Abstract
Background:
Acute mesenteric venous thrombosis (MVT) is rarely suspected as primary diagnosis in emergency departments and still carries an in-hospital mortality rate of above 20%.
Objectives:
The aim of this study was to find differences in clinical and laboratory markers between patients with acute MVT and a control group of suspected but confirmed as not having any type of acute mesenteric ischaemia (AMI).
Design:
Data was retrieved from the AMESI (Acute MESenteric Ischaemia) study. This international, multicenter prospective case-control study from 32 sites collected data on patients with suspected AMI during a 10-month period.
Methods:
Independent factors associated with acute MVT were evaluated in a multivariable logistic regression analysis and expressed as odds ratios (OR) with 95% confidence intervals (CI).
Results:
D-dimer was not significantly higher in MVT (n = 73) compared to non-AMI (n = 287) patients (median 7.0 mg/L vs 4.5 mg/L, P = .092). After entering BMI, atherosclerotic disease, history of venous thromboembolism, CRP, and D-dimer as covariates in a multi-variable logistic regression analysis, absence of atherosclerotic disease (OR 0.096, 95% CI 0.011-0.84; P = .034) and elevated D-dimer (OR 2.59/one SD increment, 95% CI 1.07-6.28; P = .034) were associated with MVT. The discriminative ability of D-dimer for MVT as assessed by area under the curve in the receiver operating characteristics analysis was 0.63 (95% CI 0.49-0.78).
Conclusion:
Elevated D-dimer was associated with MVT, but the discriminative ability of D-dimer was poor. There is an urgent need to find a more accurate plasma biomarker for this condition.
TRIAL REGISTRATION:
NCT05218863 (registered 19.01.2022).
Keywords: Mesenteric venous thrombosis, diagnosis, suspected acute mesenteric ischaemia, D-dimer
Plain Language Summary:
Acute mesenteric vein thrombosis is rare, but life-threatening. The symptoms are abdominal pain and are not distinct from other acute abdominal conditions. A blood test identifying or leading to easier diagnosis is warranted. This multicenter international study has included patients with thrombosis in the mesenteric veins as well as suspected diseases, but not confirmed conditions, of disturbed circulation to the gastrointestinal tract. Elevation of the blood marker D-dimer was found to be associated with the condition of acute mesenteric vein thrombosis, but was not enough discriminative in relation to those patients with suspected disease only. There is an urgent need to find a more accurate blood marker for deep vein thrombosis of the abdomen.
Introduction
Acute mesenteric venous thrombosis (MVT) is very rarely 1 suspected as diagnosis based on clinical and laboratory data in emergency departments, and MVT still carries an in-hospital mortality of above 20% in some reports. 2 Studies on diagnostic performance of plasma biomarkers in acute MVT have not been available since studies on biomarkers often include all patients with acute mesenteric ischaemia (AMI), where most patients have acute superior mesenteric artery (SMA) occlusion. 3 Patients with acute MVT versus SMA occlusion have very different clinical presentations, risk factor profiles, disease severity and prognosis, 4 and it is therefore not appropriate to pool data on these 2 disease entities in clinical research.
The AMESI (Acute MESenteric Ischaemia) study is an international prospective multicentre trial. 5 This study includes patients with all subtypes of AMI as well as suspected but confirmed as non-AMI. The incidence, management, and outcome of AMI in the AMESI study has recently been outlined. 6
The aim of this study was to perform a prospective case-control study to evaluate clinical data and laboratory markers of MVT among patients with suspected AMI, for potential facilitation of patient selection for definitive diagnosis with computed tomography (CT) with intravenous contrast phase enhancement and imaging in the portal phase.
Methods
Study design
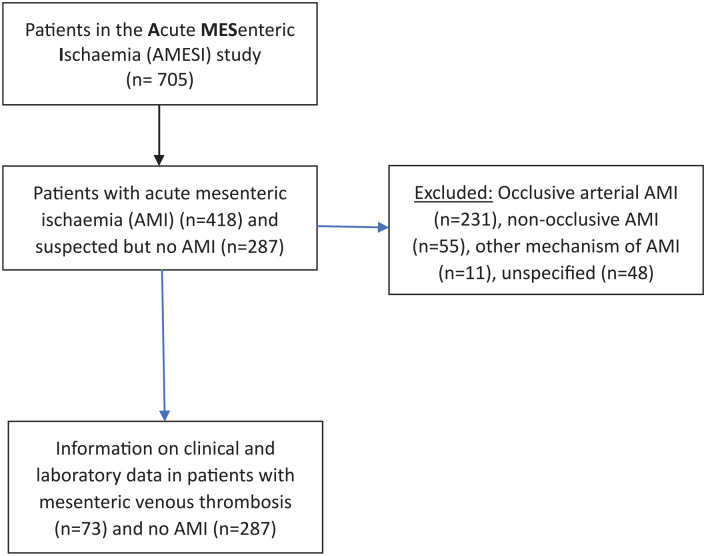
The study design has been outlined in detail. 6 The AMESI study is an international, multicenter, prospective study and included patients with all subtypes of AMI as well as suspected AMI eventually confirmed as non-AMI. If suspicion of AMI was not confirmed or mechanical bowel obstruction with local intestinal gangrene was the final diagnosis, only baseline data and hospital mortality was collected. The present sub-study is a post-hoc analysis of the AMESI study (Figure 1).
