Abstract
Although corticosteroids are an important treatment for inflammatory bowel disease (IBD) patients, many subjects develop dependence, leading to serious long-term side effects. We applied causal inference analyses to investigate the length of steroid use on reoperations in IBD patients. We identified subjects in the UK Biobank general practice dataset with at least one major GI surgery and followed them for at least 5 years to evaluate subsequent operations. We defined steroid dependence as at least 12 weeks of use (vs. acute steroid use) prior to baseline surgery. Of the 363 subjects included in our analyses, 163 (45%) were prescribed steroids on or before baseline surgery, and of these (N = 125 of 163, 77%) were dependent. Additional analyses for time-dependent data on prescriptions found a link between prescription length and reoperation. Among UC subjects with acute use, the odds of reoperation were significantly lower (OR: 0.32, 95% CI: 0.0–0.73). Steroid dependence resulted in a delay of reoperation (median 1.2 vs. 2.3 years, P = 0.01). Our findings indicate that long-term steroid use tends to increase the need for reoperation, whereas short-term use may reduce it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75215-5.
Keywords: Steroids, Inflammatory bowel disease, Causal inference, Risk prediction, Prescription patterns
Subject terms: Predictive markers, Crohn's disease, Ulcerative colitis, Epidemiology, Risk factors
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are two main subtypes of inflammatory bowel diseases (IBD) characterized by chronic, relapsing inflammation of the gastrointestinal (GI) tract, that may lead to recurrent surgeries1–7. Although advanced therapies such as biologics and small molecules have become available for management of IBD over the last two decades, corticosteroids continue to be widely used. A well-known drawback of long-term steroid use is dependence that leads to numerous systemic side effects8–12.
In this study, we analyze the long-standing United Kingdom Biobank (UK Biobank) general practice (GP) dataset, to investigate the impact of long- and short-term use of steroids and its relationship to IBD-related reoperation using machine learning (ML) methods. The UK IBD Registry13 specifies the length of oral steroids reported to the Registry between April 2016 and July 2019, including a threshold indicating a long usage duration (“Stop after 12 weeks”). Motivated by the registry’s classification of steroid prescriptions according to course lengths (e.g., short vs. long) we incorporated these definitions into our analyses to enhance understanding of long-term steroid use and its impact on IBD progression. We utilized the UK Biobank GP data, which is a subset of the UK Biobank (approximately 230,000 subjects). This resource is a subset of a well-studied, large-scale prospective biomedical database, containing detailed health information on half a million participants, which originated from multiple institutions in the UK and Scotland with well-developed methodologies to identify, extract and analyze data. In addition to socio-demographics, the GP dataset contains longitudinal records of observational data of these subjects such as comorbidities, procedures, prescriptions, and laboratory observations14. This robust dataset allows for analysis of prescription medications along with laboratory values to study reoperations. Here, we focused on the outcome of GI reoperation and prescription medication duration, particularly steroid dependence15–18. We applied a unique analytic methodology to address inherent complexity in identifying reoperation in IBD subjects from clinical data, particularly with respect to follow-up and treatment variability. Our analyses include causal inference19, subpopulation-based feature selection20, as well as traditional regression methods and therefore allowed for a more rigorous interrogation of the hypothesis that steroid dependence drives surgical outcomes in IBD.
Methods
Data source and subgroup identification
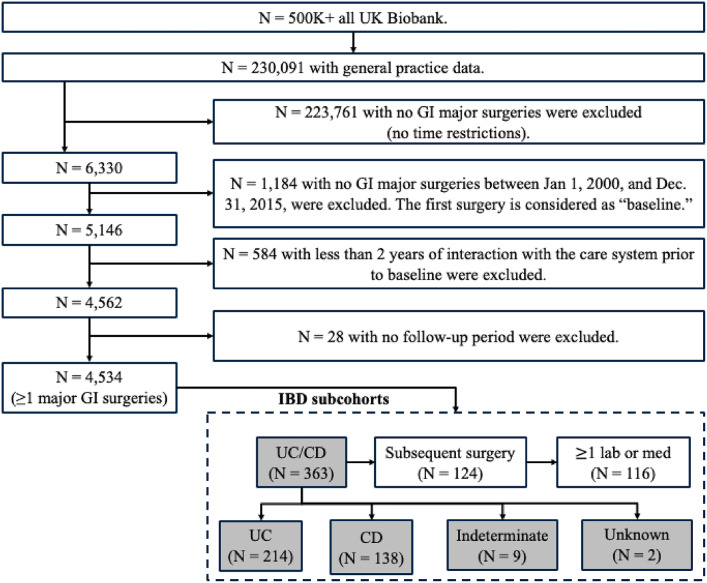
We have previously described cohort construction—please refer to Supplementary Methods and to Fig. 1 for details on processing the GP data to extract the study subcohorts14. Our first step was to determine the appropriate diagnostic and procedure codes to use, as well as to understand the limitations of the data available. We then created multiple subcohorts of subjects based on diagnosis of UC or CD and applied advanced statistical and ML methods to report unbiased estimates of outcomes based on the prescription data. In total, we analyzed 3 subcohorts (UC/CD, UC-only, and CD-only). To account for the inherent complexity of observational data from a large biomedical database, such as due to large number of factors that may affect disease progression, we incorporated several ML methods – subpopulation-based feature selection20,21, causal inference analyses19, and disease progression modeling22–25 to analyze the outcome of reoperation in each of the subcohorts. In this study, reoperation is defined as any GI surgery occurring within 5 years following the initial surgery. However, it is important to note a limitation of this definition: it may not accurately identify UC patients who undergo the second or third stage of a multistage resection procedure.
Fig. 1.
Cohort selection. Note that neither of the subjects were below 18 years’ old nor had a pregnancy status at baseline. We analyzed in total 3 subcohorts (UC/CD, UC-only, and CD-only). We used the UC/CD cohort to conduct additional analyses focused on time-series.
