Abstract
Introduction:
The treatment for Kienböck disease varies widely based on the status of the lunate. To date, there is no consensus regarding the optimal treatment for patients with coronal plane fractures of the lunate, or Lichtman Stage IIIC. Therefore, the purpose of this study was to assess whether coronal plane fractures of the lunate in Kienböck disease healed after surgical intervention, and to determine the outcomes after surgical fixation of the lunate compared with treatment with salvage procedures.
Methods:
A retrospective review of 36 patients with Lichtman IIIC Kienböck disease was conducted. Patients were classified into lunate reconstruction (vascularized bone graft [VBG] or non-VBG) or salvage procedures (proximal row carpectomy [PRC] or limited fusion). Preoperative and postoperative visual analog scale (VAS) pain, range of motion, grip strength, and Mayo Wrist Score (MWS) were analyzed. Radiographs and computed tomographic scans were reviewed for Lichtman stage, fracture location, union, modified carpal height ratio, Stahl index, and radioscaphoid angle.
Results:
Thirteen patients underwent lunate reconstruction, 13 underwent limited fusion, and 10 underwent PRC. The overall union rate was 45% after lunate reconstruction, with average time to union of 7 months. There were no differences in union rates between VBG and non-VBG. All 3 groups experienced improvement in their postoperative grip strength and MWS. There were no statistically significant differences in preoperative to postoperative changes in clinical outcome measures between the 3 cohorts; however, when we compared lunate reconstruction with all patients who underwent salvage procedures (limited carpal fusion and PRC), we noted the latter had improved functional outcomes (P = .019). Four patients (31%) in the reconstruction cohort and 2 patients (8%) in the limited carpal fusion group required reoperation at latest follow-up.
Conclusions:
Union rate of coronal plane fractures in Kienböck disease remains variable. While the proportion of patients requiring reoperation was higher in the reconstruction group, all groups of patients experienced improvement in their clinical outcomes, without a significant difference between cohorts.
Keywords: coronal, Kienböck, lunate, union
Introduction
Kienböck disease, or idiopathic osteonecrosis of the lunate, has been extensively studied in the literature1 -14 and has been classified by both radiographic analysis, such as the Lichtman classification,11,12 and an arthroscopic classification, as proposed by Bain.2,3 The Lichtman classification ranges from 0 to IV with 0 being intermittent ischemia and stage IV defined as lunate collapse with radiocarpal or midcarpal arthritis.11,12 Stage III is of particular interest as expert-based treatment recommendations change as one moves through the substages of stage III. Stage IIIC is defined as a coronal split of the lunate regardless of the lunate or wrist morphology. 11 In the Bain classification, this is described as a Bain-2b. 2 This coronal fracture often occurs centrally in the sagittal plane but can occur both dorsally and volarly as well. 1 Lichtman and colleagues 11 noted that these fractures do not heal, even after direct or indirect revascularization procedures. However, more recently, studies have suggested that Lichtman IIIC or Bain-2b Kienböck disease can be successfully treated with revascularization, open reduction internal fixation (ORIF), or a combination of these.1,4,6,9,10
Given this controversy, the primary purpose of this study was to assess whether coronal plane fractures of the lunate in Kienböck disease healed after surgical reconstruction. In addition, this study sought to determine whether there was any difference in the clinical outcomes in those patients who underwent surgical reconstruction of the lunate compared with treatment with salvage procedures, including both limited carpal fusion and proximal row carpectomy (PRC). Last, this study aimed to determine differences in reoperation rate in patients who underwent lunate reconstruction versus limited carpal fusion versus PRC for stage IIIC Kienböck disease.
Materials and Methods
Following Institutional Review Board approval, a retrospective review of patients with coronal plane fractures of the lunate in Kienböck disease from 1988 to 2020 at a single institution was performed. Patients were initially identified via diagnosis codes for Kienböck disease. This yielded 926 unique patients. Each chart was then individually reviewed for accuracy of diagnosis and these patients’ images were screened for coronal plane fractures of the lunate (Lichtman IIIC Kienböck disease) (Figure 1). Images were reviewed by 2 authors separately to ensure agreement on the diagnosis of a coronal plane fracture of the lunate. Patients with any degree of fragmentation, as identified by multiplanar fracture lines on computed tomographic (CT) imaging, were excluded from this study. Patients without a minimum of 3 months postoperative follow-up were excluded from this study. Supplemental Figure 1 shows a flow chart of patients included in this study. These patients were divided based on treatment into a lunate reconstruction cohort (with vascularized bone graft [VBG] or non-VBG) versus 1 of 2 salvage procedure cohorts: limited carpal fusion (scaphocapitate [SC] or lunocapitate arthrodesis) or PRC. The type of lunate procedure was decided based on surgeon and patient preferences, on a case-by-case basis. The surgery was performed by 1 of 8 board-certified, fellowship-trained hand surgeons.
Figure 1.
