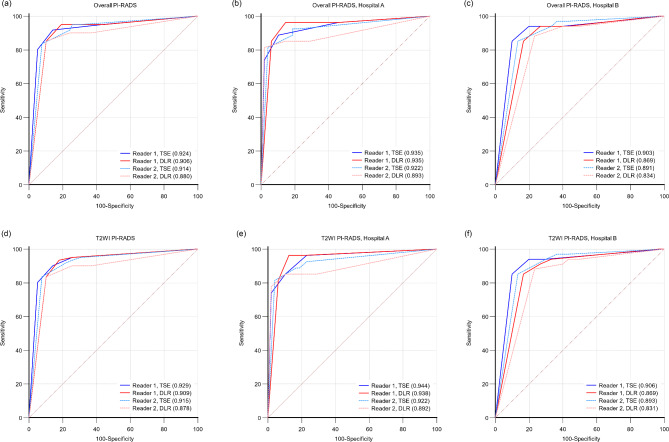
After excluding patients with PZ lesions, the AUCs for the overall PI-RADS classification in detecting csPCa were not significantly different between DLR T2WI and TSE T2WI for either reader for patients from both hospitals (Fig. 1a), from Hospital A alone (Fig. 1b), or from Hospital B alone (Fig. 1c) with P values of 0.368, 0.991, and 0.181 for reader 1 and 0.150, 0.416 and 0.142 for reader 2, respectively. Similar results were observed for the T2WI PI-RADS classification (reader 1, P = 0.248, 0.847, and 0.158; reader 2, P = 0.120, 0.402, and 0.112), as shown in Fig. 1d, e and f.
Fig. 1.
Receiver operating characteristic curves for the two readers in detecting clinically significant prostate cancer in 139 patients with TZ lesions. The results of the overall PI-RADS classification and T2WI PI-RADS classification are shown for patients in both hospitals (a, d), hospital A (b, e) and hospital B (c, f).
PI-RADS, Prostate Imaging Reporting and Data System; TSE, turbo spin echo T2-weighted imaging (T2WI); DLR, deep learning-reconstructed T2WI.
The dichotomized analysis showed that both the T2WI sequences and radiologists had a sensitivity ranging from 0.902 to 0.951 and a specificity ranging from 0.756 to 0.859. The PPV varied from 0.743 to 0.836, while the NPV was between 0.908 and 0.955 (Table 4). The sensitivity and specificity did not significantly differ between the TSE T2WI and DLR T2WI sets for either reader (reader 1: P = 0.500 for sensitivity, P = 0.219 for specificity; reader 2: P > 0.999 for both sensitivity and specificity). Figure 2 illustrates a representative case of true-positive prostate cancer, which was detected by both radiologists on both T2WI sequences.
Table 4.
Diagnostic performance of the radiologists with turbo spin echo and deep learning-based reconstructed T2-weighted imaging sequences in detecting clinically significant cancer using a PI-RADS grade > 3 in 139 patients after excluding those with peripheral zone lesions.
| Radiologist 1 | Radiologist 2 | |||
|---|---|---|---|---|
| TSE | DLR | TSE | DLR | |
| Sensitivity |
0.918 (0.819–0.973) |
0.918 (0.819–0.973) |
0.951 (0.863–0.990) |
0.902 (0.798–0.963) |
| Specificity |
0.859 (0.762–0.927) |
0.756 (0.646–0.847)) |
0.808 (0.703–0.888) |
0.756 (0.646–0.847) |
| PPV |
0.836 (0.725–0.915) |
0.747 (0.633–0.840) |
0.795 (0.684–0.880) |
0.743 (0.628–0.838) |
| NPV |
0.931 (0.845–0.977) |
0.922 (0.827–0.974) |
0.955 (0.873–0.991) |
0.908 (0.810–0.965) |
TSE, turbo spin echo T2-weighted imaging; DLR, accelerated T2-weighted imaging with deep learning reconstruction; PPV, positive predictive value; NPV, negative predictive value.
