Abstract
Objective
To assess patient perspectives on the level of shared decision making (SDM) experienced related to bariatric surgery.
Background
Severe obesity is common and has serious health implications. Yet, few eligible patients pursue bariatric surgery. Shared decision making could be a useful approach for considering treatment options.
Methods
Patients were surveyed at Kaiser Permanente and UPMC clinics providing bariatric surgical services. Cross‐sectional samples represent three time points: (a) Cohort 1 (C1): following referral; (b) Cohort 2 (C2): after initial bariatric practice appointment; (c) Cohort 3 (C3): following pre‐operative visit. Patients completed the electronic survey instruments: CollaboRATE, SDM‐Q‐9, and National Quality Forum (NQF) SDM process measures.
Results
The sample included 167 participants, half from each site. Cohort distribution was 35% C1, 33% C2, and 32% C3. Mean age was 43.8 years (SD 13.5), BMI was 48 kg/m2 (SD 8.63), 81% were female and 73% were white. Overall, 62% reported CollaboRATE top scores, with a dose‐response (C1: 54%, C2: 60%, C3: 72%). Mean (SD) SDM‐Q‐9 score (possible range: 0–100) was: 79.6 (22.5); with C1: 66.9 (26.5), C2: 83.4 (18.0), and C3: 88.4 (15.9). The average NQF score (possible range: 0–4) was 3.11 (1.14), with C1: 2.71 (1.27), C2: 3.31 (1.09), and C3: 3.28 (0.97).
Conclusions
Patients seeking bariatric care reported moderate or high levels of SDM. In general, SDM metrics were highest just before surgery.
Keywords: bariatric surgery, obesity, shared decision making
1. INTRODUCTION
Severe obesity is common and increasing, with 9.2% of US adults having a BMI ≥40 in 2017–2018, nearly double the prevalence from 1999 to 2000. 1 Nearly a quarter of US adults are predicted to have a body mass index of 35 kg/m2 or above by 2030. 2 Severe obesity is linked with substantial morbidity, mortality and reduced quality of life. 3 , 4 Considerable data support the safety and effectiveness of bariatric surgery for promoting weight loss and improved health outcomes. 5 , 6 , 7 , 8 , 9 , 10 Less than 1% of eligible patients choose to undergo bariatric surgery each year. 11 The appropriate uptake of bariatric procedures, based on informed patient decisions, is unknown. However, clinical education and support for informed decision making around bariatric surgery may be limited. One systematic review found that high‐quality online information for patients that could support shared decision making (SDM) related to bariatric surgery is lacking. 12 Furthermore, primary care providers may not introduce the option of bariatric surgery for their patients living with severe obesity. For example, one national survey of adults with severe obesity found that only 1 in 10 reported that their primary care provider had recommended bariatric surgery. 13 A survey of 161 Midwestern primary care providers found that while many PCPs believe that bariatric surgery is effective in the long term, only 65% were familiar with its indications, and only 70% felt comfortable discussing it with patients. 14 PCPs have also expressed concerns about surgical complications, long‐term side effects and ineffective weight loss. 15 , 16
The knowledge base around bariatric surgery has shifted considerably in recent years, with more long‐term data on the health outcomes of sleeve gastrectomy (SG) and Roux‐en‐Y gastric bypass (RYGB), the most commonly performed operations. These studies suggest that SG is associated with a significantly lower risk of perioperative complications and a lower risk of reoperation and subsequent hospitalization or endoscopy; however, RYGB is associated with greater weight loss and higher rates of/more frequent and durable improvements in type 2 diabetes. Despite the growing evidence base, there remains a lack of consensus in the medical community about the clinical utility of these two procedures, 17 leading to significant variation in use. 18 , 19 , 20 This variation in care is likely driven by a variety of factors, including insurance coverage, surgeon procedure preferences, and skills (e.g., not all surgeons have been trained or feel comfortable with all procedures). 21 , 22 , 23 , 24 It is important for surgeons to have a balanced discussion of the risks and benefits of all available options, not just the ones for which they have preference, expertise, or special training.
Situations in which a patient considers whether to undergo bariatric surgery, and/or which bariatric procedure to undergo may be particularly well‐suited for a SDM approach, which can support discussions when multiple reasonable treatment options may be appropriate for a given patient. 25 With SDM, clinician and patient work together, sharing information about the relevant harms and benefits of the different options, considering how patient values, goals, and preferences could influence the decision, and providing collaborative support. 26 , 27 Limited data show that, while SDM is a preferred approach to clinical care, it can be difficult to achieve in practice. 28
To better understand the current state of SDM around bariatric surgery in clinical practice, and how the perception of SDM may vary across the timeframe from first considering surgery to the immediate preoperative period, a survey assessed patient's perceptions of their conversations with providers at different stages of the bariatric decision making process.
2. METHODS
2.1. Setting
Our survey of patients' experiences with SDM was conducted in coordination with surgical clinics affiliated with two US healthcare systems: Kaiser Permanente Washington (KPWA) and UPMC. The KPWA system is an integrated health insurance and care delivery system offering bariatric surgery in Western Washington. UPMC is a large academic health care system in the mid‐Atlantic region, which includes fee‐for‐service care along with integrated care via the UPMC Insurance Services Division. The study team partnered with the UPMC Comprehensive Weight Management Center at Magee‐Womens Hospital, which offers behavioral and medical weight management approaches in addition to being the largest volume bariatric clinic of UPMC. Neither KPWA nor UPMC had undertaken any formal efforts to implement an SDM approach for bariatric surgery in the past, which can be a barrier to providing high‐quality care for patients with obesity.
The study sought to collect survey data on patients' experience of SDM by using validated measures prior to the implementation of any formal SDM approach. Therefore, this survey took place prior to the launch (i.e., at baseline) of a quality improvement SDM program implemented within the health systems that integrates a decision aid into routine care to improve fidelity of SDM around bariatric surgery in primary care and bariatric clinic settings as well as patients treated by population health diabetes nurses.
