Abstract
Introduction:
The impact of streamlining algorithms for stroke patients on process times in pre-hospital emergency medicine (PHEM) is not well investigated. We analyzed the changes in pre- and in-hospital process times after implementation of a streamlining algorithm in a physician staffed PHEM system.
Patients:
We conducted a prospective observational study and analyzed process times of adult stroke patients attended by emergency physicians (EP) of the city of Göttingen PHEM service after implementation of a streamlining algorithm including stroke triage using the FAST-ED score. Stroke patients with standard emergency treatment attended before the implementation served as a control group. All patients were transported directly to the University Medical Center Göttingen (UMG) and received endovascular therapy (EVT) and/or systemic thrombolytic therapy.
Results:
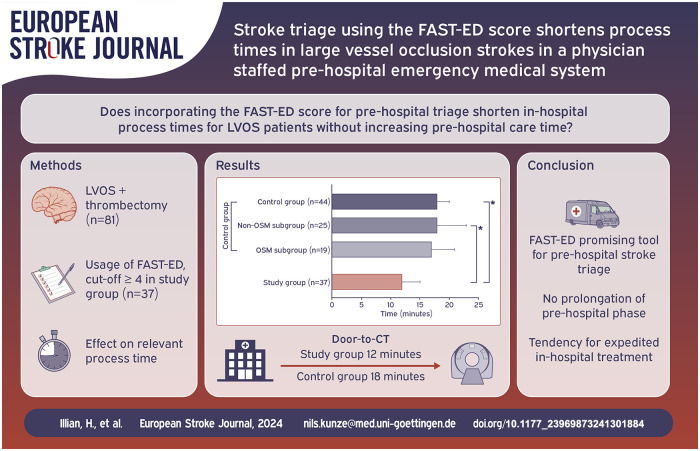
Of 75 suitable patients eligible in the study group, 37 (49.3%) received EVT and were compared to 44 patients in the control group. Pre-hospital process times did not differ significantly. Median door-to-CT time (12 vs 18 min, p = 0.017) and door-to-lysis time (20 vs 24 min, p = 0.005) were significantly shorter in the study group. Door-to-groin time was also shortened in the study group (42 vs 49 min) but not significantly (p = 0.088).
Discussion and conclusions:
Our findings indicate that a PHEM streamlining algorithm (namely the FAST-ED score) can significantly shorten in-hospital process times without delaying pre-hospital care. This improved coordination between PHEM and in-hospital emergency medicine (IHEM) may enhance neurological outcomes for stroke patients. Further research is needed to confirm these results and assess their applicability in other healthcare settings.
Keywords: Pre-hospital emergency medicine (PHEM), FAST-ED score, large vessel occlusion stroke (LVOS), process times, stroke triage
Graphical abstract.
Introduction
Systemic thrombolysis and endovascular therapy (EVT) are the first-line treatments for large vessel occlusion strokes (LVOS). 1 Efficient and accurate detection of LVOS in pre-hospital emergency medicine (PHEM) is challenging due to limited diagnostic capabilities. 2 Implementing an optimal, streamlined care framework from PHEM to the angiography suite may significantly reduce time-consuming interruptions to diagnostic and therapeutic procedures. By ensuring efficient streamlining of pre-hospital and in-hospital processes, patients could receive faster diagnosis and treatment based on correct triage in the field, which may lead to better functional outcomes.
In-hospitally the direct-to-angio (DTA) approach for stroke patients with LVOS has shown significantly improved process times and better functional outcomes at 90 days compared to conventional treatment.3,4 DTA bypasses traditional multi-detector computed tomography (MD-CT) scanning and directly evaluates the patient in the angiography suite. This process includes a rapid physical examination, NIHSS scoring, flat detector CT scanning (or direct angiography acquisition without prior CT scanning, depending on local protocols), and, if indicated, the administration of intravenous thrombolysis therapy (IVT). This is followed by definitive treatment via EVT. In some centers, the DTA approach also involves bypassing the emergency department, depending on local standards.
