Abstract
To evaluate the differences in the agreement between wedged hepatic venous pressure (WHVP) and portal venous pressure (PVP) at different hepatic venous pressure gradient (HVPG) levels to identify specific HVPG thresholds where WHVP can reliably estimate PVP, thus enhancing the accuracy of risk stratification and treatment decision-making for portal hypertension (PHT) patients. A multicenter study of 616 patients with PHT from three centers was stratified into five groups by their HVPG: HVPG < 12 (group A), 12 ≤ HVPG < 16 mmHg (group B), 16 ≤ HVPG < 20 mmHg (group C), 20 ≤ HVPG < 24 mmHg (group D), HVPG ≥ 24 mmHg (group E). Concordance was analyzed using Pearson’s correlation coefficient (R), the intraclass correlation coefficient (ICC), and Bland‒Altman analysis in each HVPG stratum. Correlation and agreement between WHVP and PVP varied by HVPG group. Highest agreement was observed in the range of 20 ≤ HVPG < 24 mmHg. (R = 0.55, ICC = 0.68). The proportion of patients with a discrepancy between WHVP and PVP that was greater than 10% of the PVP value was highest in group A (95.7%) and lowest in group D (48.4%). Overestimation of PVP was more common in group E (44.5%), and underestimation of PVP was more common in group A (94.6%). This study does not confirm the usefulness of hepatic vein pressure measurements to predict the PVP and PPG. The means of WHVP and PVP were significantly different in ranges A, B, C, and E.
Keywords: Wedged hepatic venous pressure, Portal venous pressure, Hepatic venous pressure gradient, Stratified analysis, Portal hypertension
Subject terms: Portal hypertension, Hepatic portal vein
Introduction
Portal hypertension (PHT) is one of the most common and severe complications of cirrhosis, affecting millions of patients worldwide and significantly impacting their quality of life and survival rates1. It typically signifies the progression of liver disease to an advanced stage, necessitating active monitoring and management. The clinical manifestations of PHT include gastrointestinal bleeding, ascites, and hepatic encephalopathy, making accurate assessment of portal venous pressure (PVP) crucial for the prevention and treatment of high-risk patients2,3.
WHVP has been correlated with PVP, but these investigations often overlooked the diversity of etiologies and the potential impact of disease progression on this relationship4–6. Moreover, our understanding of how HVPG affects the concordance between WHVP and PVP is superficial. HVPG ≥ 12 mmHg can indicate cirrhosis and PHT decompensation7. A measurement error of HVPG > 4 mmHg can seriously affect the judgment of clinical results and the choice of treatment8. On this foundation, the present study stratified HVPG and explored the consistency between WHVP and PVP in different HVPG strata using a large-scale, multicenter approach. Our aim is to provide a more accurate PHT assessment method for clinicians, thereby improving the treatment outcomes and prognosis of patients with PHT.
Materials and methods
Patients
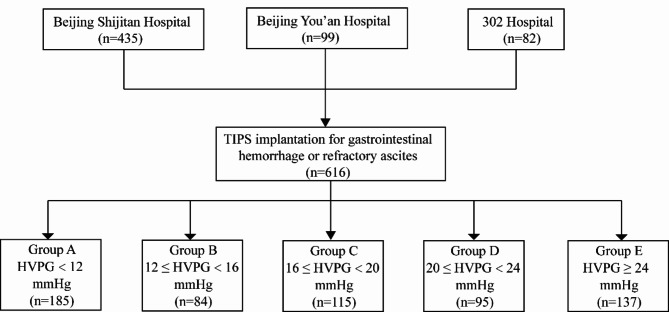
This retrospective study analyzed patients who underwent transjugular intrahepatic portosystemic shunt (TIPS) surgery at three different Beijing hospitals that have extensive experience in TIPS procedures and venous pressure measurements in Beijing Shijitan Hospital, Beijing You’an Hospital and the Fifth Medical Center of Chinese People’s Liberation Army General Hospital (302 Hospital) between January 2020 and June 2023. The study included 616 patients with PHT (Fig. 1). Inclusion criteria: (1) patients aged 18 years or older with a confirmed diagnosis of PHT, (2) patients were scheduled for TIPS treatment, in whom WHVP and PVP were successfully measured intraoperatively, (3) normal hepatic veins and inferior vena cavas (IVCs). Exclusion criteria: (1) history of malignant hepatobiliary system tumors, (2) portal vein thrombosis affecting blood flow (generally more than 1/3 of the main portal vein), (3) previous history of hepatic transplantation, (4) recent use of drugs affecting portal vein pressure, and (5) severe cardiopulmonary disease. The study was carried out in compliance with the relevant guidelines and the Declaration of Helsinki, having received approval from the ethical committees of the participating hospitals. Informed consent was obtained from all patients. TIPS was performed with local anesthesia and without sedation or general anesthesia.
Fig. 1.
Flowchart depicting the patient recruitment and stratification process. 302 Hospital: The Fifth Medical Center of Chinese People’s Liberation Army General Hospital. TIPS: transjugular intrahepatic portosystemic shunt; HVPG: hepatic venous pressure gradient.