2.2. Patient recruitment
Patient recruitment and surveying followed procedures approved by the KPWA Institutional Review Board that have been successfully applied in prior studies. 29 , 30 , 31 , 32 , 33 The goal was to use mixed modes (email, mail, phone, and tablet) to collect patient‐reported assessments of their experience with SDM from 150 patients prior to the implementation of a formal SDM approach; thus, the recruitment target was 75 patients each from KPWA and UPMC. After a year of implementation, a survey of another 150 patients will allow for a systematic evaluation of the change in fidelity of SDM in response to the intervention.
At both sites, patients were identified at three key time points in the bariatric surgery decision making process: (1) after self‐ or provider‐referral to the bariatric/weight management program, (2) after their initial visit with the bariatric/weight management program, and (3) after their pre‐operative visit with a bariatric surgeon (i.e., the last decision making step prior to surgery).
| Survey cohort | KPWA definition | UPMC definition |
|---|---|---|
| Cohort 1: Referrals a | Patients who have been referred to the bariatric surgery program for their first (not repeat/revision) bariatric procedure | Patients who have scheduled an appointment directly with the weight loss/bariatric surgery program, whether via provider referral or self‐referral. Excludes those with prior bariatric procedure(s) |
| Cohort 2: Initial visit | Patients who have completed their initial visit with the bariatric surgery program | Patients who have completed their initial visit with the weight loss/bariatric surgery program |
| Cohort 3: Pre‐operative visit | Patients who have completed their final pre‐operative visit with the bariatric surgeon | Patients who have completed their final pre‐operative visit with the bariatric surgeon |
Abbreviation: KPWA, Kaiser Permanente Washington.
KPWA requires a referral to the bariatric clinic. Referrals are not necessary at UPMC.
At KPWA, potential participants at each of the above time points were identified from the electronic health record databases and contacted initially by mail, and invited to complete a brief, 10‐min survey online or by phone. Patients who did not complete the online survey were contacted by phone. Research personnel made a maximum of five phone contacts before abandoning any case.
At UPMC, potential participants at each of the above time points were identified in several ways. A UPMC programmer identified potentially eligible patients using electronic health record data. This information was supplemented by patient lists from the bariatric surgery clinic that were generated for the study team from the coordinator in the bariatric/weight management clinic. Research personnel reached out to these individuals by phone or email or in‐person during clinic visits and invited them to complete the brief study surveys.
The online survey used Research Electronic Data Capture tools hosted at KPWA and UPMC. 34 , 35 At both KPWA and UPMC, those who completed the survey received $10 as a thank you for their time.
2.3. Survey measures
Validated, patient‐reported measures of SDM were used. Cohort 1 (C1) participants were asked to reflect on their referring provider or, if self‐referred, the provider who was most helpful in making their decision to seek care at the bariatric clinic. Cohorts 2 and 3 participants were asked to reflect on the provider they saw at their recent bariatric clinic visit. The primary outcome was assessed using the CollaboRATE tool, 36 , 37 , 38 , 39 , 40 which is a brief 3‐item process measure to assess patients' perceptions of being well‐informed and involved in decision making. The (minimally adapted) CollaboRATE items included three questions about interactions with health care providers: (1) How much effort did they make to help you understand your health issues?; (2) How much effort did they make to listen to the things that matter most to you about your health issues?; and (3) How much effort did they make to include what matters most to you in choosing what to do next? Each item was scored on a scale of 0 (“no effort was made”) to 9 (“every effort was made”). A “top score” approach to scoring was used for each survey respondent, where they were scored as “1,” if the response to all three CollaboRATE items was 9, or “0” if the response to any of the three CollaboRATE items was less than 9. The percentage of all encounters that were coded as “1” was then calculated. Higher scores represent more SDM.
Secondary measures included the SDM‐Q‐9 41 , 42 , 43 and National Quality Forum (NQF) SDM Process Survey process measures, 44 which capture patients' perceptions of other aspects of SDM, including describing options, risks and benefits. The SDM‐Q‐9 instrument consists of nine statements, rated on a six‐point scale from “completely disagree” (0) to “completely agree” (5). When required, up to two missing items were imputed using the mean of the items that were filled out to calculate the raw score. The raw score was transformed to range from 0 to 100, where 0 indicates the lowest possible level of SDM and 100 indicates the highest extent of SDM. 43
The NQF SDM Process Survey includes four items. Two items use a 4‐point scale (“A lot” “Some” “A little” “None at all”), including “how much did your healthcare provider talk with you about the reasons you might want to have bariatric surgery?” and “how much did your healthcare provider talk with you about reasons you might not want to have bariatric surgery?” Two additional items collect “Yes” or “No” responses to, “Did your healthcare provider ask you if you wanted to have bariatric surgery?” and “Did your health care provider explain that you could choose whether or not to have bariatric surgery?” To score the NQF, respondents were given one point each for answering “a lot” or “some” to questions 1 and 2 and one point each for answering yes to questions 3 and 4. The total score ranged from 0 to 4, and a higher score is indicative of better SDM. 45
2.4. Covariates
Additional information was extracted from the electronic health record databases on each participant who completed the survey at KPWA and UPMC, including age, gender, race/ethnicity, baseline body mass index (BMI; the nearest measure to the survey date), and the presence or absence of obesity‐associated comorbidities using International Classification of Diseases (ICD)‐10 diagnosis codes for the following: atrial fibrillation and other arrhythmias, congestive heart failure, coagulopathy, deficiency anemias, diabetes (complicated or uncomplicated), dyslipidemia, fatty liver disease, gastroesophageal reflux disease, hiatal hernia, hypertension, liver disease, peripheral vascular disease, renal failure, and venous thromboembolism.
