Abstract
Purpose
Immunotherapy has become the primary option for recurrent and metastatic nasopharyngeal cancer (R/M NPC) after failure of chemotherapy, but without good prognostic indicators. Our study aimed to assess the potential of the systemic immune-inflammation index (SII) in predicting the effectiveness of PD-L1 inhibitor therapy for R/M NPC.
Patients and Methods
The study cohort comprises of a prospective Phase 2 clinical trial population undergoing PD-L1 inhibitor for R/M NPC at 42 hospitals in China between 2019 and 2021. The SII is classified into high and low states based on the optimal threshold determined by the ROC curve. We assessed the relationship between SII status and objective remission rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS) using regression analyses and Kaplan–Meier method. We performed sensitivity analyses to confirm the results.
Results
Our study analyzed 153 patients from one of the largest cohorts to date of R/M NPC treated with PD-L1 inhibitor and found that SII showed a significant association with prognosis. We found higher ORR and DCR in the SII-Low group. Univariate analyses demonstrated that SII independently predicted DCR (OR, 0.43; 95% CI, 0.22–0.84; p = 0.001), PFS (HR, 1.85; 95% CI, 1.31–2.62; p < 0.001) and OS (HR, 1.92; 95% CI, 1.29–2.85; p < 0.001). After adjusting for covariates, multivariate analysis remains relevant. [DCR (OR, 0.47; 95% CI, 0.22–0.99; p = 0.048), PFS (HR, 1.72; 95% CI, 1.2–2.47; p =0.003); OS (HR, 2.08; 95% CI, 1.38–3.13; p < 0.001)]. Sensitivity analyses also support this conclusion.
Conclusion
SII may well provide predictive value for the efficacy and prognosis of patients with R/M NPC treated with PD-L1 inhibitor. Patients with high status of SII may have a poorer therapeutic effect and survival.
Keywords: systemic immune response, nasopharyngeal carcinoma prognosis, immunotherapy, efficacy
Introduction
Nasopharyngeal carcinoma (NPC) is a tumor with high malignancy and mortality rates,1 and is notably common in Southeast Asia, including southern China,2,3 where about 4.4% to 14.8% of NPC patients present with distant metastases upon initial diagnosis.4–6 Despite recent advancements in radiotherapy techniques and chemotherapeutic agents, the survival rate of NPC patients has not improved well.7–9 However, the treatment of NPC recurrence and metastasis still faces great challenges.10–12 Therefore, it has become imperative to seek more effective and promising treatment strategies.
Immunotherapy has brought about a revolution in cancer treatment in recent years., with significant breakthroughs achieved through the use of programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) immune checkpoint inhibitors (ICIs).13 Such therapeutic agents are now standard of care in the treatment of R/M NPC, marking a key advance in the field.14–16 KL-A167, a targeted PD-L1 immune checkpoint inhibitor, has been shown to be effective and safe in treating patients with R/M NPC who have failed first-line therapy.17 Despite these achievements, challenges persist in accurately predicting treatment efficacy and devising personalized therapeutic approaches for this patient cohort.
Inflammation and immune regulation’s interplay significantly influences disease progression and survival across various cancers.18 Systemic inflammation correlates with alterations in peripheral blood leukocytes, which can be gauged using the Systemic Immunoinflammatory Index (SII),19,20 calculates from neutrophil (N), lymphocyte (L), and platelet (P) counts using the formula: SII = N × P/L. The current study highlights that platelets play a crucial role in shielding circulating tumor cells (CTCs) from shear stress, aiding CTC transition, and facilitating extravasation of tumor cells to provide protection.21,22 Neutrophils promote tumor progression by releasing growth factors like vascular endothelial growth factor, which promotes adhesion of tumor cells to distant organs.23–26 Lymphocytes are essential in defending against tumors by enhancing cytotoxic cell death and restraining tumor cell proliferation and migration.27–29 SII has been established as a prognostic factor in NPC treated with chemotherapy and radiotherapy.30–32 In the present study, we further evaluated the prognostic value of SII in NPC patients receiving immunotherapy.
This study focuses on the first published and largest multicenter, prospective Phase II clinical trials of PD-L1 inhibitors in R/M NPC previously treated, to evaluate the predictive role of SII, a novel indicator that includes multiple mediators of inflammation.
Materials and Methods
Study Population
We conducted a post hoc analysis of data from a prospective phase 2 clinical trial conducted in 42 hospitals in the People’s Republic of China. The clinical trial focused on the treatment of 153 patients diagnosed with histologically confirmed R/M NPC using KL-A167, a fully humanized monoclonal antibody PD-L1. This study adheres to the principles of the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines. The patient inclusion and exclusion criteria, along with the trial profile flowchart, are provided in the Supplementary File (Supplementary Figure 1).