Measurement of WHVP and PVP
Preoperative preparation
(1) All patients stopped taking drugs that affect portal vein pressure for at least 1 week before surgery. (2) They underwent relevant laboratory tests, including complete blood count, coagulation function, liver and kidney function, electrolytes, ammonia levels, blood type, and indocyanine green retention rate at 15 min (a quantitative liver function testICG-R15). (3) They underwent an electrocardiogram, echocardiography, and chest X-ray in both anterior-posterior and lateral views to exclude severe cardiopulmonary diseases. (4) They underwent ultrasound of the portal vein, enhanced abdominal CT and MRI to clarify the conditions of the hepatic veins, portal vein, and its branches. (5) We informed the patient and the patient’s family about the patient’s condition, the expected outcomes of the procedure, the potential risks as documented, and alternative options in case of those risks. Every willing participant signed an informed consent agreement for the procedure.
Pressure measurement
The measurements were performed by experienced interventional radiologists according to a standard protocol9. After disinfection and local anesthesia, a catheter sheath was introduced into the right internal jugular vein, and pressure within the right atrium and inferior vena cava was measured using the RUPS-100 system (COOK Medical, USA). Hepatic venography is conducted to ensure that the intended vein for pressure measurement is patent and free of stenosis. If these conditions are met, the vein is selected as the vessel for pressure measurements; otherwise, an alternative hepatic vein must be chosen. After confirming venous patency with hepatic venography, a Fogarty balloon catheter (Edwards Lifesciences, USA) connected to a pressure sensor was inserted into the hepatic vein via a 10 F sheath for pressure assessment. FHVP and WHVP were determined by positioning the balloon catheter tip 3–5 cm from the inferior vena cava and achieving complete venous occlusion (5 ml of contrast medium was injected for hepatic venography to confirm the absence of contrast reflux and venous collateral flow). The measurements were adjusted for occlusion adequacy and were done three times for accuracy. Under fluoroscopic guidance, the hepatic vein was punctured into the portal vein, and PVP and inferior vena cava pressure (IVCP) were measured by advancing a pigtail catheter into the splenic vein or superior mesenteric vein. This procedure was performed three times to calculate the average pressure values. HVPG was calculated as WHVP minus FHVP, and the portal pressure gradient (PPG) was calculated as PVP minus IVCP10, after which we did the TIPS procedure.
Groups and definitions
As depicted in Fig. 1, patients were divided into 5 groups according to their HVPG values. Patients with HVPG < 12 mmHg were defined as group A, patients with 12 ≤ HVPG < 16 mmHg were defined as group B, patients with 16 ≤ HVPG < 20 mmHg were defined as group C, and patients with 20 ≤ HVPG < 24 mmHg were defined as group D, and patients with HVPG ≥ 24 mmHg were defined as group E.
WHVP and PVP were defined as in agreement when both pressures differed by ≤ 10% of the PVP value. WHVP and PVP were in disagreement when both pressures differed by > 10% of the PVP value5. WHVP was defined as underestimating PVP when WHVP was more than 10% lower than PVP. WHVP was defined as overestimating PVP when WHVP was more than 10% higher than PVP4.
Statistical analysis
SPSS 25.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad, Inc., San Diego, CA, USA) were used for statistical analysis and graphing. Continuous variables with a normal or near-normal distribution are reported as mean ± standard deviation, and nonnormally distributed variables are reported as median [interquartile range, IQR]. The chi-squared test or Fisher’s exact test was used to compare groups on categorical variables, while Student’s t test or the Mann‒Whitney U test was used for continuous ones. Within-group differences between WHVP and PVP were examined using the paired t test or Wilcoxon signed-rank test, as appropriate. Univariate and multivariate binary logistic regression analysis were used to identify characteristics that were independently linked with the disagreement between WHVP and PVP. WHVP–PVP agreement was assessed via Pearson’s correlation coefficient (R), the intraclass correlation coefficient (ICC), and Bland‒Altman analysis. A higher ICC and R along with narrower Bland‒Altman 95% limits of agreement (95% LoA, mean difference ± 1.96 standard deviation) indicated better consistency11. P < 0.05 meant a difference was statistically significant.
Results
Baseline characteristics
In this study, a cohort of 616 patients with PHT who met the inclusion criteria was recruited from three centers. As shown in Table 1, the cohort comprised 447 males (72.6%) and 169 females (27.4%), with an age distribution ranging from 18 to 80 years and a mean age of 53 ± 11 years. The etiology of PHT was categorized as follows: hepatitis B virus-related cirrhosis in 351 cases, hepatitis C virus-related cirrhosis in 42 cases, alcoholic cirrhosis in 95 cases, autoimmune liver diseases in 88 cases, and cryptogenic cirrhosis in 40 cases. Among the participants, 459 patients (74.5%) presented with gastrointestinal bleeding, 76 patients (12.3%) with refractory ascites/hydrothorax, and 81 patients (13.2%) with gastrointestinal bleeding combined with refractory ascites. All patients successfully underwent pressure measurements and the TIPS procedure without any surgery-related complications or deaths. Only WHVP, HVPG, and PPG were found to be independently associated with the disagreement between WHVP and PVP in univariate and multivariate analyses. The discrepancy between WHVP and PVP did not correspond to the etiologies included. Thus, the findings can be applied to both viral and alcoholic disease. From their HVPG measurements, the patients were stratified into five groups: group A with HVPG < 12 mmHg (185 patients, 30.0%), group B with 12 ≤ HVPG < 16 mmHg (84 patients, 13.6%), group C with 16 ≤ HVPG < 20 mmHg (115 patients, 18.7%), group D with 20 ≤ HVPG < 24 mmHg (95 patients, 15.4%), and group E with HVPG ≥ 24 mmHg (137 patients, 22.3%). Table 2 displays that univariate and multivariate analyses of parameters related to the disagreement between WHVP and PVP.