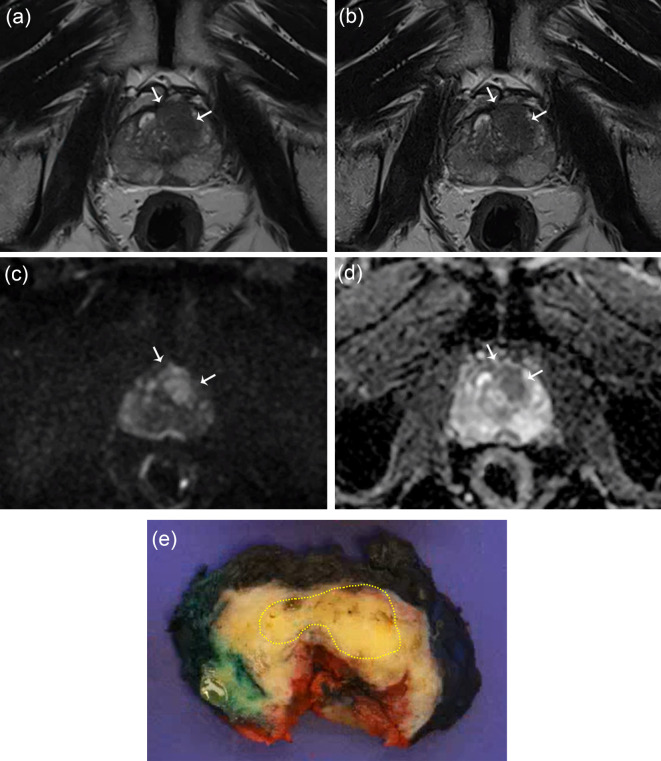
Fig. 2.
A 67-year-old man with a prostate-specific antigen level of 12 ng/mL. An ill-defined lesion with a homogeneously low signal intensity is observed in the left mid-anterior transition zone and mid-anterior peripheral zone on turbo spin echo T2-weighted imaging (T2WI) (a) and deep learning-reconstructed T2WI (b). This lesion exhibits high signal intensity on diffusion-weighted imaging (DWI) with a b value of 1400 s/mm² (c) and shows a low apparent diffusion coefficient (ADC) (d). Both radiologist 1 and radiologist 2 classified the lesion as having a PI-RADS score of 5 on both T2WI sequences and evaluated it as extraprostatic extension (EPE) grade 1. The lesion was histologically confirmed as Gleason grade group 2, and EPE was verified at the anterior aspect of the lesion (e).
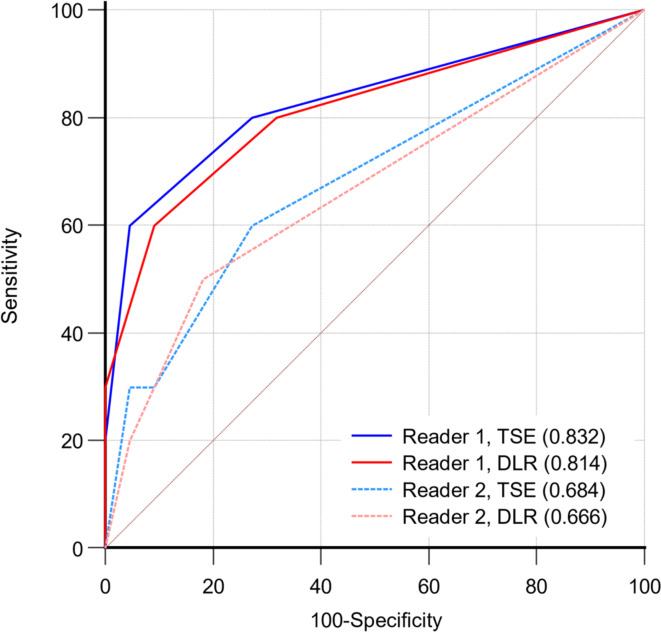
In 32 patients who underwent prostatectomy, the performance of MRI EPE grading for predicting pathologically positive EPE by the two readers is presented in Fig. 3. There was no significant difference between DLR T2WI and TSE T2WI in either reader (P = 0.255 and 0.791, respectively).