Figure 1.
Flow chart of included study patients.
Study sample
In brief, patients over 18 years of age with suspected or confirmed AMI were either admitted or transferred to 32 hospitals between 06-06-2022 and 05-04-2023. This sub-study complied with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for cohort studies (Supplemental Table S1).
Study objectives
To compare clinical and laboratory data between patients with confirmed MVT and control group of patients with suspected but confirmed not to have any subtype of AMI.
D-Dimer assays
The test principle, manufacturer, name of D-dimer assay and reference values for each respective site providing data for this sub-study is outlined in Table 1.
Table 1.
D-dimer assays in study sites including patients with acute mesenteric venous thrombosis.
| Site Nr | Site name | Country | Test principle | Manufacturer | Name of D-dimer assay | Reference values |
|---|---|---|---|---|---|---|
| 1 | Tartu Ülikooli Kliinikum | Estonia | Automated immunoassay (immunoturbidometry) | Diagnostica Stago | STA Liatest® D-Di Plus | <0.5 mg/L |
| 2 | Põhja-Eesti Regionaalhaigla | Estonia | Automated immunoassay (immunoturbidometry) | Diagnostica Stago | STA Liatest® D-Di Plus | <0.5 mg/L |
| 3 | City Hospital, Arkhangelsk, | Russia | Automated immunoassay (immunoturbidometry) | Siemens | Innovance® D-dimer | <0.45 mg/L |
| 6 | School of Medical Sciences & Hospital, University Sains Malaysia | Malaysia | Automated immunoassay (immunoturbidometry) | Diagnostica Stago | STA Liatest® D-Di Plus | <0.45 mg/L |
| 7 | Hospital Melaka | Malaysia | Site investigators did not reply | |||
| 10 | Luzerner Cantonsspital | Switzerland | Automated immunoassay (immunoturbidimetry) | Werfen | HemosIL® D-dimer HS 500 | <0.5 mg/L FEU |
| 11 | Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem | Israel | Automated immunoassay (immunoturbidimetry) | Siemens | Innovance® D-Dimer | <0.45mg/L |
| 12 | Universitätsklinikum Schleswig-Holstein, Campus Kiel | Germany | Automated immunoassay (immunoturbidimetry) | Werfen | HemosIL® D-dimer HS 500 | <0.5 mg/L |
| 15 | Virgen del Roc’o University Hospital, Seville | Spain | Automated immunoassay (immunoturbidimetry) | Werfen | HemosIL® D-Dimer HS 500 | <0.5 mg/L |
| 17 | Letterkenny University Hospital | Ireland | Automated immunoassay (immunoturbidimetry) | Siemens | Innovance® D-Dimer | <0.5 mg/L |
| 18 | Erciyes University Hospital | Turkey | Automated immunoassay (immunoturbidimetry) | Siemens | Innovance® D-Dimer | <0.5 mg/L |
| 19 | State University of Medicine and Pharmacy “Nicolae Testemitanu” | Moldova | Automated immunoassay Flourescence immunoassay | Boditech Med Inc (South Korea) | AFIAS D-Dimer test | ⩽0.5 mg/L |
| 24 | Fujian Hospital | China | Automated immunoassay | Long Island Biotech, Shanghai, (China) | Mindray ExC810 | ⩽0.55 mg/L |
| 31 | Azienda Ospedaliera Universitaria Careggi, Florence | Italy | Site investigators did not reply | |||
| 34 | Azienda Ospedaliero Universitaria Citta della Salute e della Scienza, Turin | Italy | Automated immunoassay (immunoturbidimetry) | Werfen | HemosIL® D-dimer HS 500 | <0.580 mg/l FEU |
| 36 | Royal Infirmary of Edinburgh | Scotland, United Kingdom | Automated immunoassay (immunoturbidimetry) | Siemens | Innovance® D-Dimer | ⩽0.25 mg/L |
| 37 | Beaujon Hospital, Paris | France | Automated immunoassay (immunoturbidimetry) | Diagnostica Stago | STA-Liatest® D-Di plus | <0.5 mg/L |
Abbreviation: FEU, fibrinogen equivalent units.
Definitions
Suspicion of AMI was raised by the local investigators in an adult patient with acute abdominal pain, or critically ill patients in the intensive care unit with increasing plasma lactate levels and suspicion of non-occlusive mesenteric ischaemia (NOMI). Confirmation of AMI was assured by CT scan, endoscopy, surgery, histology or autopsy. Patients with mechanical bowel obstruction comprised collection of baseline data and hospital survival. Disability was categorized as need of assistance (no/yes). Atherosclerotic disease was defined as previous ischaemic heart disease, stroke and/or peripheral arterial disease (carotid artery disease, lower extremity arterial disease). Charlson co-morbidity index 7 was calculated (mdcalc.com/calc/3917/charlson-comorbidity-index-cci). Bowel emptying 8 was defined as diarrhoea and/or vomiting.
