Abstract
Background
Ciprofol(HSK3486) is a novel 2,6-disubstituted phenol derivate, a short-acting intravenous sedative, which has similar efficacy characteristics as propofol with less incidence of side effect. Both ciprofol and propofol are often used in outpatient hysteroscopic surgery for sedation. However, the relative potency of these two drugs has not been fully determined in this context.
Objective
Our study aimed to investigate the potency-ratio of ciprofol and propofol under procedural sedation and anesthesia in restraining reaction of outpatient hysteroscopy dilatation.
Methods
The ED50 (effective dose in 50% of subjects) value for ciprofol and propofol were calculated by Up-and-Down Sequential Allocation Method. 60 healthy patients undergoing daytime hysteroscopy were randomly divided into two groups, which were intravenously injected with ciprofol at an initial dose of 0.4 mg/kg (group C) or propofol at an initial dose of 2 mg/kg (group P) at 2 min after intravenous injection of sufentanil 0.15ug/kg. A successful response is defined as the absence of patient movement in the case of cervical dilation. Conversely, the presence of patient movement is defined as failure. After successful or failed responses, each follow-up patient in the corresponding group was reduced or increased with propofol 0.5 mg/kg or ciprofol 0.1 mg/kg, respectively.
Results
The estimated ED50 value for ciprofol and propofol in restraining reaction of hysteroscopy dilatation was 0.444 mg/kg (95% CI, 0.385-0.503 mg/kg) and 1.985 mg/kg (95% CI, 1.801–2.170 mg/kg), respectively. The incidence of respiratory depression, hypoxemia and injection pain in ciprofol was significantly lower than those in propofol.
Conclusion
The ED50 of ciprofol and propofol in preventing hysteroscopy dilatation reaction was 0.444 mg/kg (95% CI, 0.385-0.503 mg/kg) and 1.985 mg/kg (95% CI, 1.801–2.170 mg/kg) for outpatient hysteroscopy. The potency-ratio of ciprofol and propofol observed in our study was 1.0:4.5(95%CI,1:3.9-1:5.1).
Trial registration
The study was registered at Chinese Clinical Trial Registry http//www.chictr.org.cn/ (Registration date19/11/22 Trial ID ChiCTR2200065954).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-024-02793-2.
Keywords: Ciprofol, Propofol, Hysteroscopy, ED50, Anethesia
Introduction
Propofol is widely used in procedural sedation and anesthesia, because of its rapid onset, short duration, and strong controllability [1–3]. However, the incidence of side effect associate with propofol include apnea, cardiovascular depression, and the incidence of injection pain cause by propofol is as high as 60% [4–6]. Ciprofol (HSK3486) is a novel 2,6-disubstituted phenol derivative has similar tolerability and efficacy characteristics as propofol. Compare to propofol, ciprofol binds more tightly to GABA receptors [7], which has better anesthetic properties and fewer side effects [8–10]. Both ciprofol and propofol demonstrate comparable efcacy and safety for anesthesia induction and maintenance in adult patients undergoing surgery. While propofol provides a faster onset of induction, ciprofol exhibits advantages in terms of pain management [11]. So it is expected to be an alternative to propofol in simple intravenous anesthesia [6, 12, 13].
A series of previous studies have established the recommended doses of ciprofol (0.4-0.5 mg/kg) and propofol(1.5-2.5 mg/kg) for sedative and anesthesia for gastroscopy and colonoscopy, fiber-optic bronchoscopy, general anesthesia in elective surgeries [6, 14, 15].Ciprofol is reported 4–5 times higher potency than propofol in previous articles [7, 16, 17]. However, the accurate potency ratio of ciprofol and propofol has not determinied yet. The aim of this study is to explore the relative potency to inhibit the dilatation response during outpatient hysteroscopy.
Materials and methods
Design and study subjects
The study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (No. IRB-20220157-R) and was registered at the Chinese Clinical Trials.gov (NO. ChiCTR2200065954) before patient enrollment. All participants signed an informed consent form.
With written informed consent, 60 Patients scheduled for elective hysteroscopy under monitored anesthesia were enrolled in the study.