Table 1.
Baseline characteristics of the included patients.
| Parameters | Group A n = 185 |
Group B n = 84 |
Group C n = 115 |
Group D n = 95 |
Group E n = 137 |
|---|---|---|---|---|---|
| Sex (male) |
128 (69.2%) |
55 (65.5%) |
88 (76.5%) |
73 (76.8%) |
103 (75.2%) |
| Age (years) | 52.4 ± 11.5 | 54.9 ± 11.2 | 54.0 ± 11.4 | 53.8 ± 12.1 | 53.2 ± 12.5 |
| Etiology | |||||
| HBV | 106 (41.1%) | 48 (46.2%) | 63 (44.7%) | 52 (46.0%) | 82 (48.5%) |
| HCV | 7 (2.7%) | 4 (3.8%) | 10 (7.1%) | 9 (8.0%) | 12 (7.1%) |
| ALC | 32 (12.4%) | 10 (9.6%) | 19 (13.5%) | 12 (10.6%) | 22 (13.0%) |
| ALD | 28 (10.9%) | 16 (15.4%) | 16 (11.3%) | 14 (12.4%) | 14 (8.3%) |
| Other | 12 (4.6%) | 6 (5.8%) | 7 (5.0%) | 8 (7.1%) | 7 (4.1%) |
| Indication | |||||
| Variceal bleeding | 138 (74.6%) | 63 (75.0%) | 88 (76.5%) | 72 (75.8%) | 98 (71.5%) |
| Ascites | 25 (13.5%) | 8 (9.5%) | 14 (12.2%) | 16 (16.8%) | 13 (9.5%) |
| Both | 22 (11.9%) | 13 (15.5%) | 13 (11.3%) | 7 (7.4%) | 26 (19.0%) |
| Laboratory values | |||||
| ALT (U/L) | 20.0 (13.5–28.0) | 18.5 (14.0–27.0) | 18.0 (12.0–26.0) | 21.0 (15.0–30.0) | 21.0 (15.0-27.5) |
| AST (U/L) | 26.0 (20.0-36.5) | 28.0 (20.3–41.8) | 26.0 (20.0–37.0) | 30.0 (22.0–47.0) | 31.0 (23.5–43.5) |
| ALP (U/L) | 89.0 (68.0-124.5) | 89.0 (64.3-140.3) | 77.0 (58.0-106.0) | 82.0 (58.0-119.0) | 90.0 (69.5–123.0) |
| GGT (U/L) | 34.0 (20.0–59.0) | 38.0 (18.0-77.3) | 29.0 (15.0–66.0) | 36.0 (17.0–83.0) | 40.0 (21.0-93.5) |
| ALB (g/L) | 37.0 ± 4.8 | 36.3 ± 4.8 | 35.2 ± 5.7 | 34.0 ± 5.8 | 34.6 ± 4.0 |
| Cr (µmol/L) | 62.0 (53.0-70.5) | 64.5 (51.3–74.8) | 63.0 (52.0–76.0) | 63.0 (52.0–74.0) | 64.0 (53.0–76.0) |
| TB (µmol/L) | 21.2 (14.3–30.6) | 21.3 (15.6–29.4) | 21.7 (16.1–30.4) | 27.3 (17.4–39.4) | 25.5 (18.6–38.2) |
| INR | 1.2 (1.1–1.4) | 1.2 (1.1–1.3) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) | 1.3 (1.2–1.5) |
| APTT (s) | 32.9 (30.2–35.3) | 32.7 (30.9–34.9) | 32.8 (30.5–35.9) | 33.4 (31.4–37.0) | 33.5 (31.3–36.5) |
| PT (s) | 13.6 (12.4–15.4) | 13.7 (12.6–14.6) | 14.6 (13.4–16.3) | 14.6 (13.2–16.2) | 14.4 (13.5–16.4) |
| Hb (g/L) | 96.4 ± 26.2 | 93.7 ± 23.6 | 85.5 ± 24.6 | 94.1 ± 24.4 | 90.0 ± 23.0 |
| WBC (×109/L) | 2.9 (1.9–4.1) | 2.8 (1.8–4.3) | 2.5 (1.6–4.2) | 2.9 (1.8–4.5) | 2.6 (1.7–3.8) |
| PLT (×109/L)) | 84.0 (49.0-131.0) | 76.0 (57.5–113.0) | 72.0 (50.0–98.0) | 75.0 (47.0-113.0) | 72.0 (48.5-102.5) |
| Child‒Pugh score | 6.0 (5.0–7.0) | 7.0 (5.3-7.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) |
| Hemodynamics (mmHg) | |||||
| WHVP | 18.0 ± 6.0 | 24.5 ± 4.4 | 28.1 ± 4.2 | 32.9 ± 4.6 | 39.2 ± 6.7 |
| PVP | 33.5 ± 7.6 | 33.9 ± 7.0 | 33.3 ± 6.5 | 33.4 ± 6.5 | 35.6 ± 6.3 |
| FHVP | 11.1 ± 4.8 | 10.8 ± 4.3 | 10.3 ± 4.0 | 11.3 ± 4.3 | 11.0 ± 4.1 |