We applied following inclusion and exclusion criteria to the UK Biobank’s entire population (over 500,000 subjects) to extract all subjects who had at least one major GI surgery. We used Office of Population Censuses and Surveys-4 (OPCS-4) codes to identify major GI surgeries (Supplementary Table 1). This process involved sequential steps (Fig. 1) to identify all subjects associated with: (1) GP data between January 1, 2000, and December 31, 2015 (N = 230,091), (2) at least one major GI surgery (N = 5,146), and (3) at least 2 years of follow-up data before the baseline surgery (N = 4,562) and a follow-up beyond 30 days after surgery (N = 4,534). We used ICD-10 codes to identify IBD subjects (K50* and K51* for CD and UC respectively).
IBD-related prescription data: duration and covariates construction
We extracted variables related to prescription of IBD-related medications (adalimumab, azathioprine, balsalazide, budesonide, dexamethasone, hydrocortisone, infliximab, mesalamine, methotrexate, olsalazine, prednisolone, prednisone, sulfasalazine, and thalidomide). Overall, we found 30,768 IBD-related prescriptions for 4,534 subjects associated with the 14 medications listed above (see Supplementary Fig. 1 for prescription distributions). All prescription entries had start dates but lacked end dates. We thus incorporated clinical expertise to assign standard daily doses for each medication based on IBD indication and widely accepted best practice use for normalization of variability in prescriptions (e.g., a tablet once a day, every 6 to 12 h, 3–4 times per day). Reference literature was reviewed for each unique prescription to confirm the standard doses used for treatment of either UC or CD. We then calculated the estimated number of tablets used per month for each prescription (e.g., once a day was 30 tablets per month, every 6 to 12 h per day as well as 3–4 times per day were considered 120 tablets per month). Combining the estimated number of tablets per month per prescription with quantity (a data entry that was available for all prescriptions), allowed us to calculate an end date for each prescription (e.g., prescription with a start date of December 8, 2008, a quantity of 40 associated with a once-a-day use, had an end date of January 17, 2009, thus was prescribed for 40 days). We created additional covariates indicating duration of medication use for each subject’s prescriptions based on practice information from the UK IBD Registry13. Overall, we extracted a total of 112 unique prescription duration variables (7 unique durations and a binary variable indicating history for each medication). Durations ranged from 1 day to 12 weeks or more for each medication type (see Supplementary Fig. 1 for the distribution of the medication durations). We further classified all IBD prescription durations for long-term use or not (i.e., 12 weeks or longer vs. short-duration use) and limited the prescription to be use of tablets and/or injections thereby excluding all others (e.g., drops, inhalers, powder). Since steroids are a general class of medications with varied use, “steroid dependence” here is defined as 12 weeks or longer use of prednisolone prescription, at least once in subject’s entire history in GP longitudinal data.
To account for IBD severity, we extracted the following covariates: age, gender, race, and smoking status, as well as common lab values such as for albumin and hemoglobin (Hgb). If no observation values were available at baseline, we considered the most recent value from the 6 months prior to baseline. To account for disease comorbidities, we used Clinical Classifications Software Refined (CCSR)26 codes to construct covariates indicating number of comorbidities at baseline, for the following conditions: digestive (N = 25 conditions), GI (N = 10), circulatory (N = 39), and respiratory (N = 17). Additionally, we incorporated two groups of inflammatory conditions (Supplementary Table 2) to reflect if the subject has possible steroid dependence (N = 7 conditions), or may require acute steroid therapy (N = 23). These two additional groups of inflammatory conditions account for conditions in which acute steroid use is typical (vs. steroid dependence).
Additionally, we included 3 covariates indicating a broad group of types of surgery performed at baseline as follows: (1) partial and/or total colectomy, (2) bypass with anastomosis after resection and/or an ostomy-related procedure, and (3) abdominal surgery and/or endoscopic procedure (Supplementary Table 1).
Outcome definition
We defined the primary outcome of reoperation as the occurrence of a subsequent major GI surgery within 5 years after the baseline surgery. In our analysis, all subjects were followed from the day after baseline surgery. Subjects were censored either at their last follow-up date or at the end of the 5-year follow-up window. Furthermore, to be considered an outcome, reoperation had to occur 30 days or more after the baseline surgery (reoperation between one month and 5 years after the index operation). This approach filtered out events that represented a continuation or complication of the baseline surgery (multiple surgeries were often observed during the same admission). The last follow-up date was determined by the date of the subjects’ last interaction with the healthcare system, such as an office visit, hospital encounter, laboratory test, or prescription for medication. We report the incidence rate for IBD per 1,000 person years (1 K PY).
Prescription duration analyses
We applied subpopulation-based feature selection14,20,21 to identify sets of the most informative features and assessed discrimination performance. Candidate features included age, sex, race, smoking status, relevant laboratory values, and UC/CD diagnosis, as well as all prescription duration variables (Supplementary Table 2). We used a randomly selected derivation set from each subcohort to identify features and used the rest as held out set to assess discrimination performance (2/3 and 1/3, respectively). As described in our previous work, we iteratively sampled subpopulations for 1,000 repetitions to identify and rank features20,21. The final list of selected features was sorted in descending order in each repeated experiment, and a concordance index was calculated for each combination of top features. The variable list associated with the highest concordance index was used as the final list of selected features. We then applied Cox regression using the selected features to obtain hazard ratios and plotted Kaplan Meier curves to obtain reoperation free survival probabilities stratified by subgroups, i.e., subjects receiving long-term steroid therapy (steroid-dependent) vs. shorter duration therapy (acute use).
Furthermore, we employed inverse probability of (treatment) weighting (IPW), a propensity based causal inference method implemented in causallib toolkit19 for estimating effects of steroid prescription duration on reoperation. IPW models the treatment assignment as weighted estimation from observed data for the study outcome and creates a pseudo randomized control trial for the treatment assignment (in our case of steroid prescription duration). Overall, we applied bootstrap procedure with 1,000 replicates to calculate 95% confidence intervals for the effect size. We studied three duration use cases i.e., long-term use (dependence), unrestricted length of use, and short-term use (acute use)13 to investigate steroidal effect on reoperation19,28. To match the treated and untreated subjects in the pseudo trials, we incorporated albumin and Hgb to account for disease severity (as in Cox regression), along with other comorbidities specified above. We also included smoking status, age and duration since diagnosis of UC/CD, extracted at baseline.
