Abstract
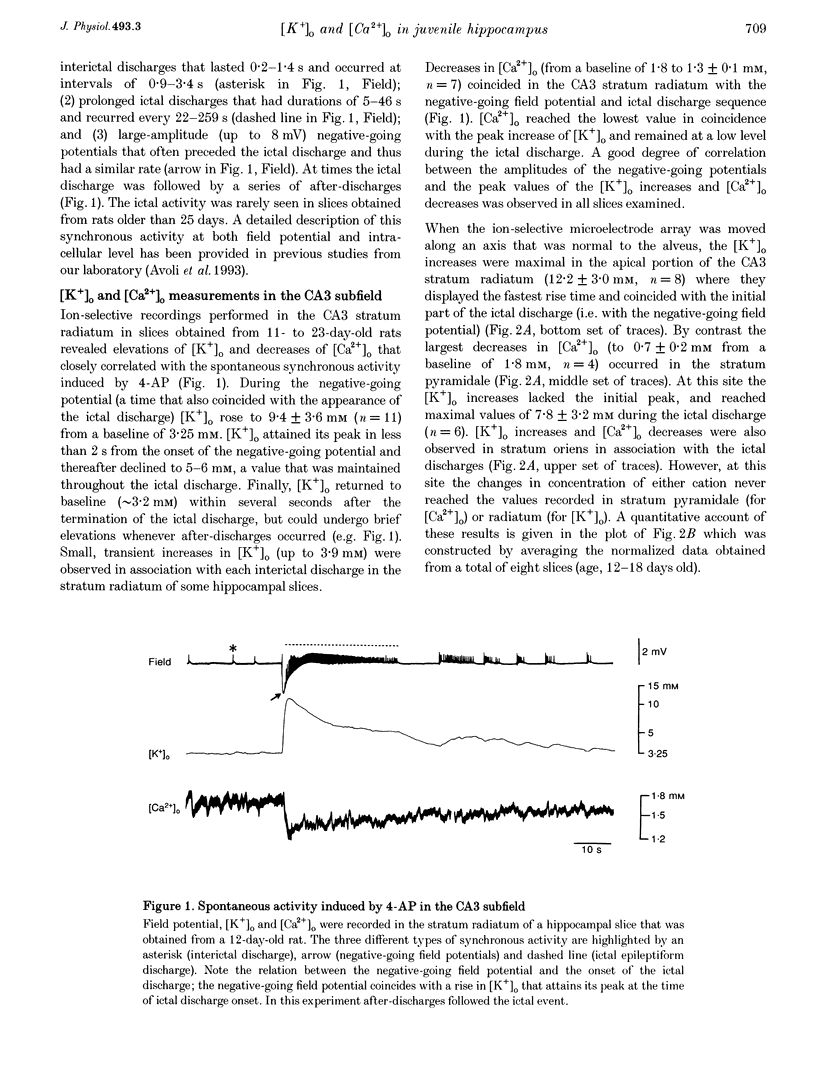
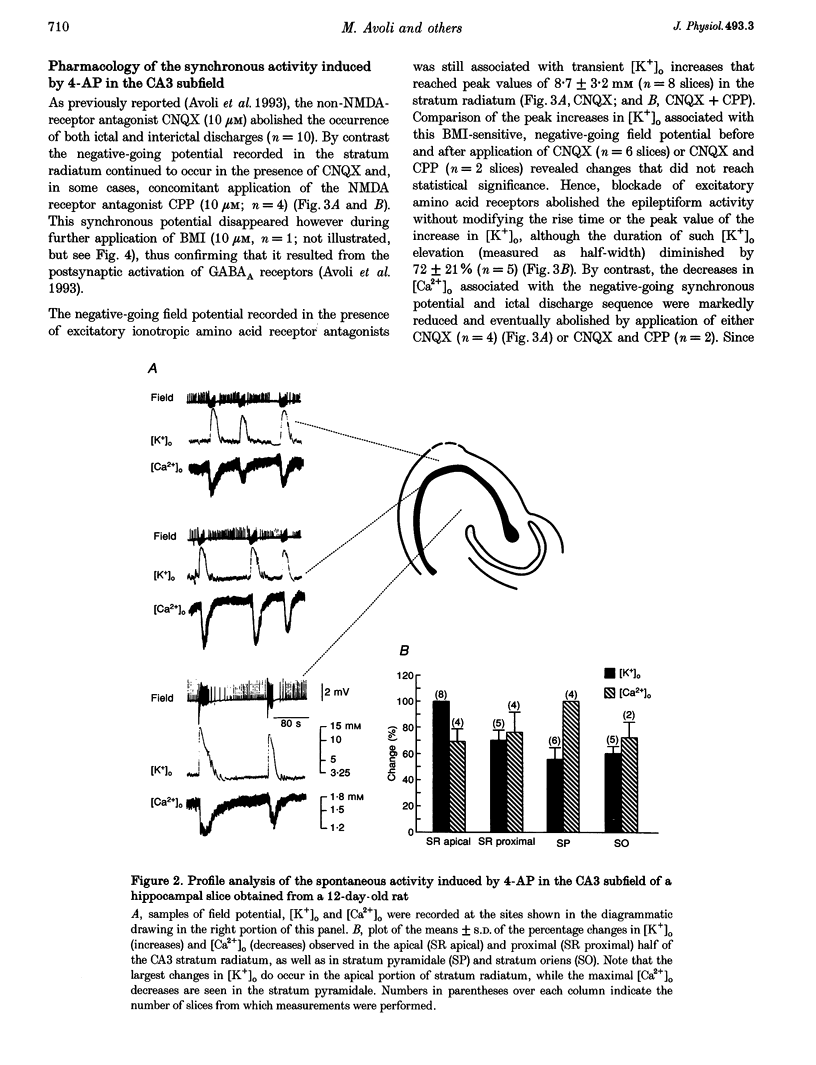
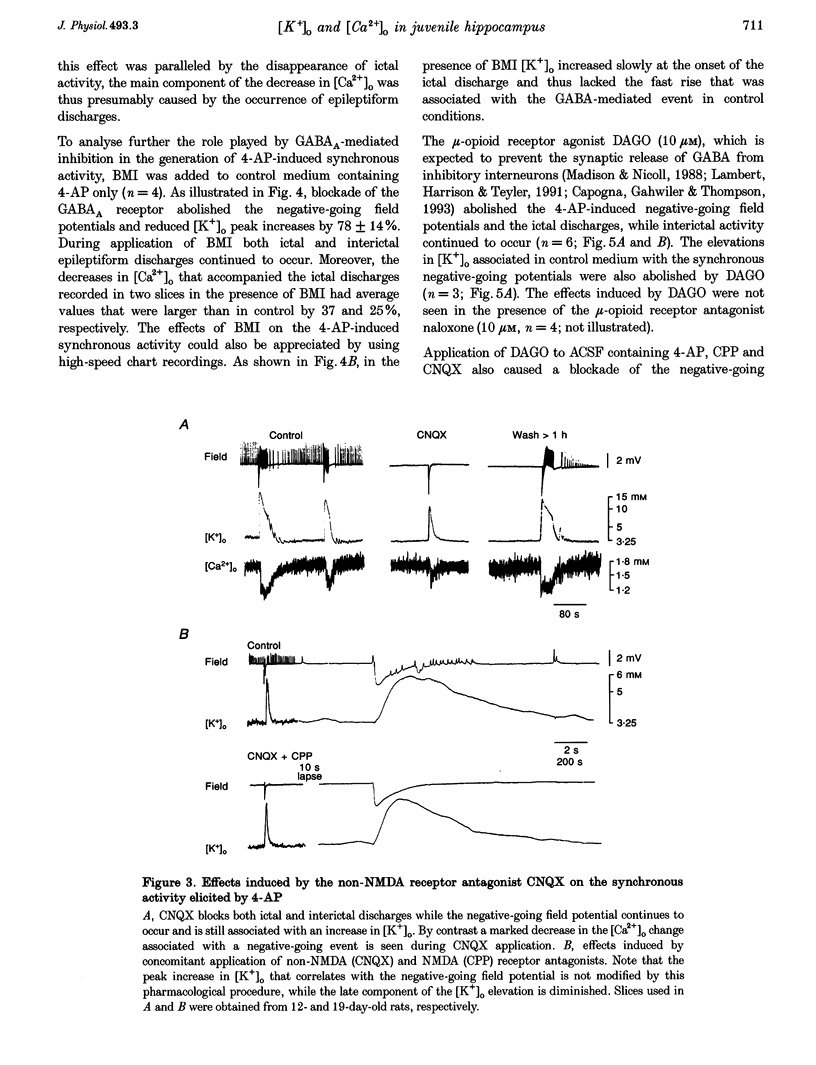
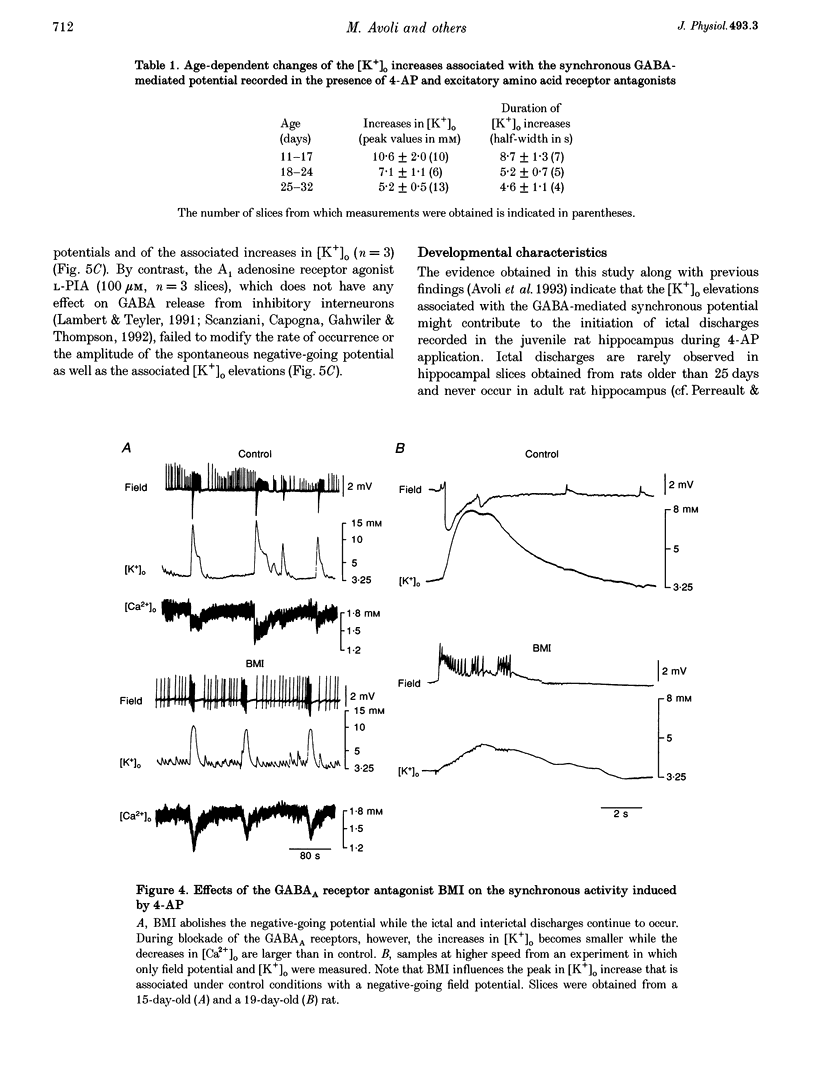
1. Field potential recordings and measurements of the extracellular concentration of free K+ ([K+]o) and Ca2+ ([Ca2+]o) were made during application of 4-aminopyridine (4-AP, 50 microM) in hippocampal slices that were obtained from 11- to 32-day-old rats. 2. Spontaneous field potentials recorded under this experimental condition in the CA3 stratum radiatum of slices from rats < 23 days old consisted of interictal (duration, 0.2-1.4 s; intervals of occurrence, 0.9-3.4 s) and ictal epileptiform discharges (duration, 5-46 