Statistics
Categorical data was presented as number and proportions (%) and continuous data as medians with interquartile ranges (IQR). Variables associated with MVT compared to non-AMI patients in univariable analysis (P < .1), were candidates for inclusion in the multi-variable logistic regression analysis, expressed in odds ratios (ORs) with 95% confidence intervals (CI). Normality of data was assessed by the Kolmogorov-Smirnov test, and all tested continuous variables were log10 transformed due to skewed distribution, and converted to Z scores, before entering as covariates into a multivariable logistic regression model. Continuous data after multi-variable testing were expressed per one standard deviation (SD) increment. A maximum of one covariate per ten MVT events was allowed in the multi-variable model. The clinical prediction multivariable model included pre-specified candidate predictors based on clinical experience. No imputation of missing data was done. The number of positive and negative D-dimer tests, according to the respective manufacturers normal reference range, in the MVT and non-AMI group, were analysed in a 2 × 2 contingency table, yielding sensitivity and specificity of D-dimer for MVT with 95% CI. Receiver operating Characteristics (ROC) analysis was performed to obtain an area under the curve (AUC) as a measurement of D-dimer, white blood cell counts and CRPs ability to discriminate between MVT and non-AMI. Level of statistical significance was P < .05. IBM SPSS Statistics, version 28 (SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Comparison of baseline demographics and risk factors between patients with acute MVT and control group
The total number of patients with confirmed acute MVT was 73, and the total number of patients with suspected but confirmed as non-AMI was 287, of which 128 suffered mechanical bowel obstruction. Compared to the control group, patients with MVT were younger (P = .008), had higher BMI (P = .009), lower Charlson comorbidity index (P = .003), less hypertension (P < .001), less atherosclerotic disease (P < .001), less atrial fibrillation (P = .006), received less antiplatelet drugs (P = .014), and had more previous venous thromboembolic event (P < .001; Table 2).
Table 2.
Comparison of clinical background data between patients with acute MVT and confirmed non-AMI.
| Patient characteristics, comorbidity and medical therapy | Acute MVT (n = 73) | Confirmed non-AMI (n = 287) | P-value |
|---|---|---|---|
| Age, years (median, IQR) | 64 (48-74) | 69 (56-80) | .008 |
| Female gender (%) | 28 (38.4) | 138/284 (48.6) | .12 |
| Body Mass Index, kg/m2 (median, IQR) | 25.9 (23.4-31.5) (n = 56) | 24.7 (22.0-27.7) (n = 216) | .009 |
| Disability (%) | 5/69 (7.2) | 75/269 (27.9) | <.001 |
| Current smoking (%) | 17/66 (25.8) | 51/225 (22.7) | .60 |
| Hypertension (%) | 27 (37.0) | 169/280 (60.4) | <.001 |
| Atherosclerotic disease (%) | 9/72 (12.5) | 96/269 (35.7) | <.001 |
| Atrial fibrillation (%) | 6 (8.2) | 65 (22.6) | .006 |
| Myocardial infarction (%) | 3/69 (4.3) | 33/274 (12.0) | .062 |
| Previous venous thromboembolic event (%) | 13/71 (18.3) | 12/272 (4.4) | <.001 |
| Charlson comorbidity index (median, IQR) | 2 (1-4) (n = 64) | 4 (2-5) (n = 268) | .003 |
| Anticoagulant drugs (%) | 20/72 (27.8) | 56/270 (20.7) | .20 |
| Antiplatelet drugs (%) | 10/72 (13.9) | 76/271 (28.0) | .014 |
| Statins (%) | 15/72 (20.8) | 85/270 (31.5) | .078 |
Comparison of clinical data at admission between acute MVTs and control group
The median pre-hospital symptom duration was 32 hours for patients with MVT compared to 24 hours for the control group (P = .040), and frequency of acute abdominal pain was higher in the MVT group (P = .042; Table 3).
Table 3.
Comparison of clinical data at admission between patients with acute MVT and confirmed non-AMI.
| Symptoms | Acute MVT (n = 73) | Confirmed non-AMI (n = 287) | P-value |
|---|---|---|---|
| Pre-hospital symptom duration, hours (median; IQR) | 32 (13-72) (n = 56) | 24 (6-48) (n = 190) | .040 |
| Acute abdominal pain (%) | 69 (94.5) | 246 (85.7) | .042 |
| Diarrhoea (%) | 12 (16.4) | 30 (10.5) | .16 |
| Bloody stool (%) (macroscopic) | 5 (6.8) | 18 (6.3) | .86 |
| Vomiting (%) | 7 (9.6) | 18 (6.3) | .32 |
| Bowel emptying (%) | 17 (23.3) | 47 (16.4) | .17 |
| Shock (%) | 4 (5.5) | 34 (11.8) | .11 |
Bowel emptying = diarrhoea and/or vomiting.
