Abstract
Introduction
Chronic kidney disease (CKD) demonstrates a complex interaction with tobacco exposure and sex differences, where females and males may experience varying risks and outcomes. This study aims to investigate how sex differences mediate the relationship between tobacco exposure and CKD development, with a secondary focus on regional variability and social determinants of health.
Study selection and criteria
Comprehensive searches on MEDLINE, EMBASE, clinicaltrials.gov, and MedRxiv until October 6, 2022, were conducted. Eligibility criteria involved any study that reported primary data on the prevalence of CKD, with information pertaining to both sex and tobacco exposure.
Data extraction
Data retrieved include patient socio-demographic characteristics, general study information, diagnostic methods, social determinants of health, and the cause of CKD (e.g., tobacco-related or non-tobacco-related).
Results
Studies were selected through a comprehensive search using key terms such as "chronic kidney disease," "smoking," and "sex differences," which identified 3,025 articles, of which 28 were selected for full texts after screening titles, abstracts. Among the 28 included studies, smoking was consistently identified as a significant risk factor for CKD, with notable disparities related to sex, socioeconomic status, race, and urban versus rural settings. Significant geographical variability in CKD prevalence was observed, ranging from 2.5% to 68.1%, with the highest prevalence in Asia. However, due to high heterogeneity and methodological limitations, a meta-analysis of CKD prevalence stratified by sex and tobacco exposure was not feasible.
Conclusions
The findings emphasize the need for further research to comprehend the intricate relationship between, tobacco exposure, sex, and CKD management, as well as the consideration of cultural, geographical, socioeconomic, political, and structural factors when understanding the pathophysiology and management of CKD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03845-y.
Keywords: SDH, Health Inequities, Kidney Disease, Socioeconomic Status, Tobacco Dependency
Introduction
Chronic Kidney Disease (CKD) affects over 800 million people with an estimated global prevalence of 13.4% [1–3]. Current guidelines identify the diagnostic criteria for CKD as (1) a glomerular filtration rate (GFR) less than 60 mL/minute/1.73 m2, and/or (2) one or more markers suggestive of kidney damage which includes albuminuria, urinary sediment abnormalities, electrolyte abnormalities, renal tubular disorders, histological or structural changes, and a history of kidney transplant, occurring for a period of greater than three months [3, 4].
In general, females have been shown to have a superior health status than males [5]. Females have a longer life expectancy of 4.4 years as shown in 2016. They have been shown to have higher rates of survival regarding chronic heart failure and myocardial infarctions [6]. Female patients also have higher cancer-specific survivals of colorectal cancer [5]. Contrary to these previous findings, female who consume tobacco have been shown to have a greater risk of chronic diseases compared to male [5].
Individuals who consume greater than 30 packs of cigarettes per year are 2.6 times more likely to develop CKD, however difference in prevalence between females and males who consume tobacco has not been elucidated [7, 8]. Furthermore, although global tobacco smoking rates are declining in higher-income countries, little decrease is observed in lower- and middle-income countries, particularly in Asia and Africa [9–11]. It has previously been shown that sex affects the causal pathway between tobacco consumption and the development of CKD, however, the studies show ambivalent results [2, 12]. These differences may be attributed to estrogen's protective effects or testosterone’s harmful effects [12]. One study examined differences in the magnitude of glomerulotubular homeostasis alteration between male and female cigarette smoke (CS)-exposed mice [13]. Both CS-exposed male and female mice experienced a significant increase in fibrosis, inflammation, and glomerulotubular damage when compared to their respective controls, but CS-exposed female mice showed a lesser effect. These observations show sex differences in inflammatory responses and cytokine production when exposed to tobacco, possibly attributed to estrogen's well-documented protective effects [13].
Projections indicate that CKD will be the fifth leading cause of death by 2040 [14, 15]. CKD burden is particularly high in lower- and middle-income countries, with India ranking eighth globally in CKD-related deaths [16, 17]. Given the potential for a sexually dimorphic response to tobacco in CKD, an improved understanding of these interactions can better help inform healthcare decision-making. Therefore, the primary objective is to investigate how sex differences mediate the causal pathway between tobacco exposure and the development of CKD. The secondary objective is to examine patterns of CKD prevalence related to regional variability and social determinants of health.
Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, and an a priori protocol was published on PROSPERO (ID: CRD42022371292) to ensure transparency and methodological rigor [18, 19].
Search strategy
A comprehensive search, assisted by a medical librarian, was conducted in MEDLINE, EMBASE, clinicaltrials.gov, and the preprint server MedvRix, from inception until October 6th, 2022. Some keywords such as “chronic kidney disease,” “smoking,” “sex differences,” were used. The full search strategies are available in the supplementary (Appendix S1).
Study selection
The search results were imported into Covidence, a systematic review management software. Pilot testing was conducted until Cohen's kappa inter-rater reliability value of 0.8 was achieved. Five reviewers (NV, AV, AX, EF, OH) were involved in the study selection process. Specifically, two reviewers were independently assigned to each study for title and abstract screening, followed by full-text review for eligibility. Exclusion reasons were documented, with discrepancies resolved by a third reviewer (NW) if necessary. Studies not reporting primary data (i.e., systematic reviews or post-hoc analyses), reviews, abstracts, conference posters, comments, editorials, or those not published in English were excluded.
Data extraction
Data extraction was independently performed by two reviewers for each study, involving the authors NV, AV, AX, EF, and OH. All discrepancies were resolved through discussion with a third author (NW) for full-text articles meeting the inclusion criteria. Extracted data encompassed patient characteristics (age, sex, smoking history including tobacco and other substances — exposure level and pack-years, comorbidities), general study information (country, journal, funding source), diagnostic methods (e.g., GOLD criteria), social determinants of health (race, education, study location — rural or urban communities), and the cause of CKD (e.g., tobacco-related or non-tobacco-related).
Quality assessment
The included literature's quality was assessed using the Newcastle–Ottawa Scale, a validated tool that assesses literature based on eight items across three categories: study group selection, group comparability, and establishment of exposure and outcomes [20–22].
Results
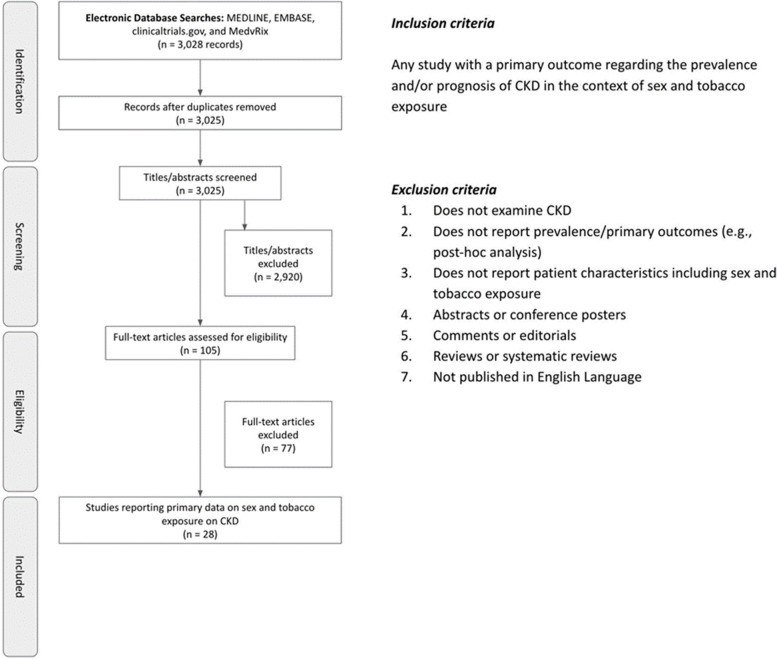
A total of 3,028 studies were identified, for which three duplicates were removed and 2,920 were excluded during the title and abstract screening. Of these, 105 studies remained for full-text screening, of which 77 were excluded for not reporting primary data stratified by both sex and tobacco exposure. Ultimately, 28 studies met the predetermined inclusion criteria (Fig. 1).
Fig. 1.
Flowchart illustrating the study selection the final number of articles included (n = 28)
Among the 28 included studies, five focused on Japanese [32–36] populations, four on Chinese [23–26] populations, three on populations from Iran [44, 87, 92] and Norway [28, 29, 31], and two on populations from India [37, 38], Taiwan [39, 40], and the United States [41, 94]. A population from Jordan [90], Australia [91], Singapore [27], the United Kingdom [30], France [42], Uganda [43], and Russia [81] were each studied once. Race information was collected in 22 studies, with reported categories including Chinese (5) [23–27], Caucasian (4) [28–31], Japanese (5) [32–36], Indian (3) [27, 37, 38], Taiwanese (2) [39, 40], South American (1) [29], Malaysian (1) [27], non-Hispanic White (1) [33], Hispanic White (1) [33], African American (1) [33], Black Caribbean (1) [41], European (1) [42], African (1) [43], Iranian (1) [44], or other (1) [27]. Of these, 11 studies reported on education [25, 26, 28, 31, 37–39, 44, 87, 90, 92], 20 reported on comorbidities [24–27, 30, 31, 34, 35, 37–44, 81, 87, 92, 94], and 17 reported on study duration[23, 25, 27–29, 31–37, 40, 41, 44, 81, 92]. Regarding funding, 10 studies received government funding [23, 24, 27, 30, 32, 35, 36, 40, 44, 94], four had institutional funding [29, 42, 43, 92], two had industry funding [37, 38], and ten reported no funding sources [25, 28, 31, 34, 39, 41, 81, 87, 90, 91], while two did not disclose their funding sources [26, 33]. Regarding study design, 15 studies were cross-sectional, eight were prospective cohort studies, two were retrospective cohort studies, and three were case–control studies (Tables 1 and 2).