Inclusion criteria: (1) Aged between 18 and 64 years, (2) BMI between 18 and 25 kg /m2, (3) ASA I-II level. Exclusion criteria: (1) pregnant and lactating women, (2) contraindications of conventional hysteroscopy, (3) contraindications to the use of ciprofol or propofol, (4) history of mental illness, (5) complicated with serious cardiopulmonary disease, (6) use analgesics within 24 h before surgery. (7) patients who were participating in other clinical studies. (8) other reasons that the researcher considers unsuitable for clinical research. (9) expansion duration is greater than 5 min.
Randomization was performed using computer-generated random number codes. The anesthesiologist, gynecologist, nurses and patients were all unaware of which drug is administered. Based on a computer-generated random number sheet, patients were randomly allocated into Group C or Group P. A nurse who did not participate in any anesthetic care or data collection opened the sealed envelopes, which contain the group assignments, and prepared the drug according to the group allocation. The anesthesiologist, who was blinded to the details of the drug, recorded the response of each patient and reported it to the nurse, who determined the dose and prepared drugs for the next case. All study drugs were drawn with 20 ml syringes and prepared by the nurse who was not involved in patient management and study data collection. The nurse also informed the anesthesiologist of the dose of the study drug to be given in each case. To ensure blinding, the syringes containing the study solution were all identical. The drugs studied were all milky in appearance, the researchers could not know the identity of the drugs used.60 patients were randomized into the ciprofol (Sishuning; Liaoning Hysco Pharmaceutical Co., Ltd.; 50 mg/20 ml): Group C (n = 30) or the propofol(Diprivan; Aspen Pharma Trading Limited;200 mg/20 ml): Group P (n = 30).The doses of ciprofol(0.4 mg/kg) and propofol(2 mg/kg) based on estimated protency-ratio of 5:1,which was best approximation according to the data of previous studies [7, 12] .
Study protocol
All patients fasted for 8 h and received no premedication. On arrival in the operating room, all participants were monitored continuous-sly with noninvasive blood pressure (NBP), electrocardiogram (ECG), heart rate (HR), respiratory rate (RR), end-tidal carbon dioxide tension (ETCO2) and pulse oxygen saturation (SpO2). All participants were given a supplementation of 6 L/min O2 by a face mask before induction of anesthesia. Oxygen of 6 L/min was administered continuously until the end of the anesthesia. Anesthesia was commenced with a bolus of sufentanil 0.15 mg/kg i.v, followed by injection of cipofol or propofol during 30 s after 2 min. Cervical dilation was started when the patient was loss response to the verbal command. A success was defined if the patient did not move during the cervical dilation, and a failure was defined if the patient moved during the cervical dilation. If the patient moved cervical dilatation, intravenous injection of half the initial dose of propofol or ciprofol should be given to deepen the anesthesia, which could be repeated until the hysteroscopy was completed. We used the up-down method of Dixon sequential allocation technique to determine the ED50 of ciprofol or propofol that would attenuate the response to cervival dilation in 50% of patients. The anesthesiologist who administer-ated the study drug was unaware of the group assignment. The initial dose of ciprofol was 0.4 mg/kg during 30s, and the initial dose of propofol was 2 mg/kg during 30s. If the previous patient was a success, the ciprofol or propofol for the subsequent patient in the same group decreased 0.1 mg/kg or 0.5 mg/kg, respectively. Whereas if the previous patient was a failure the ciprofol or propofol was increased 0.1 mg/kg or 0.5 mg/kg, respectively. NBP, ECG, HR, RR and SpO2 were recorded immediately after propofol or ciprofol injected until the end of dilation every 1 min.
The baseline of BP and HR were defined as the average of three consecutive measurements at the time when patients arrived in the operating room. Adverse reactions such as hypotension (mean blood pressure > 20% drop from the baseline MBP or systolic blood pressure < 90mmHg), bradycardia (HR < 50 beats/min), respiratory depression (RR ≤ 8 beats/min, apnea, hypoxemia (SpO2 < 93%)), injection pain, and muscle tremors were recorded. Any episode of hypotension, defined as mean blood pressure > 20% drop from the baseline MBP or systolic blood pressure < 90mmHg, was treated with a bolus of 60ug intravenous phenylephrine, repeatedly if needed. Bradycardia, defined as HR < 50 beats/min, was treated with 0.5 mg of atropine intravenously. Respiratory depression was defined as RR ≤ 8 beats/min, apnea or SpO2 < 93%.The patient was treated with face mask oxygen inhalation and respiratory support when SpO2 < 93%. Injection pain, and muscle tremors were recorded.