Prescription modeling using advanced ML
From the above-described dataset, we additionally aimed to draw insights for: (1) differentiating patterns of prescriptions with respect to the outcome (i.e. reoperation-) and (2) exploring progression trajectories to reoperation.
To investigate unique progression trajectories to reoperation based on prescriptions, we utilized a disease progression model. This model incorporates probabilistic modeling in a time-to-event framework (DPM360 toolkit)22–25 and discovers trajectory patterns as transitions between “hidden” states that are learned from longitudinal observational data of each subject. In our use case, the start of observational data was based on the first observed laboratory value and/or prescription at most 2 years prior to the baseline surgery and ended at reoperation. Therefore, only subjects with the outcome event and with observational data fitting the criteria as defined above were considered. For this modeling, the prescription medication was modeled as prescribed or not (on / off) for the observed duration. We applied this methodology to the 10 most used IBD prescriptions (Supplementary Fig. 1). We also utilized a progression model visualization tool (DPVis)29 to help interpret results and characterize the observed patterns with respect to medication usage and formed visual trajectory subgroups in consultation with domain experts. Refer to Supplementary Figs. 9 and 10 with a section for explaining supplemental ML method for additional details.
Results
There are 4,534 subjects with one major GI surgery and having at least 2 years of healthcare data before the baseline surgery (Fig. 1). All subjects were followed for 5 years after baseline surgery. We found diagnosis of UC (N = 214), CD (N = 138), indeterminant (N = 9), and unknown (N = 2) in a total of 363 subjects (Fig. 1). Of the 363 subjects, 124 (34%) had a reoperation indicating an incidence of 123.5 per 1 K PY (Table 1). The median time to reoperation after baseline surgery was 0.7 years (quartile 1: 0.4, quartile 3: 1.4) with a median follow-up of 2.6 years (quartile 1: 0.8, quartile 3: 5.0). Overall, 163 (45%) subjects had a history of steroid prescription and of these, 125 (77%) had steroid dependence. Note that this rate was higher in UC subjects than CD subjects (Table 2). For additional details regarding the UC (N = 214), and CD (N = 138) subcohorts, refer to Supplementary Tables 3 and 4, respectively. For a comprehensive comparison of surgery types, including median durations between baseline to outcome dates refer to Supplementary Table 5.
Table 1.
Characteristics of GI major surgery cohort (UC/CD subgroup).
| All | Reoperation? | P-value | ||
|---|---|---|---|---|
| Variable and category (N) | 363 | Yes (124) | No (239) | |
| Outcome | ||||
| Number of subjects with reoperation | 124 (34%) | |||
| Median time to reoperation; years (Q1, Q3) | 0.7 (0.4, 1.4) | |||
| Reoperation per 1,000 person-years | 123.5 | |||
| Median follow-up, years (Q1, Q3) | 2.6 (0.8, 5.0) | |||
| Age (years); Mean (Standard deviation) | 57.4 (8.8) | 55.3 (8.8) | 58.5 (8.6) | 0.001 |
| Male; N (%) | 183 (50.4) | 67 (54.0) | 116 (48.5) | 0.4 |
| Non-Black; N (%) | 361 (99.4) | 123 (99.2) | 238 (99.6) | 1.0 |
| Current or past smoker; N (%) | 74 (20.4) | 22 (17.7) | 52 (21.8) | 0.4 |
| Comorbid history; N (Prevalence, %) | ||||
| Recent UC diagnosis | 214 (59.0) | 135 (56.5) | 79 (63.7) | 0.2 |
| Recent CD diagnosis | 138 (38.0) | 95 (39.7) | 43 (34.7) | 0.4 |
| Indeterminate recent UC or CD | 9 (2.5) | 7 (2.9) | 2 (1.6) | 0.7 |
| UC or CD (Historical record) | 2 (0.6) | 2 (0.8) | 0 (0.0) | 0.8 |
| Comorbid history; mean number of types (standard deviation) | ||||
| Circulatory | 1.5 (2.2) | 1.2 (1.7) | 1.6 (2.4) | 0.06 |
| Digestive | 5.0 (2.8) | 4.8 (2.5) | 5.2 (2.9) | 0.2 |
| GI Cancer | 0.1 (0.3) | 0.06 (0.2) | 0.1 (0.4) | 0.01 |
| Inflammatory (acute use is typical) | 1.7 (1.5) | 1.5 (1.2) | 1.8 (1.6) | 0.05 |
| Inflammatory (steroid dependence is typical) | 0.3 (0.6) | 0.2 (0.6) | 0.3 (0.6) | 0.2 |
| Respiratory | 1.7 (2.0) | 1.4 (1.8) | 1.8 (2.1) | 0.1 |
| Medication history; N (prevalence, %) | ||||
| Prednisolone (any duration) | 163 (44.9) | 53 (42.7) | 110 (46.0) | 0.6 |
| Prednisolone (steroid dependence) | 125 (34.4) | 48 (38.7) | 77 (32.2) | 0.3 |
| Mesalamine | 106 (29.2) | 65 (33.1) | 41 (27.2) | 0.3 |
| Azathioprine | 82 (22.6) | 34 (27.4) | 48 (20.1) | 0.1 |
| Hydrocortisone | 7 (1.9) | 4 (3.2) | 3 (1.3) | 0.4 |
| Methotrexate | 7 (1.9) | 2 (1.6) | 5 (2.1) | 1.0 |
| Labs; Mean (standard deviation) | ||||
| Albumin (g/L) | 39.5 (3.5) | 39.4 (3.5) | 39.6 (3.5) | 0.6 |
| Hgb (g/dL) | 13.0 (1.1) | 12.9 (1.1) | 13.0 (1.1) | 0.6 |
P-values represent the statistical significance between subjects with and without reoperation—values below 0.05 appear in bold.
Table 2.