2.5. Statistical analysis
Patient survey and electronic health record data were summarized and assessed for validity and quality of capture. Logistic and linear regression were used to assess the significance (Chi‐square or t‐tests) of differences in binary and continuous SDM measures, respectively, across cohorts and sub‐groups. Data issues were reviewed and adjudicated by the scientific team. Information is presented both overall and by cohort. Responders were compared to nonresponders.
Consistent with prior work, 37 and the developer's recommendation, 46 the CollaboRATE score was summarized as the number and percentage of individuals indicating a top score on all three questions. The SDM‐Q‐9 and the NQF SDM Process Surveys are summarized by the mean and standard deviation. All three measures are presented both overall and by cohort. In a similar manner, the SDM measures are summarized for subgroups defined by sex (male, female), age (18–44, 45–64, 65+) and race (other, Hispanic, non‐Hispanic Black, non‐Hispanic white).
A sample size of 150 was identified based on our goal of detecting changes in CollaboRATE over one year, after implementing a quality improvement SDM program.
The Kaiser Permanente Institutional Review Board provided oversight of human research protections for this study (protocol # 1495060).
3. RESULTS
KPWA conducted its survey from 25 November 2020 through 15 June 2021, and UPMC conducted its survey from 13 October 2021 to 23 May 2022. Response rates varied by site (KPWA 23%–39%; UPMC 16%–64%), and the demographics of respondents and non‐respondents were quite similar (see Supporting Information).
The sample included 167 participants, approximately half from each site; 35% were from C1, 33% from Cohort 2 (C2)%, and 32% from Cohort 3 (C3) (Table 1). Participants were, on average 43.8 years old (SD 13.5). Most were young (57% under age 45), female (81%), and white (73%). Average BMI was severely obese [48 (SD 8.6) kg/m2), with 19% having a BMI above 55 kg/m2. Among the weight‐related comorbidities examined, the most common were dyslipidemia (37%), hypertension (29%), uncomplicated diabetes (28%), gastroesophageal reflux disease (27%), fatty liver disease (16%), and complicated diabetes (15%). Overall, the three cohorts were quite similar. Furthermore, survey responders were similar to non‐respondents (see Online Supporting Information).
TABLE 1.
Demographic and clinical characteristics of survey participants, overall and by cohort.
| Overall | Cohort 1: Referrals | Cohort 2: Initial visit | Cohort 3: Pre‐op visit | |
|---|---|---|---|---|
| Site [N (%)] | ||||
| KPWA | 83 (49.7) | 28 (48.3) | 29 (52.7) | 26 (48.2) |
| UPMC | 84 (50.3) | 30 (51.7) | 26 (47.3) | 28 (51.9) |
| Cohort [N (%)] | ||||
| 1—Referrals | 58 (34.7) | 58 (100) | ||
| 2—Initial visit | 55 (32.9) | 55 (100) | ||
| 3—Pre‐operative visit | 54 (32.3) | 54 (100) | ||
| Age [mean (SD)] | 43.8 (13.5) | 43.9 (13.8) | 45.3 (13.2) | 42.2 (13.5) |
| Age category, years [N (%)] | ||||
| 18–44 | 95 (56.9) | 33 (56.9) | 31 (56.4) | 31 (57.4) |
| 45–64 | 53 (31.7) | 16 (27.6) | 19 (34.6) | 18 (33.3) |
| 65+ | 19 (11.4) | 9 (15.5) | 5 (9.1) | 5 (9.3) |
| Gender [N (%)] | ||||
| Female | 136 (81.4) | 42 (72.4) | 50 (90.9) | 44 (81.5) |
| Male | 31 (18.6) | 16 (27.6) | 5 (9.1) | 10 (18.5) |
| Race [N (%)] | ||||
| American Indian/Alaskan native | 1 (0.6) | 0 (0) | 0 (0) | 1 (1.9) |
| Asian | 3 (1.8) | 2 (3.5) | 0 (0) | 1 (1.9) |
| Black or African American | 28 (16.8) | 10 (17.2) | 11 (20.0) | 7 (13.0) |
| Multiple race | 5 (3.0) | 2 (3.5) | 1 (1.8) | 2 (3.7) |
| Other race | 3 (1.8) | 1 (1.7) | 1 (1.8) | 1 (1.9) |
| Unknown/declined | 6 (3.6) | 3 (5.2) | 1 (1.8) | 2 (3.7) |
| White | 121 (72.5) | 40 (69.0) | 41 (74.6) | 40 (74.1) |
| Hispanic ethnicity [N (%)] | ||||
| Unknown/Declined | 9 (5.4) | 3 (5.2) | 5 (9.1) | 1 (1.9) |
| Yes, Hispanic ethnicity | 11 (6.6) | 3 (5.2) | 3 (5.5) | 5 (9.3) |
| BMI (kg/m2) [mean (SD)] | 48 (8.63) | 46 (8.0) | 48 (8.9) | 49 (9.0) |
| BMI (kg/m2) [N (%)] | ||||
| 35 to <40 | 77 (46.1) | 29 (50.0) | 28 (50.9) | 20 (37.0) |
| 40 to <45 | 58 (34.7) | 23 (39.7) | 16 (29.1) | 19 (35.2) |
| 55+ | 32 (19.2) | 6 (10.3) | 11 (20.0) | 15 (27.8) |
| Comorbidities [N (%)] | ||||
| Atrial fibrillation | 5 (3.0) | 1 (1.7) | 1 (1.8) | 3 (5. 6) |
| Arrhythmias | 22 (13.2) | 7 (12.1) | 8 (14.6) | 7 (13.0) |
| Congestive heart failure | 12 (7.19) | 5 (8.6) | 3 (5.5) | 4 (7.4) |
| Coagulopathy | 1 (0.6) | 0 (0.0) | 1 (1.8) | 0 (0.0) |
| Deficiency anemia | 22 (13.2) | 7 (12.1) | 10 (18.2) | 5 (9.3) |
| Diabetes: Complicated | 25 (15.0) | 8 (13.8) | 7 (12.7) | 10 (18.5) |
| Diabetes: Uncomplicated | 47 (28.1) | 15 (25.9) | 15 (27.3) | 17 (31.5) |
| Dyslipidemia | 61 (36.5) | 21 (36.2) | 18 (32.7) | 22 (40.7) |
| Fatty liver | 26 (15.6) | 6 (10.3) | 12 (21.8) | 8 (14.8) |
| Gastroesophageal reflux disease | 45 (27.0) | 17 (29.3) | 11 (20.0) | 17 (31.5) |
| Hiatal hernia | 14 (8.4) | 2 (3.5) | 4 (7.3) | 8 (14.8) |
| Hypertension | 48 (28.7) | 15 (25.9) | 17 (30.9) | 16 (29.6) |
| Liver disease | 22 (13.2) | 5 (8.6) | 12 (21.8) | 5 (9.3) |
| Peripheral vascular disease | 4 (2.4) | 1 (1.7) | 2 (3.6) | 1 (1.9) |
| Renal failure | 5 (3.0) | 2 (3.5) | 2 (3.6) | 1 (1.9) |
| Venous thromboembolism | 3 (1.8) | 0 (0) | 2 (3.6) | 1 (1.9) |
Abbreviation: KPWA, Kaiser Permanente Washington.