Outcomes and definitions
The primary outcome was body movement for outpatient hysteroscopy during cervical dilation. The changes of SBP, MAP, HR, SpO2, RR, ETCO2 and the occurrence of adverse reactions before and after administration were considered for evaluation as secondary outcomes.
Statistical analysis
According to the stop rule of the up-down sequential method [6, 12, 13, 18], sample size was determined by the results of simulation study suggesting that 20–40 patients will provide stable estimated of ED50, and a sample size of 30 patients for each group was determined in this study. SPSS (IBM SPSS Statistics 26.0.0) was used to perform the analyses. Normally distributed measures were expressed as mean ± SD, and comparisons between groups were made using the T test; non-normally distributed measures were expressed as median, and comparisons between groups were made using the Mann-Whitney U test; and categorical data were expressed as number (percentage), and were analyzed using the chi-square test for analysis. Logistic regression was used to analyze the success rate of sedation, to estimate the ED50 confidence interval (CI) of ciprofol and propofol. The potency-ratio with 95% CIs for ciprofol: propofol was estimated by calculating the ratio of the ED50 values using Fieller’s method via GraphPad Prism [18, 19]. Normal distribution was determined using the Kolmogorov-Smirnov test. P < 0.05 was considered statistical significant.
Result
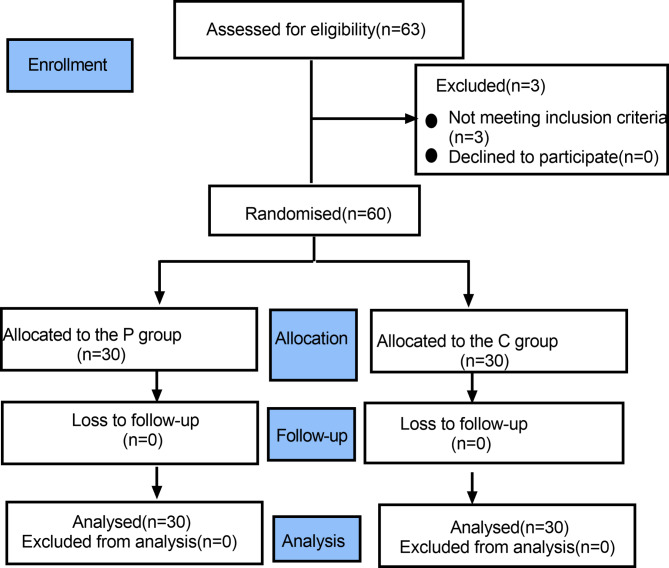
We enrolled 63 patients between November 18, 2019 and May 31, 2019. In the process, 3 patients were excluded due to cervical dilation lasting more than 5 min. 30 patients in each group were included in the final analysis. The consolidated standards of Reporting trials (CONSORT) flow diagram is shown in Fig. 1. (Fig. 1). There was no significant difference in demographic and general characteristics (Table 1).
Fig. 1.
Flow diagram of study
Table 1.