While it is possible to make a preliminary diagnosis of LVOS in PHEM this suspicion is often reevaluated in the emergency department (ED) to be then confirmed in the radiology suite at many centers, leading to avoidable prolongation of process times. Streamlining the interface between pre-hospital and in-hospital care is thus crucial to improve process times. Scoring systems like the field assessment stroke triage for emergency destination (FAST-ED) can facilitate accurate triage, with promising results in terms of specificity and sensitivity of stroke diagnosis when used by PHEM personnel.5,6 The FAST-ED score is a triage tool that assesses five key areas: facial palsy, arm weakness, speech changes, eye deviation, and denial/neglect. Each area is scored, with 0 being the lowest and 9 being the highest total score. Higher scores indicate a greater likelihood of a severe stroke due to large vessel occlusion. 5 However, the impact of DTA with the additional use of dedicated PHEM scoring systems (i.e. FAST-ED score), on process times from PHEM to the angiography suite is incompletely understood and has not been extensively studied, particularly in physician-based PHEM systems such as those used in Germany. 4 This study aimed to assess the impact of incorporating the FAST-ED score for pre-hospital triage of LVOS patients within a physician-based PHEM system on process times. We compared pre-hospital and in-hospital process times of streamlined LVOS patients with those of a cohort treated before the streamlining initiative. The primary objective was to determine if this approach shortens in-hospital processes without prolonging pre-hospital care time.
Methods
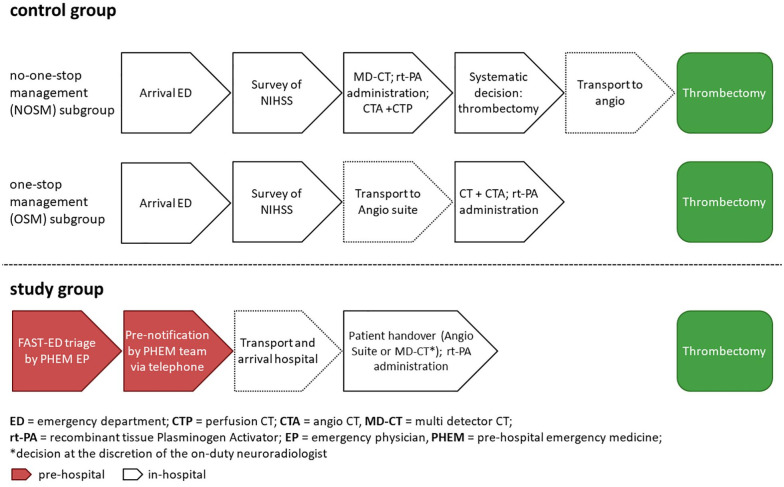
This prospective observational study was part of the ongoing interdisciplinary University Medical Center Göttingen (UMG) stroke research project which focuses on optimizing stroke therapies and understanding their pathophysiological impacts. The research group collaborates across neurology, emergency medicine, neuroradiology, anaesthesiology, and cardiology. The key interests include improving stroke management, investigating cardiac risk factors, and using advanced imaging techniques to study cerebral damage. Core of the project is a registry that collects data on UMG stroke patients for quality management and research purposes. The standard data collection is pseudonymized and includes in-hospital process times as well as selected information on patient treatment. 7 The project has been approved by the Ethics Committee of the Medical Faculty of the University of Göttingen and was amended for the inclusion of pre-hospital data (approval number 13/7/15An). According to the ethics approval informed consent was waived for this non-interventional observation. This study followed the “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.” 8 The present study was a single-center observational study that included patients attended by the physician staffed PHEM service of the city of Göttingen between August 2018 and July 2020. During the study period, two physician-staffed emergency vehicles and a helicopter were available in the city of Göttingen and were dispatched in cases of suspected stroke. Due to the study design, only patients transported by a physician-staffed emergency vehicle from Göttingen were included, which led to a smaller sample size. Annually, appromximately 200 thrombectomies are performed at UMG. All patients in both the study and control groups were transported to the hospital by one of these emergency services based in Göttingen. The PHEM standard operating procedure (SOP) for the treatment of stroke patients had been adapted just prior to this timeframe and from then on included the use of FAST-ED for stroke triage. LVOS patients treated with thrombectomy at the UMG from November 2016 to July 2018 served as the control group. This control group consisted of two subgroups: (1) the “No-One-Stop-Management” (NOSM) and the (2) “One-Stop-Management” (OSM) according to the in-hospital emergency medicine (IHEM) management. Patients of the control group were subject to a streamlining project that focused on optimizing in-hospital process times for stroke patients after ED arrival, regardless if they were brought in by ambulance or walked in. Patients in the NOSM subgroup were subsequently transported to the MD-CT. In contrast, patients in the OSM subgroup were primarily transported to the angio suite (see Figure 1 for details).