| IVCP | 8.6 ± 3.6 | 8.0 ± 3.8 | 7.9 ± 3.6 | 8.2 ± 3.2 | 8.6 ± 3.7 |
| HVPG | 6.8 ± 3.4 | 13.7 ± 1.2 | 17.8 ± 1.1 | 21.6 ± 1.2 | 28.2 ± 4.7 |
| PPG | 25.1 ± 6.6 | 26.2 ± 7.4 | 25.4 ± 6.3 | 25.4 ± 6.0 | 27.1 ± 6.2 |
HBV: hepatitis B virus–related cirrhosis; HCV: hepatitis C virus–related cirrhosis; ALC: alcoholic liver cirrhosis; ALD: autoimmune liver disease; Other: liver cirrhosis of unknown cause; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: γ-glutamyl transpeptidase; ALB: albumin; TB: total bilirubin; INR: international normalized ratio; APTT: activated partial thromboplastin time; PT: prothrombin time; Hb: hemoglobin; WBC: white blood cell; PLT: platelet; WHVP: wedged hepatic venous pressure; PVP: portal venous pressure; FHVP: free hepatic venous pressure; IVCP: inferior vena cava pressure; HVPG: hepatic venous pressure gradient; PPG: portal venous pressure gradient.
Table 2.
Uni- and multivariate analysis for the determination of factors independently associated with the disagreement between WHVP and PVP.
| Variable | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P value | OR (95% CI) | P value | |
| Sex (male) | 0.032 | 0.439–1.118 | 0.135 |
| Age (years) | 0.351 | ||
| Etiology | |||
| HBV | – | ||
| HCV | 0.371 | ||
| ALC | 0.257 | ||
| ALD | 0.154 | ||
| Other | 0.255 | ||
| Indication | |||
| Variceal bleeding | – | ||
| Ascites | 0.132 | ||
| Both | 0.137 | ||
| Laboratory values | |||
| ALT (U/L) | 0.366 | ||
| AST (U/L) | 0.934 | ||
| ALP (U/L) | 0.299 | ||
| GGT (U/L) | 0.069 | ||
| ALB (g/L) | 0.975 | ||
| Cr (µmol/L) | 0.980 | ||
| TB (µmol/L) | 0.954 | ||
| INR | 0.959 | ||
| APTT (s) | 0.792 | ||
| PT (s) | 0.234 | ||
| Hb (g/L) | 0.935 | ||
| WBC (×109/L) | 0.707 | ||
| PLT (×109/L)) | 0.658 | ||
| Child‒Pugh score | 0.359 | ||
| Hemodynamics (mmHg) | |||
| WHVP | < 0.001 | 1.004–1.100 | 0.035 |
| PVP | 0.094 | ||
| FHVP | 0.069 | ||
| IVCP | 0.203 | ||
| HVPG | < 0.001 | 1.004–1.111 | 0.035 |
| PPG | 0.012 | 0.904–0.967 | < 0.001 |
HBV: hepatitis B virus–related cirrhosis; HCV: hepatitis C virus–related cirrhosis; ALC: alcoholic liver cirrhosis; ALD: autoimmune liver disease; Other: liver cirrhosis of unknown cause; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: γ-glutamyl transpeptidase; ALB: albumin; TB: total bilirubin; INR: international normalized ratio; APTT: activated partial thromboplastin time; PT: prothrombin time; Hb: hemoglobin; WBC: white blood cell; PLT: platelet; WHVP: wedged hepatic venous pressure; PVP: portal venous pressure; FHVP: free hepatic venous pressure; IVCP: inferior vena cava pressure; HVPG: hepatic venous pressure gradient; PPG: portal venous pressure gradient.
Significant values are in bold.