On the CollaboRATE measure, 62% of the overall sample reported the highest possible scores for being informed and involved in decision making (Figure 1). Point estimates suggest that the perception of SDM was higher at each consecutive stage of the surgical preparatory process with 54% of C1, 60% of C2% and 72% of C3 reporting top scores, but the difference was not statistically significant (p = 0.1563). Average SDM‐Q‐9 scores also indicate quite high levels of other aspects of SDM [79.6 (SD 22.5) out of 100]. Like the CollaboRATE data, SDM‐Q‐9 scores were lowest for C1 and highest for C3. NQF survey data indicated moderately high levels of SDM, on average, 3.1 (SD 1.1) out of 4.0, with an increase from C1 to Cohorts 2 or 3. Both the SDM‐Q‐9 (p < 0.001) and NQF measures varied significantly across the three time points (p = 0.0069).
FIGURE 1.
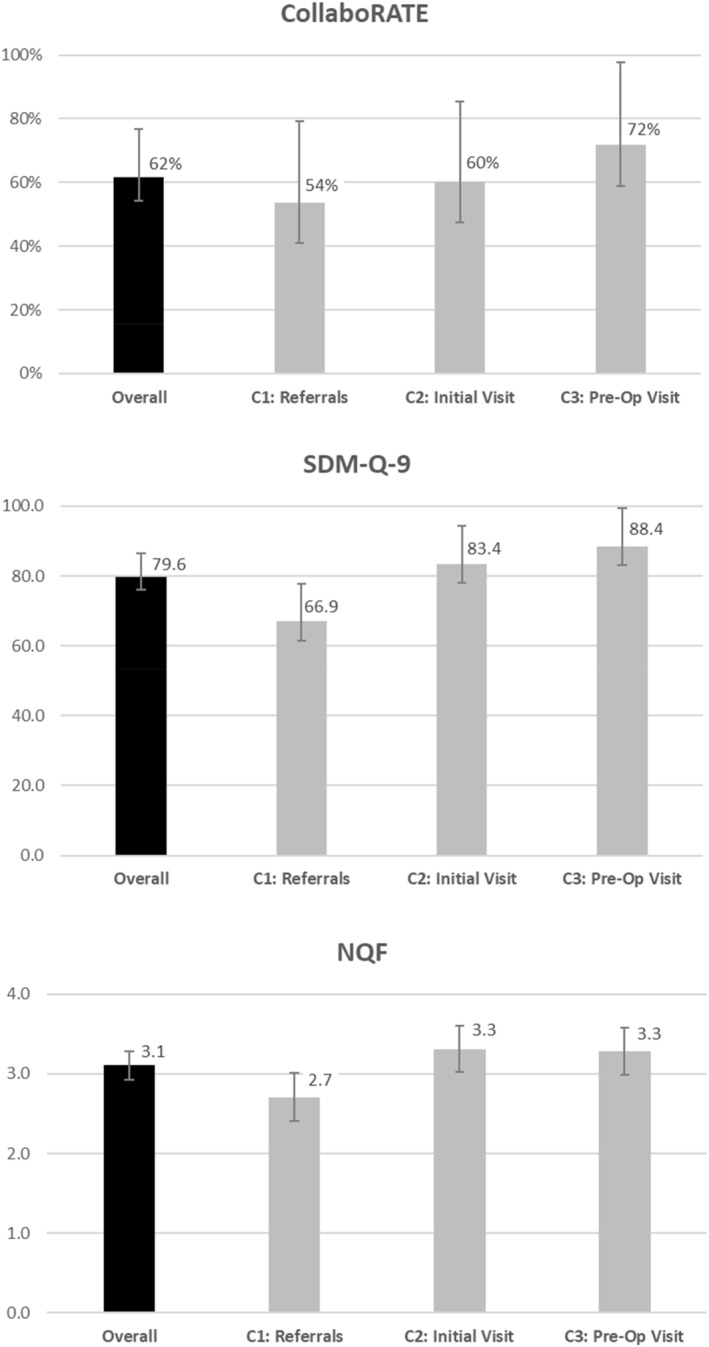
Patient‐reported experience with shared decision making, overall and by cohort.
Subgroup analyses showed no significant differences in the three SDM measures based on sex, age, race, or site (Table 2). A slightly higher proportion of women reported top scores for the CollaboRATE measure (64% vs. 50%). Point estimates for all three measures were lowest among older adults (age 65+) versus other age groups and point estimates for the CollaboRATE scores were highest for black participants.