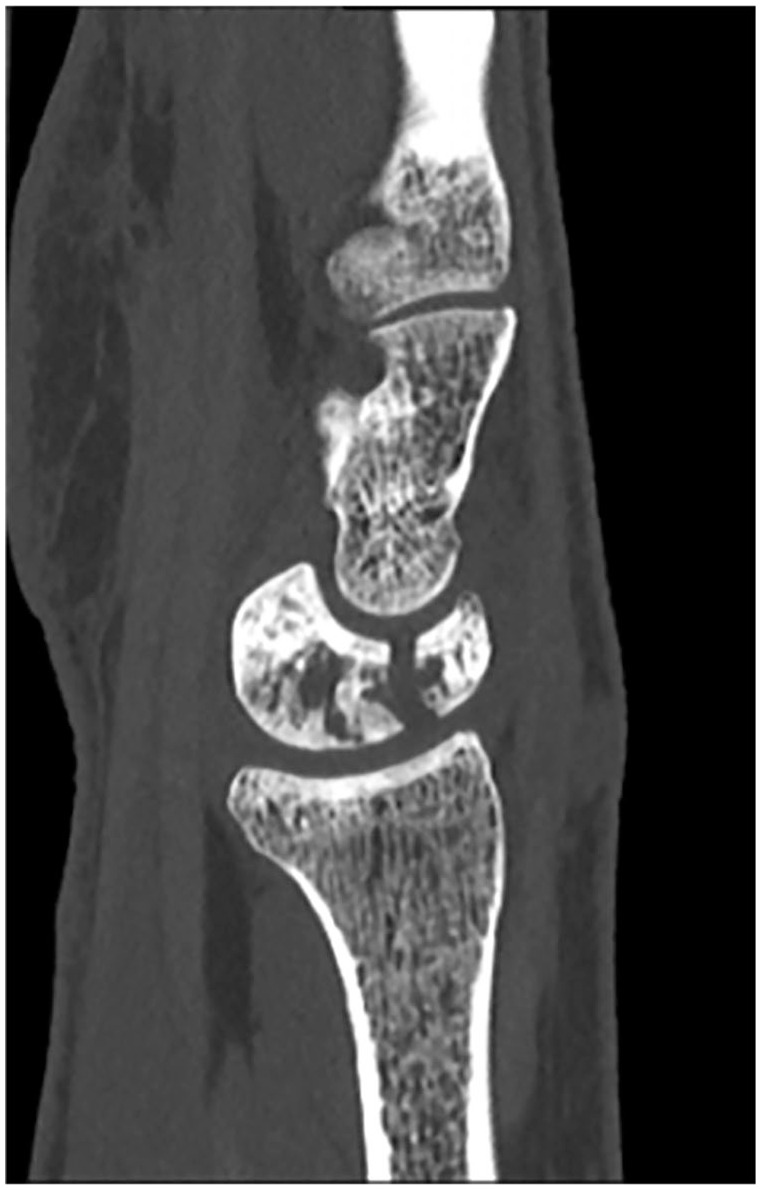
Computed tomographic scan showing a coronal plane fracture of the lunate (Lichtman IIIC) Kienböck disease.
Clinical Assessment
Medical records were reviewed to obtain patient demographic information, including smoking status, prior surgeries, type of surgery, and concomitant procedures. Pain was evaluated using patient’s self-reported pain via the visual analog scale (VAS) during each clinic visit. Other variables recorded included preoperative and postoperative range of motion (ROM), grip strength, and Mayo Wrist Score (MWS). Range of motion was measured by a provider (resident, fellow, consultant, or allied health staff) using a goniometer. Arc of motion was calculated by adding the flexion and extension measures. Three attempts at maximum grip strength were performed and the average was calculated and used for analysis. The MWS is a clinical outcome measure and is a summation of patient’s pain (25 points maximum), active flexion/extension arc as a percentage of the contralateral side (25 points maximum), grip strength as a percentage of the contralateral side (25 points maximum), and their ability to return to activities/work (25 points maximum), for a maximum total score of 100. 15 A score of 90 to 100 is categorized as excellent, 80 to 89 as good, 65 to 79 as fair, and less than 65 as poor.
Radiographic Assessment
Radiographs and CT scans were reviewed to determine the Lichtman stage, 11 coronal plane fracture location (volar 1/3, central, dorsal 1/3), lunate union (defined as evidence of bony bridging on >50% of individual sagittal CT slices), modified carpal height index 16 (MCHI), Stahl index, 17 and radioscaphoid angle (RSA). 18 Fracture location was measured on a single mid-sagittal CT slice by 2 authors concurrently to reach agreement. Volar 1/3, central 1/3, and dorsal 1/3 were measured as a proportion of the total width of the lunate on that CT slice. For lunate union, 2 authors concurrently reviewed the images to determine union versus nonunion, to ensure 100% agreement on this diagnosis. Time to union was recorded. Preoperative lunate morphology (type I vs type II) was also examined. Type I lunate refers to that which does not have a medial facet articulating with the hamate, whereas type II does have a medial facet that articulates with the hamate. 19
Reoperation Rate
Reoperations were noted for each patient, and the reoperation rate was calculated for each surgical treatment type. The second surgery that was performed and time to surgery were recorded.
