Abstract
1. Fundamental properties of Ca2+ channel currents in rat and rabbit ventricular myocytes were measured using whole cell voltage clamp. 2. In rat, as compared with rabbit myocytes, Ca2+ channel current (ICa) was half-activated at about 10 mV more negative potential, decayed slower, was half-inactivated (in steady state) at about 5 mV more positive potential, and recovered faster from inactivation. 3. These features result in a larger steady-state window current in rat, and also suggest that under comparable voltage clamp conditions, including action potential (AP) clamp, more Ca2+ influx would be expected in rat myocytes. 4. Ca2+ channel current carried by Na+ and Cs+ in the absence of divalent ions (Ins) also activated at more negative potential and decayed more slowly in rat. 5. The reversal potential for Ins was 6 mV more positive in rabbit, consistent with a larger permeability ratio (PNa/PCs) in rabbit than in rat. ICa also reversed at slightly more positive potentials in rabbit (such that PCa/PCs might also be higher). 6. Ca2+ influx was calculated by integration of ICa evoked by voltage clamp pulses (either square pulses or pulses based on recorded rabbit or rat APs). For a given clamp waveform, the Ca2+ influx was up to 25% greater in rat, as predicted from the fundamental properties of ICa and Ins. 7. However, the longer duration of the AP in rabbit myocytes compensated for the difference in influx, such that the integrated Ca2+ influx via ICa in response to the species-appropriate waveform was about twice as large as that seen in rat.
Full text
PDF

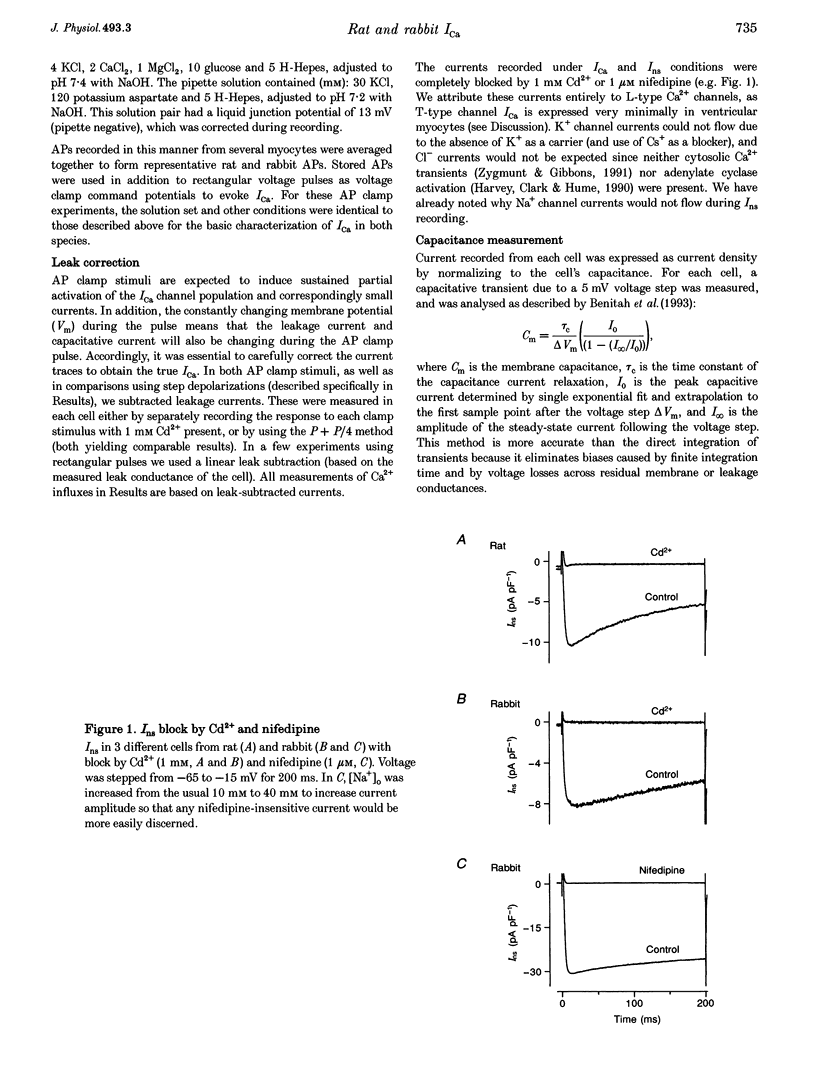

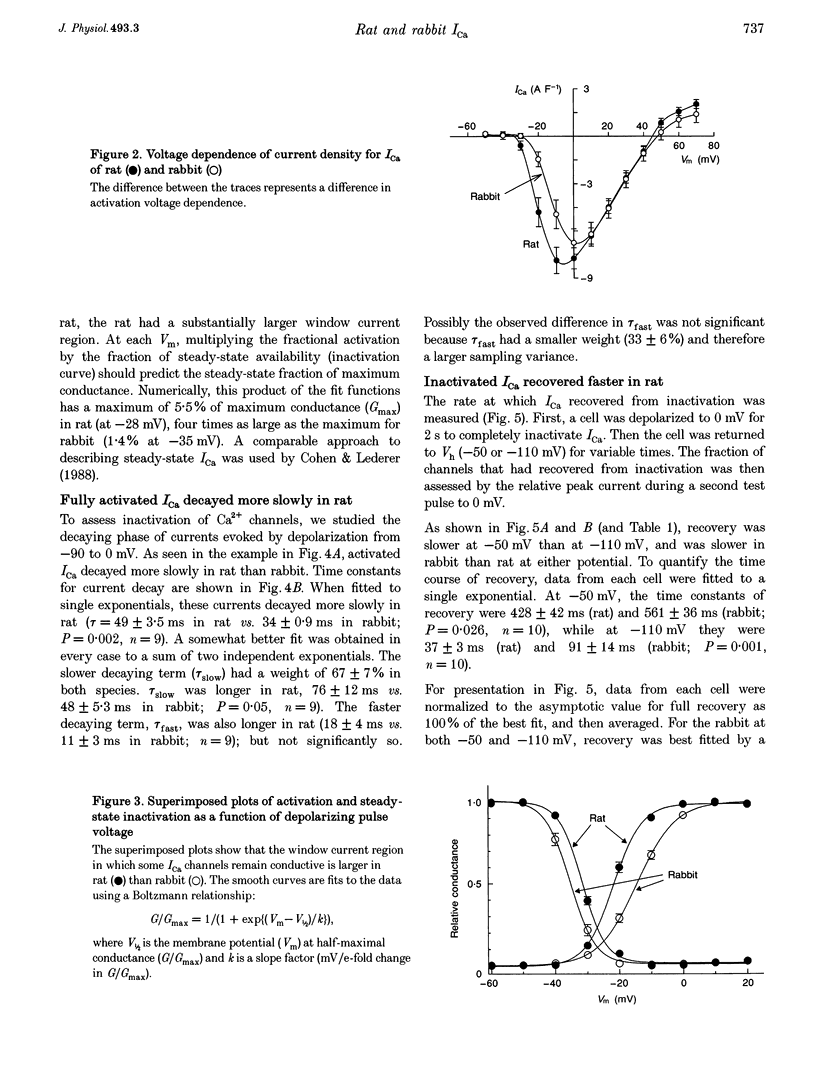
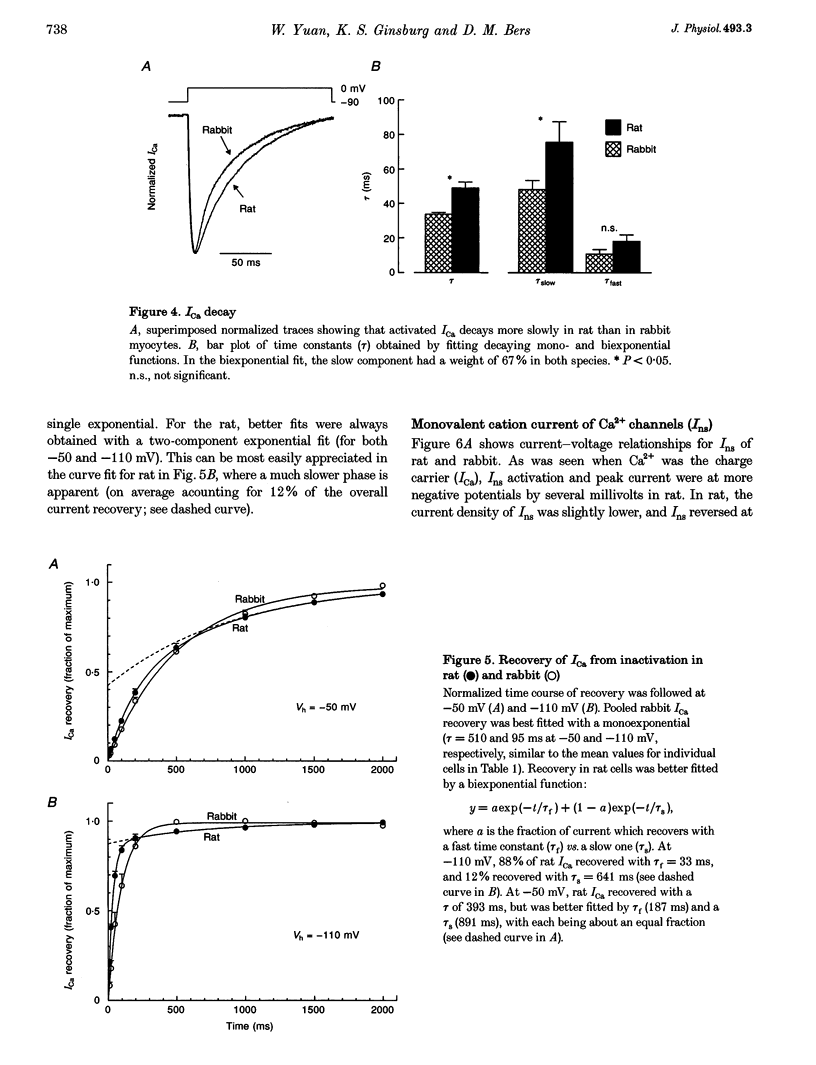
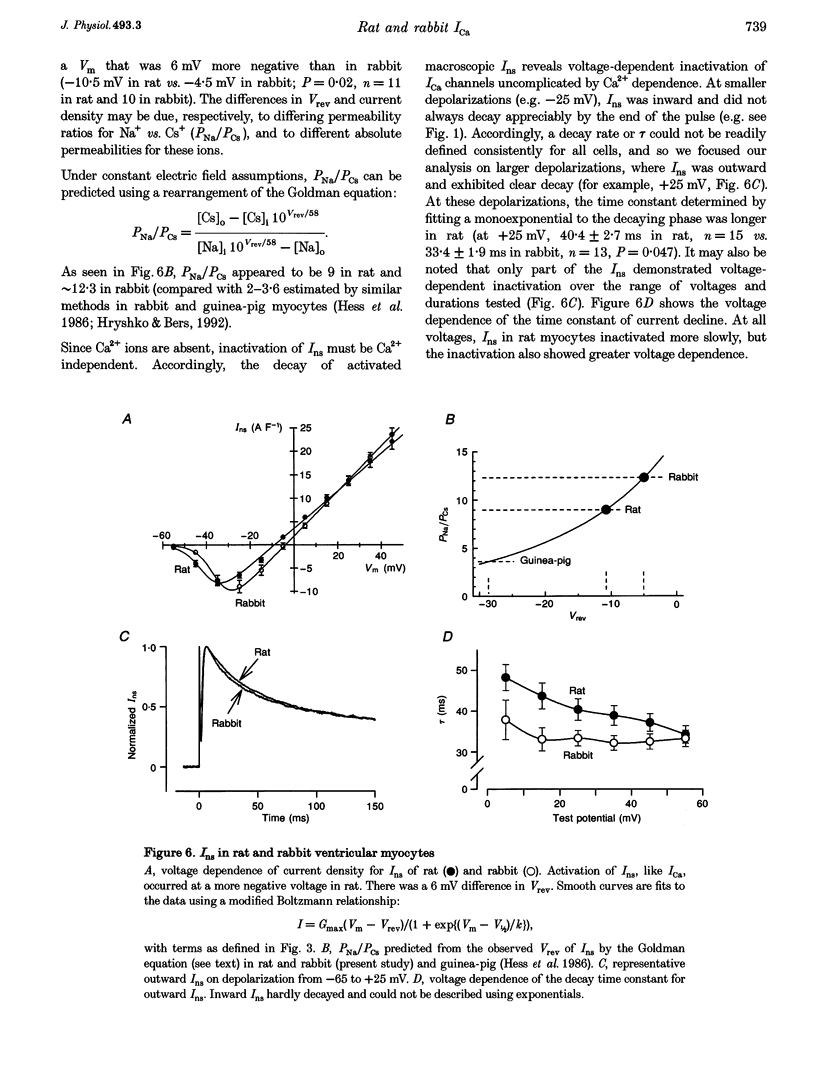
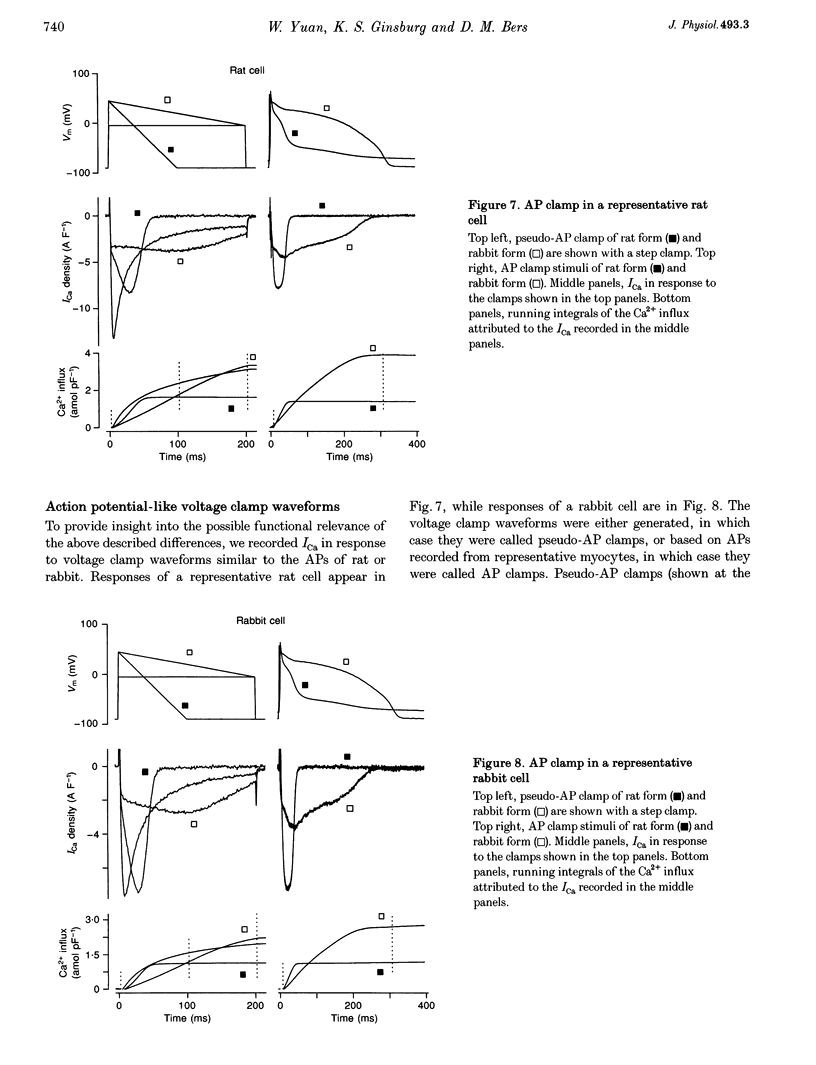
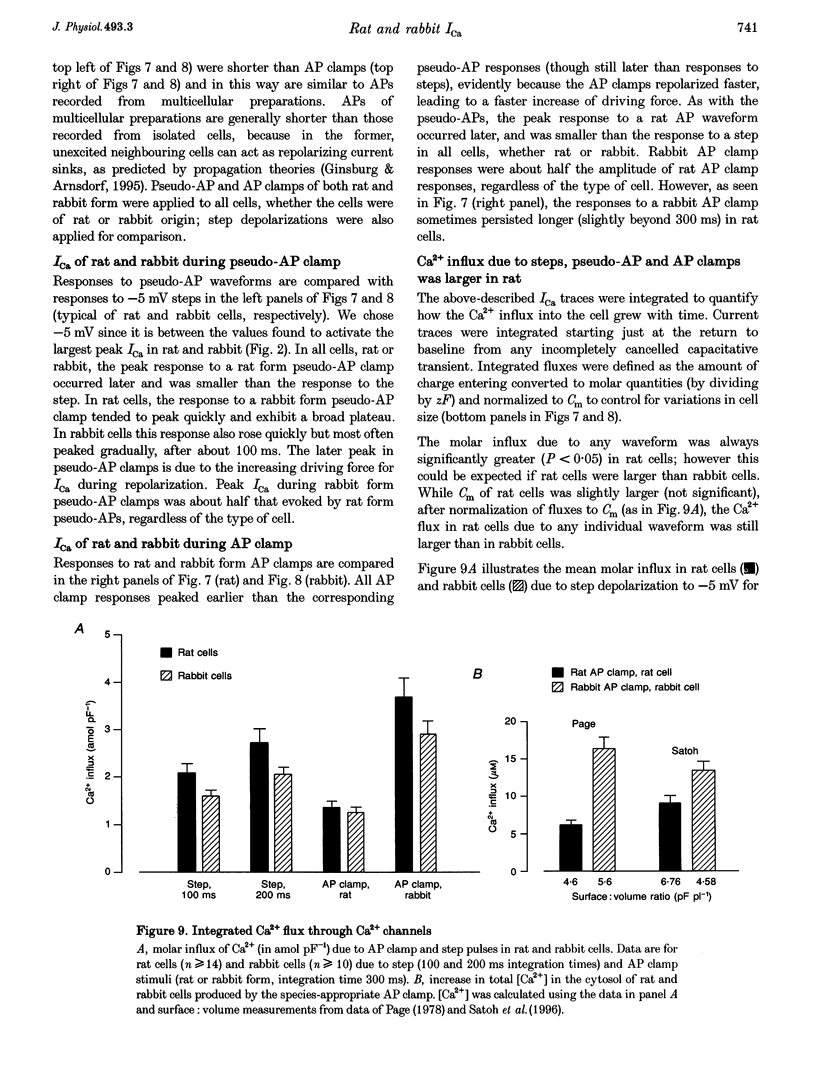





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Stiffel V. M. Ratio of ryanodine to dihydropyridine receptors in cardiac and skeletal muscle and implications for E-C coupling. Am J Physiol. 1993 Jun;264(6 Pt 1):C1587–C1593. doi: 10.1152/ajpcell.1993.264.6.C1587. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Honjo H., Harrison S. M., Zang W. J., Kirby M. S. Ultra-slow voltage-dependent inactivation of the calcium current in guinea-pig and ferret ventricular myocytes. Pflugers Arch. 1994 Aug;428(1):39–50. doi: 10.1007/BF00374750. [DOI] [PubMed] [Google Scholar]
- Bénitah J. P., Gomez A. M., Bailly P., Da Ponte J. P., Berson G., Delgado C., Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J Physiol. 1993 Sep;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Hume J. R., Noble D., Shibata E. F. Reversal potential of the calcium current in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:267–286. doi: 10.1113/jphysiol.1988.sp017249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano A., Perez-Reyes E. Molecular diversity of Ca2+ channel beta subunits. Biochem Soc Trans. 1994 May;22(2):483–488. doi: 10.1042/bst0220483. [DOI] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988 Dec;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbridge L. M., Bassani J. W., Bers D. M. Steady-state twitch Ca2+ fluxes and cytosolic Ca2+ buffering in rabbit ventricular myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C192–C199. doi: 10.1152/ajpcell.1996.270.1.C192. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Hume J. R. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987 Aug;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Intramembrane charge movement in guinea-pig and rat ventricular myocytes. J Physiol. 1989 Aug;415:601–624. doi: 10.1113/jphysiol.1989.sp017738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Moscucci A., January C. T. Direct measurement of L-type Ca2+ window current in heart cells. Circ Res. 1992 Mar;70(3):445–455. doi: 10.1161/01.res.70.3.445. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kirby M. S., Sagara Y., Gaa S., Inesi G., Lederer W. J., Rogers T. B. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem. 1992 Jun 25;267(18):12545–12551. [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflugers Arch. 1986 Nov;407(5):465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in calcium currents of rabbit ventricular cells. Circ Res. 1991 Mar;68(3):788–796. doi: 10.1161/01.res.68.3.788. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Castellano A., Kim H. S., Bertrand P., Baggstrom E., Lacerda A. E., Wei X. Y., Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992 Jan 25;267(3):1792–1797. [PubMed] [Google Scholar]
- Pérez-García M. T., Kamp T. J., Marbán E. Functional properties of cardiac L-type calcium channels transiently expressed in HEK293 cells. Roles of alpha 1 and beta subunits. J Gen Physiol. 1995 Feb;105(2):289–305. doi: 10.1085/jgp.105.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Delbridge L. M., Blatter L. A., Bers D. M. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J. 1996 Mar;70(3):1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991 Jul;7(1):45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T. Ryanodine alteration of the contractile state of rat ventricular myocardium. Comparison with dog, cat, and rabbit ventricular tissues. Circ Res. 1980 Mar;46(3):332–343. doi: 10.1161/01.res.46.3.332. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Winka L., Langer G. A. Role of calcium current and sarcoplasmic reticulum calcium release in control of myocardial contraction in rat and rabbit myocytes. J Mol Cell Cardiol. 1993 Nov;25(11):1339–1347. doi: 10.1006/jmcc.1993.1146. [DOI] [PubMed] [Google Scholar]
- Wetzel G. T., Chen F., Klitzner T. S. L- and T-type calcium channels in acutely isolated neonatal and adult cardiac myocytes. Pediatr Res. 1991 Jul;30(1):89–94. doi: 10.1203/00006450-199107000-00018. [DOI] [PubMed] [Google Scholar]
- Yuan W., Bers D. M. Protein kinase inhibitor H-89 reverses forskolin stimulation of cardiac L-type calcium current. Am J Physiol. 1995 Mar;268(3 Pt 1):C651–C659. doi: 10.1152/ajpcell.1995.268.3.C651. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- de Leon M., Wang Y., Jones L., Perez-Reyes E., Wei X., Soong T. W., Snutch T. P., Yue D. T. Essential Ca(2+)-binding motif for Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Science. 1995 Dec 1;270(5241):1502–1506. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]