Steroid dependence calculations. Refer to Fig. 1 for additional details regarding all subgroups.
| Subgroup | Total number of subjects | Total number of subjects on steroids (Prevalence %) | Total number of subjects with steroid dependence (prevalence steroid dependence, %) |
|---|---|---|---|
| UC/CD (recent, indeterminate, or historical diagnosis) | 363 | 163 (45%) | 125 (77%) |
| Recent UC diagnosis | 214 | 110 (51%) | 88 (80%) |
| Recent CD diagnosis | 138 | 49 (36%) | 34 (69%) |
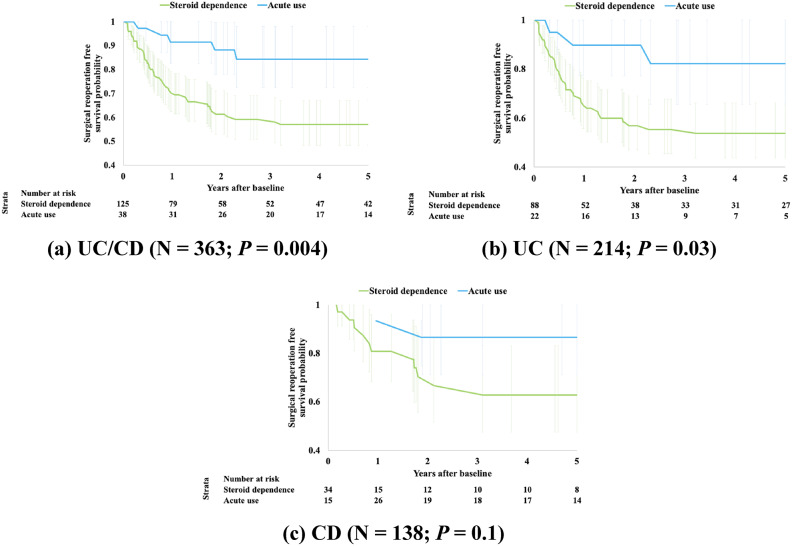
Steroid dependence vs. acute steroid use shows statistically significant difference in reoperation free survival probabilities within 5 years of baseline surgery (P = 0.004). This finding was consistent across the three analyzed subcohorts, UC, CD and UC/CD (Fig. 2).
Fig. 2.
Kaplan-Meier curves comparing subjects with a history of steroid dependence vs. acute use. (a) UC/CD (N = 363; P = 0.004). (b) UC (N = 214; P = 0.03). (c) CD (N = 138; P = 0.1)
We observed a dose-dependent relationship in steroid prescription use and reoperation using IPW. In the IBD (UC/CD) cohort, we observed increased odds with steroid dependence when compared to acute use (OR: 2.86, 95% CI: 0.84–5.45). However, when comparing any duration of steroid use to no steroid use, we observed decreased odds (OR: 0.90, 95% CI: 0.64–1.18) and much further decrease in odds between acute steroid use and no use (OR: 0.36, 95% CI: 0–1.0). Figure 3 presents odds ratios indicating consistency between the three IBD-related subcohorts (UC/CD, UC-only, and CD-only). Supplementary Figs. 2–4 highlight the validity of these findings from causal inference perspective - including weighted area under the curve (AUC) close to chance, high propensity and expected AUCs, for visibly well balanced treated and untreated subgroups, as well as covariate balancing in the pseudo-trials presented here19.
Fig. 3.
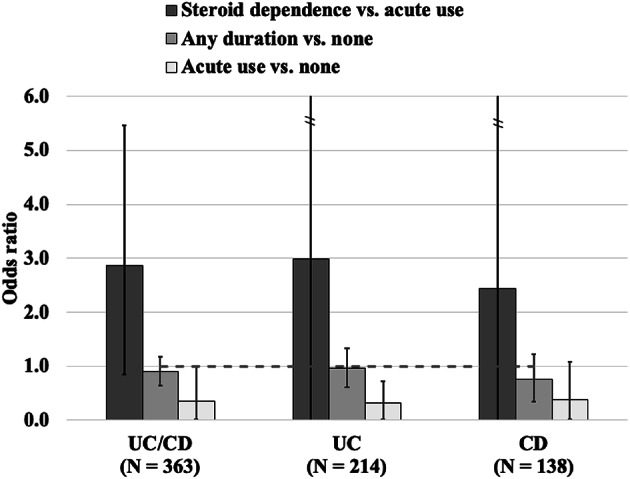
Odds ratio calculations for steroids derived from causal analyses employing inverse probability weighting (IPW).
Association analyses based on Cox regression indicated that undergoing a bypass anastomosis after a resection or ostomy surgery at baseline presents higher odds of a reoperation (OR: 2.83, 95% CI: 1.53–5.22). Use of steroids indicating dependence had increased odds of reoperation (OR: 2.54, 95% CI: 0.97–6.65), while use of steroids unrestricted to any specific duration reduced odds (OR: 0.38, 95% CI: 0.15–0.99), shown in Supplementary Fig. 5. Calculations of ORs for the subcohorts for UC and CD are presented in Supplementary Figs. 6 and 7. Additional results regarding prediction performance in the three subcohorts as well as summary tables are presented in Supplementary Fig. 8 and Supplementary Tables 3 and 4, respectively.