Table 1.
Publication characteristics of the included studies and associated risk factors of chronic kidney disease
| Author (Year) | Source of Funding | Country | Study Location (Rural/Urban) | Study Design | Study Duration | Comorbidities | Race | Risk factors for CKD |
|---|---|---|---|---|---|---|---|---|
| Dehghani (2022) [87] | None | Iran | Urban | Cross-sectional | Not reported | Obesity; Diabetes Mellitus; Cardiovascular disease; Hypertension; Hypertriglyceridemia; Hypercholesterolemia | Not reported | BMI ≥ 30 (n = 1024/9781; 10.5%) diabetes (n = 735/9781; 7.5%) hypertriglyceridemia (n = 1424/9781; 14.6%) history of cardiovascular disease (n = 349/9781; 3.6%) hypertension (n = 898/9781; 9.2%) LDL ≥ 130 (n = 1528/9781; 15.6%) history of kidney stone (n = 518/9781; 5.3%) hypercholesterolemia (n = 1167/9781; 11.9%) |
| Alramly (2013) [90] | None | Jordan | Not reported | Descriptive; Cross-sectional; Correlational | Not reported | Not reported | Not reported | Not reported |
| Briganti (2002) [91] | None | Australia | Urban; Rural | Cross-sectional | Not reported | Not reported | Not reported | Not reported |
| Dong (2021) [23] | Government | China | Urban | Observational Cohort | 10 years | Not reported | Chinese | Not reported |
| Hallan (2011) [28] | None | Norway | Urban | Prospective Cohort | 10.3 years | Not reported | Caucasian | Not reported |
| Huang (2016) [24] | Government | China | Urban | Cross-sectional | Not reported | Hyperuricemia; Hypertension; Diabetes; Cardiovascular disease | Chinese |
30 > BMI ≥ 25 (kg/m2) (n = 1003/24886; 4.0%) BMI ≥ 30 (kg/m2) (n = 134/24886; 0.5%) high triglyceride (n = 740/24886; 3.0%) high cholesterolemia (n = 1021/24886; 4.1%) Hyperuricemia (n = 1207/24886; 4.9%) Low HDL-C (n = 1110/24886; 4.5%) High LDL-C (n = 130/24886; 0.5%) High fasting plasma glucose (n = 351/24886; 1.4%) Drinking (n = 79/24886; 0.3%) Hypertension (n = 1841/24886; 7.4%) Diabetes (n = 629/24886; 2.5%) Cardiovascular disease (n = 945/24886; 3.8%) |
| Noborisaka (2013) [32] | Government | Japan | Urban | Retrospective Cohort | 6 years | Not reported | Japanese | Not reported |
| Sepanlou (2017) [92] | Institutional | Iran | Urban; Rural | Observational cohort | 2 years | Cardiovascular disease; Hypertension; Diabetes | Not reported | Not reported |
| Umesawa (2018) [33] | Not reported | Japan | Not reported | Observational cohort | 10 years | Not reported |
Japanese Non-Hispanic White Hispanic White African American |
Not reported |
| Xue (2014) [25] | None | China | Urban | Cross-sectional | 4 months | Cardiovascular disease; Diabetes mellitus; Hyperlipidemia; Hypercholesterolemia; Hyperuricemia | Chinese | Not reported |
| Yamagata (2007) [34] | None | Japan | Not reported | Prospective Cohort | 10 years | Proteinuria; Hematuria; Hypertension; Diabetes; Obesity; Hypercholesterolemia; Hypertriglyceridemia | Japanese | Not reported |
| Yang (2018) [26] | Not reported | China | Urban | Cross-sectional | Not reported | Diabetes; Stroke; Coronary heart disease; Peripheral Arterial Disease; Hypertension; | Chinese |
BMI category (n, %) Underweight (n = 113/31574; 0.4%) Normal (n = 1659/31574; 5.3%) Overweight (n = 1867/31574; 5.9%) Obese (n = 5718/31574; 18.1%) Alcohol status (n, %) Non-drinker (n = 6319/31574; 20.0%) Current drinker (n = 1299/31574; 4.1%) Ex-drinker (n = 1761/31574; 5.6%) CHD (n = 315/31574; 1.0%) Stroke (n = 608/31574; 1.9%) PAD (n, %) Yes (n = 143/31574; 0.5%) Suspected (n = 48/31574; 0.2%) DR Status (n,%) No DR (n = 5041/31574; 16.0%) Non-sight threatening (n = 1990/31574; 6.3%) Sight threatening (n = 1575/31574; 5.0%) Ungradable (n = 31/31574; 0.1%) |
| Anupama (2014) [37] | Industry | India | Rural | Cross-sectional | 11 months | Diabetes mellitus; Hypertension; Ischemic heart disease; Stroke | Indian |
BMI < 18 (n = 31/2091; 1.5%) 18–22.9 (n = 65/2091; 3.1%) 23–24.9 (n = 16/2091; 0.8%) > 25 (n = 19/2091; 0.9%) Hypertension (n = 78/2091; 3.7%) Diabetes (n = 80/2091; 3.82%) |
| Chang (2020) [39] | None | Taiwan | Not reported | Cross-sectional | Not reported | Hypertension; Diabetes; Dyslipidemia; Hyperuricemia/gout; Urinary tract stones; Cardiovascular disease; Cancer | Taiwanese |
BMI Underweight (n = 2762/297603; 0.9%) Normal (n = 36,575/297603; 12.3%) Overweight (n = 29,309/297603; 9.8%) Obese (n = 18,568/297603; 6.2%) Missing value (n = 1005/297603; 0.3%) Exercise habit No (n = 12,212/297603; 4.1%) Occasional (n = 28,282/297603; 9.5%) Regular (n = 41,105/297603; 13.8%) Missing value (n = 6620/297603; 2.2%) Alcohol drinking No (n = 86,177/297603; 29.0%) Yes (n = 1727/297603; 0.58%) Missing value (n = 315/297603; 0.1%) Hypertension (n = 47,553/297603; 16.0%) Diabetes (n = 19,802/297603; 6.7%) Dyslipidemia (n = 60,389/297603; 20.3%) Hyperuricemia/gout (n = 32,220/297603; 10.8%) Urinary tract stones (n = 1887/297603; 0.6%) Cardiovascular disease (n = 24,101/297603; 8.1%) Cancer (n = 478/297603; 0.2%) |
| Gjerde (2012) [29] | Institutional | Norway | Not reported | Cross-sectional | 1.5 years | Not reported |
Caucasian South American |
Not reported |
| Gummidi (2020) [38] | Industry | India | Rural | Prospective Cohort | Not reported | Hypertension; Diabetes; Heart disease; Obesity | Indian | Not reported |
| Lew (2017) [27] | Government | Singapore | Urban | Cross-sectional | 4 years | Hypertension; Diabetes mellitus; Cardiovascular disease; Stroke |
Chinese Indian Malaysian Other |
Not reported |
| Miguez-Burbano (2009) [41] | None | United States | Urban | Case–control | 1 year | Hypertension; Diabetes; Cancer; Hepatitis C; Hepatitis B | Black Caribbean | Not reported |
| Nakamura (2015) [35] | Government | Japan | Not reported | Observational cohort | 14.8 years | Diabetes; Proteinuria | Japanese | Not reported |
| Nitsch (2006) [30] | Government | United Kingdom | Not reported | Cross-sectional | Not reported | Obesity; Hypertriglyceridemia; Diabetes; | Caucasian | Not reported |
| Noborisaka (2013) [36] | Government | Japan | Not reported | Retrospective observational | 6 years | Not reported | Japanese | Not reported |
| Roseman (2017) [94] | Government | United States | Urban | Cross-sectional | Not reported | Hypertension; Diabetes; Albuminuria; Cardiovascular disease | Not reported | Not reported |
| Stengel (2000) [42] | Institutional | France | Urban | Case–control | Not reported | Hypertension | European | Not reported |
| Hallan (2006) [31] | None | Norway | Urban | Cross-sectional | 2 years | Diabetes mellitus; Cardiovascular disease; Hypertension; Obesity | Caucasian | Not reported |
| Kalyesubula (2017) [43] | Institutional | Uganda | Urban; Rural | Cross-sectional | Not reported | HIV-Infection; Diabetes; Hypertension; Proteinuria | African | Not reported |
| Korbut (2019) [81] | None | Russia | Not reported | Cross-sectional | 10 years | Diabetes; Diabetic retinopathy; Arterial hypertension; Coronary artery disease; Myocardial infarction in anamnesis; Chronic heart failure; Carotid atherosclerosis; Cerebrovascular event in anamnesis; Peripheral artery disease | Not reported |
Diabetic retinopathy (n = 131/360; 36.4%) Arterial hypertension (n = 184/360; 51.1%) Coronary artery disease (n = 99/360; 27.5%) Myocardial infarction in anamnesis (n = 36/360; 10.0%) Chronic heart failure (n = 11/360; 3.1%) Carotid atherosclerosis (n = 91/360; 25.3%) Cerebrovascular event in anamnesis (n = 24/360; 6.7%) Peripheral artery disease (n = 141/360; 39.2%) |
| Su (2015) [40] | Government | Taiwan | Urban | Case–control | 2 years | Obesity; Hepatitis B; Hepatitis C; Hyperuricemia; Anemia; Hyperlipidemia; | Taiwanese |
Obesity (n = 592/9138; 6.5%) Hyperuricemia (n = 1291/9138; 14.1%) Anemia (n = 863/9138; 9.4%) Hyperlipidemia (n = 1495/9138; 16.4%) Alcohol intake (ever) (n = 767/9138; 8.4%) Exercise habits (ever) (n = 3338/9138; 36.5%) Groundwater using (ever) (n = 293/9138; 3.2%) |
| Tohidi (2012) [44] | Government | Iran | Urban; Rural | Prospective Observational | 9.9 years | Diabetes mellitus; Hypertension; Cardiovascular disease | Iranian | Not reported |
Table 2.