Statistical Analysis
The primary outcome measure in this analysis was union of the lunate after surgical reconstruction. Secondary outcome measures included pain, ROM, grip strength, MWS, and radiographic outcomes as above, in both lunate reconstruction and salvage cohorts. Descriptive statistics were used to summarize the data and outcome measures. A multivariable logistic regression analysis was used to determine whether any demographic variables were associated with the outcomes of lunate union and reoperation. The Kruskal-Wallis test was used to calculate differences in continuous variables between cohorts. Fisher exact test was used to calculate differences in categorical variables. The α level was set to .05. A Kaplan-Meier survival curve to compare time to reoperation between cohorts was also calculated.
Results
A total of 36 patients with coronal plane fractures of the lunate were included in this study. Thirteen underwent lunate reconstruction with either VBG or non-VBG, 13 underwent limited carpal fusion, and 10 underwent PRC. Operative techniques are detailed in Supplemental Table 1. Patient demographics are listed in Table 1. Patients who underwent PRC were significantly older compared with those who underwent lunate reconstruction or limited carpal fusion (P = .018).
Table 1.
Patient Characteristics.
| Variable | Reconstruction | Limited fusion | PRC | P value |
|---|---|---|---|---|
| (n = 13) | (n = 13) | (n = 10) | ||
| Age a | 33 (31-40) | 35 (24-48) | 56 (48.25-64.5) | .018 |
| Female, No. (%) | 9 (69) | 10 (77) | 7 (70) | .893 |
| Smoker, No. (%) | 3 (23) | 1 (8) | 4 (40) | .130 |
| Dominant side, No. (%) | 7 (54) | 8 (62) | 5 (50) | .853 |
Note. PRC = proximal row carpectomy.
Median (IQR).
Within the reconstruction cohort, 3 underwent non-VBG and 10 received VBG (Table 2). Fixation of the graft varied, and included screws, suture anchors, temporary k-wires, external fixator, or bridge plate, or a combination of these (Table 2). Four patients (31%) also had concomitant posterior interosseous nerve (PIN) neurectomy. Median follow-up was 88 months (interquartile range [IQR]: 58-123 months) and median radiographic follow-up was 18 months (IQR: 9-26 months). Eleven of the 13 patients who underwent lunate reconstruction had postoperative CT scans, at a median of 9 months (IQR: 5-13 months).
Table 2.
Lunate Reconstruction Patients.
| Patient | Fracture location | Bone graft | Fixation | Lunate union? | Time to union, mo |
|---|---|---|---|---|---|
| 1 | Dorsal | DR non-VBG | Screw + temporary bridge plate | Yes | 4 |
| 2 | Volar | DR non-VBG | Screw + temporary k-wires | Yes | 10 |
| 3 | Middle | IC non-VBG | Screw | No | — |
| 4 | Dorsal | 4 + 5 ECA VBG | None | No | — |
| 5 | Dorsal | 4 + 5 ECA VBG | None | No | — |
| 6 | Dorsal | 4 + 5 ECA VBG | 2 screws | No | — |
| 7 | Middle | 4 + 5 ECA VBG | Screw | No | — |
| 8 | Middle | 4 + 5 ECA VBG | None | — | — |
| 9 | — | 4 + 5 ECA VBG | Temporary external fixator | — | — |
| 10 | Middle | MFC VBG | Temporary k-wires | Yes | 3 |
| 11 | Dorsal | MFT VBG | Suture anchor + temporary k-wires | Yes | 4 |
| 12 | Dorsal | 2 DMA VBG | Screw | No | — |
| 13 | Volar | Volar pedicle VBG | None | Yes | 13 |
Note. DR = distal radius; IC = iliac crest; MFC = medial femoral condyle; VBG = vascularized bone graft; ECA = extensor compartment artery; DMA = dorsal metacarpal artery; MFT = medial femoral trochlea; “—” = data not available/applicable.
Of those who underwent limited carpal fusion, 9 patients (69%) had concomitant PIN neurectomy, one of which (8%) also had anterior interosseous nerve neurectomy. Median follow-up in this cohort was 74 months (IQR: 47-84 months). Median radiographic follow-up in this cohort was 6 months (IQR: 2-14 months).
Within the PRC cohort, 8 patients (80%) had a PIN neurectomy at the time of surgery, with 4 of those (40%) also having an AIN neurectomy. Median follow-up in this cohort was 68 months (IQR: 41-85 months). Median radiographic follow-up in this cohort was 4 months (IQR: 2-25 months).