Research Endpoints
The study assessed various endpoints, including Objective Remission Rate (ORR), Disease Control Rate (DCR), Progression-Free Survival (PFS), Overall Survival (OS). ORR was evaluated by the Independent Review Committee (IRC) according to RECIST V1.1 criteria, measuring the proportion of patients achieving partial remission (PR), and stable disease (SD). DCR measured the proportion of patients achieving complete response (CR), PR and SD. PFS represented the duration from the first PD-L1 inhibitor dose to disease progression or death. And OS defined the time from PD-L1 inhibitor drug initiation to death from any cause.
Data Analysis
Patients were categorized into high SII (SII > 1139) and low SII (SII ≤ 1139) status based on the optimal threshold value of SII determined from receiver operating characteristic (ROC) curves (Supplementary Figure 2). We first assessed the prognostic significance of inflammatory biomarkers on ORR and DCR by using logistic regression and calculated the corresponding hazard ratios (HR). We employed COX regression models to examine the relationship between various SII status and PFS as well as OS. Kaplan-Meier curves were utilized to compare the differences in OS and PFS between high and low SII groups. Covariates with a p-value below 0.05 in univariate analyses were incorporated into the subsequent stepwise multivariate analysis.
We constructed multifactorial models incorporating significant and essential prognostic factors from the multivariate analyses to evaluate the predictive reliability of the SII for PD-L1 inhibitor therapy prognosis. The predictive performance of the SII and individual inflammatory mediators was evaluated using the area under the receiver operating characteristic curve (AUC-ROC) and compared with the predictive performance of component factors. We also used restricted cubic spline to flexibly model the relationship of SII with death and prognosis. Subsequently, we conducted tests to assess for potential effect modification by age, gender, smoking status, alcohol status, body mass index, ECOG PS and liver metastasis. Likelihood ratio tests were utilized to evaluate interaction on the multiplicative scale, while the relative excess risk caused by interaction was calculated to assess interaction on the additive scale.
All statistical analyses were performed using R 4.3.2 (R project, http://www.R-project.org/; accessed on 31 October 2023, version 4.3.2). Statistical significance was determined as a two-sided p-value below 0.05.
Results
Basic Clinical Information of Patients
Between February 26, 2019, and January 13, 2021, a cohort of 153 patients who had received at least one dose of PD-L1 inhibitor was analyzed across 42 medical institutions in China. The baseline characteristics of the patients are presented in Table 1. Among them, 94 individuals were categorized in SII-Low Group, and 59 in the SII-High Group. The median age of the patients was 49 years, ranging from 20 to 68. And the male proportion was relatively higher within the subpopulation (125 cases, 81.7%). At the initiation of the study, 82 out of 153 patients (53.6%) exhibited liver metastasis. ECOG scores were uniformly distributed between 0 and 1, with 94 cases (61.4%) scored as 1 (Table 1).
Table 1.
The Comparisons of the Study Population with Different SII Status at Baseline
| Variables | SII-low (n=94) | SII-high (n=59) | Overall | p value |
|---|---|---|---|---|
| N(%) | N(%) | (N=153) | ||
| Age (years) | 0.123 | |||
| Median(range) | 49.0 [20.0, 68.0] | 47.0 [26.0, 66.0] | 49.0 [20.0, 68.0] | |
| Gender | 0.465 | |||
| Male | 79 (84.0) | 46 (78.0) | 125 (81.7) | |
| Female | 15 (16.0) | 13 (22.0) | 28 (18.3) | |
| BMI | 0.606 | |||
| ≤18.5 | 16 (17.0) | 13 (22.0) | 29 (19.0) | |
| 18.5~24 | 57 (60.6) | 36 (61.0) | 93 (60.8) | |
| ≥24 | 21 (22.3) | 10 (16.9) | 31 (20.3) | |
| Tumor Stage | 0.685 | |||
| T0~2 | 30 (31.9) | 22 (37.3) | 52 (34.0) | |
