Abstract
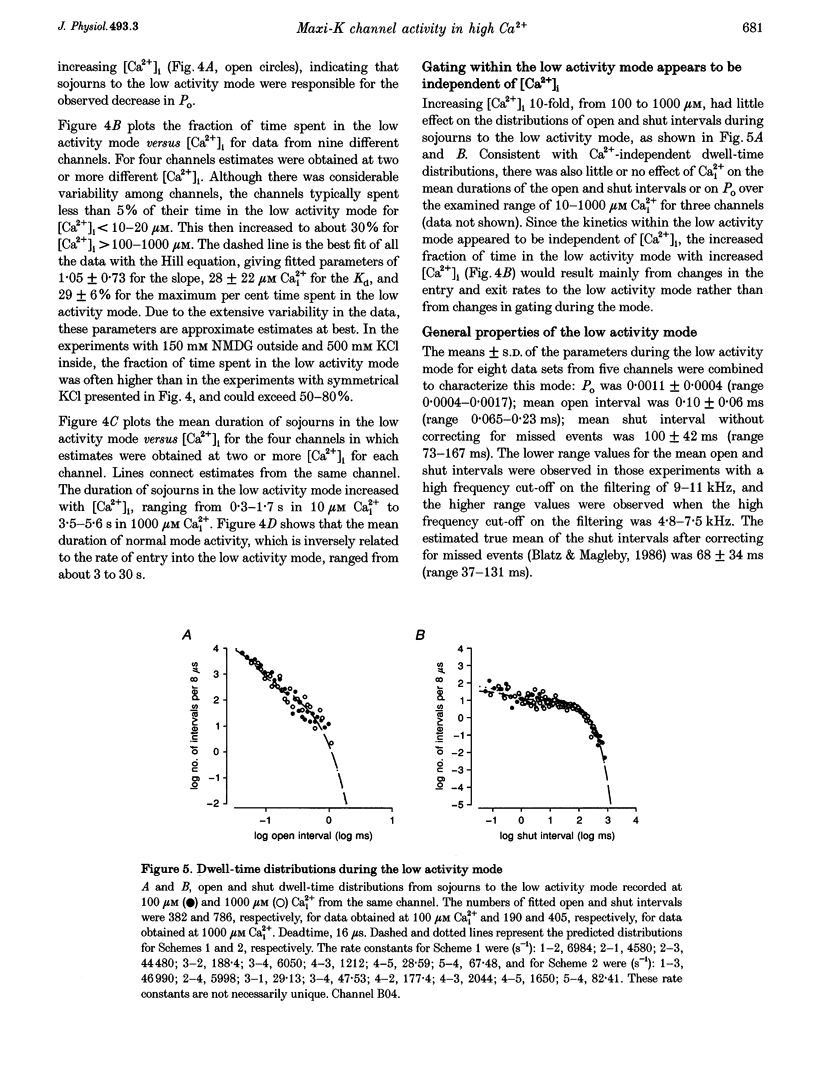
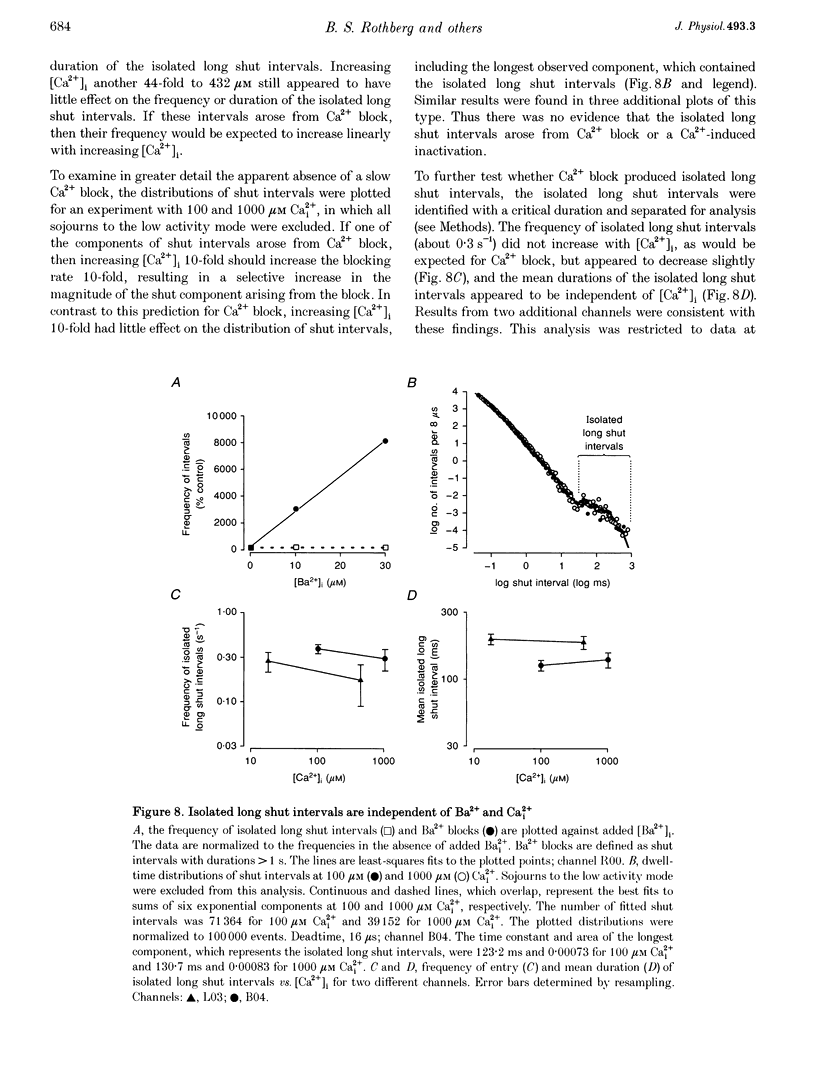
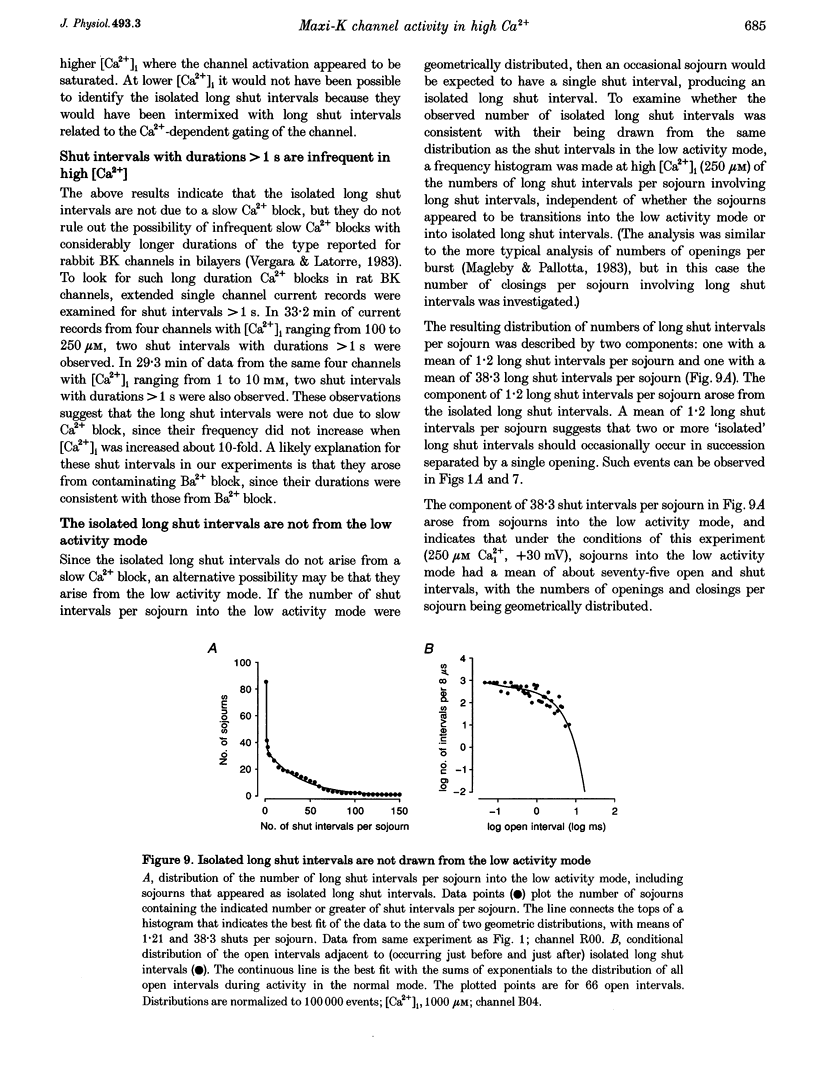
1. Large-conductance calcium-activated K+ channels (BK channels) often display long closed intervals at higher levels of Ca2+. To gain further insight into possible mechanisms for these intervals, currents were recorded from single BK channels, using the patch clamp technique, from patches of membrane excised from primary cultures of rat skeletal muscle. 2. High intracellular calcium concentrations ([Ca2+]i; 10-1000 microM) induced a low activity mode and revealed isolated long shut intervals. Neither of these phenomena were due to the Ba2+ that typically contaminates reagent grade salts. 