Agreement analysis between WHVP and PVP
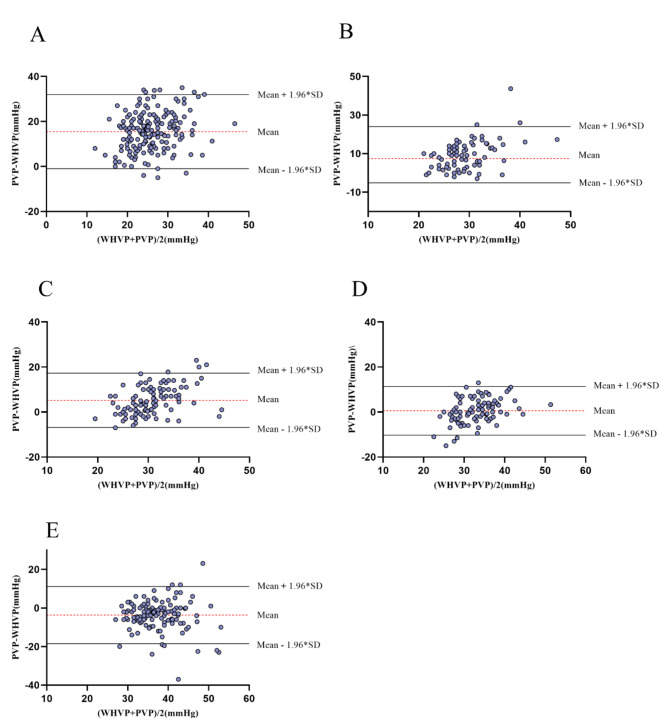
Table 3 displays the Pearson’s R, ICC, and 95%LoA for WHVP and PVP across the different groups. Group D, (20 ≤ HVPG < 24 mmHg) exhibited the strongest correlation between WHVP and PVP (R = 0.55, P < 0.001) and highest ICC (ICC = 0.68, P < 0.001), suggesting that the best concordance between WHVP and PVP was within this range. This R and ICC indicated moderate agreement. The R and ICC values in the other groups were as follows: group A: R = 0.26 (P < 0.001), ICC = 0.13 (P < 0.001); group B: R = 0.22 (P = 0.047), ICC = 0.16 (P = 0.037); group C: R = 0.41 (P < 0.001), ICC = 0.41 (P < 0.001); and group E: R = 0.33 (P < 0.001), ICC = 0.44 (P < 0.001). These four strata all showed poor agreement between WHVP and PVP.
Table 3.
Correlations between wedged hepatic venous pressure and portal venous pressure.
| R | P | ICC | P value | 95%LoA | |
|---|---|---|---|---|---|
|
Group A HVPG < 12 mmHg |
0.26 | < 0.001 | 0.13 | < 0.001 | -0.97-31.94 |
|
Group B 12 ≤ HVPG < 16 mmHg |
0.22 | 0.047 | 0.16 | 0.037 | -5.14-24.02 |
|
Group C 16 ≤ HVPG < 20 mmHg |
0.41 | < 0.001 | 0.41 | < 0.001 | -6.83-17.28 |
|
Group D 20 ≤ HVPG < 24 mmHg |
0.55 | < 0.001 | 0.68 | < 0.001 | -10.21-11.32 |
|
Group E HVPG ≥ 24 mmHg |
0.33 | < 0.001 | 0.44 | < 0.001 | -18.51-11.14 |
HVPG: hepatic venous pressure gradient; R: Pearson’s correlation coefficient; ICC: interclass correlation coefficient; 95%LoA: 95% limits of agreement (mean difference ± 1.96 standard deviation).
When assessing the concordance between WHVP and PVP using Bland‒Altman analysis, a wider 95% LoA suggested poorer agreement. Correspondingly, as revealed in Table 3, group D’s WHVP and PVP (Fig. 2) had the narrowest 95% LoA, indicating the best concordance. Group A had the widest 95% LoA, signifying the poorest agreement. These findings are generally consistent with the results of Pearson’s correlation and ICC.
Fig. 2.
Bland–Altman plots of the agreement between WHVP and PVP. A: HVPG < 12 mmHg; B: 12 ≤ HVPG < 16 mmHg; C: 16 ≤ HVPG < 20 mmHg; D: 20 ≤ HVPG < 24 mmHg; E: HVPG ≥ 24 mmHg. WHVP: wedged hepatic venous pressure; PVP: portal venous pressure; HVPG: hepatic venous pressure gradient.
Performance of WHVP in evaluating PVP
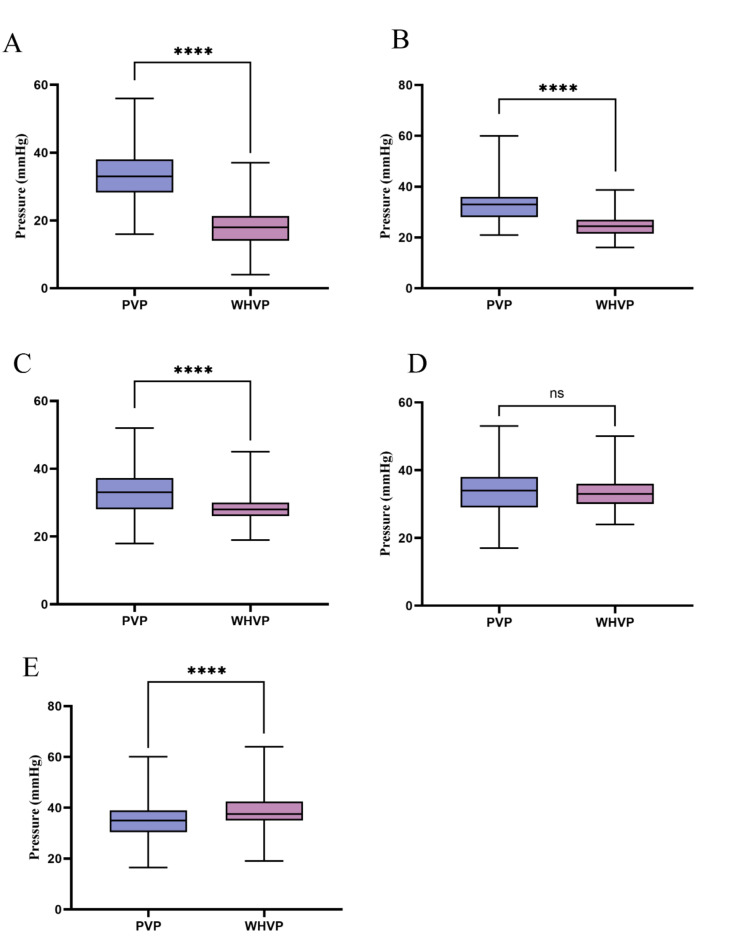
The mean values of WHVP and PVP across the different groups are presented in Table 1. In group A, the average WHVP was approximately 15 mmHg lower than the average PVP, a significant difference (P < 0.001). In group B, the average WHVP was around 9 mmHg lower than the average PVP (P < 0.001). In group C, the average WHVP was about 5 mmHg lower than the average PVP (P < 0.001). Group D exhibited the closest mean values between WHVP and PVP, with a difference of less than 1 mmHg, which were not statistically significant (P = 0.258). In group E, the average WHVP was approximately 4 mmHg higher than the average PVP (P < 0.001). The comparisons between WHVP and PVP across the groups are depicted in Fig. 3.