| T3~4 | 33 (35.1) | 17 (28.8) | 50 (32.7) | |
| Tx | 31 (33.0) | 20 (33.9) | 51 (33.3) | |
| Node Stage | 0.988 | |||
| N0~2 | 52 (55.3) | 32 (54.2) | 84 (54.9) | |
| N3 | 16 (17.0) | 10 (16.9) | 26 (17.0) | |
| Nx | 26 (27.7) | 17 (28.8) | 43 (28.1) | |
| Smoking History | 0.687 | |||
| Current and former | 36 (38.3) | 19 (32.2) | 55 (35.9) | |
| Never | 58 (61.7) | 40 (67.8) | 98 (64.1) | |
| Alcohol History | 0.982 | |||
| Current and former | 26 (27.7) | 16 (27.1) | 42 (27.5) | |
| Never | 68 (72.3) | 43 (72.9) | 111 (72.5) | |
| Prior Radiotherapy | 0.340 | |||
| Yes | 88 (93.6) | 58 (98.3) | 146 (95.4) | |
| No | 6 (6.4) | 1 (1.7) | 7 (4.6) | |
| Prior radical chemoradiotherapy | ||||
| Yes | 73 (77.7) | 44 (74.6) | 117 (76.5) | 0.215 |
| No | 21 (22.3) | 15 (25.4) | 36 (23.5) | |
| ECOG PS | 0.685 | |||
| 0 | 40 (42.6) | 19 (32.2) | 59 (38.6) | |
| 1 | 54 (57.4) | 40 (67.8) | 94 (61.4) | |
| Liver Metastasis | 0.480 | |||
| YES | 53 (56.4) | 29 (49.2) | 82 (53.6) | |
| NO | 41 (43.6) | 30 (50.8) | 71 (46.4) | |
| LYMPH (10^9/L) | <0.001 | |||
| Median(range) | 0.94 [0.40, 1.97] | 0.62 [0.12, 1.80] | 0.83 [0.12, 1.97] | |
| PLT (10^9/L) | <0.001 | |||
| Median(range) | 189 [97.0, 357] | 263 [109, 534] | 218 [97.0, 534] | |
| NEUT (10^9/L) | <0.001 | |||
| Median(range) | 3.01 [1.10, 5.72] | 4.84 [2.11, 13.8] | 3.57 [1.10, 13.8] | |
Notes: p-value was conducted with the chi-square test (categorical variables) and Mann–Whitney U-test (continuous variables), respectively.
Abbreviations: SII, systemic immune-inflammation index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; BMI, Body Mass Index (calculated as weight in kilograms divided by height in meters squared); LYMPH, Lymphocyte Counts; PLT, Platelet; NEUT, Neutrophil.
The median OS and PFS were 471 and 126 days for patients with SII ≤ 1139 and 303 and 43 days for patients with SII >1139 (Supplementary Table 1).
Correlation Between SII Status and Treatment Efficacy
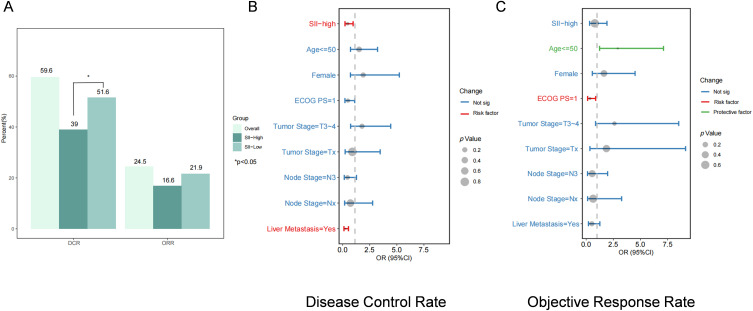
Patients classified as SII-LOW exhibited higher ORR and DCR at 21.6% and 51.6%, respectively, compared to 16.6% and 39% for those categorized as SII-High. (p=0.13 for ORR; p<0.001 for DCR, Figure 1A). We performed logistic regression analyses on the patients. The univariate analysis results revealed that patients with SII levels below 1139 (HR, 0.43; 95% CI, 0.22–0.84; P=0.001) exhibited superior disease control. In addition, ECOG score and liver metastases were risk factors for poor prognosis (Supplementary Table 2). After adjusting for other clinical characteristics, multivariate logistic regression analyses underscored the persistent significance of SII level as a potent independent prognostic factor (HR, 0.47; 95% CI, 0.22–0.99; P=0.048) (Figure 1B and C). SII was not statistically significant in predicting objective remission rates.
Figure 1.
Treatment response and its multiple logistic regression analysis. (A) Results of DCR and ORR. (B) Multiple logistic regression for DCR. (C) Multiple logistic regression for ORR.
Correlation Between SII Status and Survival
High SII significantly correlated with reduced OS in R/M NPC in the study through univariate COX regression analyses (Supplementary Table 3). We also found that ECOG PS (HR=2.79, 95% CI, 1.79–4.33, P<0.001), BMI (HR=0.44, 95% CI, 0.27–0.7 P<0.001) and liver metastasis (HR, 2.57; 95% CI, 1.73–3.81; P<0.001) were independent risk factors affecting patient survival. In addition, sex, age, smoking history, alcohol consumption history, and stage had no significant effect on survival.
After adjusting for other clinical characteristics, multivariate COX regression analysis emphasized the sustained significance of SII status as a strong independent prognostic tool in the studied population (HR, 2.08; 95% CI, 1.38–3.13; P<0.001). Similar findings were observed regarding PFS, further supporting the robustness of SII level as a prognostic marker (Table 2).