3. The low activity mode was characterized by typically single brief open intervals with mean durations of 0.1 ms, separated by long shut intervals with mean durations of 100 ms. The very low open probability of about 0.001 during the low activity mode would make a sojourn to this mode functionally equivalent to a sojourn to an inactive state. The durations of sojourns in the low activity mode were exponentially distributed, with the mean durations ranging from about 1 s in 10 microM Ca(i)2+, to 4.5 s in 1000 microM Ca(i)2+. With increased filtering, the brief open intervals would escape detection so that a sojourn to the low activity mode would appear as a single shut interval. A typical channel spent less than 5% of its time in the low activity mode for [Ca2+]i < 10 microM. This increased to about 30% for [Ca2+]i > 100-1000 microM. A kinetic model with three closed states and two open states could approximate the gating of the low activity mode. 4. The isolated long shut intervals were not from the low activity mode, suggesting a different underlying mechanism. Their frequency of occurrence of about 0.3 s-1 did not increase with increasing [Ca2+]i, indicating that they did not arise from a slow Ca2+ block. Their durations were exponentially distributed, with a mean of 127 ms, which was independent of [Ca2+]i, suggesting that a single Ca(2+)-independent closed state or block underlies the isolated long shut intervals. At higher [Ca2+]i, up to 60% of the shut time could be spent in the isolated long shut intervals. 5. These observations suggest that activation of BK channels by high [Ca2+]i can be limited by sojourns to a low activity mode and also by isolated long shut intervals, two additional phenomena that will have to be accounted for in the gating of BK channels.