Figure 1.
PHEM and IHEM management processes before (control group) and after (study group) implementation of the streamlining SOP.
LVOS algorithm
All patients received standard pre-hospital emergency care according to the valid SOPs at the time of treatment. For the study group, this included the dispatch of an emergency physician (EP) to the patient. All EPs in the city of Göttingen PHEM system are affiliated with the UMG Department of Anaesthesiology. All EPs were briefed on the procedural changes before the implementation of the streamlining LVOS algorithm. Figure 1 gives an overview over the changes in the PHEM and IHEM management of stroke patients before and after the implementation of the streamlining SOP. The implementation of pre-hospital LVOS triage using the FAST-ED score was the central aspect of the project. A FAST-ED score ⩾4 points was used as a cut-off for the new LVOS treatment path which included: the establishment of two peripheral venous cannulas, the point-of-care measurement of Quick and INR values (Coagucheck Pro II, Roche Diagnostics GmbH, Mannheim, Germany) and taking blood samples for immediate laboratory analyses. The interdisciplinary UMG stroke team was pre-notified as early as possible following positive LVOS triage to ensure optimal IHEM process management. The neurologist on call and the PHEM team met at hospital arrival in the triage room at the emergency services entrance. At the discretion of the on-duty neuroradiologist, who also performs the thrombectomy, the decision to transfer patients either directly to the angio suite or the MD-CT scanner was made based on the pre-hospital assessment, clinical conditions, and logistical circumstances. NIHSS scoring was conducted by the neurologist during intrahospital transport and was completed by the time the patients arrived at the angio suite or the MD-CT. A flat detector CT scan (FD-CT) was used to rule out hemorrhagic stroke in patients that were directly transported to the angio suite according to the approach published by Psychogios et al. 9
Inclusion and exclusion criteria
Inclusion criteria for the study group were defined as follows: age ⩾18 years, FAST-ED Score ⩾4, involvement of an EP during treatment by the PHEM service of the city of Göttingen, EVT at the UMG. Exclusion criteria for the study group were: age <18 years, FAST-ED score <4, intracerebral hemorrhage, stroke mimics (e.g. Todd’s palsy, hypoglycemia), drip-and-ship patients, contraindication for EVT. Patients treated with EVT before the implementation of the FAST-ED Scoring method comprised the control group as described above.
Data collection
EPs provided a data set for the PHEM data including symptom onset, FAST-ED score, GCS score, and time of handover for each patient. Additional data were obtained from the routine PHEM documentation and pre-hospital time stamps were taken from the digital radio system that is used to dispatch the units of the PHEM system of the City of Göttingen. In-hospital data (demographic data, NIHSS, in-hospital process times, radiological and laboratory findings) were obtained via the patient data management systems of the UMG. The following process times were analyzed: onset-to-emergency-physician (EP) contact, onset-to-computed-tomography (CT), onset-to-groin, onset-to-lysis, EP contact-to-CT, EP contact-to-groin, EP-contact-to-lysis, door-to-CT, door-to-groin, and door-to-lysis.
Statistical analysis
The normal distribution of the parameters subject to statistical analysis in this study was initially assessed using the Shapiro-Wilk test. For normally distributed parameters, the t-test for independent samples was employed to identify significant differences between the groups. Conversely, the Mann-Whitney test was conducted for non-normally distributed parameters. A significance level of p < 0.05 was considered statistically significant. Descriptive and comparative statistical analyses, as well as the generation of statistical graphs, were performed using Prism (Prism 8, GraphPad Software Inc., Boston MA, USA).