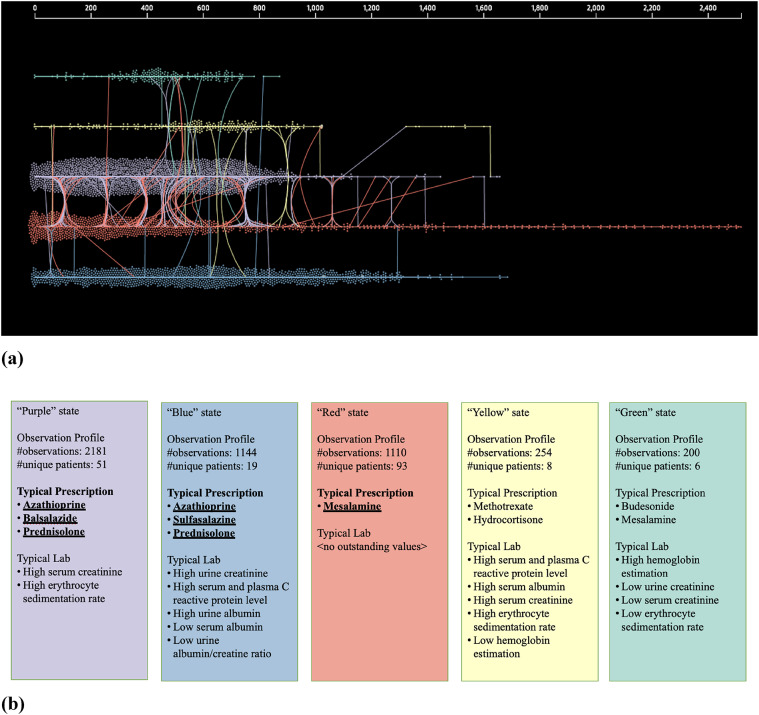
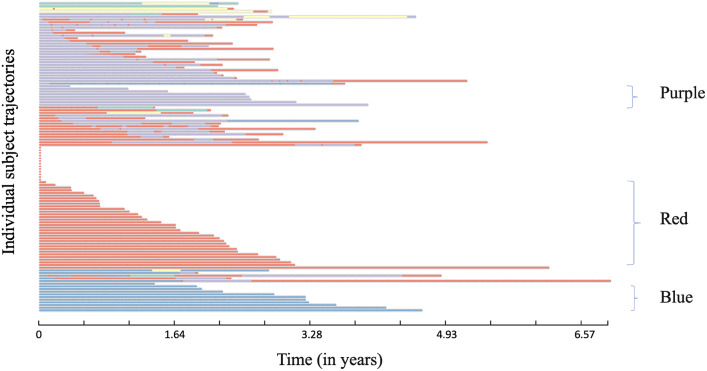
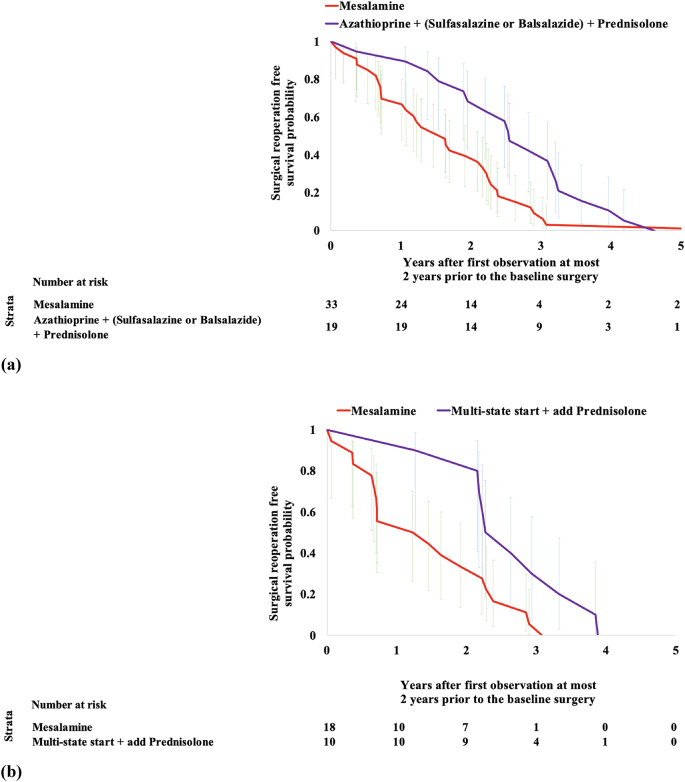
We learned several disease progression models from IBD subjects with reoperation and found learning 4 to 10 hidden states sufficed. From these models, we found 5 “hidden” states model had the highest (log) likelihood of describing the observed data (Fig. 4 and Supplementary Fig. 9). The 5-state model discovered unique prescription medication use characteristics such as use of mesalamine or azathioprine with steroid therapy and observed laboratory values in each state (Fig. 4 and Supplementary Fig. 9). These states are described as colored states: Purple, Blue, Red, Yellow, and Green (Fig. 4). We observed unique “trajectory” of medication prescriptions as described below. Specifically, there are 3 trajectory patterns that the majority (N = 60 of 116) of subjects follow until the occurrence of reoperation (Fig. 5). These are predominantly in the states for prescription medications: Purple (azathioprine, balsalazide, and prednisolone), Red (mesalamine only) and Blue (azathioprine, sulfasalazine, and prednisone). We found that the observed time to reoperation in each of the trajectories is different, i.e., from the initial observation of first medication or laboratory in the two years prior to the baseline surgery to the time of reoperation within the following 5 years. Survival time estimation for Red vs. Blue + Purple groups (Fig. 6A) showed a significant difference in time to reoperation among subjects who were prescribed mesalamine only vs. azathioprine in combination with balsalazide or sulfasalazine (1.6 vs. 2.5-year, respectively, P = 0.01). We also found faster progression to reoperation (P = 0.01) between the Red and the Blue groups (Supplementary Fig. 11). Furthermore, when analyzing a subgroup of reoperation subjects, in UC subgroup, we found significant difference if the subjects were prescribed mesalamine only (without steroids, i.e., Red state) and/or switching from it to the other two states Blue or Purple with steroids (N = 28). The median reoperation in the UC subgroup was delayed by 1.1-year with steroids, 1.2 vs. 2.3 years (P = 0.01) (Fig. 6B).
Fig. 4.
Five states discovered by DPM360 with predominant characteristics. State space modeling using Continuous-Time Hidden Markov Model (CTHMM) from DPM360 fit states over time to maximize the likelihood of observing longitudinal prescription and laboratory test events (N = 116). (a) Five states and transitions between states are discovered by CTHMM for all subjects with adequate follow-up data as defined above and confirmed reoperation (N = 116). The horizontal axis is the elapsed time (in days) of observed events (in UK Biobank of labs or prescriptions)—they are referenced from the first date of laboratory and/or prescription which are at most 2 years prior to the first GI surgery for each subject. In the figure, observed events are labeled with an inferred state by CTHMM, and then plotted according to its time of observation and inferred state color. Lines connecting dots mean transitions among the observed states. (b) Description of five states discovered by CTHMM (as above)—their predominant prescription and laboratory test value characteristics (each state has the distributions for the observations of the prescriptions and laboratory test values)—for example, if a subject moves to a Red state from a Blue state, he/she has high possibility that mesalamine is prescribed. Mean values of these distributions are shown in Supplementary Fig. 9.