Comparison of laboratory data between acute MVT and control group
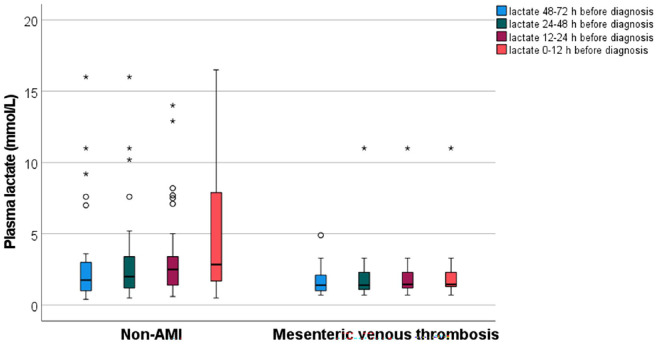
White blood cell count (P = .016) and C-reactive protein (CRP) level (P < .001) were more elevated in the acute MVT group compared to the control group. The AUC for white blood cell counts and CRP for MVT were 0.67 (95% CI 0.60-0.74) and 0.59 (95% CI 0.52-0.67), respectively. Estimated glomerular filtration rate (eGFR) at admission was higher in the MVT group (P < .001). Amylase (P = .033) and troponin T (P = .039) levels were lower in MVT patients. D-dimer was measured in 23 patients (31.5%) with MVT compared to 34 (11.8%) in the non-AMI group (P = .0001). D-dimer was not significantly higher in MVT compared to the control group (median 7.0 mg/L vs 4.5 mg/L, P = .092), whereas it was higher (P = .001) compared to patients with mechanical bowel obstruction (median 1.5 mg/L; n = 15). D-Dimer values were above the test’s respective reference value in all 23 tested patients with MVT, and in 27 out of 34 tested non-AMI patients, resulting in a sensitivity of 100% (95% CI 85.2-100) and specificity of 20.6% (95% CI 8.7-37.9). The discriminative ability of D-dimer for MVT as assessed by the AUC was 0.63 (95% CI 0.49-0.78). The optimum cut-off point for D-dimer performance of MVT diagnosis based on the ROC analysis was 5.0 mg/L. Plasma lactate levels at time point 12 to 24 hours before diagnosis (P = .007) and time point 0 to 12 hours before diagnosis (P = .005) were lower in MVT compared to the control group, and plasma lactate levels increased more between these 2 time-points in the control group (Table 4, Figure 2).
Table 4.
Comparison of laboratory data between patients with acute MVT and confirmed non-AMI.
| Laboratory data | Acute MVT (n = 73) | Confirmed non-AMI (n = 287) | P-value |
|---|---|---|---|
| White blood cell count, ×109/L (median, IQR) | 14.7 (10.6-21.9) (n = 70) | 12.5 (8.0-16.8) (n = 277) | .016 |
| CRP, mg/L (median, IQR) | 105 (41-166) (n = 60) | 44 (7.5-118.5) (n = 201) | <.001 |
| eGFR, ml/min/1.73m2 (median, IQR) | 82 (53-104) (n = 41) | 60 (36-85) (n = 206) | <.001 |
| ASAT, U/L (median, IQR) | 28 (20-37) (n = 60) | 28 (19-55) (n = 187) | .51 |
| Amylase, U/L (median, IQR) | 45 (27-55) (n = 34) | 57 (34-122) (n = 140) | .033 |
| Troponin T, ng/L (median, IQR) | 12 (10-21) (n = 14) | 38 (10-183) (n = 64) | .039 |
| pH (median, IQR) | 7.39 (7.32-7.45) (n = 47) | 7.37 (7.27-7.42) (n = 209) | .090 |
| Base excess, (median, IQR) | −1.4 (-4.3 to 1.4) (n = 28) | −1.8 (-8.0 to 1.2) (n = 203) | .33 |
| D-dimer, mg/L (median, IQR) | 7.0 (4.0-9.0) (n = 23) | 4.5 (1.3-8.2) (n = 34) | .092 |
| D-dimer above reference for normal value according to manufacturer (%) | 23/23 (100) | 27/34 (79.4) | .034 |
| Time point 1. Lactate, mmol/L (median, IQR) (48-72 hours before diagnosis) | 1.4 (1.0-2.1) (n = 28) | 1.7 (1.3-3.0) (n = 37) | .10 |
| Time point 2. Lactate, mmol/L (median, IQR) (24-48 hours before diagnosis) | 1.4 (1.1-2.3) (n = 27) | 2.2 (1.2-3.2) (n = 42) | .11 |
| Time point 3. Lactate, mmol/L (median, IQR) (12-24 hours before diagnosis) | 1.5 (1.2-2.3) (n = 34) | 2.3 (1.4-3.7) (n = 75) | .007 |
| Time point 4. Lactate, mmol/L (median, IQR) (0-12 hours before diagnosis) | 1.6 (1.3-2.9) (n = 56) | 2.4 (1.5-4.8) (n = 212) | .005 |
| Repeated Lactate measurements (at least on two occasions) (%) | 33/58 (56.9) | 79/218 (36.2) | .004 |
| Change in Lactate, mmol/L (median, IQR) between time point 4 and 1 | 0.0 (0.0-0.4) (n = 27) | 0.50 (-0.22 to 2.15) (n = 34) | .062 |
| Change in Lactate, mmol (median, IQR) between time point 4 and 3 | 0.0 (0.0-0.0) (n = 33) | 0.30 (-0.05 to 1.60) (n = 73) | .008 |
Figure 2.