Results
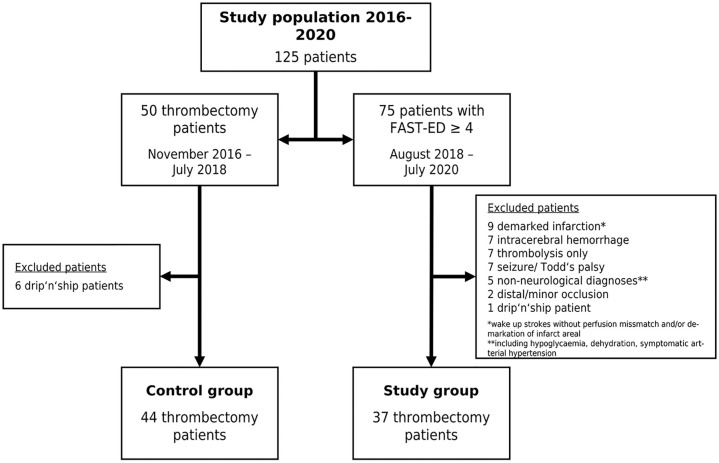
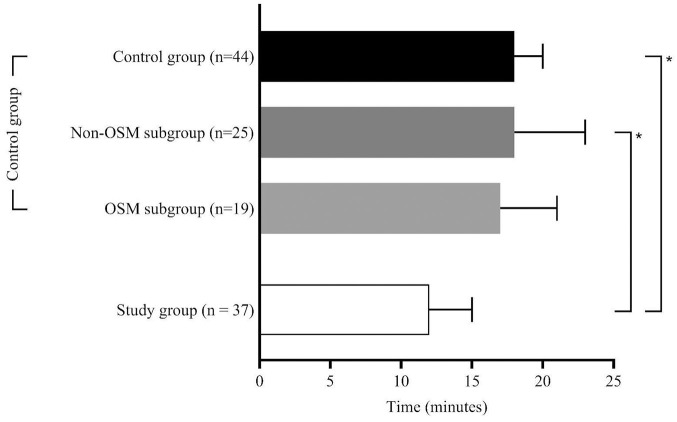
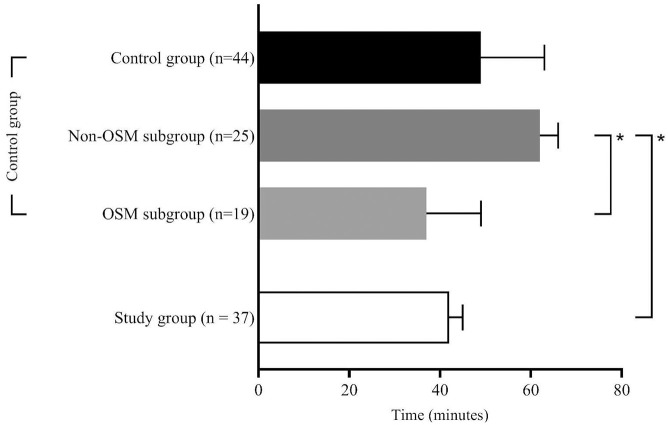
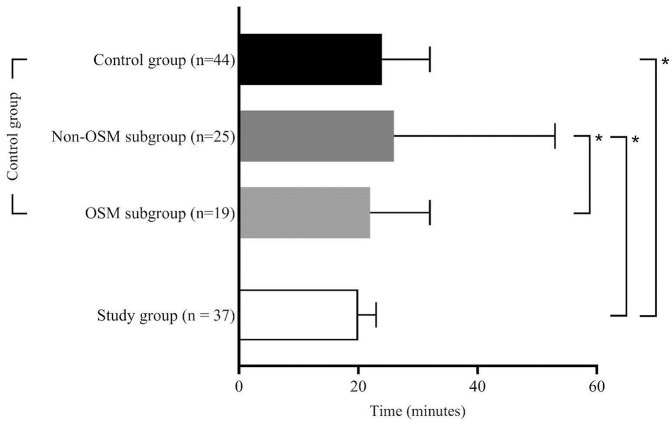
125 patients were eligible for this study. Figure 2 gives an overview over the study populations and the excluded patients. Of the 75 patients with FAST-ED ⩾4 points 54 patients (72%) actually had an LVOS. After excluding patients who did not fit the inclusion criteria 37 remained in the study group (B). The control group (A) consisted of 44 patients of whom 19 were treated according to the OSM protocol (OSM subgroup, A1) for in-hospital streamlining and 25 were treated without OSM (NOSM subgroup, A2). Table 1 summarizes the clinical and demographic characteristics of the study populations along with the statistical analyses of the process times for each group. The comparison between A and B revealed no significant difference, with the control group having a median age of 80 [28–94] years and the study group 78 [40–93] years and with 57% female patients in the control group and 43% in the study group. NIHSS scores were comparable, with a median of 13 [3–26] in the control group and 15 [3–25] in the study group. FAST-ED scores were reported only for the study group, with a median of 6 [4–9]. All 81 patients analyzed for this study underwent thrombectomy. Twenty-two patients (59%) in the study group and twenty-five patients (57%) in the control group were treated using systemic thrombolytic therapy in addition to EVT. In the study group, 16 patients (43%) were transferred directly to the angio suite, while 21 patients (57%) were first transferred to the MD-CT and then to the angio suite. That decision was made at the discretion of the on-duty neuroradiologist. Regarding treatment time intervals, the time from onset to emergency personnel contact had a median of 31 [6–210] min in the control group and 25 [9–294] min in the study group, with no significant difference. Onset-to-CT times were 76 [29–415] min in the control group and 82 [47–355] min in the study group, while onset-to-groin times were 126 [55–415] min in the control group and 109 [64–425] min in the study group, with no significant differences for these intervals. In-hospital process times showed that the door-to-CT time was significantly shorter in the study group, with a median of 12 [5–47] min compared to the overall control group, which had a median of 18 [4–46] min (A vs B; p = 0.017), and the NOSM subgroup, with a median of 18 [4–46] min (A2 vs B; p = 0.046). The door-to-groin time was significantly shorter in the study group, with a median of 42 [15–111] min, compared to 62 [27–87] min in the NOSM subgroup of the control group (A2 vs B; p = 0.001). Furthermore, the study group exhibited significantly shorter door-to-lysis times, with a median of 20 [10–51] min, compared to both the overall control group, with a median of 24 [16–141] min (A vs B; p = 0.005), and the NOSM subgroup, with a median of 26 [16–141] min (A2 vs