Fig. 3.
Comparison between WHVP and PVP using the Wilcoxon signed-rank test. ****: P < 0.0001; ns: no significance. A: HVPG < 12 mmHg; B: 12 ≤ HVPG < 16 mmHg; C: 16 ≤ HVPG < 20 mmHg; D: 20 ≤ HVPG < 24 mmHg; E: HVPG ≥ 24 mmHg. WHVP: wedged hepatic venous pressure.
As indicated in Table 4, more patients in group D had WHVP–PVP agreement (difference ≤ 10% PVP) than any other group, at 51.6%, whereas group A had the highest proportion of disagreement (difference > 10% PVP), at 95.7%. By integrating the data from Table 4 with Fig. 3, we saw that among patients whose WHVP was lower than their PVP, group A accounted for the largest proportion of 94.6%. This suggests that when HVPG is < 12 mmHg, WHVP often underestimates PVP. As the pressures gradually increased, the concordance between WHVP and PVP also increases till it reached its maximum in group D. Moreover, among patients whose WHVP was greater than their PVP, the largest proportion was found in group E, at 44.5%, indicating that when HVPG is ≥ 24 mmHg, WHVP often overestimates PVP (the differences were statistically significant after applying Bonferroni correction for multiple comparisons).
Table 4.
Performance of wedged hepatic venous pressure in assessing portal venous pressure.
| Group A n = 185 |
Group B n = 84 |
Group C n = 115 |
Group D n = 95 |
Group E n = 137 |
P value | |
|---|---|---|---|---|---|---|
|
Agreement between WHVP and PVP |
8 (4.3%) |
18 (21.4%) |
42 (36.5%) |
49 (51.6%) |
63 (46.0%) |
< 0.001 |
|
Disagreement between WHVP and PVP |
177 (95.7%) |
66 (78.6%) |
73 (63.5%) |
46 (48.4%) |
74 (54.0%) |
|
| Underestimation of PVP |
175 (94.6%) |
66 (78.6%) |
63 (54.8%) |
25 (26.3%) |
13 (9.5%) |
< 0.001 |
| Overestimation of PVP |
2 (1.1%) |
0 (0.0%) |
10 (8.7%) |
21 (22.1%) |
61 (44.5%) |
< 0.001 |
WHVP: wedged hepatic venous pressure; PVP: portal venous pressure.
Discussion
HVPG is the most commonly used clinical parameter for assessing sinusoidal PHT in patients with cirrhosis, serving as the gold standard for identifying the presence of clinically significant PHT (defined as HVPG ≥ 10 mmHg)12. Stratified management of HVPG plays a pivotal role in disease prognostic assessment13, monitoring therapeutic responses14, predicting complications15, and guiding liver transplantation decisions16. WHVP is a key variable in calculating HVPG, and accurately reflecting PVP is a prerequisite for the HVPG-based diagnosis of PHT. Although numerous studies5,17,18 have indicated that the correlation between WHVP and PVP is influenced by various factors, such as etiology and hepatic vascular anatomy, research integrating their relationship with HVPG stratification remains scarce. In this study, we conducted a multicenter analysis of HVPG strata to investigate the various correlations between WHVP and PVP. Through a detailed analysis of 616 patients, we found the significant impact of HVPG on the concordance between WHVP and PVP, providing important insights for the optimization of management and treatment strategies for PHT.
Our study indicates that the overall correlation between WHVP and PVP is relatively weak and firstly demonstrates that the concordance between WHVP and PVP varies with changes in HVPG. The best concordance was in group D (HVPG between 20 and 24 mmHg) and the worst in group A (HVPG < 12 mmHg). We set a threshold for concordance at 10% of the magnitude of PVP, calling WHVP and PVP discordant when their difference passed this threshold, in which case WHVP did not accurately reflect PVP. Accordingly, we found that the highest proportion of patients had HVPG < 12 mmHg (95.7%), and the lowest proportion had an HVPG in the 20 ~ 24 mmHg range (48.4%), providing an important reference standard for clinical assessment. Clinically, for patients undergoing secondary prevention, HVPG < 12 mmHg or a reduction of 20% from baseline indicates a decreased probability of rebleeding19. For patients with HVPG < 12 mmHg or ≥ 24 mmHg, where WHVP does not reliably estimate PVP, it is recommended to make a comprehensive assessment based on clinical manifestations, laboratory tests, and imaging studies. When necessary, PVP should be measured via the transjugular or transhepatic approach to improve clinical decision-making, particularly in assessing the risk of complications such as variceal bleeding and guiding appropriate interventions.