Fig. 3.
Receiver operating characteristic curves for the two readers in predicting extraprostatic extension (EPE) in 32 patients who underwent prostatectomy. Two readers MRI EPE grading is not significantly different between turbo spin echo (TSE) T2-weighted imaging (T2WI) and deep learning-reconstructed (DLR) T2WI (P = 0.255 and 0.791, respectively).
Discussion
In this bicenter study, a DLR algorithm for noise reduction was applied to accelerate T2WI. DLR T2WI was performed in 1 min and 6 s, achieving a 69% reduction in scan time with respect to conventional T2WI. This study demonstrated that PI-RADS classification using DLR T2WI, in conjunction with DWI, had a similar diagnostic performance in detecting csPCa as TSE T2WI with DWI in the TZ. This study focused on TZ lesions and included 139 patients after excluding those with PZ lesions. Multiple previous studies have demonstrated the similar or superior image quality and similar diagnostic performance of DLR T2WI with respect to conventional T2WI24–33. However, in those studies, the TZ lesions were not evaluated separately. Therefore, we evaluated the impact of DLR in the radiological evaluation of TZ lesions, where T2WI serves as the principal sequence for determining the PI-RADS classification.
The overall PI-RADS classification showed comparable diagnostic performance between the DLR and TSE T2WI sets in detecting csPCa according to receiver operating characteristic (ROC) curve analysis When patients with PZ lesions were excluded, similar outcomes were noted for both the overall PI-RADS and T2WI PI-RADS scores. These results imply that DLR has a minimal impact on MRI interpretation for TZ lesions. Performance metrics in a dichotomized analysis further reinforced the results, with no significant differences in sensitivity or specificity between the two T2WI sets. Notably, the sensitivity and specificity in this study align with those from a meta-analysis34, suggesting that variations in radiologist performance may not influence the results of this study.
We also investigated two additional imaging findings: EPE grade and tumor size measurement, areas in which T2WI is pivotal. Although a direct correlation between EPE status or tumor size and pathological results was not possible in all patients, we assessed the intermethod agreement between the two T2WI sequences. Our study demonstrated a high level of agreement in both EPE grade and tumor size, aligning with a prior study on patients who underwent radical prostatectomy31. In that study, prostatectomy pathology confirmed EPE status, and DLR T2WI-based EPE grading yielded AUCs between 0.73 and 0.86. Notably, the number of patients with an EPE grade of 2 was lower on DLR T2WI than on TSE T2WI, as observed by both readers. Presumably, the sharper margin of the tumor or prostate gland may influence the evaluation. Although our results suggest that accelerated DLR T2WI could be a reliable substitute for TSE T2WI for evaluating tumor aggressiveness in the TZ, further research should explore the pathologically confirmed EPE status in TZ cancers with a larger patient population.
There are several limitations to this study. First, despite our efforts to include only patients with PCa in the TZ based on clinical reports, some lesions were detected in the PZ during independent image analysis. This occurred because we did not restrict the evaluation of the PI-RADS classification solely to the TZ, as we aimed to approximate clinical practice as closely as possible with the retrospective image review. However, given the relatively low incidence of TZ cancer, our approach was the most feasible for encompassing more TZ cancers. Furthermore, since we conducted a subgroup analysis for TZ lesions with a considerable number of patients (n = 139), the aims of this study were largely met. Second, we did not confirm the EPE status of PCa in all patients with radical prostatectomy results, as surgery was performed on only a small subset of patients. Nevertheless, by comparing EPE grades across the two T2WI sequences and between the two radiologists, we were able to demonstrate little impact of DLR T2WI on the variability in MRI reporting. Third, we did not perform an image quality analysis, as the similar or superior image quality of DLR T2WI relative to TSE T2WI has already been established in several publications24–28,30,31,33. A previous study that used the same DLR T2WI sequence in the prostate gland have showed no significant differences in most image quality factors including sharpness and lesion conspicuity between TSE T2WI and DLR T2WI33. Therefore, we did not duplicate the similar evaluation in this research.