s; intervals of occurrence, 22-259 s) and negative-going potentials that often preceded the onset of ictal discharge. Ictal activity became rare in slices from rats > 25 days old. 3. The negative-going potential (which also corresponded to the ictal discharge onset) was associated with [K+]o increases to 9.4 +/- 3.6 mM (mean +/- S.D.) from 3.25 mM baseline (n = 11 slices). [K+]o remained elevated at 5-6 mM throughout the ictal event. Decreases in [Ca2+]o (from 1.8 mM baseline to 1.3 +/- 0.1 mM, n = 7) were observed during the ictal discharge. 4. Interictal and ictal discharges were abolished by the non-N-methyl-D-aspartate (NMDA) receptor antagonist 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX, 10 microM). CNQX and the NMDA receptor antagonist 3-((+/-)-2-carboxypiperazine-4-yl)propyl-1-phosphonic acid (CPP) did not influence negative-going potentials or the associated [K+]o increases (peak values were 8.7 +/- 3.2 mM, n = 8), that were blocked, however, by bicuculline methiodide (BMI, 10 microM). 5. The mu-opioid receptor agonist (D-Ala2,N-Me-Phe4,Gly5-ol)-enkephalin (DAGO, 10 microM) which inhibits GABA release from interneurons, prevented the occurrence of both GABA-mediated synchronous potentials and subsequent ictal discharges (n = 6) as well as the [K+]o elevations. DAGO effects were antagonized by naloxone (10 microM; n = 4). 6. The GABA-mediated [K+]o elevations changed as a function of age. In hippocampal slices obtained from 11- to 17-day-old rats, peak values of 10.6 +/- 2.0 mM (n = 10) and half-width durations of 8.7 +/- 1.3 s (n = 7) were observed. In slices obtained from 25- to 32-day-old animals these parameters were 5.2 +/- 0.5 mM (n = 13) and 4.6 +/- 1.1 s (n = 4), respectively. 7. This study shows that, in the juvenile rat hippocampus, 4-AP induces a glutamatergic independent synchronous potential that is due to GABA released from inhibitory terminals and is associated with an increase in [K+]o. This [K+]o elevation undergoes age-dependent changes, and is instrumental in synchronizing neurons thus initiating prolonged epileptiform discharges.