Table 2.
A Multivariate Cox Proportional Hazards Model for Overall Survival and Progression Free Survival of Patients
| Variables | Overall Survival | Progression-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 0.98 (0.96–1) | 0.126 | 0.98 (0.97–1) | 0.09 |
| SII | ||||
| High | 1.00 (Reference) | 1.00 (Reference) | ||
| Low | 2.08 (1.38–3.13) | <0.001 | 1.72 (1.2–2.47) | 0.003 |
| Gender | ||||
| Male | 1.00 (Reference) | 1.00 (Reference) | ||
| Female | 0.7 (0.41–1.19) | 0.185 | 0.79 (0.5–1.24) | 0.297 |
| Tumor Stage | ||||
| T0~2 | 1.00 (Reference) | 1.00 (Reference) | ||
| T3~4 | 1.06 (0.66–1.68) | 0.817 | 0.81 (0.53–1.23) | 0.323 |
| Tx | 1.85 (0.9–3.79) | 0.092 | 0.97 (0.5–1.9) | 0.933 |
| Node Stage | ||||
| N0~2 | 1.00 (Reference) | 1.00 (Reference) | ||
| N3 | 1.18 (0.7–1.99) | 0.531 | 1.3 (0.8–2.11) | 0.284 |
| Nx | 0.59 (0.28–1.21) | 0.149 | 1.26 (0.66–2.38) | 0.485 |
| ECOG PS | ||||
| 0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1 | 2.74 (1.66–4.52) | <0.001 | 1.58 (1.08–2.31) | 0.018 |
| Liver Metastasis | ||||
| Yes | 1.00 (Reference) | 1.00 (Reference) | ||
| No | 2.89 (1.89–4.42) | <0.001 | 1.77 (1.24–2.53) | 0.002 |
Abbreviations: SII, systemic immune-inflammation index; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Survival Analysis
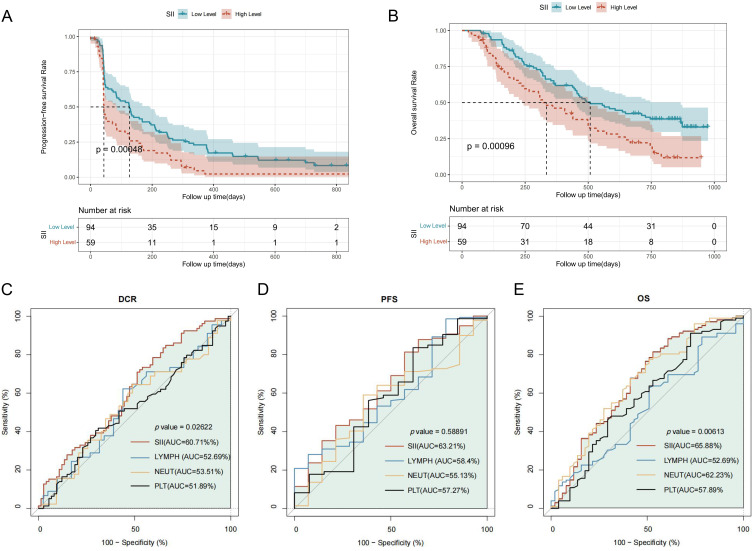
High SII status correlated with lower PFS (HR, 1.85; 95% CI, 1.31–2.62; P<0.001) in the survival analysis, and similar results were found in OS (HR, 1.93; 95% CI, 1.30–2.85; P<0.001) (Figure 2A and B).
Figure 2.
Assessment of survival based on different SII status and ROC curves. (A) The Kaplan-Meier curves show the OS of patients with different SII statuses. (B) The Kaplan-Meier curves show the PFS of patients with different SII status. (C – E) The discrimination of SII in predicting the survival and efficacy of patients. The receiver operating characteristic curve (ROC) shows the predictive value of SII with comparison to its components.
Predicting Effectiveness and Sensitive Analysis
The AUC values of the ROC curves indicated that the predictive value of SII’s in terms of DCR, PFS, OS were better than that of single indicators of inflammation (Figure 2C–E). Among the components of SII, neutrophils had the highest AUC value and may be the most important factor influencing the survival prognosis of R/M NPC.