Main findings on tobacco exposure, social determinants of health and chronic kidney disease
| Author (Year) | Total Sample Size | Sample Size (female) | Sample Size (male) | Mean (SD) or Range of Age Male in years | Mean (SD) or Range of Age Female in years | Diagnostic Method | CKD in females | CKD in males | Current/ Former/Never Smoking History (n and %) | Mean (SD) or Range of Pack Year Smoking History | Identified Risk Factors for CKD | Social Determinants of Health | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dehghani (2022) [87] | 9,781 | 4,860 | 4,921 | Not reported | Not reported | CKD (serum creatinine and eGFR < 60 ml/ min/1.73m2) | 1499 | 1186 |
Smoked (n = 2210; 22.6%) Never Smoked (n = 7571; 77.4%) |
Not reported | Smoking; Female sex |
Statistically significant protective factors against CKD: Higher education Not statistically significant association with CKD: Marital status |
|
| Alramly (2013) [90] | 161 | 69 | 92 | Not reported | Not reported | CKD (interview and medical files) | 47 | 45 |
Smoked (n = 75; 47%) Never Smoked (n = 86; 53%) |
Not reported | Male sex | Those with ESRD were significantly more likely to have a marital status of being single/divorced/widowed compared to married than those with CKD | |
| Briganti (2002) [91] | 11,247 | 5,910 | 5,337 | Not reported | Not reported | Renal impairment (eGFR < 60 mL/min/1.73 m2) | 3044 | 2588 |
Current Smokers (n = 2621; 23.3%) Non-Smokers (n = 8626; 76.7%) |
GFR ≥ 60: 18.1 ± 0.8 GFR < 60: 33.0 ± 5.7 |
Smoking; Male sex |
None | |
| Dong (2021) [23] | 141,516 | 78,078 | 63,438 | 63.07 ± 11.45 | 65.71 ± 11.78 | ESRD (ICD-9-CM or eGFR < 15 ml/min/1.73m2) | 5794 | 3315 |
Smoked (n = 5137; 10.87%) Never Smoked (n = 42,129; 89.13%) |
Not reported |
Smoking; Male sex |
None | |
| Hallan (2011) [28] | 65,589 | 34,911 | 30,678 | Not reported | Not reported | decrease of eGFR to 15 ml/min/1.73 m2 | 46 | 78 |
Current (n = 18,168; 27.7%) Former (n = 17,119; 26.1%) Never (n = 29,515; (45.0%) Unknown (n = 787; (1.2%) |
Former smoker: 11.4 ± 12.8 Current smoker: 14.5 ± 11.1 |
Smoking; Male sex |
Statistically significant risk factor for kidney failure: Higher education | |
| Huang (2016) [24] | 24,886 | 13,670 | 11,216 | Not reported | Not reported | CKD (NKF K/DOQI Guidelines) | 2409 | 1669 |
Smoked (n = 2455; 9.86%) Never Smoked (n = 22,431, 90.14%) |
Not reported | Smoking; Female sex | None | |
| Noborisaka (2013) [32] | 6,662 | 2,698 | 3,964 | 49.4 ± 7.8 | 50.2 ± 7.2 | CKD (GFR and proteinuria levels as per the new JSN criteria) | 418 | 559 |
Current (n = 2384; 35.8%) Former (n = 979; 14.7%) Never (n = 3299; 49.5%) |
Not reported | Smoking | Statistically significant risk factor for development of moderate to severe CKD: Miscellaneous job category | |
| Sepanlou (2017) [92] | 11,409 | 5,996 | 5,413 | 57.0 ± 8.3 | 55.5 ± 7.6 | CKD (eGFR < 60 ml/min/1.73m2) | 1588 | 1112 |
Smoked (n = 1871; 16.4%) Never Smoked (n = 9538; 83.6%) |
Not reported | Female sex | Statistically significant protective factors against CKD: Literacy, rural residence | |
| Umesawa (2018) [33] | 135,007 | 94,005 | 41,002 | 57.5 | 54.6 | eGFR < 45 mL/min/1.73 m2 and/or proteinuria by dipstick) | 10,358 | 6,106 |
Current (n = 23,541; 17.4%) Former (n = 12,233; 9.1%) Never (n = 99,233; 73.5%) |
Not reported | Smoking | None | |
| Xue (2014) [25] | 14,399 | 5,838 | 8,561 | 49.64 ± 16.65 | 48.13 ± 18.01 | eGFR < 60 mL/min/1.73 m2 | 619 | 747 | Not reported | Not reported | Smoking; Female sex | Statistically significant protective factors against CKD: Higher education | |
| Yamagata (2007) [34] | 123,764 | 82,752 | 41,012 | 61.8 ± 10.2 | 58.3 ± 10.0 | eGFR < 60 mL/min/1.73 m2 | 17,413 | 6,305 |
Current (n = 22,809; 18.4%) Former (n = 12,545; 10.1%) Never (n = 88,410; 71.4%) |
Not reported | Smoking | None | |
| Yang (2018) [26] | 31,574 | 15,649 | 15,925 | Not reported | Not reported | CKD (KDIGO guideline) | 4882 | 4504 |
Current (n = 4402; 13.9%) Former (n = 5574: 17.7%) Never (n = 21,572; 68.3%) |
Not reported | Smoking | Statistically significant protective factors against CKD: Higher education | |
| Anupama (2014) [37] | 2,091 | 1,138 | 953 | Not reported | Not reported | eGFR < 60 mL/min/1.73 m2 | 54 | 77 |
Smoked (n = 150; 7.2%) Never Smoked (n = 1941; 92.8%) |
Not reported |
Smoking; Male sex |
None | |
| Chang (2020) [39] | 297,603 | 144,260 | 153,343 | Not reported | Not reported | eGFR < 60 mL/min/1.73 m2 | 35,035 | 53,184 |
Smoked (n = 20,047; 6.7%) Never Smoked (n = 276,610; 93.0%) |
Not reported | Smoking | Widowed/divorced/separated or never married compared to married and poor socioeconomic status | |
| Gjerde (2012) [29] | 422 | 167 | 255 | Not reported | Not reported | eGFR < 60 mL/min/1.73 m2 | 16 | 13 |
Current (n = 190; 43.9%) Former (n = 243; 56.1%) Never (n = 0; 0%) |
Not reported | Female sex | None | |
| Gummidi (2020) [38] | 2,402 | 1,222 | 1,180 | 46.8 ± 13.99 | 44.57 ± 12.49 | CKD (KDIGO criteria) | 209 | 297 |
Smoked (n = 1032; 42.96%) Never Smoked (n = 1370; 57.04%) |
Not reported |
Smoking; Male sex |
Statistically significant protective factors against CKD: Higher education Not statistically significant association with CKD: Income, outdoor workers |
|
| Lew (2017) [27] | 88,765 | 47,331 | 41,434 | Not reported | Not reported | CKD (eGFR < 60 mL/min/1.73 m2 or 1 + dipstick proteinuria excretion) | 11,768 (95% CI: 24.5–25.3) | 11,247 (95% CI: 26.7–27.6) |
Smoked (n = 8562; 9.7%) Never Smoked (n = 80,203; 90.3%) |
Not reported | Male sex | Those of Malay ancestry were more likely and those of Indian ancestry were less likely to have CKD than those of Chinese ancestry | |
| Miguez-Burbano (2009) [41] | 536 | 230 | 306 | Not reported | Not reported | GFR < 60 ml/min/1.73 m2) | 23 | 52 |
Current (n = 296; 55.2% Former (n = 50; 9.3%) Never (n = 190; 35.5%) |
CKD: 12.7 ± 0.9 Non-CKD: 8.8 ± 1.3 |
Smoking; Male sex |
Black Caribbeans and African Americans more likely to have CKD than non-Black individuals | |
| Nakamura (2015) [35] | 34,622 | 19,154 | 15,468 | 57.4 | 58.0 | CKD (eGFR < 60 ml/min per 1.73 m(2)) and/or dipstick proteinuria) | 1054 | 1083 |
Current (n = 9685; 28.0%) Former (n = 4281; 12.4%) Never (n = 20,656; 59.6%) |
Not reported | Smoking | None | |
| Nitsch (2006) [30] | 6,317 | 3,217 | 3,100 | 51.8 ± 11.4 | 52.5 ± 11.4 | eGFR < 60 ml/min/1.73 m2 | 538 | 141 |
Current (n = 841; 27.1%) Not-current (n = 2259; 72.9%) |
Not reported |
Smoking; Female sex |
None | |
| Noborisaka (2013) [36] | 6,998 | 2,877 | 4,121 | 41.2 ± 9.7 | 42.3 ± 9.3 | Proteinuria (dipstick method) | 369 | 492 |
Current (n = 2085; 29.8%) Former (n = 537; 7.7%) Never (n = 4376; 62.5%) |
Not reported | Smoking | Occupation not associated with CKD | |
| Roseman (2017) [94] | 1,852 | 981 | 871 | 64.3 ± 9.2 | 63.9 ± 9.2 | eGFR < 45 mL/min/1.73 m2 | 58 | 62 |
Current (n = 343; 18.5%) Former (n = 573; 31.0%) Never (n = 936; 50.5%) |
Not reported |
Smoking; Male sex |
None | |
| Stengel (2000) [42] | 537 | 197 | 340 | Not reported | Not reported | serum creatinine > 150 micromole/L | 17 | 57 |
Smoked (n = 272; 50.6%) Never smoked (n = 265; 49.4%) |
Not reported |
Smoking; Male sex |
None | |
| Hallan (2006) [31] | 65,193 | 34,708 | 30,485 | Not reported | Not reported | eGFR < 45 mL/min/1.73 m2 | 375 | 246 |
Smoked (n = 36,358; 55.8%) Never smoked (n = 28,835; 44.2%) |
GFR ≥ 45: 6.9 ± 10.8 GFR < 45: 8.5 ± 16.1 |