Demographic data
| Group P(n = 30) | Group C(n = 30) | P-value | |
|---|---|---|---|
| Age(years) | 38.7 ± 1.5(24–54) | 39.0 ± 1.6(28–56) | 0.902 |
| Weight(kg) | 57.1 ± 1.5(46–78) | 56.9 ± 1.3(48-72.5) | 0.941 |
| Height(cm) | 159.8 ± 1.2(146–170) | 160.6 ± 1.0(147–172) | 0.600 |
| BMI(kg/m2) | 22.3 ± 0.5(18–27) | 22.0 ± 0.5(18–27) | 0.712 |
Data are presented as a number or mean ± SD
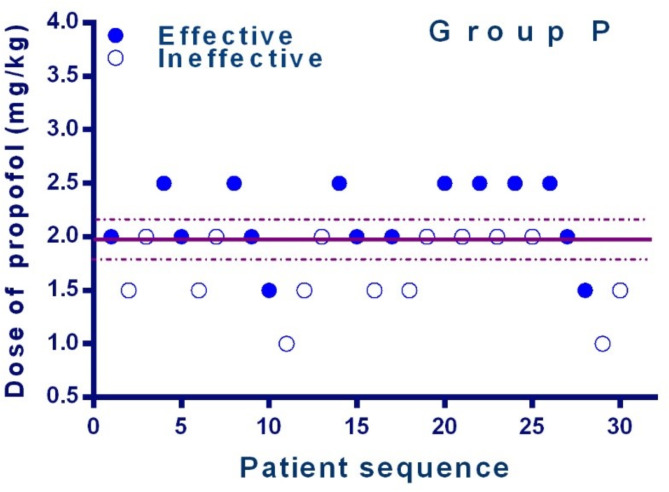
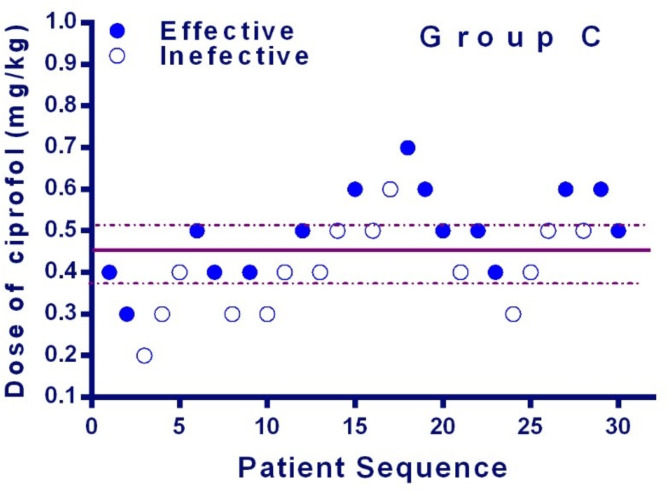
The sequences of all the cases are presented in Figs. 2 and 3. The ED50 of ciprofol and propofol in preventing hysteroscopy dilatation reaction was 0.444 mg/kg (95% CI, 0.385-0.503 mg/kg) and 1.985 mg/kg (95% CI, 1.801–2.170 mg/kg) for daytime hysteroscopy. The potency-ratio of ciprofol and propofol observed was 1.0:4.5. (95%CI,1:3.9-1:5.1)
Fig. 2.
Individual response to Cervical dilation at corresponding dose of propofol(mg/kg). Solid circles indicate successful anesthesia and hollow circles indicate unsuccessful anesthesia. The solid line represents the ED50 and the dashed line represents the 95% CI
Fig. 3 .
Individual response to Cervical dilation at corresponding dose of ciprofol (mg/kg). Solid circles indicate successful anesthesia and hollow circles indicate unsuccessful anesthesia. The solid line represents the ED50 and the dashed line represents the 95% CI
The occurrence of adverse reactions during anesthesia is shown in Table 2. The incidence of respiratory depression, apnea and the occurrence of hypoxemia in Group ciprofol was significantly lower than those in Group propofol (P < 0.05). Moreover, the incidence of injection pain and numbers of hypotension was also significantly lower in ciprofol than in Group ciprofol than that in Group propofol. (P < 0.05). There was no significant difference in the occurrence of muscle tremor between the two groups. No cases of bradycardia were occurred in two groups (P > 0.05). (Table 2).
Table 2.