Full text
PDF

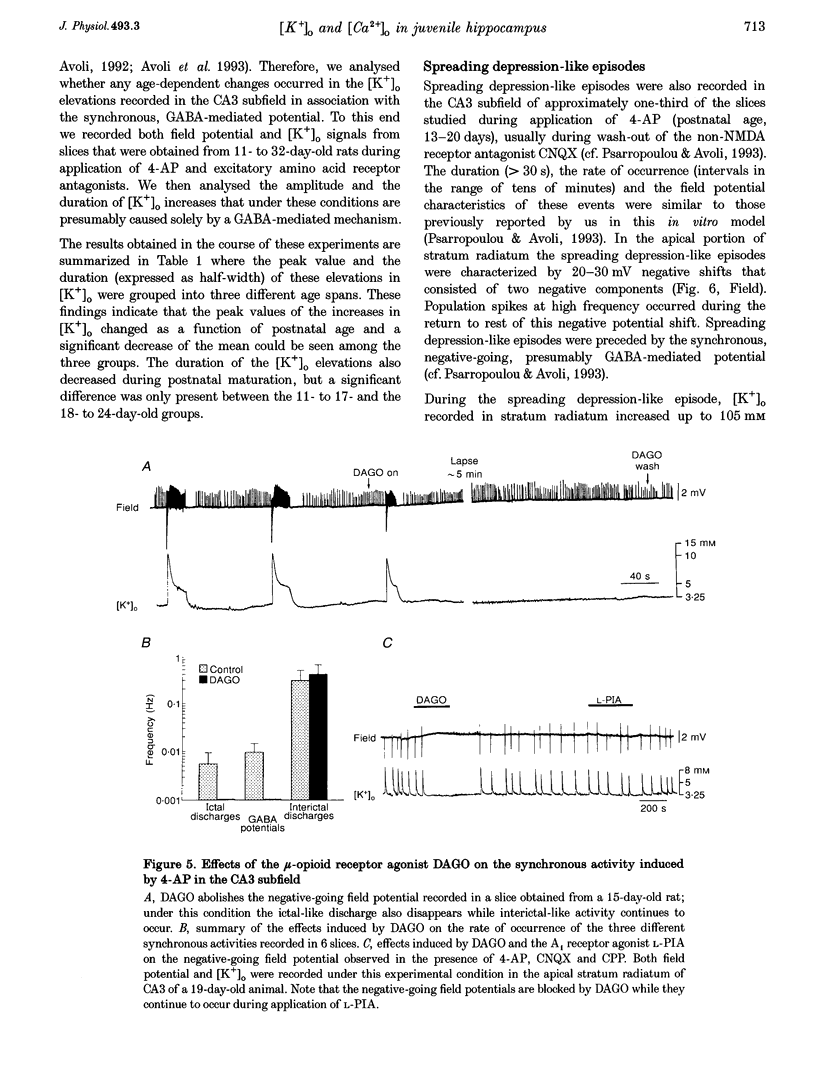
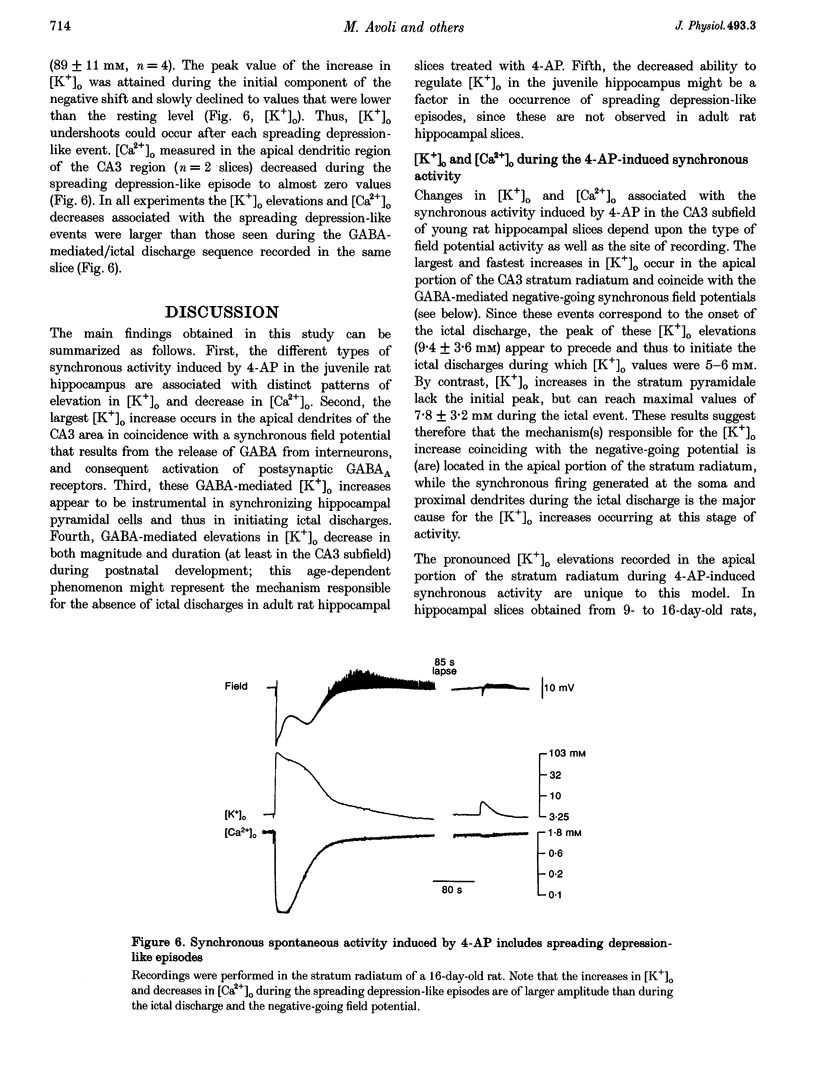



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avoli M., Psarropoulou C., Tancredi V., Fueta Y. On the synchronous activity induced by 4-aminopyridine in the CA3 subfield of juvenile rat hippocampus. J Neurophysiol. 1993 Sep;70(3):1018–1029. doi: 10.1152/jn.1993.70.3.1018. [DOI] [PubMed] [Google Scholar]
- Barolet A. W., Morris M. E. Changes in extracellular K+ evoked by GABA, THIP and baclofen in the guinea-pig hippocampal slice. Exp Brain Res. 1991;84(3):591–598. doi: 10.1007/BF00230971. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Ransom B. R., Kunis D. M., Gutnick M. J. Activity-dependent K+ accumulation in the developing rat optic nerve. Science. 1982 Jun 18;216(4552):1341–1343. doi: 10.1126/science.7079771. [DOI] [PubMed] [Google Scholar]
- Ellenberg J. H., Hirtz D. G., Nelson K. B. Age at onset of seizures in young children. Ann Neurol. 1984 Feb;15(2):127–134. doi: 10.1002/ana.410150204. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Heinemann U. Alterations in the microenvironment during spreading depression associated with epileptiform activity in the immature neocortex. Brain Res Dev Brain Res. 1989 Apr 1;46(2):243–252. doi: 10.1016/0165-3806(89)90288-5. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Heinemann U. Extracellular K+ and Ca2+ changes during epileptiform discharges in the immature rat neocortex. Brain Res. 1987 Dec 1;433(2):299–303. doi: 10.1016/0165-3806(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Haglund M. M., Schwartzkroin P. A. Role of Na-K pump potassium regulation and IPSPs in seizures and spreading depression in immature rabbit hippocampal slices. J Neurophysiol. 1990 Feb;63(2):225–239. doi: 10.1152/jn.1990.63.2.225. [DOI] [PubMed] [Google Scholar]
- Haglund M. M., Stahl W. L., Kunkel D. D., Schwartzkroin P. A. Developmental and regional differences in the localization of Na,K-ATPase activity in the rabbit hippocampus. Brain Res. 1985 Sep 16;343(1):198–203. doi: 10.1016/0006-8993(85)91180-1. [DOI] [PubMed] [Google Scholar]