Box plot graph showing plasma lactate levels in time intervals prior to diagnosis. Temporal plasma lactate values prior to diagnosis of acute MVT versus non-AMI (acute mesenteric ischaemia) patients. Box plot graph showing median and interquartile range values of plasma lactate at different time ranges before diagnosis of acute MVT and non-AMI patients. The line across the box indicates the median, the box represents interquartile range, and the whiskers are lines that extend from the box edges to the highest and lowest values, excluding outliers. Values more than 1.5 IQR’s but less than 3 IQR’s (o), and more than 3 IQR’s (*), from the box edges are labelled as outliers and extremes, respectively
Clinical and laboratory variables associated with acute MVT
After entering body mass index, atherosclerotic disease, history of venous thromboembolism, CRP and D-dimer as covariates in a multi-variable logistic regression analysis, absence of previous atherosclerotic disease (OR 0.096, 95% CI 0.011-0.84; P = .034) and D-dimer (OR 2.59/one SD increment, 95% CI 1.07-6.28; P = .034) were associated with MVT (Table 5).
Table 5.
Clinical and laboratory variables associated with acute MVT compared to confirmed non-AMI.
| Variable | Multi-variable logistic regression | |
|---|---|---|
| OR (95%CI) | P-value | |
| Body mass index | 2.42 a (0.87-6.76) | .090 |
| Atherosclerotic disease | 0.096 (0.011-0.84) | .034 |
| History of venous thromboembolism | 4.36 (0.20-93.50) | .35 |
| C-reactive protein | 1.65 a (0.41-6.57) | .48 |
| D-dimer | 2.59 a (1.07-6.28) | .034 |
All 5 variables were entered into the model.
OR was expressed per one standard deviation increment.
Discussion
This is the first ever prospective case-control study on patients with acute MVT. This is very difficult to achieve, due to the low incidence and awareness of the disease, preventing researchers from performing studies of higher quality, but was made possible thanks to data from the AMESI study. This sub-study on MVT patients, with a control group of patients, with similar symptoms but with proven non-AMI, has shown that elevated D-dimer and absence of previous atherosclerotic disease were independently associated with MVT in the adjusted analysis, which should alert clinicians to the possibility of MVT in a patient with acute abdomen. However, a clinical decision tool such as the Wells score 9 for pre-test probability of deep vein thrombosis 10 does not exist for MVT, and it is unlikely to ever be developed. Even if the Wells score cannot be used as a standalone test to confirm or exclude deep vein thrombosis, it is a valuable tool in combination with D-dimer measurement for evidence-based decision making. 11 D-dimer had, however, a poor discriminative ability for diagnosis of MVT in the present study. This may be explained by the fact that only 23/73 patients with MVT had their D-dimer tested, and that the median time elapsed from debut of symptoms until presentation was 32 hours. D-dimer is an acute phase protein that will be more elevated close to the thrombotic event.
Although a history of atherosclerotic disease was independently inversely associated with MVT in the present study, this factor is of very little value in the clinical setting. There are, however, other factors not studied, such as known inherited thrombophilia, especially factor V Leiden mutation, that can give a clue towards the diagnosis of MVT. The prevalence of factor V Leiden mutation without presence of cancer was reported to be high in both MVT and systemic venous thromboembolism in a population-based study, suggesting that screening for thrombophilia should be considered in unscreened patients with these diagnoses. 12 It is useful to be aware of the relation between intra-abdominal solid organ malignancy and increased risk of MVT. Thus, intra-abdominal cancer should be excluded in patients with MVT. 12 Although there was no difference in medication with anticoagulation between patients with MVT and no AMI, it is of great concern that 28% of patients with MVT were on anticoagulation therapy at onset of disease. The history of anticoagulation therapy in patients with MVT was seldom reported in a recent systematic review of eleven contemporary retrospective studies, 2 and the anticoagulation failure rate was found to be 1.7% in one of those studies. 12 However, the high anticoagulation therapy failure rate presented in this prospective international study is more generalizable in different settings. The reasons behind this real-life scenario can only be speculated upon, but it is not unlikely that a large proportion of these MVT patients on anticoagulation have no or poor adherence to this preventive medication as adherence to medication in chronic diseases commonly is reported to be around 50%. 13
There is an urgent need to find an accurate plasma biomarker for all subtypes of AMI. 14 Even though D-dimer performed better than the inflammatory marker CRP in diagnosing MVT in the adjusted analysis, D-dimer does not seem to be sufficiently predictive to be of diagnostic aid based on the results of this matched case-control study. Even if 100% of patients with MVT had values above the upper reference value, 79% of patients had elevated levels also in the control group. Thus, normal D-dimer might act as a test of exclusion for MVT in the present study, whereas the lack of specificity greatly reduces the D-dimer test’s utility as a useful diagnostic modality. Appropriately, a prospective multi-centre study on biomarkers in prediction of acute mesenteric ischaemia (BIPAMI study) with collection of sequential blood samples after onset of suspected AMI is in progress (ClinicalTrials.gov: NCT06212921).
The AMESI Study was not designed primarily as a laboratory study, and consequently, there existed various commercially available D-dimer assays, calibrators, and monoclonal antibodies, provided by different manufacturers 15 (Table 1), and the results had to be pooled. Hence, the results should be interpreted cautiously due to inconsistencies in performance characteristics of the different D-Dimer assays. However, the use of automated immunoassays, of which the majority used immunoturbidimetry assay technology, with rapid sample turnaround time at all sites which provided information on the D-dimer assays, was favoured compared to more manual methods such as immunofiltration or enzyme-linked immunosorbent assay (ELISA). Analysis of plasma D-dimer in one core lab using one assay technology and calibrator, would, nevertheless introduce scientific rigour and provide more high-quality data.