Fig. 5.
Individual state-transition view overtime and cluster identification. This view shows transition patterns of individual subjects (N = 116). Three main trajectories of prescriptions are noted: (1) predominantly on balsalazide (Purple: N = 8) or (2) sulfasalazine (Blue: N = 11) along with prednisolone and azathioprine or (3) predominantly on mesalamine prescription only (Red: N = 33).
Fig. 6.
Kaplan-Meier curves for the UC/CD subgroup with reoperation (N = 116) considering events between the first observation of laboratory and/or prescription at most 2 years prior to the baseline surgery and up to subsequent 5 years. (a) Comparison of subjects with prescription of mesalamine only (Red: N = 33) vs. any immunosuppressive such as azathioprine + (sulfasalazine or balsalazide) (Purple + Blue: N = 19) in combination with prednisolone (P = 0.01). Median time to reoperation was 1.6 and 2.5 years for the Red vs. Purple + Blue subgroups, respectively (0.9-year difference). (b) Comparison of UC only subjects with prescription of mesalamine only (stating with Red: N = 18) vs. the ones starting with mesalamine only and switch to prednisolone combination with azathioprine + sulfasalazine or azathioprine + sulfasalazine + balsalazide (switched to Purple + Blue: N = 10) (P = 0.01). Median reoperation was delayed − 1.2 vs. 2.3 years for the Red vs. Purple + Blue subgroups, respectively (1.1-year difference).
Discussion
Our findings suggest that steroid dependence at the time of initial IBD-related surgery is associated with a higher rate of reoperation within 5 years. This is consistent with previous reports that the use of steroids is associated with worse postoperative patient outcomes30–32. Preoperative steroid use may be a surrogate marker for higher baseline disease severity, and thus may contribute to the increased risk of reoperation.
Our findings also suggest that the acute use of steroids (less than 12 weeks) is generally associated with a lower rate of reoperation. In contrast, steroid dependence (use of 12 weeks or more) was associated with delays in reoperation within 5 years following baseline surgery. There was a significant difference in reoperation free survival between acute use vs. steroid dependent groups in IBD subjects. Causal inference analysis found a link between the length of steroid prescriptions and reoperation in all subgroups. This link was particularly statistically significant in UC subjects who were on acute steroid use. Our analysis also suggests that acute steroid therapy may confer some delay with respect to reoperation, especially in UC subjects as previously reported12,33.
Using advanced methods for disease progression model, we found three unique prescription-use trajectory subgroups not only suggesting variation in practice but also difference in outcome when steroid therapy is used with other medications. Interestingly, two of these trajectories had significant differences in time to the reoperation, with mesalamine showing a faster progression when compared to azathioprine and steroid use showing a somewhat slower progression to reoperation. The latter also supports that use of any acute steroid therapy is beneficial and that azathioprine use may be more beneficial then mesalamine after conservative surgery34.
Although repeat disease-related surgeries are more common in CD patients1, UC patients may undergo subsequent IBD related surgery as such revision of pouch. Post-operative management including careful follow-up and smoking cessation have been adovcated2–4. Benefits of biologics such as anti-TNF therapy3 in post-op patients remain unclear. In that regard, our study findings highlight the effects of oral steroid therapy which are still widely used in clinical practice.
Our study has several limitations. The study cohort consisted of subjects from the UK Biobank GP, which may not be representative of the general population, specifically in the US. The final subcohorts that we analyzed were relatively small (hundreds of subjects) limiting statistical significance of our findings. The prevalence of Whites in our cohort was over 99.0% representing a potential for bias and limiting the generalizability of our findings to broader populations. Further, our study only included subjects who underwent one or more major GI surgeries, which may limit the generalizability of our findings further to subjects with less severe disease or other conditions indicating systemic inflammation (e.g., rheumatoid arthritis). Our dataset lacked also clinical disease activity indices (Crohn’s Disease Activity Index or Harvey-Bradshaw Index), therefore, we elected to use pertinent laboratory markers as proxies for disease severity in our models. The UK Biobank GP dataset also lacked information pertaining to actual prescription use, i.e., if they were a routine auto-script or partially or entirely consumed, resulting possibly elevated number of subjects who were steroid-dependent. Our analysis, also, did not incorporate dose values which were broadly missing or not straightforward to reliably extract. Finally, although our study considered a large set of covariates, additional unknown predictors that if included could affect our findings, for example, covariates that rely on information found in clinical notes or social determinants of health35. In conclusion, we found that the majority of subjects in the UK Biobank with a diagnosis of IBD and a baseline GI surgery were prescribed steroids, especially indicating dependence, often starting long before the baseline surgery. Our data-driven analyses also show that steroid use indicating dependence is not only associated with much higher risk of reoperation but may have a causal link. These relationships, however, must be confirmed in additional independent datasets (e.g., All Of Us36). Our findings indicating variation in time to reoperation for mesalamine vs. azathioprine use in IBD subjects may inform future trials for combination therapies (e.g., with biologics36). In that regard, our work provides positive controls for future studies37.
Our study results confirmed known findings about the impact of duration of steroid use (chronic vs. acute use) on post-operative patient outcomes30–32 while enhanced and extended current knowledge for several reasons: (1) a unique data source, the UK Biobank GP dataset, providing a much larger study cohort than previously available; (2) a unique methodology to evaluate GI reoperation incorporating clinician expertise and study design as described under Methods; (3) analyses that were not restricted to any particular age range; (4) the comparison of findings from two distinct methodologies (regression and causality inference38) which were consistent with each other; (5) a methodology to create high quality analytic subcohorts (e.g., including 2 or more years of care prior to the index surgery, and 6) extended patient follow-up − 5 years – after the index surgery39. Finally, we report findings on multiple distinct subcohorts which has allowed us to compare distinct subsets within the context of steroid dependence vs. acute use in IBD reoperation.