Smoking | None | |
| Kalyesubula (2017) [43] | 955 | 640 | 315 | 30 (24–40) | 32 (25–43) | creatinine clearance < 60 mls/min/1.73 m2 | 104 | 41 |
Current (n = 54; 5.6%) Former (n = 39; 4.1%) Never (n = 862; 90.3%) |
Not reported | None | Statistically significant risk factors for kidney disease: High socioeconomic status | |
| Korbut (2019)[81] | 360 | 260 | 100 | Not reported | Not reported | eGFR < 45 mL/min/1.73 m2 | 149 | 35 |
Smoked (n = 34; 9.4%) Never Smoked (n = 326; 90.6%) |
Not reported | Smoking; Female sex; Male sex | Not reported | |
| Su (2015) [40] | 10,463 | 5,245 | 5,218 | Not reported | Not reported | eGFR < 60 mL/min | 2302 | 3026 |
Smoked (n = 2090; 20.6%) Never Smoked (n = 8035; 79.4%) |
Not reported | Male sex | Low income | |
| Tohidi (2012) [44] | 3,313 | 1,859 | 1,454 |
Non-CKD: 39.28 ± 12.56 CKD: 53.87 ± 11.64 |
Non-CKD: 35.52 ± 10.67 CKD: 45.63 ± 12.17 |
eGFR < 45 mL/min/1.73m2 | 517 (95% CI: 25.77–29.85) | 206 (95% CI: 12.38–15.96) |
Current (n = 322; 18.2%) Former (n = 135; 7.6%) Never (n = 1312; 74.2%) |
Not reported |
Smoking; Female sex |
None | |
Additionally, seven studies reported on CKD etiology [24, 26, 37, 39, 40, 81, 87]. The reported prevalence of diabetic-related CKD ranged from 2.5% to 36.4% [24, 26, 37, 39, 81, 87], while CKD related to hypertension ranged from 3.7% to 51.1% [24, 37, 39, 81, 87]. Only one study explored IgA nephropathy as a potential cause of CKD (Table 1) [42]. A total of 22 studies identified smoking as a risk factor for CKD [23–26, 28, 30–39, 41, 42, 44, 81, 87, 91, 94]. Disparity in tobacco smoking prevalence among participants is evident, with people who currently consume tobacco, ranging from 5.6% to 55.2%, people who formerly consumed tobacco, from 4.1% to 56.1%, and people who never consumed tobacco, from 6.7% to 55.8% (Table 2) [23, 24, 26–44, 81, 87, 90–92, 94].
Regarding social determinants of health, 18 studies reported primary outcome measures [25–28, 32, 36, 38–41, 43, 87, 90, 92]. Several studies found no statistically significant impact of factors like relationship status, family structure, occupation, social class, or education on CKD prevalence [36, 38, 87]. Several studies found significant associations between CKD prevalence and socioeconomic status [39, 43].Higher education was associated with a lower likelihood of CKD in some studies but linked to kidney failure in others [25, 26, 28, 38, 87, 92]. Higher income was generally associated with lower CKD prevalence, while lower income and poverty were linked to higher CKD risk [38, 40]. Certain occupations such as security guard, farmer, or housekeeper were associated with higher rates of proteinuria compared to clerical work [32, 36, 38]. Relationship status also played a role, with widowed, divorced, separated, or never-married individuals more likely to have decreased kidney function compared to those in relationships [39, 90]. Some studies noted racial and ethnic disparities in CKD prevalence [27, 41]. Black individuals from the Caribbeans and African Americans had higher CKD rates compared to non-Black individuals [41]. Malaysian ancestries were more likely to have CKD, while individuals of Indian ancestry were less likely to have CKD compared to those of Chinese ancestry (Table 2) [27].
Four studies looked at CKD prevalence in both urban and rural populations [43, 44, 91, 92], 13 studies focused on urban setting [23–28, 31, 32, 40–42, 87, 94], and two studies focused on rural populations [37, 38]. Among the two studies, CKD prevalence in females ranged from 4.7% to 17.1%, and in males from 8.1% to 25.2% [37, 38]. Studies exclusively focused on urban populations displayed wider ranges, with CKD prevalence in females from 0.1% to 43.9%, and in males from 0.3% to 58% [23–28, 31, 32, 40–42, 87, 94]. For studies encompassing both settings, CKD prevalence in females ranged from 16.3% to 51.5%, and in males from 13.0% to 48.5%, suggesting CKD prevalence varies based on urban and rural contexts (shown in Table 1) [43, 44, 91, 92].
There was significant geographical diversity in CKD prevalence. In Asia, rates varied widely, with East Asia reporting prevalence ranging from 5.5% to 43.9% in females and 5.2% to 58.0% in males, with Taiwanese males exhibiting the highest CKD prevalence [23–26, 32–36, 39, 40]. South and Southeast Asia reported prevalence ranging from 4.7% to 24.9% in females and 8.1% to 27.1% in males [27, 37, 38]. West Asia had rates from 26.5% to 68.1% in females and 14.2% to 48.9% in males, with Jordanian females exhibiting the highest CKD prevalence [44, 87, 90, 92]. Lower rates were seen in Western and Northern Europe (0.1–16.7% in females, 0.3–16.7% in males) [28–31, 42], while Central and Western Europe showed high rates (57.4% in females, 35.0% in males) [81]. The Norwegian population had the lowest CKD prevalence for both males and females [28, 29, 31]. North America reported rates from 5.9–10.0% in females and 7.1–17.0% in males [41, 94]. One study from Africa reported prevalence of 16.2% in females and 13.0% in males, while Australia showed rates similar to Western Asia (51.5% in females, 48.5% in males) [43, 91] (Table 3).
Table 3.
CKD prevalence in males and females per region and country
| Region (n = number of studies) | Prevalence of CKD – Female (%) | Prevalence of CKD – Male (%) |
|---|---|---|
| East Asia (11) | ||
| China (4) [23–26] | 7.4–31.2 | 5.2–28.3 |
| Japan (5) [32–36] | 5.5–21.0 | 7.0–15.4 |
| Taiwan (2) [39, 40] | 24.3–43.9 | 34.7–58.0 |
| South Asia (2) | ||
| India (2) [37, 38] | 4.7–17.1 | 8.1–25.2 |
| South-East Asia (1) | ||
| Singapore (1) [27] | 24.9 | 27.1 |
| West Asia (4) | ||
| Jordan (1) [90] | 68.1 | 48.9 |
| Iran (3) [44, 87, 92] | 26.5–30.8 | 14.2–24.1 |
| Western Europe (2) | ||
| United Kingdom (1) [30] | 16.7 | 4.5 |
| France (1) [42] | 8.6 | 16.7 |
| Northern Europe (3) | ||
| Norway (3) [28, 29, 31] | 0.1–9.6 | 0.3–5.1 |
| Central and Western Europe (1) | ||
| Russia (1) [81] | 57.4 | 35.0 |
| Australia (1) [91] | 51.5 | 48.5 |
| North America (2) | ||
| United States (2) [41, 94] | 5.9–10.0 | 7.1–17.0 |
| Africa (1) | ||
| Uganda (1) [43] | 16.2 | 13.0 |
The quality of included literature was assessed as Good and Poor, as per the Newcastle–Ottawa Scale (Table 4). Ten studies demonstrated high methodological rigor and were rated as Good [28, 30, 32–36, 41, 44, 93], while 18 were rated as Poor due to a risk of bias [23–27, 29, 31, 37, 39, 40, 42, 43, 81, 87, 90–92, 94]. Participant selection scores ranged from two to four, indicating moderate to low risk of bias. Participant comparability scores ranged from zero to two, indicating high to low risk of bias. Outcome determination scores ranged from one to three, indicating high to low risk of bias. Regarding funding, 10 studies received government funding [23, 24, 27, 30, 32, 35, 36, 40, 44, 94], four had institutional funding [29, 42, 43, 92], two had industry funding [37, 38], and ten reported no funding sources [25, 28, 31, 34, 39, 41, 81, 87, 90, 91], while two did not disclose their funding sources [26, 33]. Regarding study design, 15 studies were cross-sectional [24–27, 29–31, 37, 39, 43, 81, 87, 90, 91, 94], eight were prospective cohort studies [23, 28, 33–35, 38, 44, 92], two were retrospective cohort studies [32, 36], and three were case–control studies [40–42] (Tables 1 and 2).