B; p = 0.001). After Bonferroni correction, only the door-to-groin and door-to-lysis times between the control group and the NOSM subgroup remained significant (corrected p-value = 0.0046) Figures 3 to 5 collectively highlight the differences in door-to-CT, door-to-lysis, and door-to-groin times between the study group and the control group, including its OSM and NOSM subgroups. In all three figures, the study group consistently shows shorter times compared to the control group especially the NOSM subgroup. The door-to-CT time in Figure 3 was notably reduced in the study group compared to the control group and particularly the NOSM subgroup. Similarly, in Figure 4, the door-to-lysis times were shortest in the study group, showing improvements over the control group and the NOSM subgroup. Figure 5 shows that the study group also had the shortest door-to-groin times, outperforming the NOSM subgroup and the control group overall.
Figure 2.
Flow-chart of study populations and excluded patients.
Table 1.
Main results comparing pre-FAST-ED and FAST-ED group.
| Parameter | (A) Control group n = 44 | (A1) Control group | (A2) Control group | (B) Study group n = 37 | p Values | ||
|---|---|---|---|---|---|---|---|
| OSM subgroup n = 19 | Non-OSM subgroup n = 25 | A vs B | A1 vs B | A2 vs B | |||
| Age, median [min–max], years | 80 [28–94] | 78 [51–94] | 82 [28–94] | 78 [40–93] | 0.349 | 0.236 | 0.164 |
| Female, n (%) | 25 (57) | 12 (63) | 13 (52) | 16 (43) | – | – | – |
| NIHSS, median [min–max], points | 14 [3–26] | 13 [7–21] | 16 [3–26] | 15 [3–25] | 0.223 | 0.190 | 0.409 |
| FAST-ED, median [min–max], points | n.a. | n.a. | n.a. | 6 [4–9] | – | – | – |
| Wake up stroke, n (%) | 5 (11) | 0 (0) | 5 (20) | 6 (16) | – | – | – |
| Systemic thrombolysis, n (%) | 25 (57) | 11 (58) | 14 (56) | 22 (59) | – | – | – |
| Onset-to-EP contact | 31 [6–210] | 40 [7–210] | 25 [6–342] | 25 [9–294] | 0.829 | 0.246 | 0.920 |
| Onset-to-CT | 76 [29–415] | 96 [40–286] | 73 [29–385] | 82 [47–355] | 0.567 | 0.177 | 0.987 |
| Onset-to-groin | 126 [55–415] | 126 [55–330] | 127 [64–415] | 109 [64–425] | 0.271 | 0.436 | 0.177 |
| Onset-to-lysis a | 80 [45–230] | 80 [45–225] | 90 [50–230] | 85 [55–220] | 0.839 | 0.914 | 0.664 |
| EP contact-to-CT | 50 [23–76] | 52 [33–76] | 49 [23–73] | 49 [27–82] | 0.714 | 0.665 | 0.858 |
| EP contact-to-groin | 79 [48–190] | 69 [48–190] | 91 [58–122] | 79 [37–142] | 0.551 | 0.243 | 0.098 |
| EP contact-to-lysis a | 60 [38–192] | 58 [38–80] | 64 [44–192] | 57 [35–92] | 0.201 | 0.883 | 0.118 |
| PHEM period (incl, transport) | 30 [7–54] | 18 [8–41] | 29 [7–51] | 31 [18–71] | 0.232 | 0.484 | 0.222 |
| Door-to-CT | 18 [4–46] | 17 [6–35] | 18 [4–46] | 12 [5–47] | 0.017* | 0.052 | 0.046* |
| Door-to-groin | 49 [16–133] | 37 [16–133] | 62 [27–87] | 42 [15–111] | 0.088 | 0.468 | 0.001* |
| Door-to-lysis a | 24 [16–141] | 22 [16–33] | 26 [16–141] | 20 [11–51] | 0.005* | 0.206 | 0.001* |
All durations presented as median [min–max], minutes. *p<0.05.aif eligable.