Finally, our approach may serve as a template to address common issues when using population-based datasets for clinical research to study IBD subjects. We addressed numerous aspects of this process in ways that ultimately enabled us to both validate existing knowledge regarding steroid use in IBD and derive new insights that may have important implications for clinical practice with respect to delaying or preventing reoperations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Kenney Ng PhD for his continuous critical input. We would also like to thank Michiharu Kudo PhD for providing support and resources.
Abbreviations
- 1K PY
1000 person years
- AUC
Area under the curve
- CD
Crohn’s disease
- CI
Confidence interval
- Hgb
Hemoglobin
- IBD
Inflammatory bowel disease
- GI
Gastrointestinal
- GP
General practice
- IPW
Inverse probability weighting
- OR
Odds ratio
- UC
Ulcerative colitis
- UK Biobank
United Kingdom Biobank
- ML
Machine learning
Author contributions
Uri Kartoun: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Validation; Writing original draft.Akira Koseki: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Review & editing.Akihiro Kosugi: Data curation; Formal analysis; Methodology; Validation.Kingsley Njoku: Data curation; Formal analysis; Investigation; Methodology; Validation; Review & editing.Tesfaye Yadete: Data curation; Formal analysis; Investigation; Methodology; Validation; Review & editing.Eileen Koski: Data curation; Formal analysis; Investigation; Methodology; Validation; Review & editing.Joao Bettencourt-Silva: Methodology; Review & editing.Natasha Mulligan: Methodology; Review & editing.Jianying Hu: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Supervision; Validation; Review & editing.Julia Liu: Conceptualization; Formal analysis; Investigation; Methodology; Validation; Review & editing.Thaddeus Stappenbeck: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Validation; Review & editing.Vibha Anand: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Supervision; Validation; Visualization; Review & editing.
Funding
This work was supported by a collaboration grant between IBM and Cleveland Clinic, and an internal grant from Morehouse School of Medicine.
Data availability
Note that data access was provided under UK Biobank application #95318. The data that support the findings of this study are available from the UK Biobank Access Management Team, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request (contact Uri Kartoun at Uri.Kartoun@ibm.com) and with permission of the UK Biobank Access Management Team.
Declarations
Competing interests
Potential competing interests: The authors declare that this work was conducted free of commercial, financial, or non-financial relationships that could be interpreted as a potential conflict of interest. Uri Kartoun, Akira Koseki, Akira Koseki, Akihiro Kosugi, Eileen Koski, Joao Bettencourt-Silva, Natasha Mulligan, Jianying Hu, and Vibha Anand are employees of IBM. Dr. Julia Liu: Advisory Board Member for Janssen, Takeda, Bristol Myers Squibb, and Pfizer; Consultant to Janssen, Takeda, Bristol Myers Squibb, Eli Lilly, and Pfizer. Dr. Thad Stappenbeck is an advisor for Janssen and Abbvie and is a founder of Mobius Care. The remaining authors have no disclosures.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Uri Kartoun and Akira Koseki.
Contributor Information
Julia Liu, Email: jjliu@msm.edu.
Thaddeus Stappenbeck, Email: stappet@ccf.org.
Vibha Anand, Email: anand@us.ibm.com.
References
- 1.Bernell, O., Lapidus, A. & Hellers, G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann. Surg.231(1), 38–45. 10.1097/00000658-200001000-00006 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee, K. E., Cantrell, S., Shen, B. & Faye, A. S. Post-operative prevention and monitoring of Crohn’s disease recurrence. Gastroenterol. Rep. (Oxf). 10, goac070. 10.1093/gastro/goac070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang, K. M. et al. Risk factors for postoperative recurrence after primary bowel resection in patients with Crohn’s disease. World J. Gastroenterol.23(38), 7016–7024. 10.3748/wjg.v23.i38.7016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valibouze, C., Desreumaux, P. & Zerbib, P. Post-surgical recurrence of Crohn’s disease: Situational analysis and future prospects. J. Visc. Surg.158(5), 401–410. 10.1016/j.jviscsurg.2021.03.012 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Candia, R., Naimark, D., Sander, B. & Nguyen, G. C. Cost-utility analysis: Thiopurines plus endoscopy-guided biological step-up therapy is the optimal management of postoperative Crohn’s disease. Inflamm. Bowel Dis.23(11), 1930–1940. 10.1097/MIB.0000000000001233 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein, G. R. et al. ACG Clinical Guideline: Management of Crohn’s disease in adults. Am. J. Gastroenterol.113(4), 481–517. 10.1038/ajg.2018.27 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Joustra, V. et al. Natural history and risk stratification of recurrent Crohn’s Disease after Ileocolonic Resection: A Multicenter Retrospective Cohort Study. Inflamm. Bowel Dis.28(1), 1–8. 10.1093/ibd/izab044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, X. Q. et al. Study of disease phenotype and its association with prognosis of paediatric inflammatory bowel disease in China. BMC Pediatr.18(1), 229. 10.1186/s12887-018-1212-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Cristofaro, E. et al. Long-term risk of Colectomy in patients with severe Ulcerative Colitis responding to Intravenous corticosteroids or Infliximab. J. Clin. Med.11(6), 1679. 10.3390/jcm11061679 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akintimehin, A. O., O’Neill, R. S., Ring, C., Raftery, T. & Hussey, S. Outcomes of a National Cohort of children with acute severe Ulcerative Colitis. Front. Pediatr.6, 48. 10.3389/fped.2018.00048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu, Y. et al. Risk factors and long-term outcome of disease extent progression in Asian patients with ulcerative colitis: A retrospective cohort study. BMC Gastroenterol.19(1), 7. 10.1186/s12876-018-0928-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, C. Y., Cham, C. M. & Chang, E. B. Epithelial wound healing in inflammatory bowel diseases: The next therapeutic frontier. Translational Res.236, 35–51. 10.1016/j.trsl.2021.06.001 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annual-Report-on-the-Use-of-Biologics-for-Inflammatory-Bowel-Diseases-2018-19-IBD-Registry-Oct-2019.pdf. Accessed July 6, 2023. http://ibdregistry.org.uk/wp-content/uploads/2021/11/Annual-Report-on-the-Use-of-Biologics-for-Inflammatory-Bowel-Diseases-2018-19-IBD-Registry-Oct-2019.pdf
- 14.Kartoun, U. et al. Subtyping gastrointestinal surgical outcomes from real world data: A comprehensive analysis of UK Biobank. AMIA Annu. Symp. Proc. 2023, 426–435. (2023). [PMC free article] [PubMed]
- 15.Denson, L. A. et al. Challenges in IBD research: Precision medicine. Inflamm. Bowel Dis.25(Suppl 2), S31–S39. 10.1093/ibd/izz078 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Li, J. & Qian, J. M. Artificial intelligence in inflammatory bowel disease: Current status and opportunities. Chin. Med. J. (Engl). 133(7), 757–759. 10.1097/CM9.0000000000000714 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivera, P., Danese, S., Jay, N., Natoli, G. & Peyrin-Biroulet, L. Big data in IBD: A look into the future. Nat. Rev. Gastroenterol. Hepatol.16(5), 312–321. 10.1038/s41575-019-0102-5 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Ogallo, W., Tadesse, G. A., Speakman, S. & Walcott-Bryant, A.Detection of anomalous patterns associated with the impact of medications on 30-day hospital readmission rates in diabetes care. AMIA Jt. Summits Transl. Sci. Proc. 2021, 495–504. (2021). [PMC free article] [PubMed]
- 19.Shimoni, Y. et al. An evaluation toolkit to Guide Model Selection and Cohort Definition in Causal Inference. 10.48550/arXiv.1906.00442 (2019).