Table 4.
Quality of the 28 included articles as per the Newcastle–Ottawa Scale
| Author (Year) | Selection | Comparability | Outcome | Quality |
|---|---|---|---|---|
| Dehghani (2022) [87] | 2 | 0 | 1 | Poor |
| Alramly (2013) [90] | 2 | 0 | 1 | Poor |
| Briganti (2002) [91] | 2 | 1 | 1 | Poor |
| Dong (2021) [23] | 3 | 0 | 2 | Poor |
| Hallan (2011) [28] | 3 | 1 | 3 | Good |
| Huang (2016) [24] | 3 | 1 | 1 | Poor |
| Noborisaka (2013) [32] | 3 | 1 | 2 | Good |
| Sepanlou (2017) [92] | 2 | 1 | 1 | Poor |
| Umesawa (2018) [33] | 4 | 1 | 2 | Good |
| Xue (2014) [25] | 3 | 0 | 1 | Poor |
| Yamagata (2007) [34] | 4 | 1 | 2 | Good |
| Yang (2018) [26] | 2 | 1 | 1 | Poor |
| Anupama (2014) [37] | 3 | 1 | 1 | Poor |
| Chang (2020) [39] | 2 | 1 | 1 | Poor |
| Gjerde (2012) [29] | 2 | 1 | 1 | Poor |
| Gummidi (2020) [38] | 3 | 1 | 2 | Good |
| Lew (2017) [27] | 3 | 1 | 1 | Poor |
| Miguez-Burbano (2009) [41] | 3 | 1 | 2 | Good |
| Nakamura (2015) [35] | 3 | 2 | 2 | Good |
| Nitsch (2006) [30] | 4 | 2 | 2 | Good |
| Noborisaka (2013) [36] | 4 | 2 | 2 | Good |
| Roseman (2017) [94] | 3 | 2 | 1 | Poor |
| Stengel (2000) [42] | 3 | 1 | 1 | Poor |
| Hallan (2006) [31] | 3 | 2 | 1 | Poor |
| Kalyesubula (2017) [43] | 3 | 2 | 1 | Poor |
| Korbut (2019) [81] | 3 | 1 | 1 | Poor |
| Su (2015) [40] | 3 | 2 | 1 | Poor |
| Tohidi (2012) [44] | 4 | 2 | 2 | Good |
None of the included studies provided suitable data for meta-analysis on CKD prevalence stratified by both sex and tobacco exposure.
Discussion
This systematic review reveals significant variability in study results and quality across the literature, including differences in exposure and outcome definitions, study designs, methodologies, sourced populations, and follow-up durations. While aiming to explore the association between sex, tobacco exposure, and CKD development through a meta-analysis of CKD prevalence, none of the included studies provided data on all three components. Two studies reporting CKD prevalence stratified by sex showed high heterogeneity, precluding meta-analysis [27, 44]. Among the 28 included studies, 22 identified smoking as a CKD risk factor, while 12 and eight studies recognized male and female sex as risk factors, respectively (Table 2). Despite these limitations, this analysis hints at a potential sexually dimorphic relationship between smoking and CKD development, emphasizing the need for further research on CKD prevalence and sex-specific risk factors.
Tobacco exposure has been identified as a primary contributor to CKD development, with evidence suggesting a dose-dependent relationship [45, 46]. Risk factors for tobacco exposure and dependence include age, sex, genetics, substance use, education, income, race, and geographic location [47]. It is crucial to explore the association between sex and CKD, while accounting for tobacco exposure and other influential variables such as older age, diabetes mellitus, and hypertension [48]. Sex-related disparities in CKD progression reveal that factors like smoking may contribute to sex differences, with females more likely to abstain from smoking and experience lower rates of end-stage renal disease and death compared to males [49]. Similarly, there are lower total smoking doses and rare occurrences of kidney failure progression in females, despite equal current smoking prevalence in males [28]. Additionally, there is higher CKD prevalence in females but faster disease progression in males [2]. The reasons for these sex differences remain unclear and could involve a combination of intersectional factors such as biological differences, socioeconomic, political, and structural inequities, and cultural differences. While tobacco use is declining among males according to the World Health Organization, there is a notable increase among females in low- and low-middle countries [50]. Gender norms further influence smoking behaviors, with women often associating smoking with femininity, attractiveness, and rebellion, while men see it as a symbol of strength, virility, independence, and mystery [51–53]. Women typically start smoking later out of curiosity, while men tend to imitate [54]. This gender disparity in smoking initiation may contribute to the higher prevalence of CKD in men, given their longer and heavier smoking habits compared to women.
Estrogen has been extensively documented to exert multiple beneficial effects on kidney structure and function, mediated through both genomic and non-genomic pathways involving estrogen receptors (ERs). A 15-year prospective population-based study revealed a 2.66 hazard ratio of CKD incidence in females with lower endogenous estrogen exposure (EEE) during later stages of life when compared to females with higher EEE [55]. Estrogen significantly mitigates glomerulosclerosis, a key pathological feature of CKD characterized by the sclerosis of glomeruli and subsequent impairment of renal function. The anti-sclerotic effects of estrogen are likely mediated through the attenuation of mesangial cell proliferation and the suppression of extracellular matrix protein deposition [56]. Similarly, estrogen plays a crucial role in reducing tubulointerstitial fibrosis. These anti-fibrotic effects are thought to be mediated through the modulation of inflammatory responses and the regulation of key fibrotic mediators such as transforming growth factor-beta (TGF-β) [56]. A critical aspect of estrogen's role in renal physiology is its regulation of phosphorus-calcium balance, a process predominantly occurring in the proximal renal tubules. Estrogen promotes the reabsorption of calcium and phosphate, which helps maintain serum levels within physiological norms and prevents complications such as renal osteodystrophy. The regulation of these minerals is particularly crucial in CKD, where dysregulation can lead to significant morbidity [56]. Estrogens are also pivotal in maintaining mitochondrial integrity and function within renal cells, potentially influencing cellular energy dynamics and apoptosis pathways [57]. Moreover, estrogen modulates the endothelin-1 system, which is integral to maintaining vascular tone and ensuring adequate renal blood flow and glomerular filtration rate (GFR), thus supporting overall kidney function [57]. Emerging research highlights the significance of ERα polymorphisms in influencing the susceptibility and progression of renal diseases. These genetic variations may alter the normal signaling pathways of estrogen and its receptors, potentially affecting the individual's response to endogenous or exogenous estrogens [57]. Men, lacking the protective effects of estrogen, might experience more pronounced renal tissue damage under similar conditions of stress or disease, leading to a higher prevalence and faster progression of CKD. Understanding these sex-based differences in CKD, mediated through estrogenic effects, is crucial for developing targeted therapies that could leverage the protective effects of estrogen or its analogs.
The literature on the impact of testosterone on kidney function presents conflicting findings. Animal studies have indicated detrimental effects of testosterone on the kidney, including glomerular and tubular damage, kidney fibrosis, proteinuria, and hypertensive effects [58–65]. Conversely, testosterone has also been associated with positive effects on the kidney, such as renal vasodilation, reduced inflammation, and decreased kidney injury, as observed in both animal and human studies [58–65]. Additionally, the testosterone precursor hormone dehydroepiandrosterone sulfate is believed to influence kidney function through various mechanisms, although its overall impact on kidney function remains uncertain [66]. A meta-analysis revealed that lower testosterone levels may increase the risk of CKD in the general population and elevate the risk of all-cause mortality and cardiovascular events in males with CKD [66]. These findings are consistent with a prospective population-based study that identified a higher hazard ratio of CKD progression in male adults with hypogonadism compared to those with normal testosterone levels in later life [67, 68].