Our study further revealed that when HVPG is < 12 mmHg, WHVP often underestimates PVP, which may be related to the presence of anatomical shunting in hepatic vessels and changes in hepatic hemodynamics. Extensive research by our group20–23 has indicated that hepatic venous collaterals can be detected more efficiently when the dose (15 ml) and pressure (200–300 psi) of contrast medium are increased, and the presence of hepatic venous collaterals is a key factor contributing to the underestimation of PPG by HVPG. Specifically, when the hepatic vein is occluded with a balloon to measure WHVP, the presence of collaterals allows a portion of the hepatic venous blood flow to return to the systemic circulation through these collaterals, resulting in a lower measured pressure. Therefore, in conjunction with the findings of this study, we can infer that the lower the HVPG is, the more pronounced the underestimation of PVP by WHVP becomes, suggesting that at this point, diversion through collaterals plays a dominant role. One study24 has shown that the underestimation of PVP by WHVP could be attributed to presinusoidal PHT, yet the underlying pathophysiological mechanisms remain unclear. Whether these patients with presinusoidal PHT predominantly have HVPG < 12 mmHg warrants further investigation.
WHVP often overestimated PVP in group E (HVPG ≥ 24 mmHg), which may be associated with their abnormal perfusion of hepatic blood flow. Our previous studies20–23 indicated that the absence of collaterals between hepatic veins and between hepatic and portal veins could lead HVPG to overestimate PPG. In such scenarios, the blood flow and sinusoidal pressure in the liver might depend mostly on hepatic arterial perfusion, possibly even resulting in a reversal of the direction of portal vein blood flow. The findings of the current study suggest that the higher the HVPG is, particularly above 24 mmHg, the more pronounced the overestimation of PVP becomes, indicating that the blood supply to the sinusoids may come mostly from the hepatic artery under these conditions. Other studies have shown that an WHVP often overestimates PVP when the PHT includes a postsinusoidal component3,4. This may be related to the presence of reversed hepatic blood flow, the opening of paraumbilical veins, and the formation of portocaval anastomoses. Whether this phenomenon of postsinusoidal PHT occurs more in patients with HVPG ≥ 24 mmHg requires further research.
Despite the valuable insights provided by our study, it has some limitations. First, it did not delve into the physiological mechanisms underlying the discrepancies in concordance between WHVP and PVP. This will be an important direction for future research. Second, although the patients had a wide range of etiologies of PHT, these causes might not have been subdivided finely enough. Specific etiologies, such as hepatitis B virus, hepatitis C virus, and alcoholic liver disease, may have different impacts on the physiological effects of PHT and thus on the concordance between WHVP and PVP. The failure to finely distinguish these causes may obscure the impact of etiology-specific factors. Third, given that our study is based on a cohort of patients who underwent TIPS across three hospitals, there is a possibility that the inclusion criteria may have favored patients who had more severe cases of PHT requiring intervention. This could limit the generalizability of our findings to all patients with PHT. Moreover, as a retrospective study, we were limited by the available data and the inherent constraints of non-randomized data collection. This means we could not control for all confounding variables or obtain prospective measures, which might reduce the strength of causal conclusions.
Future research should aim to overcome these limitations, enhancing the generalizability of the results, through multicentric, international collaborative and prospective studies. We also highlight that future research could focus on the development of more accurate, non-invasive measurement tools and predictive models to help reconcile the differences between WHVP and PVP and reduce the reliance on invasive procedures. In summary, through a large-scale, multicentric design, this study conducted an in-depth analysis of the concordance between WHVP and PVP in patients with PHT and revealed the impact of HVPG on their concordance. These findings underscore the importance of considering HVPG when evaluating patients with PHT, as well as the necessity of identifying potential discrepancies between WHVP and PVP when formulating treatment strategies. Despite certain limitations, our research provides valuable information and new directions for future clinical practice, which we hope will help optimize management strategies for PHT and improve patient outcomes.
Acknowledgements
We would like to express our gratitude to all the medical staff of the Liver disease Minimally Invasive Diagnosis and Treatment Center of Beijing Shijitan Hospital affiliated to Capital Medical University, Liver Vascular disease Diagnosis and Treatment Center of Fifth Medical Center of Chinese PLA General Hospital and Department of Gastroenterology and Hepatology of Beijing You’an Hospital affiliated to Capital Medical University for assisting in the collection of clinical data.