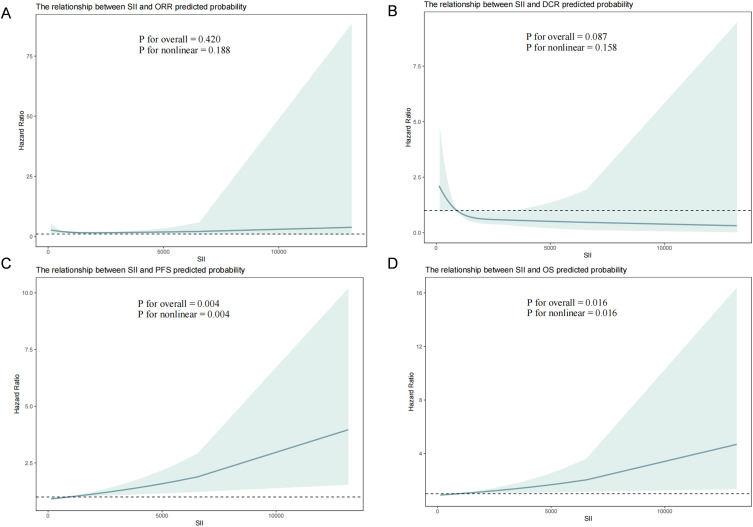
We used restricted spline plots to flexibly model and visualize the relationship between SII predicting ORR, DCR, PFS and OS. The Hazard Ratio of ORR fluctuated around 1 and was relatively insignificant, as shown in Figure 3A. With respect to the DCR with predicted treatment, Figure 3B shows that the DCR was good in the lower range of the SII, after which the rate of disease control declined (linear P = 0.158). The risk of PFS and OS was relatively flat until an SII of about 1330, after which it began to rise rapidly (nonlinear P = 0.016), similar to the findings for progression-free survival, as in Figures Figure 3C and D (Figure 3).
Figure 3.
The restricted cubic spline (RCS) curves for SII and different endpoints. (A) SII Predicts RCS Curves for ORR. (B) SII Predicts RCS Curves for DCR. (C) SII Predicts RCS Curves for PFS. (D) SII Predicts RCS Curves for OS.
Subgroup and Interaction Analysis
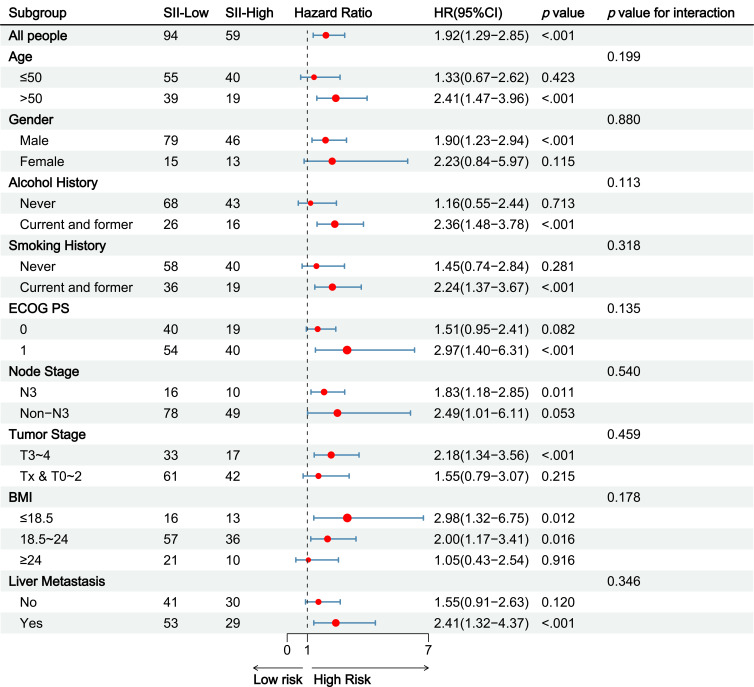
Subgroup analyses indicated significant differences of the effect of some factors on the OS outcome (Figure 4). The OS was lower in men (HR: 2.41; 95% CI: 1.47–3.96) and individuals aged over 50 (HR: 0.47; 95% CI: 0.22–0.99). Additionally, lower survival rates were observed in individuals with a history of smoking and alcohol consumption, an ECOG score of 1, advanced Tumor Stage and Node Stage, BMI <24, and liver metastases. And there were no significant interactions between in these subgroups. Similar results were obtained from subgroup analysis of DCR and PFS. (Supplementary Figures 3 and 4)
Figure 4.
Subgroup analysis of Overall survival.
Discussion
NPC is a common malignant tumor in the head and neck region, with a relatively high incidence.1 Owing to its deep-seated location and the absence of early symptomatic manifestations, numerous cases are diagnosed in advanced stages, thereby presenting a formidable challenge for effective intervention.6,33 Despite the historical efficacy of conventional radiotherapy and chemotherapy in managing nasopharyngeal carcinoma, their long-term and progression-free survival outcomes remain unsatisfactory.8,10,11 In addressing this clinical impasse, immunotherapy has emerged as the established treatment approach for patients with recurrent or metastatic NPC as the second-line option. Notably, among the arsenal of immunotherapeutic agents, PD-L1 inhibitors have garnered considerable attention. The quest for robust biomarkers capable of delineating treatment response assumes paramount importance, thereby facilitating the stratification of patients and the tailoring of individualized treatment regimens.