DM is considered the most common cause of CKD and ESRD with type 2 DM (T2DM) accounting for 30–50% of cases and type 1 DM (T1DM) accounting for 3.9% of cases [69–72]. The most common medication for diabetes management is metformin. A previous long-term study, the DPP Outcomes Study, showed that metformin induces a greater effect in reducing coronary artery calcium in men that can suggest a protective cardiovascular effect. This can suggest that despite the use of metformin, women are at a greater risk of renal infarct or progressive CKD compared to men [73]. Another study evaluated creatinine levels in patients with acute myocardial infarction with diabetes. Females were found to have higher creatinine levels, which was an independent predictor for a longer stay in the hospital [74]. Furthermore, a longitudinal study, the REGARDS trial, showed that being male was associated with a relative risk (RR) of 0.95 (95% CI: 0.84–1.09) risk of developing CKD. This is compared to diabetes and smoking which had a RR of 1.91 (95% CI: 1.65–2.20) and 1.30 (95% CI: 1.08–1.57) respectively [75]. On the contrary, a study examining 8413 individuals with T2DM and CKD in the UK showed a hazard ratio of 0.84 (95% confidence interval: 0.77 to 0.92) for all-cause mortality for female compared to male. The hazard ratio for smoking, regardless of sex, was 1.62 (95% CI: 1.39 to 1.88) [76]. These studies show that females may be at risk for worse renal function in the context of diabetes, but other systemic factors are still protective against mortality. One thing is for certain, risk factor modification, especially tobacco cessation, is an important component of managing CKD in the context of diabetes.
One included study looked at IgAN and found a dose–effect relationship in chronic renal failure. It reported odds ratios (OR) of 1.9 vs 1.3 for ≤ 20 cigarettes/day and an OR of 5.2 vs 3.0 for > 20 cigarettes/day, and an OR of 1.9 vs 1.4 for ≤ 15 pack years and 3.9 vs 2.0 for > 15 pack years [42]. IgAN was focused in this study due to its higher prevalence relative to other glomerular diseases such as IgG4-related disease, lupus nephritis, ANCA-associated vasculitis, and amyloidosis. IgAN is the most common primary glomerulonephritis worldwide, with significant implications for patient outcomes. Additionally, potential sex-specific differences in the incidence and progression of IgAN further justify its inclusion in this discussion [77]. IgAN has an estimated incidence of 2.5/100,000 people, with a greater burden observed in Asian populations [6, 78, 79]. A higher risk of major susceptible loci in mucosal immunity, IgA production, and complement activation pathways were found in Chinese patients [80]. White patients with minimally symptomatic IgAN showed slower disease progression, with only 4% experiencing significant proteinuria (> 1 g/day) over a span of 108 months, compared to 33% in Chinese and Japanese cohorts [81]. Considering IgAN's heavier burden in Asian nations, particularly in populous countries like India, where it has a prevalence of 16.5%, its impact on global CKD rates must be recognized [17].
In India, the bidi industry constitutes a significant segment of the tobacco market. Bidis are manually crafted cigarettes formed using dried tendu leaves encasing tobacco. This sector not only sustains millions financially but also presents substantial health risks to its workforce, which predominantly consists of females and children [82–85]. These workers, originating from socioeconomically vulnerable demographics, are exposed to occupational hazards that are often under-recognized and poorly addressed, thereby emphasizing profound disparities in workplace health and safety standards. During bidi production, workers are subjected to both direct and passive inhalation of nicotine and tobacco dust [83, 86]. This exposure leads to the transdermal and respiratory absorption of nicotine [83, 86]. Prolonged exposure to these substances has been documented to precipitate a multitude of health issues. Specifically, the ingestion and dermal absorption of nephrotoxic substances, such as heavy metals found in tobacco, are implicated in various forms of kidney damage [83, 85–89]. This exposure could heighten the risk of chronic kidney disease (CKD) and is exacerbated by the socioeconomic status of these workers, who frequently lack adequate access to healthcare, thereby delaying the diagnosis and management of CKD and other health issues. The majority of bidi rollers are women and children, drawn to this home-based, labor-intensive employment as it allows for the concurrent management of household responsibilities [82–85]. There is a critical need for targeted research to elucidate the long-term health effects of tobacco exposure among bidi rollers, particularly regarding renal and overall health. Policy initiatives to enhance health equity among bidi rollers must incorporate preventive and remedial strategies. Preventive measures should include the enforcement of stringent regulations on occupational exposure to tobacco, enhancement of workplace safety, and provision of protective equipment. Remedial strategies should concentrate on providing accessible and affordable healthcare to these workers. Health interventions might include regular health screenings, subsidized healthcare services tailored to the specific needs of bidi rollers (especially concerning renal health), and educational programs to raise awareness about occupational risks. Addressing the intricate health, social, and economic challenges faced by bidi rollers, and others in similar circumstances, requires a comprehensive approach that involves governmental action, community support, and international attention to reform labor conditions, enhance healthcare access, and ensure equitable economic opportunities for this vulnerable population.
Study limitations include inconsistent control of second-hand smoke exposure quantity, variability in reporting smoking status, bias towards urban populations in included studies impacting generalizability, differences in CKD diagnostic criteria, study design, follow-up period, and tobacco exposure definition across included studies. Additionally, CKD rates varied due to cultural, genetic, and environmental factors, and 18 of the included studies were rated as 'poor' quality according to the Newcastle–Ottawa Scale. Moreover, the included studies did not stratify results by caste, which is significant given the higher disease burden and mortality rates observed in lower caste groups due to factors such as poverty, poor sanitation, and limited access to healthcare, potentially introducing bias into the results. However, this systematic review offers a holistic analysis of the complex interplay between tobacco exposure, sex differences, and CKD development, providing an in-depth understanding of how these factors collectively influence disease risk and aiding in the development of tailored prevention and intervention strategies. By including studies from diverse geographic regions and accounting for social determinants of health, it provides a well-rounded perspective on the global and socio-economic factors influencing CKD prevalence.
Conclusion
This comprehensive analysis of CKD encompasses diverse contributors to its development and progression. The roles of sex hormones, cultural influences, and socio-economic factors add layers of complexity, necessitating continued research to unravel the intricacies of CKD etiology and pathogenesis. From the geographical variations in IgA nephropathy prevalence to the intricate relationship between hypertension, tobacco exposure, and CKD, the multifaceted nature of these factors underscores the need for nuanced, context-specific interventions. This knowledge is vital for developing targeted strategies, especially in vulnerable populations. This review provides evidence supporting male sex and tobacco exposure as risk factors for CKD development, and further research is needed to assess the strength of the association between tobacco exposure, sex, and CKD.
N.W. contributed to the study investigation, data curation, original draft writing, review & editing writing, project administration, and visualization. R.C. contributed to the study conceptualization, methodology, data curation, original draft writing, review & editing writing, and project administration. N.V. contributed to the study investigation and review & editing writing. A.V. contributed to the study investigation and review & editing writing. A.X. contributed to the study investigation. E.F. contributed to the study investigation. O.H. contributed to the study investigation. R.S. contributed to the study methodology. S.J. contributed to the study methodology, review & editing writing,. A.C. contributed to the study methodology, review & editing writing. S.P. contributed to the study conceptualization, methodology, review & editing writing, supervision, and project administration. N.V and A.V contributed equally.
Supplementary Information
Abbreviations
- CI
Confidence Interval
- CKD
Chronic Kidney Disease
- CRD
Centre for Reviews and Dissemination
- CS
Cigarette Smoke
- DM
Diabetes Mellitus
- DPP
Diabetes Prevention Program
- EEE
Endogenous Estrogen Exposure
- EMBASE
Excerpta Medica Database
- ER
Estrogen Receptor
- ESRD
End-Stage Renal Disease
- GFR
Glomerular Filtration Rate
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- IgA
Immunoglobulin A
- IgAN
Immunoglobulin A Nephropathy
- NOS
Newcastle-Ottawa Scale
- OR
Odds Ratio
- RR
Relative Risk
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- TGF-β
Transforming Growth Factor-beta
Authors’ contributions
N.W. contributed to the study investigation, data curation, original draft writing, review & editing writing, project administration, and visualization. R.C. contributed to the study conceptualization, methodology, data curation, original draft writing, review & editing writing, and project administration. N.V. contributed to the study investigation and review & editing writing. A.V. contributed to the study investigation and review & editing writing. A.X. contributed to the study investigation. E.F. contributed to the study investigation. O.H. contributed to the study investigation. R.S. contributed to the study methodology. S.J. contributed to the study methodology, review & editing writing,. A.C. contributed to the study methodology, review & editing writing. S.P. contributed to the study conceptualization, methodology, review & editing writing, supervision, and project administration. N.V and A.V contributed equally.
Funding
None.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study does not require ethics approval as this is a synthesis of existing data, there are no human or animal participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Natasha Verhoeff and Aditi Venkatraman contributed equally to this work.