Adverse events of anesthesia
| Group P(n = 30) | Group C(n = 30) | P-value | |
|---|---|---|---|
| Hypotension(n) | 18 | 9 | 0.037* |
| bradycardia(n) | 0 | 0 | 0.999 |
| respiratory depression(n) | 26 | 17 | 0.010* |
| Duration of respiratory depression(s) | 60.0(37.8,120) | 35.0(0.0,85.8) | 0.019* |
| apnea(n) | 11 | 3 | 0.015* |
| hypoxemia(n) | 4 | 0 | 0.043* |
| injection pain(n) | 9 | 0 | 0.001* |
| muscle tremor(n) | 0 | 1 | 0.326 |
Data are presented as a number or median (IQR). * indicates p < 0.05
Discussion
The main finding of our study is that the ED50 of ciprofol and propofol in preventing hysteroscopy dilatation reaction was 0.444 mg/kg and 1.985 mg/kg for outpatient hysteroscopy. The definite potency-ratio of ciprofol and propofol under procedural sedation and anesthesia in restraining reaction of outpatient hysteroscopy dilatation was 1.0:4.5(95%CI,1:3.9-1:5.1) by up-down sequential allocation method.
Ciprofol (HSK3486) is a new type of intravenous anesthesia drug independently developed in China. It is an isomer of propofol and a receptor enhancer of gamma-aminobutyrate (GABA). The combination of propofol and opioids for intravenous anesthesia is commonly used in clinical anesthesiology [20–22]. Propofol may cause bradycardia, severe hypotension and respiratory depression. Ciprofol can make chloride ions flow inside and cause hyperpolarization of nerve cell membrane, thus achieving central nervous system inhibition. Ciprofol is efficient, easy to control, safe and comfortable. Its advantages mainly lie in its rapid onset, rapid recovery, less dosage, wider safety window, less respiratory depression, and less injection pain. Compared with propofol, cyclopofol has stronger sedative and anesthetic effects, and less residue after a single administration.
Previous clinical studies showed that 0.4–0.9 mg/kg of ciprofol exhibited a similar onset time of propofol and provided effective and well tolerated sedation in adults [7, 23–25]. Phase III clinical trials reported that 0.4 mg/kg of ciprofol was non-inferior to propofol of 1.5 mg/kg and 2.0 mg/kg in gastroscopy and colonoscopy [6, 12]. The potency ratio of ciprofol to propofol obtained in previous studies was within a certain range, and the exact ratio was not obtained. In our study, the up-and-down method was adopted to calculate the accurate titer ratio of ciprofol to propofol during hysteroscopic surgery. The result of the present study showed that the potency-ratio of ciprofol and propofol under procedural sedation and anesthesia in restraining reaction of outpatient hysteroscopy dilatation was 1.0:4.5. Our study provides important reference for the clinical use of ciprofol. Furthermore, it provides a reliable reference for later studies on the effects of ciprofol and propofol on respiratory circulation, recovery time and injection pain based on equivalent measurement.
We chose hysteroscopic surgery because it was one of the most common outpatient surgery in the field of gynecology. This type of surgery requires high quality anesthesia: induction and recovery are required to be fast, and the anesthetic drugs used must be safe, effective, and have few side effects. In hysteroscopic surgery, pain stimulation comes from cervical dilation, and the patient’s body movement is the most intuitive indicator of the depth of anesthesia analgesia. Therefore, in this study, the success or failure of anesthesia and sedation were measured by the occurrence of body movement during cervical dilation.
We found that the respiratory complications including respiratory depression, apnoea, hypoxemia in ciprofol group were significantly lower compared to propofol group, which were consistent with the previous studies [3, 5, 6, 12, 14]. Our result also found that ciprofol had less negative effects on hemodynamics, which is similar to the results of a previous study [26]. Due to its lipid solubility, injection pain becomes one of the most common adverse effects of propofol, and the incidence of injection pain with propofol is about 50-90% in adults [14, 27, 28]. Ciprofol is an isomer of propofol, which has improved pharmacological and physicochemical properties, leading to lower probability of causing injection pain [7, 12].
There are still some limitations of our study. Firstly, this study was only conducted in female patients with ASAI-II for outpatient hysteroscopy during cervical dilation, and further discussion was needed in male patients, obese patients, elderly patients, ASA III-IV patients or the other types of surgery. Secondly, we only explored the combination of ciprofol combined with sufentanil, still there were groups of ciprofol with other analgesics that needed to be further investigated. Thirdly, our study didn’t monitor the depth of anesthesia, BIS or entropy, which could evaluate sedation accurately.