Full text
PDF


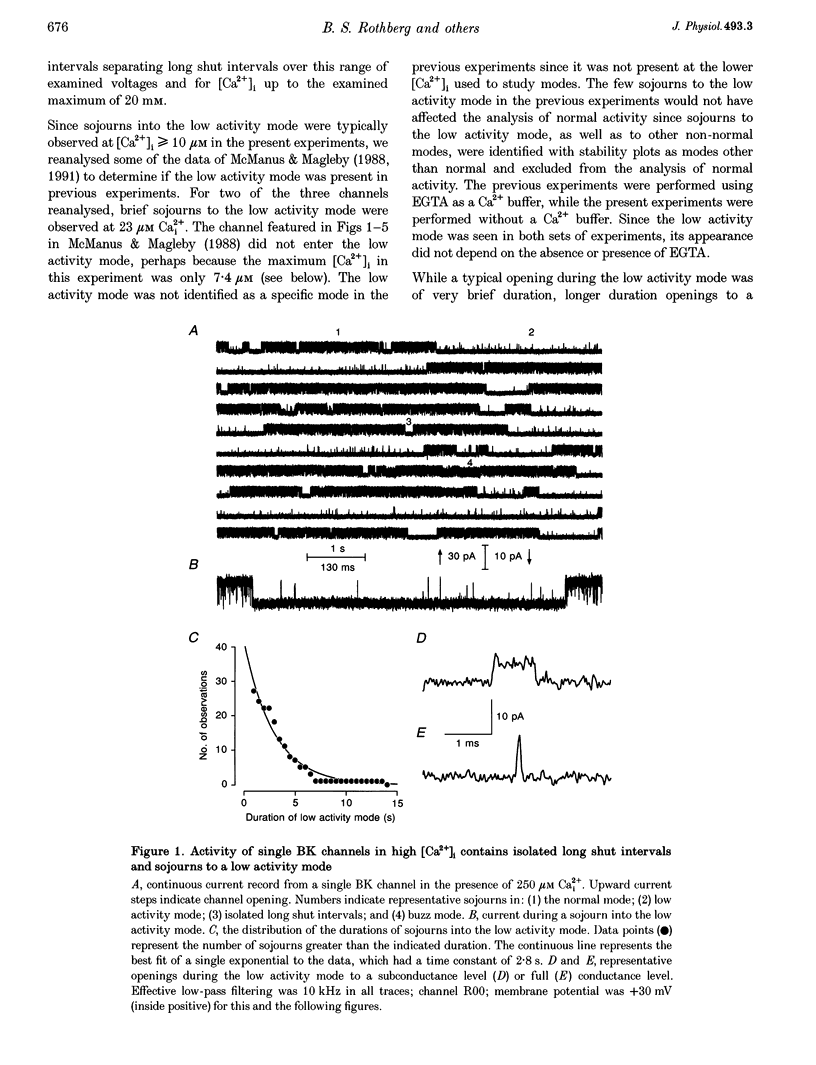
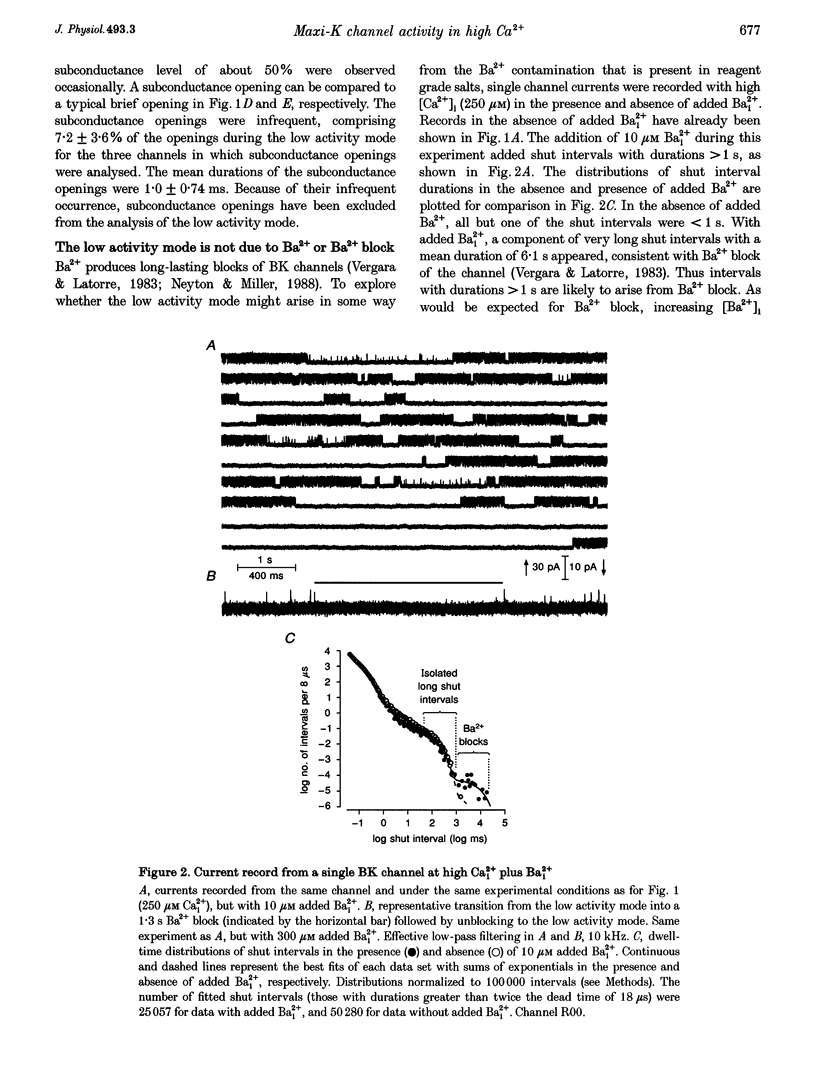

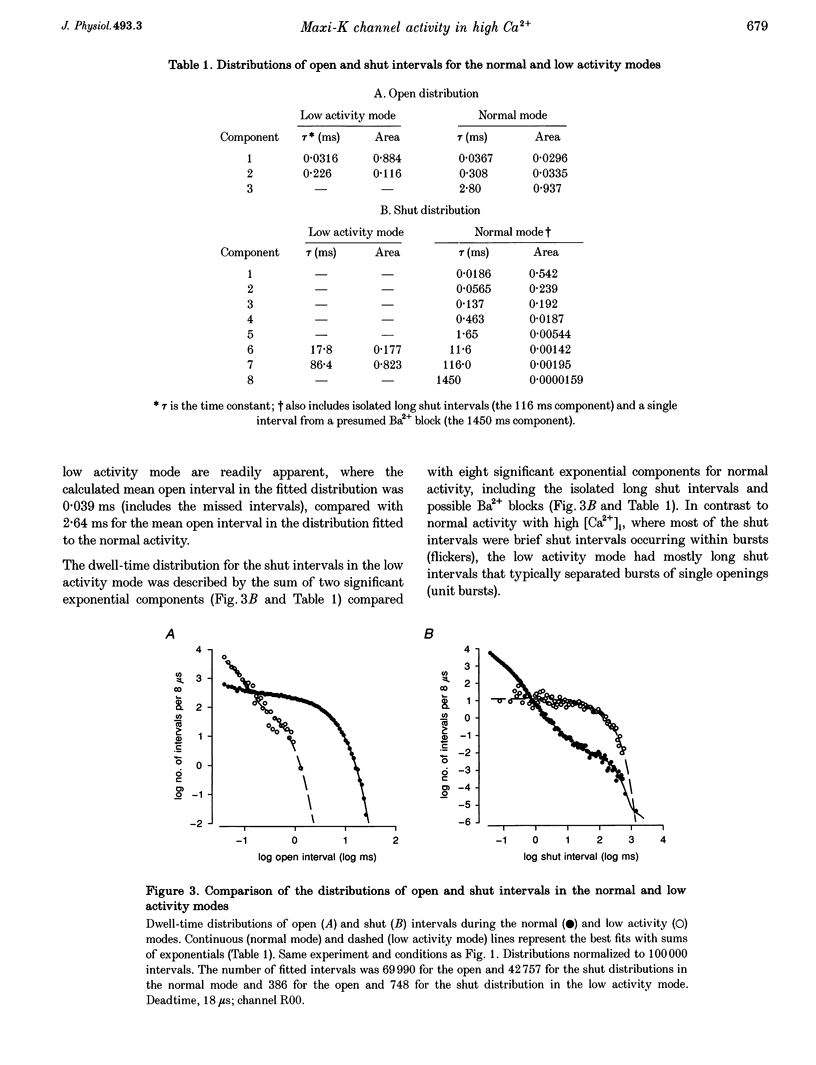
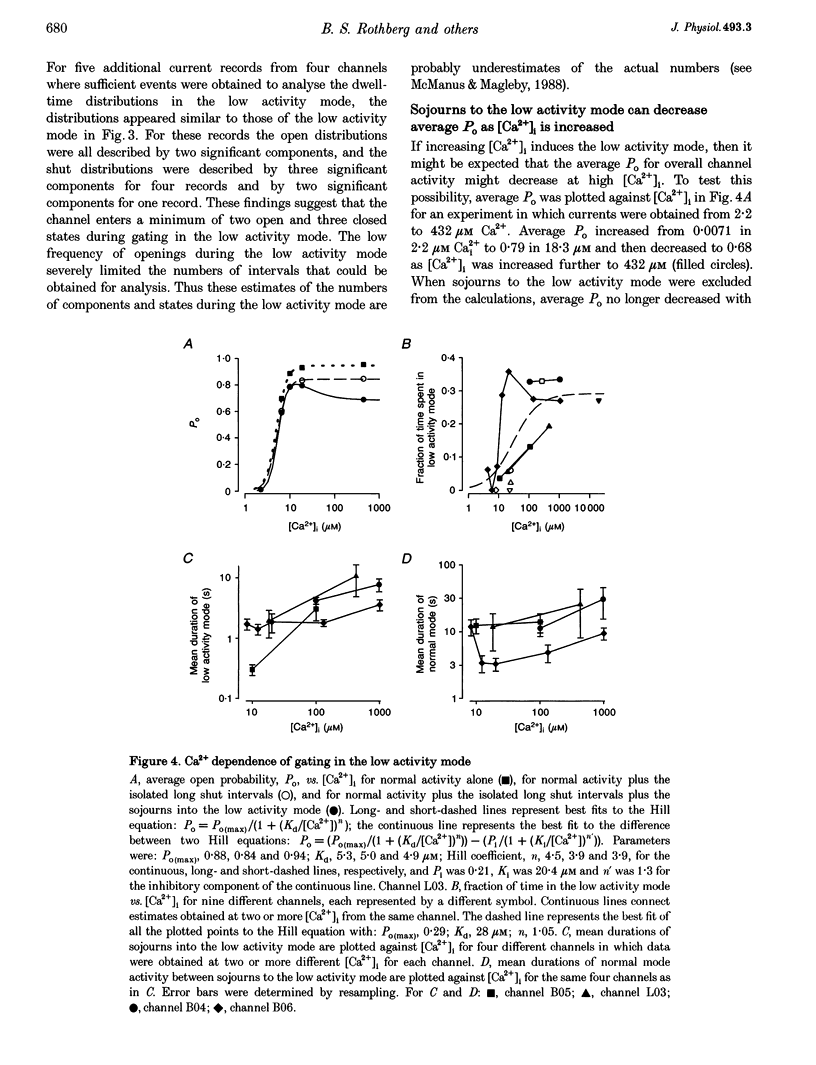






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Identifying kinetic gating mechanisms for ion channels by using two-dimensional distributions of simulated dwell times. Proc Biol Sci. 1990 Sep 22;241(1302):220–228. doi: 10.1098/rspb.1990.0089. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Boheim G. The gating of single calcium-dependent potassium channels is described by an activation/blockade mechanism. Biophys Struct Mech. 1982;9(1):35–60. doi: 10.1007/BF00536014. [DOI] [PubMed] [Google Scholar]
- Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988 Nov;92(5):549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M., Horn R. Opentime heterogeneity during bursting of sodium channels in frog skeletal muscle. Biophys J. 1986 Mar;49(3):773–777. doi: 10.1016/S0006-3495(86)83704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C. R., Lingle C. J. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 1992 Sep 18;257(5077):1694–1698. doi: 10.1126/science.1529355. [DOI] [PubMed] [Google Scholar]
- Song L., Magleby K. L. Testing for microscopic reversibility in the gating of maxi K+ channels using two-dimensional dwell-time distributions. Biophys J. 1994 Jul;67(1):91–104. doi: 10.1016/S0006-3495(94)80458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Latorre R. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers. Evidence for a Ca2+ and Ba2+ blockade. J Gen Physiol. 1983 Oct;82(4):543–568. doi: 10.1085/jgp.82.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]