This cohort represents the first and largest population examined to evaluate the prognosis and efficacy of PD-L1 inhibitors in R/M NPC who has been previously treated. In our multicenter retrospective cohort study, we have unveiled a significant association between baseline levels of inflammation-associated cells and survival and efficacy in the treated population. This underscores the intricate interplay between the tumor microenvironment and the response to immunotherapy. Emphasizing the pivotal roles of lymphocytes, platelets, and neutrophils in prognosticating survival and efficacy post-immunotherapy in R/M NPC, we highlight we highlight the promise of SII, combining these inflammatory markers, as a valuable prognostic tool. The SII may contribute to achieving equilibrium between tumor microenvironment inflammation and immune response. Moreover, the detection of biomarkers in blood holds promise in circumventing the limitations of tumor tissue testing. Blood testing necessitates only a simple blood draw, boasts minimal invasiveness, permits multiple assessments, and facilitates dynamic monitoring of changes.
The conclusive demonstration of heightened inflammatory mediators’ association with the heightened proliferation of cancerous cells, immune system modulation impacting adaptive responses, and diminished treatment efficacy collectively underscore their pivotal role in predicting mortality rates and prognosis among cancer patients.18,21,34 Consequently, evaluating inflammatory mediators becomes a pivotal aspect of a comprehensive assessment aimed at predicting the outcome of immunotherapy for recurrent/metastatic NPC patients. Despite validating the predictive potential of SII in such patients, its practical application in daily clinical practice remains challenging.
In our current investigation, we observed a satisfactory predictive value of SII for both survival and efficacy in R/M NPC patients undergoing KL-A167. The ROC and RCS curves illustrated that SII exhibited superior predictive ability compared to inflammatory cells alone in identifying patients at high risk for NPC. The derived high SII linked to increased risk of from R/M NPC, as well as poor prognosis from immunotherapy, remained robust even after adjusting for a spectrum of potential confounders.
Furthermore, our analysis revealed that, among the components of SII, neutrophils and lymphocytes exhibited the highest discriminatory value in predicting survival in cancer patients. Previous studies have elucidated that neutrophils release immunosuppressive substances and promote the growth of blood vessels, supporting tumor cell invasion, and migration.23–25,35 On the contrary, platelets shield tumors from immune cytotoxicity, promote epithelial-mesenchymal transition and enhance tumor cell movement and spread.22,36 Meanwhile, lymphocytes play a pivotal role in immunotherapy by slowing tumor growth and promoting cell death.29 Thus, the composite indicator SII, which combines three inflammatory mediators, correlates strongly with prognosis in patients receiving immunotherapy.
While there’s a recognized link between SII and survival in NPC patients across different clinical settings,31,32 only a few studies have investigated the correlation between SII during immunotherapy and mortality risk, as well as treatment effectiveness. Monitoring SII metrics in R/M NPC upon hospital admission may yield tangible clinical benefits, enhancing the survival rates of cancer patients. However, existing evidence primarily stems from studies of NPC patients receiving radiotherapy or a variety of different immunotherapies.32,37–39 For example, a study based on SII and survival of nasopharyngeal cancer patients treated with PD-1 inhibitors showed a more significant association between SII status at cycle 3 after PD-1 inhibitor treatment and patient prognosis.40 In addition, another study evaluating ICI-treated NPC patients found that baseline SII and the immunotherapy-related SII reduction was independent prognostic factor for PFS in advanced NPC patients receiving ICIs.37
Our study presents several noteworthy novelties. Firstly, it stands as the world’s inaugural and largest investigation into assessing prognosis and the effectiveness of PD-L1 inhibitors in treating R/M NPC that has been previously treated. This pioneering endeavor validates the feasibility of employing the SII as a predictive tool for R/M NPC survival and furnishes a benchmark for clinical practice within Chinese medical centers. Secondly, we embark on exploring, for the first time in a Chinese population, the correlation between SII status and the efficacy of immunotherapy in R/M NPC. Moreover, advocating clinical intervention for cancer patients exhibiting elevated SII levels could significantly ameliorate SII status and prolong overall survival.