Clinical Outcomes
Supplemental Table 2 details the baseline preoperative clinical variables between the 3 cohorts. The only variable that was different between cohorts preoperatively was the MWS, which was significantly lower in the PRC group (P = .026). Postoperative clinical outcomes are listed in Table 3. Patients who underwent lunate reconstruction were immobilized for a significantly longer period postoperatively compared with the other 2 cohorts (P = .001). Postoperatively, patients who underwent reconstruction had the highest grip strength. This difference approached, but did not reach, statistical significance (P = .061). The changes in clinical outcomes from preoperative to postoperative were calculated for each variable and are listed in Table 4. There were no significant differences in the postoperative changes in clinical outcome measures when comparing between 3 groups: reconstruction, limited fusion, and PRC. When lunate reconstruction was compared against all patients who underwent a salvage procedure (both limited fusion and PRC), there was a statistically significant improvement in MWS for those who underwent any salvage procedure (P = .019). There were no differences in postoperative pain (P = .142), arc of motion (P = .733), or grip strength (P = .294), when comparing these 2 cohorts.
Table 3.
Postoperative Clinical Assessment by Treatment Type.
| Variable | Reconstruction (n = 13) | Limited fusion (n = 13) | PRC (n = 10) | P value | |||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| Immobilization, wk | 8 | 6.5-12 | 6 | 6-7 | 4 | 3-5 | .001 |
| VAS pain | 1 | 1-3 | 3 | 0-7 | 0 | 0-2 | .219 |
| Flexion, ° | 41.5 | 20.8-52.5 | 29.5 | 20-38.8 | 30 | 30-35 | .365 |
| Extension, ° | 44 | 38-45 | 40 | 30-44 | 40 | 35-45 | .597 |
| Radial deviation, ° | 10 | 7-12.5 | 10 | 7.5-15 | 5 | 5-10 | .387 |
| Ulnar deviation, ° | 20 | 19.5-23 | 26 | 20-34.3 | 25 | 15-30 | .722 |
| Arc of motion | 71.5 | 65-86 | 74 | 35-81.5 | 75 | 63-75 | .785 |
| Grip, kg | 21 | 18-26.5 | 19 | 15.3-28 | 11 | 9.9-12 | .061 |
| MWS | 60 | 56.3-68.8 | 57.5 | 47.5-67.5 | 65 | 50-75 | .707 |
Note. PRC = proximal row carpectomy; IQR = interquartile range; VAS = visual analog scale; MWS = Mayo Wrist Score.
Table 4.
Preoperative to Postoperative Changes in Clinical Outcomes by Treatment Type.
| Variable | Reconstruction (n = 13) | Limited fusion (n = 13) | PRC (n = 10) | P value | |||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| VAS pain | 3 | 0 to 5 | 0 | −2 to 4 | 0 | 0 to 2.5 | .222 |
| Flexion, ° | −3 | −14 to 1.5 | −17.5 | −25 to 7.5 | −4 | −17.5 to 2.5 | .950 |
| Extension, ° | −3 | −13.5 to 5 | 0 | −19.8 to 12.3 | 0 | −7.5 to 2.5 | .864 |
| Radial deviation, ° | −5 | −6.3 to −3.8 | −2.5 | −5 to 0 | −15 | −17.5 to −5 | .683 |
| Ulnar deviation, ° | 4.5 | −7.8 to 5.5 | 5 | 0 to 10.5 | 7.5 | 6.25 to 8.75 | .542 |
| Arc of motion | 0 | −25.5 to 19 | −3.5 | −26.3 to 7.5 | −10 | −26 to 2.5 | .891 |
| Grip, kg | 8 | 5 to 15.5 | 11 | 6 to 13 | 4 | 3.3 to 6.5 | .601 |
| MWS | 15 | −10 to 25 | 22.5 | 13.8 to 38.8 | 30 | 17.5 to 55 | .176 |
Note. PRC = proximal row carpectomy; IQR = interquartile range; VAS = visual analog scale; MWS = Mayo Wrist Score.
Radiographic Outcomes
Eleven of the 13 patients who underwent lunate reconstruction had postoperative CT scans. These patients are detailed in Table 1. Of those with CT scans, the overall union rate was 45% (5 of 11). The union rate was 38% (3 of 8) in patients who underwent vascularized bone grafting and 67% (2 of 3) in patients who had a non-VBG. This difference was not statistically significant (P = .749). The 2 patients who underwent medial femoral condyle/medial femoral trochlea (MFC/MFT) VBG went on to union (100%), along with the 1 patient who underwent a volar pedicle VBG. None of the patients who had a 4 + 5 extensor compartment artery (ECA) VBG achieved union. Average time to union was 6.9 months in the VBG group (range: 3.3-13.2 months) and 7.0 months in the non-VBG group (range: 4.0-10.1 months). Of those who did not achieve lunate union, the latest postoperative CT scan was obtained at an average of 11.0 months (range: 2.6-27.4 months). Of note, the most recent CT scans in those who did not undergo reoperation were obtained at 9.2 and 27.4 months postoperatively. Average age for those who did not achieve union was 39 years (range: 24-49 years). Three patients (50%) had their dominant hand affected and 2 patients (33%) were smokers. Multivariable logistic regression analysis did not find any association between age, sex, dominant hand, or smoking status with lunate union. Lunate morphology did not have an impact on union rate (P = 1.000).