In conclusion, DLR T2WI reduced the scan time by 69% without compromising the diagnostic performance for TZ lesions, suggesting its potential as an efficient alternative for prostate lesion assessment.
Methods
Patients
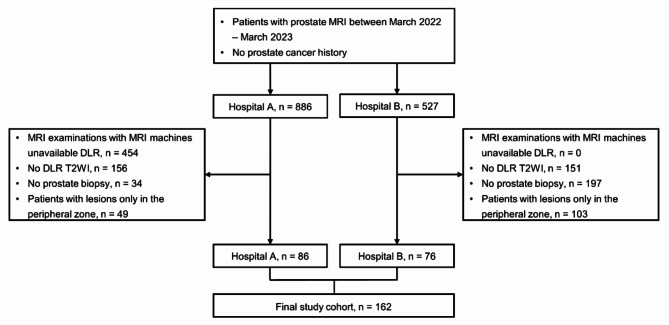
This retrospective bicenter study was approved by the institutional review boards of both participating hospitals, and was conducted according to the Helsinki Declaration guidelines. The requirement for informed consent was waived. PCa-naive patients who underwent prebiopsy prostate MRI due to a clinical suspicion of PCa between March 2022 and March 2023 were eligible. The exclusion criteria were as follows: (1) MRI examinations performed on machines in which DLR was unavailable, (2) MRI examinations without DLR T2WI, (3) no prostate biopsy, and (4) focal lesions only in the PZ based on clinical MRI reports. Poor image quality did not affect the inclusion of patients in the study. The final study cohort comprised 162 patients (86 from Hospital A and 76 from Hospital B, as depicted in Fig. 4). Patient age, prostate-specific antigen (PSA) level, and biopsy and prostatectomy results were obtained from electronic medical records. csPCa was defined as PCa with a Gleason grade group of 2 or higher according to the biopsy results.
Fig. 4.
Patient enrollment process.
MRI acquisition
MRI was performed using clinical 3 T scanners (MAGNETOM Vida, Siemens Healthcare, Erlangen, Germany) with a 30-channel body coil and a 32-channel or 72-channel spine coil, without an endorectal coil. Both mpMRI and bpMRI protocols adhered to PI-RADS guidelines. Sagittal T2WI was replaced with axial DLR T2WI, as we DLR to yield more information given its ability to illustrate sharper margins and produce less noise according to multiple studies25,26,30,32,33. For the conventional TSE T2WI, we utilized generalized autocalibrating partially parallel acquisitions (GRAPPA) with an acceleration factor of 2, whereas for the DLR T2WI, we used an acceleration factor of 4 and set the sequence to achieve equivalent spatial resolution to that of TSE T2WI. The scan times for TSE T2WI and DLR T2WI were 3 min 32 s and 1 min 6 s, respectively. The detailed parameters of the T2WI and DWI sequences used in the image analysis are shown in Table 5.
Table 5.
MRI parameters.
| Hospital A | Hospital B | |||||
|---|---|---|---|---|---|---|
| Sequence | TSE T2WI | DLR T2WI | DWI | TSE T2WI | DLR T2WI | DWI |
| TR (ms) | 2500–3000 | 2500–3000 | 4100–5300 | 2500–3000 | 2500–3000 | 5200–6000 |
| TE (ms) | 100–103 | 103 | 69–85 | 103 | 103 | 76 |
| Field of view (mm) | 200 × 200 | 180 × 180 |
220 × 220 or 200 × 180 |
180 × 180 | 180 × 180 | 200 × 180 |
| Matrix | 320 × 320 | 320 × 320 |
120 × 108 or 220 × 198 |
320 × 320 | 320 × 320 | 120 × 108 |
| Flip angle (°) | 136–148 | 136 | 90 | 136 | 136 | 90 |
| Slice thickness (mm) | 3 | 3 | 3 | 3 | 3 | 3 |
| Gap | 0 | 0 | 0 | 0 | 0 | 0 |
| B values (s/mm2) | - | - | 0, 100, 1000, 1500 | - | - | 0, 100, 1000, 1500 |
| NEX | 2 | 1 | 5, 5, 10, 10 | 2 | 1 | 2, 2, 9, 9 |
T2WI, T2-weighted imaging; TSE, turbo spin echo; DWI, diffusion-weighted imaging; TR, repetition time; TE, echo time; NEX, number of excitations.