An interesting observation was that plasma lactate levels were lower at measurement points 12 to 24 hours and 0 to 12 hours before diagnosis of MVT compared to non-AMI patients (Figure 2). In addition, there was a significant increase in plasma lactate levels between these 2 time points in the non-AMI group compared to the MVT group. Hence, monitoring dynamics of plasma lactate appears to be of little value in diagnosing MVT early. MVT may be secondary to acute pancreatitis in a minor proportion of patients. 16 The finding of lower amylase levels in the MVT group may, nevertheless, be related to lower frequency of acute pancreatitis, compared to the non-AMI group. In contrast to AMI of arterial origin, 17 it is unlikely that AMI of venous origin may lead to significant ischaemic injury to pancreas with elevation of amylase. Troponin T is a marker of myocardial stress and was reported to be elevated in 42% of patients with acute abdomen. 18 The lower troponin T levels in MVT patients, compared to non-AMI patients, may reflect less cardiomyocyte injury due to lower age, low proportion of atherosclerotic disease, and better renal function. 17
Even though CT of abdomen with intravenous contrast enhancement has developed as the diagnostic modality of choice for MVT, 19 there are concerns of overuse of emergency CT scans in some healthcare systems 20 and of its great cost. 21 In addition, a large proportion of patients in different settings around the world must pay for diagnostic workup and treatment, and many patients cannot themselves afford expensive investigations. Therefore, laboratory tests that accurately indicate either acute vessel occlusion or intestinal ischaemia, and subsequent need of emergency CT scan, are highly warranted.
There are additional limitations of the study besides the variability of D-dimer assays. One is the unknown exact timing of blood sampling in relation to symptom duration, influencing levels of plasma biomarkers. The laboratory data was not prespecified in the AMESI Study, and this post-hoc analysis study was based on available data. Even though the number of patients with MVT tested for D-Dimer in each group was larger than ever previously reported, the sample sizes in each group, 23 in the MVT group and 34 patients in the non-AMI group, respectively, are still low, with risk of type II statistical error. Testing of D-dimer was higher in the MVT group, which may be influenced by testing of D-dimer immediately after a diagnostic CT of the abdomen in some patients. Complete data on final diagnosis was not available in the non-AMI group. The major strengths are related to the prospective case-control study design on patients with AMI, a notably very difficult-to-study group of patients, the control group consisting of patients with suspected but confirmed non-AMI patients, and involvement of study sites in various countries increasing generalizability of the findings.
In conclusion, elevated D-dimer was associated with MVT, but the specificity of D-dimer was, however, poor. Currently, diagnosis of MVT is almost exclusively dependent on CT of the abdomen with intravenous contrast enhancement and imaging in the portal phase. There is an urgent need to find a more accurate plasma biomarker for this condition.
Supplemental Material
Supplemental material, sj-docx-1-bmi-10.1177_11772719241296631 for D-Dimer in Acute Mesenteric Venous Thrombosis: A Prospective Case-Control International Multicenter Study by Stefan Acosta, Annika Reintam Blaser, Alexandre Nuzzo, Yasmin Soltanzadeh-Naderi, Joel Starkopf, Alastair Forbes, Marko Murruste, Kadri Tamme, Anna-Liisa Voomets, Merli Koitmäe, Miklosh Bala, Zsolt Bodnar, Dumitru Casian, Zaza Demetrashvili, Alan Biloslavo, Virginia Dúran Muñoz-Cruzado, Benjamin Hess, Karri Kase, Mikhail Kirov, Matthias Lindner, Cecilia I Loudet, Dimitrios Damaskos and Martin Björck in Biomarker Insights
Acknowledgments
Sites and Collaborators (AMESI Investigators):
Intestinal Stroke Center, Department of Gastroenterology, IBD and Intestinal Failure, AP-HP. Nord, Beaujon Hospital, Paris Cité University, Paris, France
• Olivier Corcos
• Yves Castier
• Maxime Ronot
Division of General Surgery, University Hospital of Trieste ASUGI, Trieste, Italy
• Mario D’Oria
• Lucia Paiano
Universitätsklinikum Schleswig-Holstein, Campus Kiel, Kiel, Germany
• Gunnar Elke
• Denise Nagel
• David I. Radke
Hospital General San Martin de La Plata, Buenos Aires, Argentina
• Dr Jacqueline Vilca Becerra
• Dr María Elina Abeleyra
Lucerne Cantonal Hospital, Lucerne, Switzerland
• Martin Cahenzli