When looking at the location of fractures, there was 100% union rate in the 2 patients with volar 1/3 fractures (Supplemental Table 3). One of these patients had a non-VBG with distal radius autograft, and the other patient had a VBG with a volar pedicle graft. This approached but did not reach statistical significance compared with dorsal 1/3 fractures (P = .073). There were no significant differences in the postoperative changes in radiographic measures, including the MCHI, Stahl index, and RSA in those patients who achieved union and those who did not (P = .672, .578, and .483).
Reoperation Rate
Six patients (16%) underwent reoperation at latest follow-up. This included 4 patients (31%) in the reconstruction cohort and 2 patients (8%) in the limited carpal fusion group. None of the patients who underwent PRC required reoperation. These differences in reoperation did not reach statistical significance (P = .196). Multivariable logistic regression analysis did not find any significant association between age, sex, dominant hand, or smoking status with the need for reoperation. Reoperation occurred at an average of 10 months (range: 6-13 months) and 6 months (range: 4-8 months), in the reconstruction and limited carpal fusion groups, respectively. The Kaplan-Meier survivorship free from reoperation for all 3 cohorts is noted in Figure 2. At 1 year, there was a 100% survival in the PRC cohort, 82% survival in the limited fusion cohort, and 75% survival in the reconstruction cohort. The details of the patients who underwent reoperation are listed in Table 5. Within the reconstruction cohort, none of the patients with CT evidence of lunate union went on to require reoperation, whereas 4 of the 6 patients (67%) without evidence of union required reoperation. There were 2 additional patients who required a second surgery. This included a patient who required irrigation and debridement for a pin site infection of an external fixator that was temporarily placed after VBG, as well as a patient who underwent excision of a granuloma along the incision from prior PRC.
Figure 2.
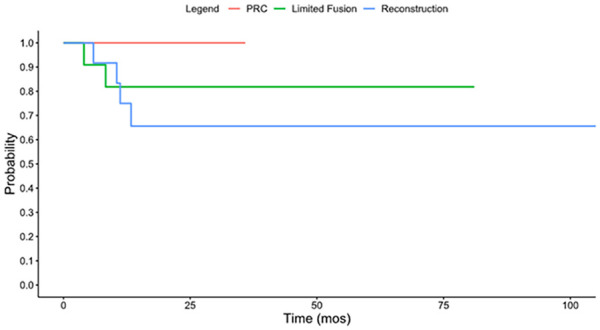
Kaplan-Meier survivorship free from reoperation.
Note. PRC = proximal row carpectomy.
Table 5.
Patients Requiring Reoperation.
| Patient | Age/gender | Initial treatment | Lunate union? | Revision surgery | Time to reoperation, mo |
|---|---|---|---|---|---|
| 1 | 43 M | Non-VBG with ICBG + PIN neurectomy | No | Total wrist arthrodesis | 10 |
| 2 | 40 M | 4 + 5 ECA VBG | No | Partial lunate excision with SC arthrodesis | 6 |
| 3 | 31 F | 2 DMA VBG | No | Partial lunate excision and PIN neurectomy | 13 |
| 4 | 49 F | 4 + 5 ECA VBG + PIN neurectomy | No | Partial lunate excision with SC arthrodesis | 11 |
| 5 | 37 M | SC arthrodesis with ICBG | N/A | Excision of lunate and total wrist arthrodesis | 4 |
| 6 | 48 M | SC arthrodesis with DRBG + PIN neurectomy | N/A | Excision of lunate and extensor tenolysis | 8 |
Note. VBG = vascularized bone graft; ICBG = iliac crest bone graft; PIN = posterior interosseous nerve; ECA = extensor compartment artery; DMA = dorsal metacarpal artery; SC = scaphocapitate; DRBG = distal radius bone graft.
Discussion
Controversy exists regarding the treatment for Lichtman Grade IIIC or Bain Grade-2B Kienböck disease. In this situation, many have deemed the lunate as non-salvageable and advocate for wrist salvage procedures such as PRC.2,10,17 There has been minimal literature to guide surgical decision-making in this scenario.
The overall union rate after lunate fracture reconstruction is widely variable. One previous study by Barrera-Ochoa et al 4 examined the outcomes after performing a radial osteotomy along with lunate reconstruction specifically in Lichtman Grade IIIC Kienböck disease. They used a single headless compression screw without additional bone grafting and achieved a union rate of 73%. 4 Another study by Chou and colleagues 6 reported on 5 patients with fractured lunates using various fixation techniques and found a 100% union rate in this cohort. On the contrary, a previous study by Arimitsu et al 1 had a union rate of 15% (2 of 13) for all fractures in the coronal plane of the lunate that were treated with capitate shortening osteotomy alone. Our study’s results report an overall union rate of 45% in patients who underwent lunate reconstruction. These results are closest to those of Tatebe et al, 20 who found a 50% union rate after radial osteotomy for displaced lunate fractures.