References
- 1.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7–11. 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–304. 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52. 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 5.Rawla P, Limaiem F, Hashmi MF. IgA Nephropathy. 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538214/
- 6.Sakata Y, Miyata S, Nochioka K, Miura M, Takada T, Tadaki S, et al. Gender Differences in Clinical Characteristics, Treatment and Long-Term Outcome in Patients With Stage C/D Heart Failure in Japan: Report From the Chart-2 Study. Circ J. 2014;78(2):428–35. 10.1253/circj.CJ-13-1009. [DOI] [PubMed] [Google Scholar]
- 7.Yacoub R, Habib H, Lahdo A, Al Ali R, Varjabedian L, Atalla G, KassisAkl N, Aldakheel S, Alahdab S, Albitar S. Association between smoking and chronic kidney disease: a case control study. BMC Public Health. 2010;25(10):731. 10.1186/1471-2458-10-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapolyai M, Forró M, Lengvárszky Z, Fülöp T. Dialysis patients who smoke are more hypertensive, more fluid overloaded and take more antihypertensive medications than nonsmokers. Ren Fail. 2020;42(1):413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakkarwar VA. Smoking in diabetic nephropathy: sparks in the fuel tank? World J Diabetes. 2012;3(12):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Qin A, Pei G, Jiang Z, Dong L, Tan J, Tan L, Tang Y, Qin W. Cigarette smoking may accelerate the progression of IgA nephropathy. BMC Nephrol. 2021;22(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai X, Gakidou E, Lopez AD. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. 2022;31(2):129–37. [DOI] [PubMed] [Google Scholar]
- 12.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–64. 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan A, Abidi E, Habeichi NJ, Ghali R, Alawasi H, Fakih C, Zibara K, Kobeissy F, Husari A, Booz GW, Zouein FA. Gender-biased kidney damage in mice following exposure to tobacco cigarette smoke: More protection in premenopausal females. Physiol Rep. 2020;8(2):e14339. 10.14814/phy2.14339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher BR, Damery S, Aiyegbusi OL, Anderson N, Calvert M, Cockwell P, Ferguson J, Horton M, Paap MCS, Sidey-Gibbons C, Slade A, Turner N, Kyte D. Symptom burden and health-related quality of life in chronic kidney disease: A global systematic review and meta-analysis. PLoS Med. 2022;19(4):e1003954. 10.1371/journal.pmed.1003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manns B, Hemmelgarn B, Tonelli M, Au F, So H, Weaver R, Quinn AE, Klarenbach S; for Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease. The Cost of Care for People With Chronic Kidney Disease. Can J Kidney Health Dis. 2019;6:2054358119835521. 10.1177/2054358119835521. [DOI] [PMC free article] [PubMed]
- 16.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander S, Varughese S, Franklin R, Rebekah G, Roy S, Yusuf S, Thomas A, Eapen JJ, John EE, Valson AT, David VG. Three-year clinical outcomes of the first South Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2022;7(2):305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Wu N, Chow R, Verhoeff N, Fong E, Venkatraman A, Xiang A, et al. Sexually Dimorphic Response To Tobacco in the Development of Chronic Kidney Disease: A Systematic Review 2022. osf.io/dps2t. [DOI] [PMC free article] [PubMed]
- 20.Newcastle Ottawa Scale (February 2023) Ottawa Hospital Research Institute. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed: March 2, 2023).
- 21.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed]
- 22.Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions.
- 23.Dong W, Wan EY, Fong DY, Kwok RL, Chao DV, Tan KC, Hui EM, Tsui WW, Chan KH, Fung CS, Lam CL. Prediction models and nomograms for 10-year risk of end-stage renal disease in Chinese type 2 diabetes mellitus patients in primary care. Diabetes Obes Metab. 2021;23(4):897–909. [DOI] [PubMed] [Google Scholar]
- 24.Huang YP, Zheng T, Zhang DH, Chen LY, Mao PJ. Community-based study on elderly CKD subjects and the associated risk factors. Ren Fail. 2016;38(10):1672–6. [DOI] [PubMed] [Google Scholar]
- 25.Xue L, Lou Y, Feng X, Wang C, Ran Z, Zhang X. Prevalence of chronic kidney disease and associated factors among the Chinese population in Taian. China BMC nephrology. 2014;15(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Chu TK, Lian J, Lo CW, Lau PK, Nan H, Liang J. Risk factors of chronic kidney diseases in Chinese adults with type 2 diabetes. Sci Rep. 2018;8(1):14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lew QL, Allen JC, Nguyen F, Tan NC, Jafar TH. Factors associated with chronic kidney disease and their clinical utility in primary care clinics in a multi-ethnic Southeast Asian population. Nephron. 2018;138(3):202–13. [DOI] [PubMed] [Google Scholar]
- 28.Hallan SI, Orth SR. Smoking is a risk factor in the progression to kidney failure. Kidney Int. 2011;80(5):516–23. [DOI] [PubMed] [Google Scholar]
- 29.Gjerde B, Bakke PS, Ueland T, Hardie JA, Eagan TM. The prevalence of undiagnosed renal failure in a cohort of COPD patients in western Norway. Respir Med. 2012;106(3):361–6. [DOI] [PubMed] [Google Scholar]
- 30.Nitsch D, Felber DD. von EA, Gaspoz JM, Downs SH, Leuenberger P, Tschopp JM, Brandli O, Keller R, Gerbase MW, Probst-Hensch NM, Stutz EZ, ckermann-Liebrich U. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant. 2006;21:935–44. [DOI] [PubMed]
- 31.Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarød K, Holmen J. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis. 2006;47(3):396–405. [DOI] [PubMed] [Google Scholar]
- 32.Noborisaka Y, Ishizaki M, Yamada Y, Honda R, Yokoyama H, Miyao M, Tabata M. Distribution of and factors contributing to chronic kidney disease in a middle-aged working population. Environ Health Prev Med. 2013;18(6):466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesawa M, Sairenchi T, Haruyama Y, Nagao M, Yamagishi K, Irie F, Watanabe H, Kobashi G, Iso H, Ota H. Validity of a risk prediction equation for CKD after 10 years of follow-up in a Japanese population: the Ibaraki prefectural health study. Am J Kidney Dis. 2018;71(6):842–50. [DOI] [PubMed] [Google Scholar]
- 34.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71(2):159–66. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Nakagawa H, Murakami Y, Kitamura A, Kiyama M, Sakata K, Tsuji I, Miura K, Ueshima H, Okamura T, EPOCH-JAPAN research group. Smoking increases the risk of all-cause and cardiovascular mortality in patients with chronic kidney disease. Kidney international. 2015;88(5):1144–52. [DOI] [PubMed] [Google Scholar]
- 36.Noborisaka Y, Ishizaki M, Yamada Y, Honda R, Yokoyama H, Miyao M, Tabata M. The effects of continuing and discontinuing smoking on the development of chronic kidney disease (CKD) in the healthy middle-aged working population in Japan. Environ Health Prev Med. 2013;18:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anupama YJ, Uma G. Prevalence of chronic kidney disease among adults in a rural community in South India: Results from the kidney disease screening (KIDS) project. Indian J Nephrol. 2014;24(4):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gummidi B, John O, Ghosh A, Modi GK, Sehgal M, Kalra OP, Kher V, Muliyil J, Thakur JS, Ramakrishnan L, Pandey CM. A systematic study of the prevalence and risk factors of CKD in Uddanam. India Kidney Int Rep. 2020;5(12):2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang HJ, Lin KR, Lin MT, Chang JL. Association between lifestyle factors and decreased kidney function in older adults: a community-based cross-sectional analysis of the Taipei City elderly health examination database. BMC Nephrol. 2020;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su SL, Lin C, Kao S, Wu CC, Lu KC, Lai CH, Yang HY, Chiu YL, Chen JS, Sung FC, Ko YC. Risk factors and their interaction on chronic kidney disease: a multi-centre case control study in Taiwan. BMC Nephrol. 2015;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Míguez-Burbano MJ, Wyatt C, Lewis JE, Rodríguez A, Duncan R. Ignoring the obvious missing piece of chronic kidney disease in HIV: cigarette smoking. J Assoc Nurses AIDS Care. 2010;21(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stengel B, Couchoud C, Cénée S, Hémon D. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int. 2000;57(6):2519–26. [DOI] [PubMed] [Google Scholar]
- 43.Kalyesubula R, Nankabirwa JI, Ssinabulya I, Siddharthan T, Kayima J, Nakibuuka J, Salata RA, Mondo C, Kamya MR, Hricik D. Kidney disease in Uganda: a community based study. BMC Nephrol. 2017;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tohidi M, Hasheminia M, Mohebi R, Khalili D, Hosseinpanah F, Yazdani B, Nasiri AA, Azizi F, Hadaegh F. Incidence of chronic kidney disease and its risk factors, results of over 10 year follow up in an Iranian cohort. [DOI] [PMC free article] [PubMed]
- 45.Bleyer AJ, Shemanski LR, Burke GL, Hansen KJ, Appel RG. Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int. 2000;57(5):2072–9. [DOI] [PubMed] [Google Scholar]
- 46.Roehm B, Simoni J, Pruszynski J, Wesson DE. Cigarette smoking attenuates kidney protection by angiotensin-converting enzyme inhibition in nondiabetic chronic kidney disease. Am J Nephrol. 2017;46(4):260–7. [DOI] [PubMed] [Google Scholar]
- 47.Substance Abuse Treatment: Addressing the Specific Needs of Women. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2009. (Treatment Improvement Protocol (TIP) Series, No. 51.) 6 Substance Abuse Among Specific Population Groups and Settings. Available from: https://www.ncbi.nlm.nih.gov/books/NBK83240/ [PubMed]
- 48.Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, He L, Su X. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017;32(3):475–87. [DOI] [PubMed] [Google Scholar]
- 49.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, Manoharan A, Steigerwalt S, Wright J, Rahman M, Rosas SE. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora M, Datta P, Barman A, Sinha P, Munish VG, Bahl D, Bhaumik S, Nazar GP, Tullu F. The Indian bidi industry: trends in employment and wage differentials. Front Public Health. 2020;7(8):572638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization (WHO) . (2012). Global report: Mortality attributable to tobacco. Geneva, Switzerland: Author. Retrieved July 15, 2020, from http://www.who.int/tobacco/publications/surveillance/rep_mortality_attributable/en/index.html
- 52.World Health Organization. Gender and health. World Health Organization; 1998.