Image analysis and gold standard
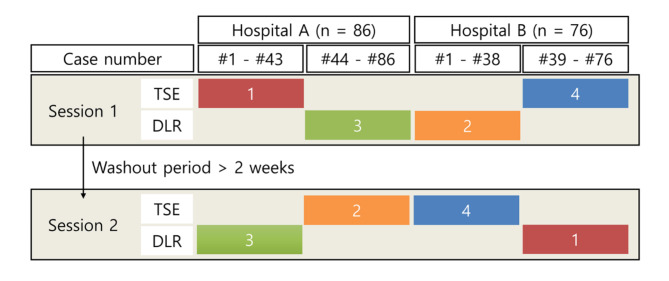
Two radiologists with more than 10 years of experience in urologic imaging independently reviewed the MRI scans. Due to differences in image texture, it was impossible to blind the reviewers to the T2WI technique. The two image sets were acquired for each patient: (1) axial TSE T2WI and axial DWI and (2) axial DLR T2WI and axial DWI. To avoid bias, the two radiologists reviewed these images in a predetermined sequence illustrated in Fig. 5, consisting of alternating between the T2WI techniques and the hospitals where the scans were performed.
Fig. 5.
Predetermined sequence of image analysis. Patients with TSE T2WI and DLR T2WI sets were reviewed in two sessions with a 2-week washout period. The review order alternated between hospitals and T2WI sequences, following a predetermined sequence of red (1), orange (2), green (3), and blue (4) blocks of MRI examinations including the half of patients from each hospital.
The radiologists identified an index lesion with the highest PI-RADS classification and the largest diameter, assigned the T2WI and overall PI-RADS class, measured the lesion diameter on T2WI and evaluated EPE using a previously suggested grading system, described as follows: (1) grade 1: curvilinear contact length 1.5 cm or capsular bulge/irregularity, (2) grade 2: curvilinear contact length 1.5 cm and capsular bulge/irregularity, and (3) grade 3: frank breach of the prostatic capsule or invasion of adjacent structures35.
The gold standard for lesion diagnosis was determined based on the biopsy results. Although the biopsy methods varied, both hospitals implemented a combination of targeted and systematic biopsies. Hospital A utilized a dedicated MRI-transrectal ultrasound (TRUS) fusion navigation system to perform a 12-core systematic and targeted biopsy. Hospital B used an electromagnetic MRI-US fusion approach to perform a modified targeted biopsy and a systematic biopsy, omitting systematic cores from areas that included the target lesion. A third radiologist, not involved in image analysis, matched the image review and biopsy results. When a lesion detected in the study was already reported on the clinical MRI and targeted during the biopsy, the targeted core results were used. If a detected lesion was not identified in the clinical report, the systematic core results closest to the target lesion were used, based on the six sections of the prostate gland (right/left, base/mid/apex).
Statistical analysis
Clinical variables are summarized using medians and interquartile ranges (IQRs) or frequencies with proportions. The differences in clinical variables between the two hospitals were evaluated using independent t tests and chi-square tests. Interreader agreement (radiologist 1 versus 2) and intermeathod agreement (TSE T2WI versus DLR T2WI) in the PI-RADS classification, EPE evaluation and tumor size were analyzed using intraclass correlation coefficients (ICCs) with a two-way model. The results were interpreted with the following standards: < 0.5, poor; 0.5–0.75, moderate; 0.75–0.9, good; and > 0.9, excellent agreement.
The diagnostic performance of each T2WI image set in detecting csPCa and predicting EPE was analyzed using receiver operating characteristic (ROC) curves, and differences in the areas under the curve (AUCs) were evaluated using DeLong’s method. The patients in the cohort were divided into two groups depending on whether the PI-RADS score was greater than or equal to or less than 4 (n = 139) after individuals with lesions identified within the PZ by either radiologist were excluded. Diagnostic metrics, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), were calculated along with 95% confidence intervals. McNemar’s tests were utilized to assess differences between DLR and TSE T2WI.