Recent case reports have shown promising results with vascularized bone grafting options to treat Lichtman IIIC Kienböck disease. Higgins et al 18 used a free MFT graft along to achieve union of the volar fragment, although the dorsal fragment showed a persistent fracture line. The midcarpal joint maintained congruency at 2 years and clinical outcomes were improved compared with before surgery. One of the patients in the current study was treated with a similar technique and went on to bony union. In addition, the patient in this study treated with an MFC graft also went on to union, suggesting that this may be an option for VBG in lunate fractures. Havulinna et al used a “Keyhole revascularization” technique (a pedicled distal radius bone graft based on the 4th extensor compartmental artery) that showed near full healing of a coronal plane lunate fracture. 10 Chou et al 6 treated 5 patients with a variety of vascularized bone grafting options with ORIF and SC pinning (pins removed at 6 weeks) ± radial shortening osteotomy and observed a 100% union rate. Their study included 5 total patients, 4 of which were treated with 1,2 intercompartmental supraretinacular artery (1,2 ICSRA) VBG and 1 with 4 + 5 ECA VBG. 6 The current study differed from this as it had 6 patients who underwent 4 + 5 ECA VBG, none of which achieved union. There were no patients who had a 1,2 ICSRA VBG performed in this study for comparison. Moran et al 13 noted a 71% successful revascularization rate after 4 + 5 ECA VBG to the lunate; however, it is important to note that these patients were Lichtman Grade II, IIIA, or IIIB and none were Grade IIIC. Similarly, Gillis and colleagues reported on the outcomes of 20 patients 18 years or below (stages II to IIIB) who underwent either revascularization or lunate offloading procedures. At an average follow-up of just over than 5 years, there was an improvement in clinical outcomes in both cohorts, although none of the patients included had a lunate fracture. 21
This suggests that these results are not applicable to all patients with Kienböck disease and there are likely significant differences in the lunate’s innate biology to heal once it goes on to fracture. These overall small numbers make it difficult to draw conclusions on the efficacy of different types of VBG for lunate fractures; however, this study shows the potential promise for the utility of MFC/MFT over 4 + 5 ECA VBG in these patients.
The aforementioned study by Arimitsu et al 1 analyzed union rate of coronal plane lunate fractures in Kienböck disease after a capitate shortening alone. They found that location of the coronal plane fracture affected union rate, with central fractures having a 0% union rate, volar a 20% union rate, and dorsal a 33% union rate. 1 In our study, we noted a 33% central fracture union rate, 100% volar union rate in 2 patients, and a dorsal union rate of 29% (P = .073). It is difficult to determine the precise impact that fracture location plays on lunate union rate given the overall small sample size. While not specifically examined in this study, the size of both the dorsal and volar fragments likely contributes significantly to the fracture healing, in addition to the fracture location. In addition, pattern of disruption of blood supply to the lunate fracture would impact its ability to heal, which may be location-dependent. Future studies would help to elucidate the role that fragment size or vascular supply play on lunate union.
Despite the relatively low union rate after lunate reconstruction, clinical outcomes, including VAS, grip strength, and MWS, improved postoperatively for this cohort as well as both of the salvage cohorts. To date, there are no data comparing the clinical outcomes between these groups. Arimitsu et al 1 reported significantly improved VAS pain, ROM, and grip strength after partial capitate shortening for lunate fractures in Kienböck disease. Similarly, Tatebe and colleagues 20 showed improved grip strength and ROM in their cohort of 31 patients who underwent radial osteotomy for lunate fracture. Salvage procedures, such as PRC, are well-documented in the literature for advanced stages of Kienböck disease. The PRC, in particular, has shown to improve pain and grip strength, while also preserving wrist ROM.22 -24 The current study supports both reconstruction and salvage surgery as reasonable options to improve clinical outcomes, with improved functionality in those patients who underwent salvage procedures (MWS: P = .019).
There was a lower reoperation rate compared with the nonunion rate in patients who underwent lunate reconstruction. This suggests that not all nonunions are symptomatic. The aforementioned study by Tatebe et al 20 drew similar conclusions, finding no significant difference in clinical outcomes based on whether or not the lunate fracture went on to union. This calls into question the role for nonoperative management in patients with Kienböck disease, including those with Lichtman Stage IIIC lunates. Perhaps, the natural history of Kienböck disease is such that some patients become less symptomatic over time, regardless of whether or not they undergo surgical intervention. 25 Prior literature has shown that the rate of incidental Kienböck disease in asymptomatic patients ranges anywhere from 0.27% to 1.9% in the general population.26 -28 One study by Kristensen et al 29 reported that 77% of patients with Kienböck disease who did not undergo any treatment had spontaneous resolution of their symptoms. A more recent study by DeGeorge and colleagues followed 25 patients with Kienböck disease who were treated nonoperatively and followed for 4 years. They found that there was significant improvement in pain, grip strength, and functional status at final follow-up, even though they had radiographic disease progression. 25 With this in mind, surgeons should discuss both operative and nonoperative treatment options with their patients.