Conclusions
The definite potency-ratio of ciprofol and propofol under procedural sedation and anesthesia in restraining reaction of outpatient hysteroscopy dilatation was1.0:4.5(95%CI,1:3.9-1:5.1) by up-down sequential allocation method.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the colleagues in the Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Author contributions
Lin jin, Xiaowei Qian, Jinzhong Wang and Xinzhong Chen supervised the study and revised the manuscript.Lin jin, Yu Zhang and Xiaoping Chen conceived and designed the study. Lin Jin, Cuicui Jiao and Lihong Sun interpreted the data. Lin Jin, Cuicui jiao and Xiaoping Chen drafted the manuscript.Lin Jin, Yu Zhang, Xiaoping Chen recruited patients and performed the study. Lin jin, Yu Zhang and Xiaoping Chen acquired the data. Xiaowei Qian, Jinzhong Wang and Xin-zhong Chen revised the manuscript.Cuicui Jiao, Yu Zhang, Xiaoping Chen and Lihong Sun checked the data and reanalyzed part of the outcomes. All authors made substantial contributions to study conception and design, data acquisition, data analysis and interpretation and drafting, revising or critically reviewing the article.
Funding
There was no funding for this study.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Women’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) (No. IRB-20220157-R) and was registered at the Chinese Clinical Trials.gov (NO. ChiCTR2200065954). All patients provided written informed consent. We confirm our study complies with the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
conflicts of interest
The authors declared no funding sources or conflicts of interest to support this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lin Jin and Cui-cui Jiao contributed equally to this work.
Contributor Information
Xin-zhong Cheng, Email: chenxinz@zju.edu.cn.
Jin-zhong Wang, Email: wjz3512@163.com.
Xiao-wei Qian, Email: qianxw@zju.edu.cn.
References
- 1.Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of Propofol. Clin Pharmacokinet. 2018;57(12):1539–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanaya N, Gable B, Wickley PJ, Murray PA, Damron DS. Experimental conditions are important determinants of cardiac inotropic effects of propofol. Anesthesiology. 2005;103(5):1026–34. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Guo P, Yang L, Liu Z, Yu D. Comparison and Clinical Value of Ciprofol and Propofol in Intraoperative Adverse Reactions, Operation, Resuscitation, and Satisfaction of Patients under Painless Gastroenteroscopy Anesthesia. Contrast Media Mol Imaging 2022, 2022:9541060. [DOI] [PMC free article] [PubMed]
- 4.Baker MT, Naguib M. Propofol: the challenges of formulation. Anesthesiology. 2005;103(4):860–76. [DOI] [PubMed] [Google Scholar]
- 5.Hu C, Ou X, Teng Y, Shu S, Wang Y, Zhu X, Kang Y, Miao J. Sedation effects produced by a Ciprofol Initial Infusion or Bolus Dose followed by Continuous Maintenance Infusion in healthy subjects: a phase 1 trial. Adv Ther. 2021;38(11):5484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wang X, Liu J, Wang X, Li X, Wang Y, Ouyang W, Li J, Yao S, Zhu Z, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131(2):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin L, Ren L, Wan S, Liu G, Luo X, Liu Z, Li F, Yu Y, Liu J, Wei Y. Design, synthesis, and evaluation of Novel 2,6-Disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606–17. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Q, Luo Z, Wang X, Wang D, Li J, Wei X, Tang J, Yao S, Ouyang W, Zhang W, et al. Efficacy and safety of ciprofol versus propofol for the induction of anesthesia in adult patients: a multicenter phase 2a clinical trial. Int J Clin Pharm. 2023;45(2):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhtar SMM, Fareed A, Ali M, Khan MS, Ali A, Mumtaz M, Kirchoff R, Asghar MS. Efficacy and safety of Ciprofol compared with propofol during general anesthesia induction: a systematic review and meta-analysis of randomized controlled trials (RCT). J Clin Anesth. 2024;94:111425. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Bertoch T, Habib AS, Yan P, Zhou R, Lai YL, Liu X, Essandoh M, Daley WL, Gelb AW. Comparison of the efficacy of HSK3486 and propofol for induction of General Anesthesia in adults: a Multicenter, Randomized, Double-blind, controlled, phase 3 Noninferiority Trial. Anesthesiology. 2024;140(4):690–700. [DOI] [PubMed] [Google Scholar]