- 53.Kågesten A, Gibbs S, Blum RW, Moreau C, Chandra-Mouli V, Herbert A, Amln A. Understanding factors that shape gender attitudes in early adolescence globally: A mixed-methods systematic review. PLoS ONE. 2016;11(6):e0157805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hafez N, Ling PM. How Philip Morris built Marlboro into a global brand for young adults: Implications for international tobacco control. Tob Control. 2005;14(4):262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahimkar MB, Bhisey RA. Occupational exposure to bidi tobacco increases chromosomal aberrations in tobacco processors. Mutat Res. 1995;334(2):139–44. [DOI] [PubMed] [Google Scholar]
- 56.Farahmand M, Ramezani Tehrani F, Khalili D, Cheraghi L, Azizi F. Endogenous estrogen exposure and chronic kidney disease; a 15-year prospective cohort study. BMC Endocr Disord. 2021;21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrica LI, Gluhovschi CR, Velciov SI. Chronic kidney disease and the involvement of estrogen hormones in its pathogenesis and progression. Rom J Intern Med. 2012;50(2):135–44. [PubMed] [Google Scholar]
- 58.Ma HY, Chen S, Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43(1):619–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho MH, Jung KJ, Jang HS, Kim JI, Park KM. Orchiectomy attenuates kidney fibrosis after ureteral obstruction by reduction of oxidative stress in mice. Am J Nephrol. 2012;35:7–16. [DOI] [PubMed] [Google Scholar]
- 60.Ji H, Menini S, Mok K, Zheng W, Pesce C, Kim J, Mulroney S, Sandberg K. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol. 2005;288:F513–20. [DOI] [PubMed] [Google Scholar]
- 61.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M. Gender-specific effects of endogenous testosterone: female alpha-estrogen receptor-deficient C57BL/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–72. [DOI] [PubMed] [Google Scholar]
- 62.Metcalfe PD, Leslie JA, Campbell MT, Meldrum DR, Hile KL, Meldrum KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab. 2008;294:E435–43. [DOI] [PubMed] [Google Scholar]
- 63.Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, Juncos LA. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol. 2013;304:R951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paller CJ, Shiels MS, Rohrmann S, Menke A, Rifai N, Nelson WG, Platz EA, Dobs AS. Association between sex steroid hormones and hematocrit in a nationally representative sample of men. J Androl. 2012;33:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrero JJ, Barany P, Yilmaz MI, Qureshi AR, Sonmez A, Heimburger O, Ozgurtas T, Yenicesu M, Lindholm B, Stenvinkel P. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant. 2012;27:709–15. [DOI] [PubMed] [Google Scholar]
- 66.Kurita N, Horie S, Yamazaki S, Otani K, Sekiguchi M, Onishi Y, Takegami M, Ono R, Konno S, Kikuchi S, et al. Low testosterone levels and reduced kidney function in Japanese adult men: the locomotive syndrome and health outcome in Aizu cohort study. J Am Med Dir Assoc. 2016;17(371):e1-371.e6. [DOI] [PubMed] [Google Scholar]
- 67.van der Burgh AC, Khan SR, Neggers SJ, Hoorn EJ, Chaker L. The role of serum testosterone and dehydroepiandrosterone sulfate in kidney function and clinical outcomes in chronic kidney disease: a systematic review and meta-analysis. Endocrine Connections. 2022;11(6). [DOI] [PMC free article] [PubMed]
- 68.Amiri M, Ramezani Tehrani F, Rahmati M, Amanollahi Soudmand S, Behboudi-Gandevani S, Sabet Z, Azizi F. Low serum testosterone levels and the incidence of chronic kidney disease among male adults: A prospective population-based study. Andrology. 2020;8(3):575–82. [DOI] [PubMed] [Google Scholar]
- 69.Diabetes [Internet]. World Health Organization; 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- 70.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, De Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoogeveen EK. The epidemiology of diabetic kidney disease. Kidney Dialysis. 2022;2(3):433–42. [Google Scholar]
- 73.Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37(10):1705–17. [DOI] [PubMed] [Google Scholar]
- 74.Shalaby G, Sabri S, Alsilami AN, Alhassani RY, Alsayed SH, Alhazmi MA, Aoudallah MT, Khaled S. Predictors of prolonged hospital stay and in-hospital mortality in female patients with acute myocardial infarction with specific reference to diabetes. Int J Cardiol. 2024;17:131785. [DOI] [PubMed] [Google Scholar]
- 75.Cheung KL, Crews DC, Cushman M, Yuan Y, Wilkinson K, Long DL, Judd SE, Shlipak MG, Ix JH, Bullen AL, Warnock DG. Risk factors for incident CKD in Black and White Americans: the REGARDS study. Am J Kidney Dis. 2023;82(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.González-Pérez A, Saez M, Vizcaya D, Lind M, Rodriguez LG. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: a population-based cohort study in the UK. BMJ Open Diabetes Res Care. 2021;9(1):e002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: core curriculum 2021. Am J Kidney Dis. 2021;78(3):429–41. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Zhang Y, Zhang H. IgA nephropathy: a Chinese perspective. Glomerular Dis. 2022;2(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Storrar J, Chinnadurai R, Sinha S, Kalra PA. The epidemiology and evolution of IgA nephropathy over two decades: A single centre experience. PLoS ONE. 2022;17(9):e0268421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shukla P, Khanna A, Jain SK. Working condition: A key factor in increasing occupational hazard among bidi rollers: A population health research with respect to DNA damage. Indian J Occup Environ Med. 2011;15(3):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korbut AI, Klimontov VV, Vinogradov IV, Romanov VV. Risk factors and urinary biomarkers of non-albuminuric and albuminuric chronic kidney disease in patients with type 2 diabetes. World J Diabetes. 2019;10(11):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khanna A, Gautam DS, Gokhale M, Jain SK. Tobacco dust induced genotoxicity as an occupational hazard in workers of bidi making cottage industry of central India. Toxicol Int. 2014;21(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bidi rolling is an occupational health hazard: Who study. 2022. Available from: https://www.who.int/india/news/feature-stories/detail/bidi-rolling-is-an-occupational-health-hazard--who-study
- 84.Bhisey RA, Govekar RB. Biological monitoring of bidi rollers with respect to genotoxic hazards of occupational tobacco exposure. Mutat Res. 1991;261(2):139–47. [DOI] [PubMed] [Google Scholar]
- 85.India’s tobacco girls. BBC; 2012. Available from: https://www.bbc.com/news/world-asia-india-18391652
- 86.Shukla P, Khanna A, Jain SK. Working condition: A key factor in increasing occupational hazard among bidi rollers: A population health research with respect to DNA damage. Indian J Occup Environ Med. 2011;15(3):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dehghani A, Alishavandi S, Nourimajalan N, Fallahzadeh H, Rahmanian V. Prevalence of chronic kidney diseases and its determinants among Iranian adults: results of the first phase of Shahedieh cohort study. BMC Nephrol. 2022;23(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.The Prevalence and Characteristics of Circulating IgA Anti-Glomerular Basement Membrane Autoantibodies in Anti-Glomerular Basement Membrane Disease Yang, Xue-fen et al. Kidney International Reports, 8(11):2395-2402 [DOI] [PMC free article] [PubMed]
- 89.Yang L, Zhou Y, Jiang M, Wen W, Guo Y, Pakhale S, Wen SW. Why Female Smokers Have Poorer Long-Term Health Outcomes than Male Smokers: The Role of Cigarette Smoking During Pregnancy. Public Health Rev. 2024;45:1605579. 10.3389/phrs.2024.1605579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alramly M, Darawad MW, Khalil AA. Slowing the progression of chronic kidney disease: Comparison between predialysis and dialysis Jordanian patients. Ren Fail. 2013;35(10):1348–52. [DOI] [PubMed] [Google Scholar]
- 91.Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, Atkins RC. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab Kidney Study. Am J Kidney Dis. 2002;40(4):704–12. [DOI] [PubMed] [Google Scholar]
- 92.Sepanlou SG, Barahimi H, Najafi I, Kamangar F, Poustchi H, Shakeri R, Hakemi MS, Pourshams A, Khoshnia M, Gharravi A, Broumand B. Prevalence and determinants of chronic kidney disease in northeast of Iran: Results of the Golestan cohort study. PLoS ONE. 2017;12(5):e0176540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gummidi B, John O, Ghosh A, Modi GK, Sehgal M, Kalra OP, Kher V, Muliyil J, Thakur JS, Ramakrishnan L, Pandey CM. A systematic study of the prevalence and risk factors of CKD in Uddanam. India Kidney Int Rep. 2020;5(12):2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roseman DA, Hwang SJ, Oyama-Manabe N, Chuang ML, O’Donnell CJ, Manning WJ, Fox CS. Clinical associations of total kidney volume: the Framingham Heart Study. Nephrol Dial Transplant. 2017;32(8):1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.