TABLE 2.
Subgroup comparisons of patient reported experience with shared decision making.
| CollaboRATE a | SDM‐Q‐9 | NQF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Mn | SD | Mn | SD | ||||
| Sex | |||||||||
| Female | 86 | 64 | 0.1637 | 79.7 | 22.8 | 0.9402 | 3.1 | 1.2 | 0.8690 |
| Male | 14 | 50 | 79.3 | 21.1 | 3.1 | 1.0 | |||
| Age | |||||||||
| <45 | 55 | 61 | 0.5806 | 80.9 | 22.5 | 0.3307 | 3.0 | 1.2 | 0.1541 |
| 45–64 | 35 | 66 | 80.0 | 21.3 | 3.4 | 0.9 | |||
| 65+ | 10 | 53 | 72.6 | 25.1 | 2.9 | 1.2 | |||
| Race | |||||||||
| Other Non‐Hispanic race | 7 | 58 | 0.9021 | 70.2 | 34.1 | 0.3376 | 3.2 | 0.9 | 0.9127 |
| Hispanic ethnicity | 7 | 64 | 87.5 | 22.0 | 3.2 | 1.3 | |||
| Non‐Hispanic Black | 17 | 71 | 83.0 | 17.4 | 3.3 | 1.0 | |||
| Non‐Hispanic White | 66 | 60 | 79.4 | 22.1 | 3.1 | 1.2 | |||
| Unrecorded | 3 | 60 | 73.3 | 15.9 | 3.0 | 1.7 | |||
| Site | |||||||||
| KPWA | 48 | 58 | 0.2962 | 78.5 | 21.5 | 0.5031 | 3.0 | 1.2 | 0.5048 |
| UPMC | 52 | 66 | 80.8 | 23.5 | 3.2 | 1.1 | |||
Abbreviations: KPWA, Kaiser Permanente Washington; NQF, National Quality Forum; SDM, shared decision making.
Number of survey respondents that gave their provider the top score on CollaboRATE.
The frequency of respondents reporting a top score for each CollaboRATE item was quite consistent across the three individual items (Table 3). A similar pattern was seen for the SDM‐Q‐9 scores. Within the NQF responses, the item addressing frequency of conversation with providers about reasons for not wanting to have surgery (i.e., “a lot” or “some”) showed a somewhat higher raw score point estimate compared with the other three items.
TABLE 3.
Item‐level response for the overall survey population, N = 167.
| CollaboRATE Top Score, N (%) | N | % |
|---|---|---|
| How much effort did they make to help you understand your health issues? | 113 | 69% |
| How much effort did they make to listen to the things that matter most to you about your health issues? | 115 | 71% |
| How much effort did they make to include what matters most to you in choosing what to do next? | 112 | 69% |
| SDM‐Q‐9 | Mean | SD |
|---|---|---|
| My provider made clear that a decision needs to be made | 3.92 | 1.36 |
| My provider wanted to know exactly how I want to be involved in making the decision | 3.93 | 1.31 |
| My provider told me that there are different options for treating my medical condition | 4.03 | 1.26 |
| My provider precisely explained the advantages and disadvantages of the treatment options | 4.10 | 1.21 |
| My provider helped me understand all of the information | 4.19 | 1.09 |
| My provider asked me which treatment option I prefer | 4.21 | 1.18 |
| My provider and I thoroughly weighed the different treatment options | 3.92 | 1.36 |
| My provider and I selected a treatment option together | 3.85 | 1.50 |
| My provider and I reached an agreement on how to proceed | 4.13 | 1.27 |
| NQF | Mean | (SD) |
|---|---|---|
| How much did your healthcare provider talk with you about the reasons you might want to have bariatric surgery? | 1.63 | 0.89 |
| How much did your healthcare provider talk with you about reasons you might not want to have bariatric surgery | 2.37 | 1.12 |
| Did your healthcare provider ask if you wanted to have bariatric surgery? | 1.25 | 0.43 |
| Did your healthcare provider explain that you could choose whether or not to have bariatric surgery? | 1.10 | 0.30 |
Abbreviations: NQF, National Quality Forum; SDM, shared decision making.
4. DISCUSSION
In this sample of patients at two large health systems, patients perceived moderate or high levels of SDM when assessed at the time of referral/self‐referral, after initial evaluation, and following a pre‐operative visit. In general, levels of SDM were higher in patients who had moved further through the preparatory process leading up to bariatric surgery. While sub‐group analyses were limited by sample size, they did not identify groups with clearly divergent patterns of experience. Likewise, there was limited heterogeneity across individual items in the measures.
Based on the CollaboRATE data, approximately 75% reported the highest possible level of feeling informed and involved in decision making at the final assessment (C3)—consistent with levels that have followed SDM interventions. 39 At the same time point, the average SDM‐Q9 score was 88.4 (SD 15.9) out of a possible 100, indicating that patients endorsed having experienced communication with the provider that included key SDM components such as discussion of various treatment options, risks and benefits, and that s/he and the physician jointly selected the treatment option. Scores on the NQF assessing patient preferences and awareness of choices were relatively lower at the C3 time point [mean 3.3 (SD 1.0) out of 4.0]. For both the SDM‐Q9 and NQF, the variability in responses narrowed quite a bit across cohorts.
Shared decision‐making has been proposed as a strategy for improving communication between patients and providers and for countering weight bias—two factors that are likely contributors to the underutilization of bariatric surgery. 47 Weight bias is common and has concerning workplace and health implications, 48 , 49 and negative stances are evident in attitudes toward surgical weight loss treatment. 50 Healthcare providers may be unlikely to discuss surgical weight loss options, 47 reflecting an emphasis on surgical risks, despite strong efficacy and safety data. 16 , 51 , 52 The high levels of SDM seen in this study do not ameliorate the need to address these concerns. Furthermore, provider communication challenges related to bariatric surgery have typically been documented in primary care settings; our assessment at sites providing surgical services increases the likelihood that providers are comfortable communicating risks and benefits related to bariatric procedures.