- 11.Hudaib M, Malik H, Zakir SJ, Rabbani S, Gnanendran D, Syed ARS, Suri NF, Khan J, Iqbal A, Hussain N, et al. Efficacy and safety of ciprofol versus propofol for induction and maintenance of general anesthesia: a systematic review and meta-analysis. J Anesth Analg Crit Care. 2024;4(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng Y, Ou M, Wang X, Zhang W, Liu X, Liang Y, Li K, Wang Y, Ouyang W, Weng H, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164:105904. [DOI] [PubMed] [Google Scholar]
- 13.Ding YY, Long YQ, Yang HT, Zhuang K, Ji FH, Peng K. Efficacy and safety of ciprofol for general anaesthesia induction in elderly patients undergoing major noncardiac surgery: a randomised controlled pilot trial. Eur J Anaesthesiol. 2022;39(12):960–3. [DOI] [PubMed] [Google Scholar]
- 14.Wu B, Zhu W, Wang Q, Ren C, Wang L, Xie G. Efficacy and safety of ciprofol-remifentanil versus propofol-remifentanil during fiberoptic bronchoscopy: a prospective, randomized, double-blind, non-inferiority trial. Front Pharmacol. 2022;13:1091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Wang X, Liu J, Zuo YX, Zhu QM, Wei XC, Zou XH, Luo AL, Zhang FX, Li YL, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26(5):1607–17. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Liu J, Wu X, Zhang Z. Ciprofol: A Novel Alternative to Propofol in Clinical Intravenous Anesthesia? Biomed Res Int 2023, 2023:7443226. [DOI] [PMC free article] [PubMed]
- 17.Bian Y, Zhang H, Ma S, Jiao Y, Yan P, Liu X, Ma S, Xiong Y, Gu Z, Yu Z, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87(1):93–105. [DOI] [PubMed] [Google Scholar]
- 18.Ngan Kee WD, Ng FF, Khaw KS, Lee A, Gin T. Determination and comparison of graded dose-response curves for epidural bupivacaine and ropivacaine for analgesia in laboring nulliparous women. Anesthesiology. 2010;113(2):445–53. [DOI] [PubMed] [Google Scholar]
- 19.Naguib M, Sari-Kouzel A, Seraj M, el-Gammal M, Gomma M. Induction dose-responses studies with propofol and thiopentone. Br J Anaesth. 1992;68(3):308–10. [DOI] [PubMed] [Google Scholar]
- 20.Yin N, Xia J, Cao YZ, Lu X, Yuan J, Xie J. Effect of propofol combined with opioids on cough reflex suppression in gastroscopy: study protocol for a double-blind randomized controlled trial. BMJ Open. 2017;7(9):e014881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zbytovska J, Gallusova J, Vidlarova L, Prochazkova K, Simek J, Stepanek F. Physical compatibility of Propofol-Sufentanil mixtures. Anesth Analg. 2017;124(3):776–81. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low-dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. 2019;125(5):460–5. [DOI] [PubMed] [Google Scholar]
- 23.Teng Y, Ou MC, Wang X, Zhang WS, Liu X, Liang Y, Zuo YX, Zhu T, Liu B, Liu J. Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open-label, single-arm dose-escalation phase 1 study. Am J Transl Res. 2021;13(12):13791–802. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, Jin X, Wang Q, Lyu J, Wang S, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12(8):4594–603. [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia Guzzo ME, Fernandez MS, Sanchez Novas D, Salgado SS, Terrasa SA, Domenech G, Teijido CA. Deep sedation using propofol target-controlled infusion for gastrointestinal endoscopic procedures: a retrospective cohort study. BMC Anesthesiol. 2020;20(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man Y, Xiao H, Zhu T, Ji F. Study on the effectiveness and safety of ciprofol in anesthesia in gynecological day surgery: a randomized double-blind controlled study. BMC Anesthesiol. 2023;23(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53(5):468–76. [DOI] [PubMed] [Google Scholar]
- 28.Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC. Perioperative Clinical Research C: Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.