The final consideration during surgical decision-making is the risk of reoperation. As expected, there was a higher rate of reoperation in the reconstruction cohort (31%) compared with the limited fusion (8%) and PRC groups (0%); however, this did not reach statistical significance. In addition, the ability to achieve union was protective against reoperation.
There are several limitations to this study. First, this was retrospectively examined data with relatively small numbers of patients for a given stage of Kienböck disease, which makes it difficult to attain adequate statistical power. As a tertiary referral center, however, we feel that this number reflects the rarity of this condition. This also limits in-person follow-up, as many patients followed up locally, and as such, given the inability to directly measure their clinical outcomes and review their postoperative images, some patients were excluded from this study. In addition, within each group, there was heterogeneity between the patients and also the various surgical techniques used. Due to the retrospective nature of this study, the rationale behind surgical technique decision-making is limited. Given the nature of Kienböck disease, we are unable to pinpoint the chronicity of each fracture, which may play a role in its ability to heal. Furthermore, there is a wide age range of patients in this cohort. We acknowledge that union and clinical outcomes may be impacted by each patient’s age, and this may be better elucidated with a larger cohort of patients.
In conclusion, union rate of coronal plane fractures in Kienböck disease remains variable, despite treatment with both VBG and non-VBG. As such, consideration needs to be paid to the type of bone graft if one is to consider reconstruction of the lunate. Nonunion after lunate reconstruction is not always symptomatic and does not necessitate reoperation. While the risk of reoperation is slightly higher in the reconstruction cohort, both groups of patients experienced improvement in their clinical outcomes, with greater functional outcome scores in those patients who underwent salvage procedures. It is imperative that surgeons have a thorough discussion with patients regarding their goals of care and potential outcomes to come to a mutual treatment decision.
Supplemental Material
Supplemental material, sj-docx-1-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-2-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-3-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-4-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-tif-5-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Acknowledgments
We would like to thank Dirk Larson for his expertise and guidance regarding the statistics in this project.
Footnotes
Contributorship: All the named authors were actively involved in the planning, enactment, and writing up of the study.
Ethical Approval: Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008 (IRB 20-004053).
Statement of Animal and Human Rights: This article does not contains any studies with human or animal subjects.
Statement of Informed Consent: Informed consent was obtained for this study. Informed consent for research purposes was obtained per institutional protocol.
Trial Registration: Not applicable
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SK is a consultant for Arthrex. The other author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Lauren E. Tagliero  https://orcid.org/0000-0001-6917-6248
https://orcid.org/0000-0001-6917-6248
Nicholas Munaretto  https://orcid.org/0000-0003-1791-0943
https://orcid.org/0000-0003-1791-0943
Alexander Shin  https://orcid.org/0000-0001-9658-8192
https://orcid.org/0000-0001-9658-8192
Sanjeev Kakar  https://orcid.org/0000-0002-2886-1510
https://orcid.org/0000-0002-2886-1510
Supplemental material is available in the online version of the article.
References
- 1. Arimitsu S, Shimada K, Moritomo H. Lunate fracture healing after partial capitate shortening in Kienböck disease. J Orthop Sci. 2020;25(3):428-434. [DOI] [PubMed] [Google Scholar]
- 2. Bain GI, Begg M. Arthroscopic assessment and classification of Kienbock’s disease. Tech Hand Up Extrem Surg. 2006;10(1):8-13. [DOI] [PubMed] [Google Scholar]
- 3. Bain GI, MacLean SB, Yeo CJ, et al. The etiology and pathogenesis of Kienböck disease. J Wrist Surg. 2016;5:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrera-Ochoa S, Campillo-Recio D, Muñoz-Perdomo T, et al. Dorsolateral biplane closing radial osteotomy and lunate fixation for stage IIIC Kienböck disease: a new surgical approach. Tech Hand Up Extrem Surg. 2018;22(3):74-80. [DOI] [PubMed] [Google Scholar]
- 5. Bürger HK, Windhofer C, Gaggl AJ, et al. Vascularized medial femoral trochlea osteochondral flap reconstruction of advanced Kienböck disease. J Hand Surg Am. 2014;39(7):1313-1322. [DOI] [PubMed] [Google Scholar]