Northern State Medical University and City Hospital #1, Arkhangelsk, Russia
• Tatjana Semenkova
• Anton Nikonov
• Alexey Smetkin
University Hospital North Norway, Tromsø, Norway
• Kristoffer Lein
• Geir Ivar Nedredal
• Øivind Irtun
Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel
• Oded Cohen-Arazi
• Asaf Keda
Nicolae Testemitanu State University of Medicine and Pharmacy of the Republic of Moldova, Chisinau, Moldova
• Tatiana Malcova
Virgen del Rocío University Hospital, Sevilla, Spain
• Felipe Pareja Ciuró
• Anabel García-Leon
• Carlos Javier García-Sánchez
Sarawak General Hospital, Kuching, Malaysia
• Mohammad Alif Yunus
• Lim Jia Hui
• Loy Yuan Ling
Rabin Medical Center, University of Tel Aviv, Petah Tikva, Israel
• Moran Hellerman Itzhaki
• Ilya Kagan
• Pierre Singer
N. Kipshidze Central University Hospital, Tbilisi, Georgia
• Ana Tvaladze
Royal Infirmary of Edinburgh, Edinburgh, United Kingdom
• Damian J Mole
• Darja Clinch
Hospital Melaka, Malacca, Malaysia
• Too Xiao Qing
Stavanger University Hospital, Stavanger, Norway
• Hanne Fuglseth
• Morten Vetrhus
Azienda Ospedaliera Universitaria Careggi, Firenze, Italy
• Maximilian Scheiterle
• Jacopo Martellucci
• Giulia Cerino
Fujian Provincial Hospital, Fuzhou, China
• Donghuang Hong
• Jinsheng Liu
Hospital Bintulu, Bintulu, Malaysia
• Ernest Ong
Erciyes University Hospital, Kayseri, Turkey
• Kursat Kundogan
• Tutkun Talih
Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
• Lovenish Bains
AOU Cittá della Salute e della Scienza, Turin, Italy
• Diego Visconti
• Lorenzo Gibello
Hospital Ampang, Ampang, Malaysia
• Ruhi Fadzlyana Jailani
• Muhammad Amirul Ashra
School of Medical Sciences & Hospital Universiti Sains Malaysia, Kota Bharu, Malaysia
• Andee Dzulkarnaen Zakaria
• Ahmad Faiz Najmuddin Mohd Ghazi
Hospital Pengajar Universiti Putra, Serdang, Malaysia
• Nur Suriyana Abd Ghani
Hospital Sultanah Nur Zahirah, Kuala Terengganu, Malaysia
• Mohd Fadliyazid Ab Rahim
University Hospital Centre Zagreb, Zagreb, Croatia
• Goran Augustin
• Damir Halužan
Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India
• Mohan Gurjar
• Rahul Rahul
Queen Elisabeth Hospital, Kota Kinabalu, Malaysia
• Firdaus Hayati
• Jin-Jiun Mah
Footnotes
ORCID iDs: Kadri Tamme  https://orcid.org/0000-0001-6110-0417
https://orcid.org/0000-0001-6110-0417
Dumitru Casian  https://orcid.org/0000-0002-4823-0804
https://orcid.org/0000-0002-4823-0804
Zaza Demetrashvili  https://orcid.org/0000-0002-5020-9684
https://orcid.org/0000-0002-5020-9684
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
AMESI Investigators (Collaborators):
Olivier Corcos, Yves Castier, Maxime Ronot, Mario D’Oria, Lucia Paiano, Gunnar Elke, Denise Nagel, David I. Radke, Dr Jacqueline Vilca Becerra, Dr María Elina Abeleyra, Martin Cahenzli, Tatjana Semenkova, Anton Nikonov, Alexey Smetkin, Kristoffer Lein, Geir Ivar Nedredal, Øivind Irtun, Oded Cohen-Arazi, Asaf Keda, Tatiana Malcova, Felipe Pareja Ciuró, Anabel García-Leon, Carlos Javier García-Sánchez, Mohammad Alif Yunus, Lim Jia Hui, Loy Yuan Ling, Moran Hellerman Itzhaki, Ilya Kagan, Pierre Singer, Ana Tvaladze, Damian J Mole, Darja Clinch, Too Xiao Qing, Hanne Fuglseth, Morten Vetrhus, Maximilian Scheiterle, Jacopo Martellucci, Giulia Cerino, Donghuang Hong, Jinsheng Liu, Ernest Ong, Kursat Kundogan, Tutkun Talih, Lovenish Bains, Diego Visconti, Lorenzo Gibello, Ruhi Fadzlyana Jailani, Muhammad Amirul Ashra, Andee Dzulkarnaen Zakaria, Ahmad Faiz Najmuddin Mohd Ghazi, Nur Suriyana Abd Ghani, Mohd Fadliyazid Ab Rahim, Goran Augustin, Damir Halužan, Mohan Gurjar, Rahul Rahul, Firdaus Hayati, and Jin-Jiun Mah
Declarations
Ethics approval and consent to participate: Primary ethical approval was obtained from the Ethics Committee of the University of Tartu (357/T-8 and 364M-7). Each participating site obtained local Ethics Committee approval according to site, country, and institutional regulations. Delayed written informed consent was obtained from the patient or patient’s next of kin/proxy at the first possibility if requested by the local ethics committee.
Consent for publication: Written informed consent for publication was provided by the participant(s) or a legally authorized representative.
Author contributions: All authors contributed to the study conception and design. Data collection was performed by all authors and analysis by Stefan Acosta. The first draft of the manuscript was written by Stefan Acosta and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Estonian Research Council, Grant PRG1255. The funding body had no insight into or intellectual role of the present study.