Admittedly, our study has some limitations. We note that despite being retrospective, the sample size from the prospective clinical trial as well as the follow up time remained constrained. While we observed differences in ORR post-PD-L1 inhibitor treatment for R/M NPC, subsequent regression analyses did not yield statistically significant results, likely due to sample size constraints or the period of observation. Furthermore, although we investigated the association between baseline SII levels and survival, we did not dynamically monitor SII trends during treatment, precluding assessment of its impact on patient prognosis. Additionally, our reliance solely on the composite inflammatory index overlooks potential synergies with other markers. The effect of EBV-mediated inflammatory response in NPC,21,41 which is now also partially investigated by combining both types of metrics, EBV and inflammation,42 in the prediction of survival. And uncontrolled covariates, such as detailed medical history, including prior surgeries and radiotherapy, may also influence cancer patient survival. Moreover, the majority of our study population presented with early to intermediate-stage cancers upon admission, necessitating further inquiry into whether advanced cancer patients could derive greater immunotherapy benefits. Future endeavors should prioritize prospective, large-scale, multicenter randomized controlled trials to delve deeper into the immunotherapeutic mechanisms across varying SII inflammation indicators. Such efforts are critical for refining treatment strategies and enhancing patient survival outcomes.
Conclusion
SII holds promise as a predictive marker for both the efficacy and prognosis of patients with R/M NPC undergoing treatment with PD-L1 inhibitors. Elevated SII levels may signify a diminished therapeutic response and poorer survival outcomes. This finding underscores the clinical significance of incorporating SII assessment into treatment protocols for R/M NPC. By identifying patients with high SII levels early in the treatment process, clinicians can potentially tailor interventions to optimize therapeutic efficacy and improve patient survival. Therefore, integrating SII evaluation into routine clinical practice may enhance treatment decision-making and ultimately enhance outcomes for R/M NPC population receiving PD-L1 inhibitor therapy.
Funding Statement
The work was supported by the National Key Research and Development Program of China. (2021YFE0206600), National Natural Science Foundation of China (82172842 and 81672386) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
AUC-ROC, Area under the receiver operating characteristic curve; BTC, Biliary tract cancer; BMI, Body Mass Index; CTCs, Circulating tumor cells; CR, Complete response; DCR, Disease control rate; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, Hazard ratios; IRC, Independent Review Committee; ICIs, Immune checkpoint inhibitors; L/LYMPH, Lymphocyte; N/ NEUT, Neutrophil; ORR, Objective remission rate; OS, Overall survival; PFS, Progression-free survival; PD-1, Programmed cell death protein 1; PD-L1, Programmed cell death ligand 1; PR, Partial remission; P/Platelet, PLT; R/M NPC, Recurrent and metastatic nasopharyngeal cancer; ROC, Receiver operating characteristic; RCS, Restricted cubic spline; SII, Systemic immune-inflammation index; SD, Stable disease.
Data Sharing Statement
The data that support the findings of this study are available from Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China).
Ethics Approval and Informed Consent
Ethical approval for the collection of human data was obtained from the Institutional Review Board (HX-IRB-AF-12-V4.0) of West China Hospital. Prior to enrollment, all participants provided written informed consent. Registry and the Registration No. of the study/trial: KL-A167 clinical trial (NCT03848286). This study adheres to the principles of the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines.
Consent for Publication
Not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Junyou Ge, Yan Qing and Youneng Wei are employees of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China). All other authors declare no potential conflicts of interest.
References
- 1.Chen Y-P, Chan ATC, Quynh-Thu L, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- 2.Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):39. doi: 10.1186/s40880-019-0385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353 [DOI] [PubMed] [Google Scholar]
- 4.Chua MLK, Ong SC, Wee JTS, et al. Comparison of 4 modalities for distant metastasis staging in endemic nasopharyngeal carcinoma. Head Neck J Sci Specialties Head Neck. 2009;31(3):346–354. doi: 10.1002/hed.20974 [DOI] [PubMed] [Google Scholar]
- 5.Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur. J. Cancer. 2017;77:117–126. doi: 10.1016/j.ejca.2017.02.029 [DOI] [PubMed] [Google Scholar]
- 6.Ng S-H, Chan S-C, Yen T-C, et al. Pretreatment evaluation of distant-site status in patients with nasopharyngeal carcinoma: accuracy of whole-body MRI at 3-Tesla and FDG-PET-CT. Eur Radiol. 2009;19(12):2965–2976. doi: 10.1007/s00330-009-1504-5 [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, Phase 3 trial. Lancet Oncol. 2021;22(8):1162–1174. doi: 10.1016/S1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 8.Al-Sarraf M, LeBlanc M, Giri PGS, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized intergroup study 0099. J clin oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310 [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi: 10.1016/S0140-6736(16)31388-5 [DOI] [PubMed] [Google Scholar]