Statistical analyses were performed using SPSS software version 24.0 (IBM, Armonk, NY, USA), MedCalc version 20.014 (MedCalc Software Ltd., Ostend, Belgium) and GraphPad Prism version 10.0 (GraphPad Software, Inc., La Jolla, CA, USA). A P value < 0.05 was considered to indicate statistical significance.
Author contributions
D.K. and M.C. analyzed the results and wrote the main manuscript text. Y.L. and S.R. prepared figures and supervised the research. M.D.N., H.L., and D.H. provided the resources and software for MRI acquisition and M.C. received the research grant. All authors reviewed the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. NRF-2022R1F1A1069476). The funders were not involvd in the study design, data collection, analysis, or interpretation of the data.
Data availability
The data generated during and/or analysed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Competing interests
Marcel Dominik Nickel, Hyun-Soo Lee and Dongyeob Han are employees of Siemens Healthineers, but they did not influence the results of this study. Moon Hyung Choi is currently receiving a research grant from Company Siemens Healthineers and the research is not related to prostate MRI. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This retrospective study involving human participants was performed in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board of the Catholic Medical Center approved this study (XC23WIDI0064).
Informed consent
Informed consent was waived due to the retrospective design of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernandes, M. C., Yildirim, O., Woo, S., Vargas, H. A. & Hricak, H. The role of MRI in prostate cancer: current and future directions. MAGMA. 35, 503–521 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park, J. J. & Kim, C. K. Paradigm shift in prostate Cancer Diagnosis: pre-biopsy prostate magnetic resonance imaging and targeted Biopsy. Korean J. Radiol.23, 625–637 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaur, S., Turkbey, B. & Prostate, M. R. Imaging for posttreatment evaluation and recurrence. Radiol. Clin. North. Am.56, 263–275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, C. H., Tan, T. W. & Tan, C. H. Multiparametric MRI in active surveillance of prostate Cancer: an overview and a practical Approach. Korean J. Radiol.22, 1087–1099 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajwa, P. et al. Reliability of serial prostate magnetic resonance imaging to detect prostate Cancer progression during active surveillance: a systematic review and Meta-analysis. Eur. Urol.80, 549–563 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Oberlin, D. T., Casalino, D. D., Miller, F. H. & Meeks, J. J. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom. Radiol. (NY). 42, 1255–1258 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kuhl, C. K. et al. Abbreviated biparametric prostate MR Imaging in men with elevated prostate-specific Antigen. Radiology. 285, 493–505 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Turkbey, B. et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2, Eur. Urol. 76, 340–351 (2019). [DOI] [PubMed]
- 9.Gupta, R. T., Spilseth, B., Patel, N., Brown, A. F. & Yu, J. Multiparametric prostate MRI: focus on T2-weighted imaging and role in staging of prostate cancer. Abdom. Radiol. (NY). 41, 831–843 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Onay, A. et al. The role of T2-weighted images in assessing the grade of extraprostatic extension of the prostate carcinoma. Abdom. Radiol. (NY). 45, 3293–3300 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Bass, E. J. et al. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis.24, 596–611 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Cuocolo, R. et al. Clinically significant prostate Cancer detection with biparametric MRI: a systematic review and Meta-analysis. AJR Am. J. Roentgenol.216, 608–621 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Alabousi, M. et al. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naive patients: a diagnostic test accuracy systematic review and meta-analysis. BJU Int.124, 209–220 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Schoots, I. G. et al. PI-RADS Committee position on MRI without contrast medium in biopsy-naive men with suspected prostate Cancer: Narrative Review. AJR Am. J. Roentgenol.216, 3–19 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Tavakoli, A. A. et al. Contribution of dynamic contrast-enhanced and Diffusion MRI to PI-RADS for detecting clinically significant prostate Cancer. Radiology. 