Figure 3.
Door-to-CT times.
Figure 5.
Door-to-groin times.
Figure 4.
Door-to-lysis times.
Discussion
Our study demonstrates that the adoption of a pre-hospital LVOS triage algorithm in the region of southern Lower Saxony significantly reduces in-hospital process times compared to the previously used NOSM treatment of LVOS patients. These findings align with previous research indicating that streamlining the management of LVOS patients can expedite procedures and potentially improve patient outcomes.10,11 Our results support the hypothesis that a structured algorithm, like FAST-ED, can improve the efficiency of stroke care. However, it is noteworthy that when comparing the FAST-ED group to the OSM control group, no significant differences in process times were observed. Looking more closely at the different procedural time elements, it appears that the OSM protocol likely reduces procedure time in a different part of the workflow. Specifically, while the intervention group shows similar door-to-lysis times compared to the OSM group, the OSM group has faster door-to-groin times. This indicates that both approaches may save time at different stages of the stroke-triage process, potentially leading to overall time savings. The intervention group possibly saves time through direct transport to the CT or angio suite, while the OSM group may save time by streamlining logistics. These findings align with the general principle observed in previous studies by Ribo et al., 12 which demonstrated significant reductions in hospital workflow times through direct transfer to the angiosuite. On this observation one could suggest that prior streamlining efforts might have already optimized the in-hospital workflow, and the addition of the FAST-ED score did not further accelerate the process even when the number of cases might be too small for this statement. The discrepancy in the expected versus observed time savings in the OSM versus the study group indicates potential bottlenecks that warrant further investigation. It is possible that logistical or procedural delays within the angiography suite itself may have counteracted the expected time advantage. Additionally, variations in the clinical workflow, such as staff readiness or equipment availability, could have played a role in neutralizing the anticipated time savings. Future studies with larger cohorts should focus on identifying and mitigating these specific factors to enhance the overall efficiency of stroke treatment protocols. The implementation of the FAST-ED score did not significantly impact any of the PHEM process times such as PHEM-EP contact-to-groin, -lysis, or -CT. Our results show that the additional pre-hospital measures taken after a positive LVOS evaluation using the FAST-ED score (such as establishing a second peripheral venous access, drawing blood samples, and point-of-care INR testing) did not prolong pre-hospital process times including transportation to a comprehensive stroke centers (CSC). This is an important finding as it supports the feasibility of using the FAST-ED score in the pre-hospital setting without delaying patient transport and that measures usually undertaken in the emergency department can be implemented into PHEM care.