Our documentation of CollaboRATE levels that are similar to prior findings following SDM interventions may reflect particularly high‐quality care in these two large academic medical centers or signify growing interest in SDM in surgical specialties, which is suggested by recent literature in this area. 53 , 54 Alternatively, the timing of the survey shortly after the acute phase of the COVID‐19 pandemic may reflect higher levels of satisfaction than are typical—for example, if some survey participants were resuming surgical plans that had experienced a pandemic‐related delay. Further research is needed to distinguish between these possibilities.
The current analyses are limited to establishing a baseline level of SDM in two clinical settings using validated tools; no data were collected on the contents of those conversations. While Roux‐en‐Y gastric bypass and sleeve are commonly used at both sites, UPMC offers a broader range of procedures, and procedure choice varied somewhat by site. For example, the proportion of bariatric operations using sleeve gastrectromy and the proportion using Roux‐en‐Y gastric bypass during the timeframe of this survey were 48% and 52%, respectively, at Kaiser Permanent and 41% and 51% at UPMC. There is no “right” procedure choice, and the documented high levels of SDM suggest that decisions have been well‐informed; however, it is possible that the implementation of decision‐aids designed to support balanced discussion of key risks and benefits, could shift patient choices and thus alter the case mix of procedures.
The SDM measures did not differ significantly across the two sites, despite differences in factors such as geographic distribution, payer structure, and procedures offered. While the order in which topics are typically discussed varies between the two sites for patients considering bariatric surgery, both sites address a similar set of topics, which may be reflected in the similar SDM metrics. At KPWA, initial visits are group‐based and focus on general education about bariatric surgery outcomes and procedure types, and expectation‐setting around the process of surgery, including preparation and follow‐up. UPMC initial visits are typical individualized meetings with a non‐surgical provider, and focus on assessment of the patient's surgical eligibility review of his/her health history, provision of education about the process of bariatric surgery, and discussion of the commitment needed for reaching the patient's weight and health goals. The first provider visit at KPWA addresses patient goals in terms of weight loss and health, while at UPMC the focus is often on the pre‐operative process, surgical risks and benefits. At both sites, the pre‐operative visit focuses on answering questions, addressing informed consent, and confirming the patient's decision for bariatric surgery.
It is important to note that this is not a longitudinal study, but instead assesses three cross‐sectional samples, and variation between cohorts 1–3 not only reflect their different positions in the process of considering and/or planning for bariatric surgery, but also any secular trends that occurred between the time that C3 and C1 first accessed the bariatric clinics. It is possible that higher metrics of SDM among C3 reflect experiences that occurred during their appointments at the bariatric clinics. It is also possible that patients with sub‐optimal clinical interactions (and thus lower SDM metrics) who might have been eligible for C3 were more likely to seek alternative treatment options/venues, so their lower scores may not be well represented. While the C1 survey assesses decision making experiences prior to seeking surgical weight loss services, the sample only includes those who have been referred or self‐referred to these clinics. The perspectives of other patients with severe obesity who had not been referred or self‐referred are omitted, so inference about their experiences with SDM related to severe obesity treatment is not possible. Furthermore, this convenience sample enrolled a minority of patients who were seen in the bariatric clinics during this time, reflecting both patient interest and the fact that clinical staff were not able to approach every patient. The limited sample size makes subgroup analyses difficult to interpret. It is also useful to recall that a provider referral was required to be seen at the Kaiser Permanente surgical clinic while UPMC patients were primarily self‐referred, which may have impacted conversations about weight treatment decisions. Furthermore, some UPMC patients, particularly in C1, may have sought out the bariatric practice specifically for the lifestyle and/or medication services offered for weight management, rather than to seek information about bariatric surgery. Findings reflect two distinct models of bariatric care delivery, but may not generalize to other health systems.
In summary, in two large health systems, patients self‐reported moderate to high scores on three measures of SDM, particularly just before surgery. Relatively lower scores on the CollaboRATE and NQF measures, compared with the SDM‐Q9 suggest that decision making related to bariatric surgery could benefit from additional focus on patient preferences, assistance with awareness of treatment options for severe obesity, and emphasis on patient involvement in decision making. However, the scores suggest that intervention may face a ceiling effect. Future research by this team will assess whether implementation of a more formal SDM process around bariatric surgery can lead to further improvements in patients' perceptions of their SDM experience.
CONFLICT OF INTEREST STATEMENT
Dr. Courcoulas reported receiving grants from Allurion Technologies outside the submitted work. Dr. Arterburn reported receiving a contract from Sharecare Inc outside the submitted work. Dr. McTigue has received research contracts from Pfizer and Eli Lilly for work related to obesity. Dr. Glyn Elwyn has been responsible for the development of Option Grid patient decision aids, now licensed to EBSCO Health, where he acts as a consultant. Dr Ahmed received grants from Allurion Technologies outside the submitted work. Diana L. Stilwell is employed by Healthwise, a not‐for‐profit developer of patient education solutions. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Supporting information
Table S1
ACKNOWLEDGMENTS
The views, statements and opinions presented in this editorial are solely the responsibility of the author (s) and do not necessarily represent the views of the Patient‐Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee. The funder did not participate in data collection or analyses. The authors are most grateful to the KPWA Survey Program and UPMC Bariatric team for their support of this work.