- 6. Chou J, Bacle G, Ek ETH, et al. Fixation of the fractured lunate in Kienböck disease. J Hand Surg Am. 2019;44:67.e1-67.e8. [DOI] [PubMed] [Google Scholar]
- 7. Collon S, Tham SKY, McCombe D, et al. Scaphocapitate fusion for the treatment of Lichtman stage III Kienböck’s disease. Hand Surg Rehabil. 2020;39(3):201-206. [DOI] [PubMed] [Google Scholar]
- 8. Elhassan BT, Shin AY. Vascularized bone grafting for treatment of Kienböck’s disease. J Hand Surg Am. 2009;34:146-154. [DOI] [PubMed] [Google Scholar]
- 9. Gillis JA, Higgins JP. Coronal fracture of the lunate in advanced Kienböck disease: reestablishing midcarpal congruency to enable osteochondral reconstruction: a case report. JBJS Case Connect. 2018;8(2):e37. [DOI] [PubMed] [Google Scholar]
- 10. Havulinna J, Jokihaara J, Paavilainen P, et al. Keyhole revascularization for treatment of coronal plane fracture of the lunate in Kienböck disease. J Hand Surg Am. 2016;41(11):e441-e445. [DOI] [PubMed] [Google Scholar]
- 11. Lichtman DM, Lesley NE, Simmons SP. The classification and treatment of Kienbock’s disease: the state of the art and a look at the future. J Hand Surg Eur Vol. 2010;35(7):549-554. [DOI] [PubMed] [Google Scholar]
- 12. Lichtman DM, Pientka WF, 2nd, Bain GI. Kienböck disease: a new algorithm for the 21st century. J Wrist Surg. 2017;6:2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moran SL, Cooney WP, Berger RA, et al. The use of the 4 + 5 extensor compartmental vascularized bone graft for the treatment of Kienböck’s disease. J Hand Surg Am. 2005;30(1):50-58. [DOI] [PubMed] [Google Scholar]
- 14. Rhee PC, Jones DB, Moran SL, et al. The effect of lunate morphology in Kienböck disease. J Hand Surg Am. 2015;40(4):738-744. [DOI] [PubMed] [Google Scholar]
- 15. Cooney WP, Bussey R, Dobyns JH, et al. Difficult wrist fractures. Perilunate fracture-dislocations of the wrist. Clin Orthop Relat Res. 1987;214:136-147. [PubMed] [Google Scholar]
- 16. Nattrass GR, King GJ, McMurtry RY, et al. An alternative method for determination of the carpal height ratio. J Bone Joint Surg Am. 1994;76(1):88-94. [DOI] [PubMed] [Google Scholar]
- 17. Stahl S, Hentschel PJ, Santos Stahl A, et al. Comparison of clinical and radiologic treatment outcomes of Kienböck’s disease. J Orthop Surg Res. 2015;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen CF, Mathiesen FK, Lindequist S. Measurements of carpal bone angles on lateral wrist radiographs. J Hand Surg Am. 1991;16:888-893. [DOI] [PubMed] [Google Scholar]
- 19. Viegas SF. The lunatohamate articulation of the midcarpal joint. Arthroscopy. 1990;6(1):5-10. [DOI] [PubMed] [Google Scholar]
- 20. Tatebe M, Horii E, Majima M, et al. Radial osteotomy for Kienböck’s disease with displaced fracture of the lunate. J Hand Surg Am. 2007;32(9):1343-1347. [DOI] [PubMed] [Google Scholar]
- 21. Gillis JA, Khouri JS, Moran SL. Adolescent Kienböck’s disease: a comparison between lunate offloading and revascularization procedures. J Wrist Surg. 2020;9(3):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buluç L, Gündeş H, Baran T, et al. Proximal row carpectomy for Lichtman stage III Kienböck’s disease. Acta Orthop Traumatol Turc. 2015;49(6):641-647. [DOI] [PubMed] [Google Scholar]
- 23. Croog AS, Stern PJ. Proximal row carpectomy for advanced Kienböck’s disease: average 10-year follow-up. J Hand Surg Am. 2008;33(7):1122-1130. [DOI] [PubMed] [Google Scholar]
- 24. De Smet L, Robijns P, Degreef I. Proximal row carpectomy in advanced Kienbock’s disease. J Hand Surg Br. 2005;30:585-587. [DOI] [PubMed] [Google Scholar]
- 25. DeGeorge BR, Jr, Chawla SS, Lewallen L, et al. Functional and radiographic disease progression in nonoperatively managed Kienböck disease. Plast Reconstr Surg. 2021;147:1117-1123. [DOI] [PubMed] [Google Scholar]
- 26. Mennen U, Sithebe H. The incidence of asymptomatic Kienböck’s disease. J Hand Surg Eur Vol. 2009;34(3):348-350. [DOI] [PubMed] [Google Scholar]
- 27. Tsujimoto R, Maeda J, Abe Y, et al. Epidemiology of Kienböck’s disease in middle-aged and elderly Japanese women. Orthopedics. 2015;38(1):e14-e18. [DOI] [PubMed] [Google Scholar]
- 28. van Leeuwen WF, Janssen SJ, ter Meulen DP, et al. What is the radiographic prevalence of incidental Kienböck disease? Clin Orthop Relat Res. 2016;474:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristensen SS, Thomassen E, Christensen F. Kienböck’s disease—late results by non-surgical treatment. J Hand Surg Br. 1986;11(3):422-425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-2-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-3-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-docx-4-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND
Supplemental material, sj-tif-5-han-10.1177_15589447241298722 for Treatment Options for Coronal Plane Fractures of the Lunate in Kienböck Disease by Lauren E. Tagliero, Nicholas Munaretto, Karina Lenartowicz, Alexander Shin, Allen Bishop, Steven L. Moran and Sanjeev Kakar in HAND