- Heinemann U., Lux H. D., Gutnick M. J. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977 Mar 30;27(3-4):237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994 Mar;42(4):489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaila K., Rydqvist B., Pasternack M., Voipio J. Inward current caused by sodium-dependent uptake of GABA in the crayfish stretch receptor neurone. J Physiol. 1992;453:627–645. doi: 10.1113/jphysiol.1992.sp019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Nicholson C. Extracellular ionic variations during spreading depression. Neuroscience. 1978;3(11):1045–1059. doi: 10.1016/0306-4522(78)90122-7. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E., Reiffenstein R. J. Stimulation-evoked changes in extracellular K+ and Ca2+ in pyramidal layers of the rat's hippocampus. Can J Physiol Pharmacol. 1982 Dec;60(12):1643–1657. doi: 10.1139/y82-243. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Harrison N. L., Teyler T. J. Evidence for mu opiate receptors on inhibitory terminals in area CA1 of rat hippocampus. Neurosci Lett. 1991 Mar 11;124(1):101–104. doi: 10.1016/0304-3940(91)90831-d. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Teyler T. J. Adenosine depresses excitatory but not fast inhibitory synaptic transmission in area CA1 of the rat hippocampus. Neurosci Lett. 1991 Jan 14;122(1):50–52. doi: 10.1016/0304-3940(91)90190-5. [DOI] [PubMed] [Google Scholar]
- Louvel J., Avoli M., Kurcewicz I., Pumain R. Extracellular free potassium during synchronous activity induced by 4-aminopyridine in the juvenile rat hippocampus. Neurosci Lett. 1994 Feb 14;167(1-2):97–100. doi: 10.1016/0304-3940(94)91036-7. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Heinemann U., Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol. 1986;44:619–639. [PubMed] [Google Scholar]
- Lücke A., Nagao T., Köhling R., Avoli M. Synchronous potentials and elevations in [K+]o in the adult rat entorhinal cortex maintained in vitro. Neurosci Lett. 1995 Feb 13;185(3):155–158. doi: 10.1016/0304-3940(95)11248-u. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Tse F. W., Crichton S. A., Kettenmann H. GABA-activated Cl- channels in astrocytes of hippocampal slices. J Neurosci. 