McTigue K, Courcoulas A, Wellman R, et al. Exploring patient perspectives on shared decision making about bariatric surgery in two healthcare systems. Obes Sci Pract. 2024;e779. 10.1002/osp4.779
REFERENCES
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsveille, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State‐level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381 (25):2440‐2450. 10.1056/nejmsa1909301 [DOI] [PubMed] [Google Scholar]
- 3. Arterburn DE, McDonell MB, Hedrick SC, Diehr P, Fihn SD. Association of body weight with condition‐specific quality of life in male veterans. Am J Med. 2004;117 (10):738‐746. 10.1016/j.amjmed.2004.06.031 [DOI] [PubMed] [Google Scholar]
- 4. McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296 (1):79‐86. 10.1001/jama.296.1.79 [DOI] [PubMed] [Google Scholar]
- 5. Sarkhosh K, Switzer NJ, El‐Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23 (3):414‐423. 10.1007/s11695-012-0862-2 [DOI] [PubMed] [Google Scholar]
- 6. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta‐analysis. Am J Med. 2009;122 (3):248‐256.e245. 10.1016/j.amjmed.2008.09.041 [DOI] [PubMed] [Google Scholar]
- 7. Maggard MA, Shugarman LR, Suttorp M, et al. Meta‐analysis: surgical treatment of obesity. Ann Intern Med. 2005;142 (7):547‐559. 10.7326/0003-4819-142-7-200504050-00013 [DOI] [PubMed] [Google Scholar]
- 8. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ. 2013;347 (oct22 1):f5934. 10.1136/bmj.f5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puzziferri N, Roshek TB, 3rd , Mayo HG, Gallagher R, Belle SH, Livingston EH. Long‐term follow‐up after bariatric surgery: a systematic review. JAMA. 2014;312 (9):934‐942. 10.1001/jama.2014.10706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324 (9):879‐887. 10.1001/jama.2020.12567 [DOI] [PubMed] [Google Scholar]
- 11. Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg. 2020;271 (2):201‐209. 10.1097/sla.0000000000003554 [DOI] [PubMed] [Google Scholar]
- 12. Musbahi A, Brown LR, Reddy A, Viswanath YKS, Rao M, Gopinath BR. Systematic review of online patient resources to support shared decision making for bariatric surgery. Int J Surg. 2020;74:34‐38. 10.1016/j.ijsu.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 13. Still CD. Creating bariatric surgery advocates: why it is critical to educate primary care physicians. In: Bariatric Times; 2011. [Google Scholar]
- 14. Tork S, Meister KM, Uebele AL, et al. Factors influencing primary care physicians' referral for bariatric surgery. J Soc Laparoendosc Surg. 2015;19 (3):e2015. 10.4293/jsls.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stolberg CR, Hepp N, Juhl AJA, B CD, Juhl CB. Primary care physician decision making regarding referral for bariatric surgery: a national survey. Surg Obes Relat Dis. 2017;13 (5):807‐813. 10.1016/j.soard.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 16. Conaty EA, Denham W, Haggerty SP, Linn JG, Joehl RJ, Ujiki MB. Primary care physicians' perceptions of bariatric surgery and major barriers to referral. Obes Surg. 2020;30 (2):521‐526. 10.1007/s11695-019-04204-9 [DOI] [PubMed] [Google Scholar]
- 17. Smetana GW, Jones DB, Wee CC. Should this patient have weight loss surgery? Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. 2017;166 (11):808‐817. 10.7326/m17-0698 [DOI] [PubMed] [Google Scholar]
- 18. Toh S, Rasmussen‐Torvik LJ, Harmata EE, et al. The national patient‐centered clinical research network (PCORnet) bariatric study cohort: rationale, methods, and baseline Characteristics. JMIR Res Protoc. 2017;6 (12):e222. 10.2196/resprot.8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reames BN, Birkmeyer NJ, Dimick JB, et al. Variation in the Care of Surgical Conditions: Obesity. The Dartmouth Institute for Health Policy and Clinical Practice; 2014. [PubMed] [Google Scholar]
- 20. Macht R, Rosen A, Horn G, Carmine B, Hess D. An exploration of system‐level factors and the geographic variation in bariatric surgery utilization. Obes Surg. 2016;26 (7):1635‐1638. 10.1007/s11695-016-2164-6 [DOI] [PubMed] [Google Scholar]
- 21. Weinstein AL, Marascalchi BJ, Spiegel MA, Saunders JK, Fagerlin A, Parikh M. Patient preferences and bariatric surgery procedure selection; the need for shared decision‐making. Obes Surg. 2014;24 (11):1933‐1939. 10.1007/s11695-014-1270-6 [DOI] [PubMed] [Google Scholar]
- 22. Courcoulas A, Coley RY, Clark JM, et al. Interventions and operations 5 Years after bariatric surgery in a cohort from the US national patient‐centered clinical research network bariatric study. JAMA Surg. 2020;155 (3):194‐204. 10.1001/jamasurg.2019.5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arterburn D, Wellman R, Emiliano A, et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. 2018;169 (11):741‐750. 10.7326/m17-2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McTigue KM, Wellman R, Nauman E, et al. Comparing the 5‐year diabetes outcomes of sleeve gastrectomy and gastric bypass: the national patient‐centered clinical research network (PCORNet) bariatric study. JAMA Surg. 2020;155 (5):e200087. 10.1001/jamasurg.2020.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140 (1):54‐59. 10.7326/0003-4819-140-1-200401060-00012 [DOI] [PubMed] [Google Scholar]
- 26. Armstrong KA, Metlay JP. Annals clinical decision making: communicating risk and engaging patients in shared decision making. Ann Intern Med. 2020;172 (10):688‐692. 10.7326/m19-3495 [DOI] [PubMed] [Google Scholar]
- 27. Elwyn G, Cochran N, Pignone M. Shared decision making‐the importance of diagnosing preferences. JAMA Intern Med. 2017;177 (9):1239‐1240. 10.1001/jamainternmed.2017.1923 [DOI] [PubMed] [Google Scholar]
- 28. Gillick MR. Re‐engineering shared decision‐making. J Med Ethics. 2015;41 (9):785‐788. 10.1136/medethics-2014-102618 [DOI] [PubMed] [Google Scholar]