- 20.Feature Selection Based on Subpopulations and Propensity Score Matching: A Coronary Artery Disease Use Case using the UK Biobank. Accessed February 27. (2023). https://knowledge.amia.org/76677-amia-1.4637602/f007-1.4641746/f007-1.4641747/580-1.4642000/269-1.4641997?qr=1
- 21.Sub-population-based feature selection (SBPFS) | Computational Cardiovascular Research Group. Accessed February 24. (2023). https://www.rle.mit.edu/cb/sub-population-based-feature-selection-sbpfs/
- 22.Sun, Z. et al. A probabilistic disease progression modeling approach and its application to integrated Huntington’s disease observational data. JAMIA Open.2(1), 123–130. 10.1093/jamiaopen/ooy060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan, A. et al. A machine-learning derived Huntington’s Disease Progression Model: insights for clinical Trial Design. Mov. Disord.37(3), 553–562. 10.1002/mds.28866 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Kwon, B. C. et al. Modeling disease progression trajectories from longitudinal observational data. AMIA Annu Symp Proc. 2020, 668–676. (2020). [PMC free article] [PubMed]
- 25.Kwon, B. C. et al. Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat. Commun.13(1), 1514. 10.1038/s41467-022-28909-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical Classifications Software Refined (CCSR). Accessed March 2. (2023). https://hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
- 27.Kartoun, U. et al. Subtyping gastrointestinal surgical outcomes from real world data: A comprehensive analysis of UK Biobank. AMIA 2023 Annual Symposium (to appear). (2023). [PMC free article] [PubMed]
- 28.causallib/examples/ipw.ipynb at master · BiomedSciAI/causallib. GitHub. Accessed July 28. (2023). https://github.com/BiomedSciAI/causallib/blob/master/examples/ipw.ipynb
- 29.Kwon, B. C. et al. DPVis: Visual analytics with hidden Markov models for disease progression pathways. IEEE Trans. Vis. Comput. Graph. 27(9), 3685–3700. 10.1109/TVCG.2020.2985689 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Chouairi, F., Torabi, S. J., Mercier, M. R., Gabrick, K. S. & Alperovich, M. Chronic steroid use as an independent risk factor for perioperative complications. Surgery. 165(5), 990–995. 10.1016/j.surg.2018.12.016 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, G. C., Elnahas, A. & Jackson, T. D. The impact of preoperative steroid use on short-term outcomes following surgery for inflammatory bowel disease. J. Crohns Colitis. 8(12), 1661–1667. 10.1016/j.crohns.2014.07.007 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Moghadamyeghaneh, Z. et al. Impact of chronic steroid use on outcomes of colorectal surgery. Am. J. Surg.210(6), 1003–1009. 10.1016/j.amjsurg.2015.07.002 (2015). discussion 1009. [DOI] [PubMed] [Google Scholar]
- 33.Jung, S., Fehr, S., Harder-d’Heureuse, J., Wiedenmann, B. & Dignass, A. U. Corticosteroids impair intestinal epithelial wound repair mechanisms in vitro. Scand. J. Gastroenterol.36(9), 963–970. 10.1080/003655201750305495 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Ardizzone, S. et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn’s disease. Gastroenterology. 127(3), 730–740. 10.1053/j.gastro.2004.06.051 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Bettencourt-Silva, J. H. et al. Discovering New Social determinants of Health concepts from Unstructured Data: Framework and evaluation. Stud. Health Technol. Inf.270, 173–177. 10.3233/SHTI200145 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Ramirez, A. H. et al. The all of us research program: Data quality, utility, and diversity. Patterns (N Y). 3(8), 100570. 10.1016/j.patter.2022.100570 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl. J. Med.362(15), 1383–1395. 10.1056/NEJMoa0904492 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Buisson, A. et al. Ustekinumab is more effective than azathioprine to prevent endoscopic postoperative recurrence in Crohn’s disease. United Eur. Gastroenterol. J.9(5), 552–560. 10.1002/ueg2.12068 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kartoun, U. et al. Prediction performance and fairness heterogeneity in cardiovascular risk models. Sci. Rep.12(1), 12542. 10.1038/s41598-022-16615-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Note that data access was provided under UK Biobank application #95318. The data that support the findings of this study are available from the UK Biobank Access Management Team, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request (contact Uri Kartoun at Uri.Kartoun@ibm.com) and with permission of the UK Biobank Access Management Team.