- 10.Lee AWM, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Sem rad oncol. 2012;22(3):233–244. doi: 10.1016/j.semradonc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Lee AWM, Ng WT, Chan LLK, et al. Evolution of treatment for nasopharyngeal cancer - Success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi: 10.1016/j.radonc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Tang L, Liu N, et al. Famitinib in combination with concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a Phase 1, open-label, dose-escalation Study. Cancer Commun. 2018;38(1):38. doi: 10.1186/s40880-018-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. in: aster JC, Feany MB, editors. annual review of pathology: mechanisms of disease, vol 16, 2021. Ann Rev Pathol Mechanisms Dis. 2021;16(1):223–249. doi: 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 14.Chan AT, Lee VHF, Hong RL, et al. Results of KEYNOTE-122: a phase III study of pembrolizumab (pembro) monotherapy vs chemotherapy (chemo) for platinum-pretreated, recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC). Ann Oncol. 2021;32:S786–S. [Google Scholar]
- 15.Hsu C, Lee S-H, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J clin oncol. 2017;35(36):4050–+. doi: 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 16.Mai H-Q, Chen Q-Y, Chen D, et al. Toripalimab plus chemotherapy for recurrent or metastatic nasopharyngeal carcinoma the Jupiter-02 randomized clinical trial. JAMA J Am Med Assoc. 2023;330(20):1961–1970. doi: 10.1001/jama.2023.20181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Qin X, Peng X, et al. Efficacy and safety of KL-A167 in previously treated recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm, phase 2 study. Lancet Regional Health-Western Pacific. 2023;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):E493–E503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 19.Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi: 10.1097/SLA.0000000000003370 [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J hematol oncol. 2018;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quail DF, Amulic B, Aziz M, et al. Neutrophil phenotypes and functions in cancer: a consensus statement. J Exp Med. 2022;219(6). doi: 10.1084/jem.20220011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J hematol oncol. 2021;14(1). doi: 10.1186/s13045-021-01187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173–187. doi: 10.1038/s41577-021-00571-6 [DOI] [PubMed] [Google Scholar]
- 26.Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1). doi: 10.1186/s12943-017-0707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer. 2016;2(12):747–757. doi: 10.1016/j.trecan.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for Immunotherapy of Cancer. 2016;4(1). doi: 10.1186/s40425-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021;18(4):842–859. doi: 10.1038/s41423-020-00565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y-H, Chang K-P, Lin Y-S, Chang T-S. Pretreatment combination of platelet counts and neutrophil-lymphocyte ratio predicts survival of nasopharyngeal cancer patients receiving intensity-modulated radiotherapy. Onco Targets Ther. 2017;10:2751–2760. doi: 10.2147/OTT.S137000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu A, Li H, Zheng Y, et al. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int. 2017;2017:1–6. doi: 10.1155/2017/3047802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Liu L, Huang X, et al. Pretreatment systemic immune-inflammation index predicts survival for non-metastatic nasopharyngeal carcinoma: two independent institutional studies. J Nat Cancer Center. 2022;2(1):60–67. doi: 10.1016/j.jncc.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee AWM, Fee WE Jr, Ng WT, Chan LK. Nasopharyngeal carcinoma: salvage of local recurrence. Oral Oncol. 2012;48(9):768–774. doi: 10.1016/j.oraloncology.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 35.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig. 2013;123(8):3446–3458. doi: 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orellana R, Kato S, Erices R, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. Bmc Cancer. 2015;15:15. doi: 10.1186/s12885-015-1014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao J, Chen Q, Bai X, et al. Predictive value of immunotherapy-induced inflammation indexes: dynamic changes in patients with nasopharyngeal carcinoma receiving immune checkpoint inhibitors. Ann Med. 2023;55(2). doi: 10.1080/07853890.2023.2280002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Chen D, Wang R, Xie S, Wang X, Huang H. Development and validation of a nomogram to predicting the efficacy of PD-1/PD-L1 inhibitors in patients with nasopharyngeal carcinoma. Clin Transl Oncol. 2024;26(10):2601–2607. doi: 10.1007/s12094-024-03504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J-R, Shen G-P, Ren Z-F, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck J Sci Specialties Head Neck. 2012;34(12):1769–1776. doi: 10.1002/hed.22008 [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Liang W, Wu X, et al. A nomogram based on the SII3 and clinical indicators predicts survival in patients with nasopharyngeal carcinoma treated with PD-1 inhibitors. Medicine. 2024;103(19): e38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Duan Y, Cheng S, et al. EBV-encoded RNA via TLR3 induces inflammation in nasopharyngeal carcinoma. Oncotarget. 2015;6(27):24291–24303. doi: 10.18632/oncotarget.4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Y, Shi -L-L, Zhu L-S, Ding Q, Ba L, Peng G. Prognostic efficacy of the combination of the pretreatment systemic immune-inflammation index and Epstein-Barr virus DNA status in locally advanced nasopharyngeal carcinoma patients. J Cancer. 2021;12(8):2275–2284. doi: 10.7150/jca.52539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (Chengdu, China).