1989 Oct;9(10):3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol. 1988 Apr;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J., Traynelis S. F., Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990 Aug 10;249(4969):674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Futamachi K. J., Prince D. A. Extracellular potassium activity during epileptogenesis. Exp Neurol. 1974 Feb;42(2):248–263. doi: 10.1016/0014-4886(74)90023-5. [DOI] [PubMed] [Google Scholar]
- Mutani R., Futamachi K. J., Prince D. A. Potassium activity in immature cortex. Brain Res. 1974 Jul 19;75(1):27–39. doi: 10.1016/0006-8993(74)90768-9. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 1992 Jan;12(1):104–115. doi: 10.1523/JNEUROSCI.12-01-00104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarropoulou C., Avoli M. 4-Aminopyridine-induced spreading depression episodes in immature hippocampus: developmental and pharmacological characteristics. Neuroscience. 1993 Jul;55(1):57–68. doi: 10.1016/0306-4522(93)90454-n. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992 Nov;9(5):919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Giacchino J. L. Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. J Neurophysiol. 1985 Apr;53(4):1098–1108. doi: 10.1152/jn.1985.53.4.1098. [DOI] [PubMed] [Google Scholar]
- Steinhäuser C., Jabs R., Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994 Feb;4(1):19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Swann J. W., Smith K. L., Brady R. J. Extracellular K+ accumulation during penicillin-induced epileptogenesis in the CA3 region of immature rat hippocampus. Brain Res. 1986 Dec;395(2):243–255. doi: 10.1016/s0006-8993(86)80203-7. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988 Jan;59(1):259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Dingledine R. Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J Neurophysiol. 1989 May;61(5):927–938. doi: 10.1152/jn.1989.61.5.927. [DOI] [PubMed] [Google Scholar]
- Zuckermann E. C., Glaser G. H. Hippocampal epileptic activity induced by localized ventricular perfusion with high-potassium cerebrospinal fluid. Exp Neurol. 1968 Jan;20(1):87–110. doi: 10.1016/0014-4886(68)90126-x. [DOI] [PubMed] [Google Scholar]