306, 186–199 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Christophe, C. et al. Prostate cancer local staging using biparametric MRI: assessment and comparison with multiparametric MRI. Eur. J. Radiol.132, 109350 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Abreu-Gomez, J. et al. Pharmacokinetic modeling of dynamic contrast-enhanced (DCE)-MRI in PI-RADS category 3 peripheral zone lesions: preliminary study evaluating DCE-MRI as an imaging biomarker for detection of clinically significant prostate cancers. Abdom. Radiol. (NY). 46, 4370–4380 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Winkel, D. J. et al. Compressed sensing Radial Sampling MRI of prostate perfusion: utility for detection of prostate Cancer. Radiology. 290, 702–708 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caglic, I. et al. Comparison of biparametric versus multiparametric prostate MRI for the detection of extracapsular extension and seminal vesicle invasion in biopsy naive patients. Eur. J. Radiol.141, 109804 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Zawaideh, J. P. et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: assessment of contrast benefit in clinical practice. Eur. Radiol.30, 4039–4049 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Kiryu, S. et al. Clinical impact of Deep Learning Reconstruction in MRI. Radiographics: Rev. Publication Radiological Soc. North. Am. Inc. 43, e220133 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Gassenmaier, S. et al. Deep Learning Applications in Magnetic Resonance Imaging: Has the Future Become Present? Diagnostics (Basel). 11, 2181 (2021). [DOI] [PMC free article] [PubMed]
- 23.Lin, D. J., Johnson, P. M., Knoll, F. & Lui, Y. W. Artificial Intelligence for MR Image Reconstruction: an overview for clinicians. J. Magn. Reson. Imaging. 53, 1015–1028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bischoff, L. M. et al. Deep Learning Super-resolution Reconstruction for fast and motion-robust T2-weighted prostate MRI. Radiology. 308, e230427 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Gassenmaier, S. et al. Deep learning-accelerated T2-weighted imaging of the prostate: reduction of acquisition time and improvement of image quality. Eur. J. Radiol.137, 109600 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Gassenmaier, S. et al. Accelerated T2-Weighted TSE Imaging of the Prostate Using Deep Learning Image Reconstruction: A Prospective Comparison with Standard T2-Weighted TSE Imaging, Cancers. 13, (2021). [DOI] [PMC free article] [PubMed]
- 27.Harder, F. N. et al. Prospectively Accelerated T2-Weighted Imaging of the Prostate by Combining Compressed SENSE and Deep Learning in Patients with Histologically Proven Prostate Cancer, Cancers. 14, (2022). [DOI] [PMC free article] [PubMed]
- 28.Johnson, P. M. et al. Deep Learning Reconstruction enables highly accelerated biparametric MR Imaging of the prostate. J. Magn. Reson. Imaging. 56, 184–195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung, W., Kim, E. H., Ko, J., Jeong, G. & Choi, M. H. Convolutional neural network-based reconstruction for acceleration of prostate T(2) weighted MR imaging: a retro- and prospective study. Br. J. Radiol.95, 20211378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong, A. et al. Comparison of a deep learning-accelerated vs. conventional T2-Weighted sequence in Biparametric MRI of the prostate. J. Magn. Reson. Imaging. 58, 1055–1064 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, J. C., Park, K. J., Park, M. Y., Kim, M. H. & Kim, J. K. Fast T2-Weighted imaging with Deep Learning-Based Reconstruction: evaluation of Image Quality and Diagnostic performance in patients undergoing radical prostatectomy. J. Magn. Reson. Imaging. 55, 1735–1744 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Gassenmaier, S. et al. Thin-slice prostate MRI enabled by Deep Learning Image Reconstruction. Cancers. 15, 578 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, E. H. et al. Deep learning-accelerated T2-weighted imaging of the prostate: impact of further acceleration with lower spatial resolution on image quality. Eur. J. Radiol.145, 110012 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Park, K. J., Choi, S. H., Kim, M. H., Kim, J. K. & Jeong, I. G. Performance of prostate imaging reporting and Data System Version 2.1 for diagnosis of prostate Cancer: a systematic review and Meta-analysis. J. Magn. Reson. Imaging. 54, 103–112 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Mehralivand, S. et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI, Radiology. 290, 709–719 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during and/or analysed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.