Competing interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ARB has received speaker or consultancy fees from Nestlé, VIPUN Medical, Nutricia and Fresenius Kabi, and is holding a grant from Estonian Research Council (PRG1255). AF has received speaker fees from B Braun and Fresenius Kabi. AN has received speaker or consultancy fees from Abbvie and Janssen, research funding from MSD-Avenir, and PhD grants from Fondation de l’Avenir and SNFGE. SA, YSN, JS, MM, KT, ALV, MK, MBa, ZB, DC, ZD, AB, VDMC, BH, KK, MK, ML, CIL, DD and MBj declare no conflicts of interest.
Availability of data and materials: The raw data supporting the conclusions of this article will be made available on request by the authors.
References
- 1. Salim S, Ekberg O, Elf J, et al. Clinical implications of CT findings in mesenteric venous thrombosis at admission. Emerg Radiol. 2018;25:407-413. [DOI] [PubMed] [Google Scholar]
- 2. Acosta S, Salim S. Management of acute mesenteric venous thrombosis: a systematic review of contemporary studies. Scand J Surg. 2021;110:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zafirovski A, Zafirovska M, Kuhelj D, Pintar T. The impact of biomarkers on the early detection of acute mesenteric ischemia. Biomedicines. 2023;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu W, Yang L, Zhou Z. Clinical features and factors affecting postoperative mortality for obstructive acute mesenteric ischemia in China: a hospital-based study. Vasc Health Risk Manag. 2020;16:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reintam Blaser A, Forbes A, Acosta S, et al. The acute MESenteric ischaemia (AMESI) study: a call to participate in an international prospective multicentre study. Eur J Vasc Endovasc Surg. 2022;63:902-903. [DOI] [PubMed] [Google Scholar]
- 6. Reintam Blaser A, Mändul M, Björck M, et al. Incidence, diagnosis, management and outcome of acute MESenteric ischaemia: a prospective, multicentre observational study (AMESI Study). Crit Care. 2024;28:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32:382-387. [PubMed] [Google Scholar]
- 8. Schneider TA, Longo WE, Ure T, Vernava AM. 3rd. Mesenteric ischemia. Acute arterial syndromes. Dis Colon Rectum. 1994;37:1163-1174. [DOI] [PubMed] [Google Scholar]
- 9. Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207. [DOI] [PubMed] [Google Scholar]
- 10. Kakkos SK, Gohel M, Baekgaard N, et al. European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61:9-82. [DOI] [PubMed] [Google Scholar]
- 11. Geersing GJ, Zuithoff NP, Kearon C, et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ. 2014;348:g1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salim S, Zarrouk M, Elf J, et al. Clinical implications of different risk factor profiles in patients with mesenteric venous thrombosis and systemic venous thromboembolism: a population-based study. J Thromb Thrombolysis. 2019;47:572-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdou JK, Auyeung V, Patel JP, Arya R. Adherence to long-term anticoagulation treatment, what is known and what the future might hold. Br J Haematol. 2016;174:30-42. [DOI] [PubMed] [Google Scholar]
- 14. Reintam Blaser A, Starkopf J, Björck M, et al. Diagnostic accuracy of biomarkers to detect acute mesenteric ischaemia in adult patients: a systematic review and meta-analysis. World J Emerg Surg. 2023;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dempfle CE. D-dimer assays: the current status and new assay technologies. Thromb Res. 2006;118:569-571. [DOI] [PubMed] [Google Scholar]
- 16. Salim S, Zarrouk M, Elf J, et al. Improved prognosis and low failure rate with anticoagulation as first-line therapy in mesenteric venous thrombosis. World J Surg. 2018;42:3803-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acosta S, Block T, Björnsson S, et al. Diagnostic pitfalls at admission in patients with acute superior mesenteric artery occlusion. J Emerg Med. 2012;42:635-641. [DOI] [PubMed] [Google Scholar]
- 18. Keskpaik T, Talving P, Kirsimägi Ü, et al. Associations between elevated high-sensitive cardiac troponin t and outcomes in patients with acute abdominal pain. Eur J Trauma Emerg Surg. 2023;49:281-288. [DOI] [PubMed] [Google Scholar]
- 19. Björck M, Koelemay M, Acosta S, et al. Oderich G. Management of the diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53:460-510. [DOI] [PubMed] [Google Scholar]
- 20. Systermans BJ, Devitt PG. Computed tomography in acute abdominal pain: an overused investigation? ANZ J Surg. 2014;84:155-159. [DOI] [PubMed] [Google Scholar]
- 21. Berge P, Darsonval A, Nedelcu C, Paisant A, Aubé C. Incidental findings on emergency CT scans: predictive factors and medico-economic impact. Eur J Radiol. 2020;129:109072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-bmi-10.1177_11772719241296631 for D-Dimer in Acute Mesenteric Venous Thrombosis: A Prospective Case-Control International Multicenter Study by Stefan Acosta, Annika Reintam Blaser, Alexandre Nuzzo, Yasmin Soltanzadeh-Naderi, Joel Starkopf, Alastair Forbes, Marko Murruste, Kadri Tamme, Anna-Liisa Voomets, Merli Koitmäe, Miklosh Bala, Zsolt Bodnar, Dumitru Casian, Zaza Demetrashvili, Alan Biloslavo, Virginia Dúran Muñoz-Cruzado, Benjamin Hess, Karri Kase, Mikhail Kirov, Matthias Lindner, Cecilia I Loudet, Dimitrios Damaskos and Martin Björck in Biomarker Insights