Abbreviations
- HVPG
Hepatic venous pressure gradient
- ICC
Intraclass correlation coefficient
- PHT
Portal hypertension
- PVP
Portal venous pressure
- R
Pearson’s correlation coefficient
- WHVP
Wedged hepatic venous pressure
Author contributions
Fuquan Liu, Yongping Yang and Huiguo Ding designed the research; Bing Zhu, Dongze Li, Hua Tian, Shaoli You, Fuchuan Wang, Sa Lv, Yifan Wu, Chengbin Dong and Yu Zhang performed the research; Yifan Lv analyzed the data and wrote the paper; Fuquan Liu reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
The study was supported by the Talent Training Plan during the “14th Five-Year Plan” period of Beijing Shijitan Hospital affiliated to Capital Medical University (2023LJRCLFQ) and National Multi-Center Clinical Research Project of Peking University First Hospital (2022cz020301) to Fuquan Liu.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical statements
The study was approved by the Ethics Committee and Institutional Review Board of Beijing Shijitan Hospital, Beijing You’an Hospital and the Fifth Medical Center of Chinese PLA General Hospital.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saab S. Portal hypertension. Clin. Liver Dis., 23(4): xiii–xiv. (2019). [DOI] [PubMed]
- 2.Simonetto D. A., Liu, M. & Kamath, P. S. Portal hypertension and related complications: Diagnosis and management. Mayo Clin. Proc.94 (4), 714–726 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Turco, L. & Garcia-Tsao, G. Portal hypertension: Pathogenesis and diagnosis. Clin. Liver Dis.23 (4), 573–587 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Cheng, Y. et al. Agreement between wedged hepatic venous pressure and portal pressure in hepatic sinusoidal obstruction syndrome . J. Pers. Med., 13(1). (2022). [DOI] [PMC free article] [PubMed]
- 5.Ferrusquia-acosta, J. & Bassegoda, O. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis . J. Hepatol.74 (4), 811–818 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Perello, A. et al. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis . Hepatology30 (6), 1393–1397 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Veldhuijzen van Zanten, D., Buganza, E & Abraldes, J. G. The role of hepatic venous pressure gradient in the management of cirrhosis. Clin. Liver Dis.25 (2), 327–343 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Ma, J. & Gong, X. Impact of Intrahepatic Venovenous Shunt on hepatic venous pressure gradient measurement . J. Vasc Interv Radiol.31 (12), 2081–2088 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Alliance C. P. H., Group M I I, C. Consensus on clinical application of hepatic venous pressure gradient in China (2023 edition) . Zhonghua Yi Xue Za Zhi, 103(48): 3885–3895. (2023). [DOI] [PubMed]
- 10.Bosch, J., Abraldes J. G., Berzigotti, A. & Garcia-pagan J. C. The clinical use of HVPG measurements in chronic liver disease . Nat. Rev. Gastroenterol. Hepatol.6 (10), 573–582 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement . Lancet1 (8476), 307–310 (1986). [PubMed] [Google Scholar]
- 12.De Franchis, R., Bosch, J. et al. Baveno VII - renewing consensus in portal hypertension . J. Hepatol.76 (4), 959–974 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paternostro, R. et al. The prognostic value of HVPG-response to non-selective beta-blockers in patients with NASH cirrhosis and varices . Dig. Liver Dis.54 (4), 500–508 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Albillos, A. et al. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: A meta-analysis . Am. J. Gastroenterol.102 (5), 1116–1126 (2007). [DOI] [PubMed] [Google Scholar]
- 15.La Mura V, Garcia-Guix M, Berzigotti, A. et al. A prognostic strategy based on stage of cirrhosis and HVPG to Improve Risk Stratification after Variceal bleeding . Hepatology72 (4), 1353–1365 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Giabicani, M., Joly, P., SigauT S, et al. Predictive role of hepatic venous pressure gradient in bleeding events among cirrhotic patients undergoing Orthotopic Liver transplantation . JHEP Rep., 101051. (2024). [DOI] [PMC free article] [PubMed]
- 17.Osada, Y. et al. Wedged hepatic venous pressure does not reflect portal pressure in patients with cirrhosis and hepatic veno-venous communications . Dig. Dis. Sci.53 (1), 7–13 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Pomier-Layrargues G, & Kusielewicz, D. Presinusoidal portal hypertension in non-alcoholic cirrhosis . Hepatology5 (3), 415–418 (1985). [DOI] [PubMed] [Google Scholar]
- 19.Baiges, A., Hernandez-gea, V, & Bosch, J. Pharmacologic prevention of variceal bleeding and rebleeding . Hepatol. Int.12 (Suppl 1), 68–80 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Liu, B. & Zhang, D. Correlation between hepatic venous pressure gradient and portal pressure gradient in patients with autoimmune cirrhotic portal hypertension and collateral branches of the hepatic vein . Hepatol. Res.53 (11), 1084–1095 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Lv, Y. et al. Correlation between hepatic venous pressure gradient and portal venous pressure gradient in hepatitis B cirrhosis with different hepatic veins anatomy . Eur. J. Radiol.155, 110463 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Zhang, D. et al. Hepatic venous pressure gradient: Inaccurately estimates portal venous pressure gradient in alcoholic cirrhosis and portal hypertension . World J. Gastrointest. Surg.15 (11), 2490–2499 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WANG, L. et al. Study on the correlation between PPG and HVPG in patients with portal hypertension. Zhonghua Gan Zang Bing Za Zhi. 30 (7), 722–727 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Madir, A., Grgurevic I, Tsochatzis, E. A., & Pinzani, M. Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges . World J. Gastroenterol.30 (4), 290–307 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.