- 29. Arterburn D, Flum DR, Westbrook EO, et al. A population‐based, shared decision‐making approach to recruit for a randomized trial of bariatric surgery versus lifestyle for type 2 diabetes. Surg Obes Relat Dis. 2013;9 (6):837‐844. 10.1016/j.soard.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arterburn D, Westbrook EO, Terrell A. Weight control practices of severely obese patients who are not seeking bariatric surgery. Obesity. 2013;21 (8):1509‐1513. 10.1002/oby.20488 [DOI] [PubMed] [Google Scholar]
- 31. Arterburn DE, Westbrook EO, Bogart TA, Sepucha KR, Bock SN, Weppner WG. Randomized trial of a video‐based patient decision aid for bariatric surgery. Obesity. 2011;19 (8):1669‐1675. 10.1038/oby.2011.65 [DOI] [PubMed] [Google Scholar]
- 32. Coleman KJ, Brookey J. Gender and racial/ethnic background predict weight loss after roux‐en‐Y gastric bypass independent of health and lifestyle behaviors. Obes Surg. 2014;24 (10):1729‐1736. 10.1007/s11695-014-1268-0 [DOI] [PubMed] [Google Scholar]
- 33. Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310 (22):2416‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42 (2):377‐381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient‐reported measure of shared decision making in clinical encounters. Patient Educ Counsel. 2013;93 (1):102‐107. 10.1016/j.pec.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 37. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient‐reported measure of the shared decision‐making process. J Med Internet Res. 2014;16 (1):e2. 10.2196/jmir.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barr PJ, Forcino RC, Thompson R, et al. Evaluating CollaboRATE in a clinical setting: analysis of mode effects on scores, response rates and costs of data collection. BMJ Open. 2017;7 (3):e014681. 10.1136/bmjopen-2016-014681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tai‐Seale M, Elwyn G, Wilson CJ, et al. Enhancing shared decision making through carefully designed interventions that target patient and provider behavior. Health Aff. 2016;35 (4):605‐612. 10.1377/hlthaff.2015.1398 [DOI] [PubMed] [Google Scholar]
- 40. Forcino RC, Barr PJ, O'Malley AJ, et al. Using CollaboRATE, a brief patient‐reported measure of shared decision making: results from three clinical settings in the United States. Health Expect. 2018;21 (1):82‐89. 10.1111/hex.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ballesteros J, Moral E, Brieva L, Ruiz‐Beato E, Prefasi D, Maurino J. Psychometric properties of the SDM‐Q‐9 questionnaire for shared decision‐making in multiple sclerosis: item response theory modelling and confirmatory factor analysis. Health Qual Life Outcome. 2017;15 (1):79. 10.1186/s12955-017-0656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doherr H, Christalle E, Kriston L, Harter M, Scholl I. Use of the 9‐item shared decision making questionnaire (SDM‐Q‐9 and SDM‐Q‐Doc) in intervention studies‐A systematic review. PLoS One. 2017;12 (3):e0173904. 10.1371/journal.pone.0173904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kriston L, Scholl I, Holzel L, Simon D, Loh A, Harter M. The 9‐item Shared Decision Making Questionnaire (SDM‐Q‐9). Development and psychometric properties in a primary care sample. Patient Educ Counsel. 2010;80 (1):94‐99. 10.1016/j.pec.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 44. Barry MJ, Edgman‐Levitan S, Sepucha K. Shared decision‐making: staying focused on the ultimate goal [serial on the Internet]. NEJM Catalyst; 2018. https://catalyst.nejm.org/shared‐decision‐making‐patient‐decision‐aids/ [Google Scholar]
- 45. National Quality Forum . #2962 Shared Decision Making Process: Measure Information; 2017. Accessed September, 25, 2023.
- 46. Elwyn G. Scoring CollaboRATE; 2024. Accessed March 21, 2024. http://www.glynelwyn.com/scoring‐collaborate.html
- 47. Sarwer DB, Gasoyan H, Bauerle Bass S, Spitzer JC, Soans R, Rubin DJ. Role of weight bias and patient‐physician communication in the underutilization of bariatric surgery. Surg Obes Relat Dis. 2021;17 (11):1926‐1932. 10.1016/j.soard.2021.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17 (5):941‐964. 10.1038/oby.2008.636 [DOI] [PubMed] [Google Scholar]
- 49. Phelan SM, Burgess DJ, Yeazel MW, Hellerstedt WL, Griffin JM, van Ryn M. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes Rev. 2015;16 (4):319‐326. 10.1111/obr.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dolan P, Afaneh C, Symer M, Dakin GF, Pomp A, Yeo H. Assessment of public attitudes toward weight loss surgery in the United States. JAMA Surg. 2019;154 (3):264‐266. 10.1001/jamasurg.2018.4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Funk LM, Jolles SA, Greenberg CC, et al. Primary care physician decision making regarding severe obesity treatment and bariatric surgery: a qualitative study. Surg Obes Relat Dis. 2016;12 (4):893‐901. 10.1016/j.soard.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stolberg CR, Hepp N, Juhl AJA, Deepti BC, Juhl CB. Primary care physician decision making regarding referral for bariatric surgery: a national survey. Surg Obes Relat Dis. 2017;13 (5):807‐813. 10.1016/j.soard.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 53. Niburski K, Guadagno E, Mohtashami S, Poenaru D. Shared decision making in surgery: a scoping review of the literature. Health Expect. 2020;23 (5):1241‐1249. 10.1111/hex.13105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shaw S, Hughes G, Stephens T, et al. Understanding decision making about major surgery: protocol for a qualitative study of shared decision making by high‐risk patients and their clinical teams. BMJ Open. 2020;10 (5):e033703. 10.1136/bmjopen-2019-033703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