Our study confirmed that a FAST-ED score ⩾4 accurately predicts LVOS with a 72% probability, further validating it as a reliable pre-hospital diagnostic tool consistent with the existing literature. 5 Several studies implicate that LVOS patients might benefit most from direct transport to a CSC where thrombectomy can be performed immediately after diagnosis.13–15 However, this is an area of ongoing scientific debate. 16 In regions with multiple hospitals utilizing the FAST-ED score in PHEM could provide significant outcome benefits for LVOS patients by reducing secondary transports. When comparing our findings to other studies on DTA approaches, we found limited data available. A recent narrative review 4 noted that out of approximately 2,000 patients in 12 DTA studies, only 150 were directly transferred to a CSC’s angio suite with all other patients undergoing secondary transport. Notably, 74 of these 150 cases came from previous work conducted by our own research group. 11 Studies focusing on PHEM-to-angiography process times are scarce. This finding highlights the need for further research, particularly within various PHEM systems, including both physician-based and paramedic-based models. This additional research could provide valuable insights into the effectiveness of different algorithms across diverse emergency medical frameworks, ultimately enhancing the generalizability and applicability of our findings.
Limitations
The trial did not directly assess patient outcomes, focusing instead on process times as surrogates. While process times are an important measure of efficiency, they cannot fully capture the quality of care provided. Few studies, if any, investigate patient outcomes based on pre-hospital triage scores. 17 When considering LVOS triage in PHEM, it is crucial to incorporate comprehensive patient outcome measures. These metrics should, among others, include the degree of haemodynamic stability during endovascular treatment (EVT), the need for mechanical ventilation, and variations in performance during different working hours as well as long-term neurological outcomes. Based on our analysis, we are unable to determine how many patients with a FAST-ED score <4 still had an LVOS. Additionally, the impact of DTA protocols on the outcome of non-LVOS patients, such as those with intracerebral hemorrhage, remains unclear. There is a notable lack of analyses addressing this issue in the current literature. An interesting study in this context is the DIRECT ANGIO trial from France, which, according to its protocol, includes both LVOS and non-LVOS patients. However, the results of this trial are still pending. 18 Future research should provide a more holistic understanding of the impact of LVOS triage algorithms on both the efficiency and quality of stroke care, ultimately offering a clearer picture of its benefits and potential areas for improvement. Another limitation regarding the analyzed times is the large number of statistical tests that were conducted. After applying Bonferroni correction to the p-values, only the comparison between the NOSM subgroup and the intervention group showed a statistically significant difference in the door-to-lysis and door-to-CT times. Nevertheless, the other values still indicate a trend toward shorter times in the study group. Furthermore, our study was conducted in an urban region with short travel distances and only one CSC, which limits the generalizability of the findings. Research in more complex hospital landscapes, particularly regions with multiple transportation options and primary stroke centers, is necessary to determine the broader applicability of the FAST-ED algorithm and its impact on core process times in these contexts.
Conclusions
In conclusion, while our findings suggest that the implementation of a pre-hospital LVOS triage algorithm may help reduce process times, the potential impact on stroke care in the PHEM and IHEM sectors remains uncertain. The FAST-ED score shows promise as a tool for LVOS triage by PHEM physicians without specialized neurological training. However, further research is needed to evaluate patient outcomes and to explore whether this algorithm can effectively reduce secondary transports, particularly in more complex hospital environments. In conclusion, while our findings suggest that the implementation of a pre-hospital LVOS triage algorithm may help reduce process times, the potential impact on stroke care in the PHEM and IHEM sectors remains uncertain. The FAST-ED score shows promise as a tool for LVOS triage and appears to be usable by PHEM physicians without specialized neurological training. However, further research is needed to evaluate patient outcomes and to explore whether this algorithm can effectively reduce secondary transports, particularly in more complex hospital environments.
Footnotes
Authors’ note: Prof. Dr. med. Markus Roessler is now affiliated to Center of Anaesthesiology, Intensive Care, Emergency Medicine and Pain Therapy Universitätsklinikum OWL der Universität Bielefeld Campus Klinikum Mitte Teutoburger Strasse 50, 33604 Bielefeld, Germany.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JL has received advisory grants from Siemens Healthineers.
MNP received Grants from the Swiss National Science Foundation (SNF) for the DISTAL trial (33IC30_198783), ICARUS (32003B_220118) and TECNO trial (32003B_204977), Grant from Bangerter-Rhyner Stiftung for the DISTAL trial.Unrestricted Grants for the DISTAL trial from Stryker Neurovascular Inc., Medtronic Inc., Phenox GmbH, Penumbra Inc. and Rapid Medical Inc., Sponsor-PI SPINNERS trial (Funded by a Siemens Healthineers AG Grant), Research agreement with Siemens Healthineers AG, Local PI for the ASSIST, EXCELLENT, ACT in STROKE, TENSION, COATING, SURF and ESCAPE-NEXT trials.Speaker fees: Stryker Neurovascular Inc., Medtronic Inc., Penumbra Inc., Acandis GmbH, Phenox GmbH, Siemens Healthineers AG.
The remaining authors confirm that there are no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Ethics Committee of the Medical Faculty of the University of Göttingen (approval number 13/7/15An).
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Guarantor: NKS
Contributorship: All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Hanna Illian  https://orcid.org/0009-0004-5886-5143
https://orcid.org/0009-0004-5886-5143
Clemens Grimm  https://orcid.org/0000-0002-2903-8454
https://orcid.org/0000-0002-2903-8454
Jan Liman  https://orcid.org/0000-0002-7465-9655
https://orcid.org/0000-0002-7465-9655
Nils Kunze-Szikszay  https://orcid.org/0000-0001-9260-6939
https://orcid.org/0000-0001-9260-6939
References
- 1. Mistry EA, Mistry AM, Nakawah MO, et al. Mechanical thrombectomy outcomes with and without intravenous thrombolysis in stroke patients. Stroke 2017; 48: 2450–2456. [DOI] [PubMed] [Google Scholar]
- 2. Schwamm LH. Optimizing prehospital triage for patients with stroke involving large vessel occlusion: the road less traveled. JAMA Neurol 2018; 75: 1467–1469. [DOI] [PubMed] [Google Scholar]
- 3. Sarraj A, Goyal N, Chen M, et al. Direct to angiography vs repeated imaging approaches in transferred patients undergoing endovascular thrombectomy. JAMA Neurol 2021; 78: 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desai SM, Psychogios M, Khatri P, et al. Direct transfer to the neuroangiography suite for patients with stroke. Stroke 2023; 54: 1674–1684. [DOI] [PubMed] [Google Scholar]
- 5. Lima FO, Silva GS, Furie KL, et al. Field assessment stroke triage for emergency destination. Stroke 2016; 47: 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puolakka T, Virtanen P, Kinnunen J, et al. Prehospital identification of large vessel occlusion using the FAST-ED score. Acta Neurol Scand 2021; 144: 400–407. [DOI] [PubMed] [Google Scholar]
- 7. UMG. Neurologie: Klinische Schlaganfallforschung, https://neurologie.umg.eu/forschung/arbeitsgruppen/klinische-schlaganfallforschung/ (2024, accessed 18 Novemeber 2024).
- 8. Von Elm E. Strobe initiative. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 9. Psychogios MN, Behme D, Schregel K, et al. One-stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke 2017; 48: 3152–3155. [DOI] [PubMed] [Google Scholar]
- 10. Schregel K, Behme D, Tsogkas I, et al. Effects of workflow optimization in endovascularly treated stroke patients – a pre-post effectiveness study. PLoS One 2016; 11: e0169192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Psychogios MN, Maier IL, Tsogkas I, et al. One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med 2019; 8: 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribo M, Boned S, Rubiera M, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J Neurointerv Surg 2018; 10: 221–224. [DOI] [PubMed] [Google Scholar]
- 13. Froehler MT, Saver JL, Zaidat OO, et al. Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation 2017; 136: 2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019; 11: 14–19. [DOI] [PubMed] [Google Scholar]
- 15. Weisenburger-Lile D, Blanc R, Kyheng M, et al. Direct admission versus secondary transfer for acute stroke patients treated with intravenous thrombolysis and thrombectomy: insights from the endovascular treatment in Ischemic Stroke Registry. Cerebrovasc Dis 2019; 47: 112–120. [DOI] [PubMed] [Google Scholar]
- 16. Pérez de la Ossa N, Abilleira S, Jovin TG, et al. Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in nonurban areas: the RACECAT randomized clinical trial. JAMA 2022; 327: 1782–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ospel JM, Dmytriw AA, Regenhardt RW, et al. Recent developments in pre-hospital and in-hospital triage for endovascular stroke treatment. J NeuroIntervent Surg 2023; 15: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 18. Riou-Comte N, Zhu F, Cherifi A, et al. Direct transfer to angiosuite for patients with severe acute stroke treated with thrombectomy: the multicentre randomised controlled DIRECT ANGIO trial protocol. BMJ Open 2021; 11: e040522. [DOI] [PMC free article] [PubMed] [Google Scholar]