Abstract
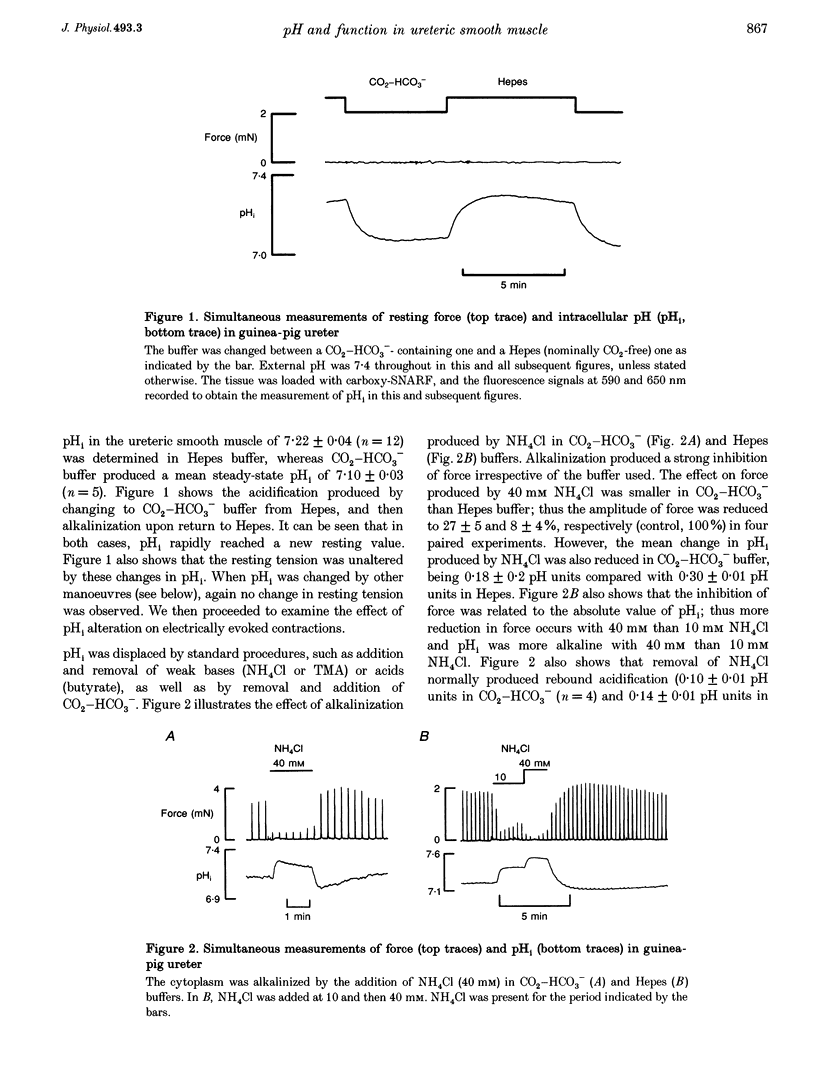
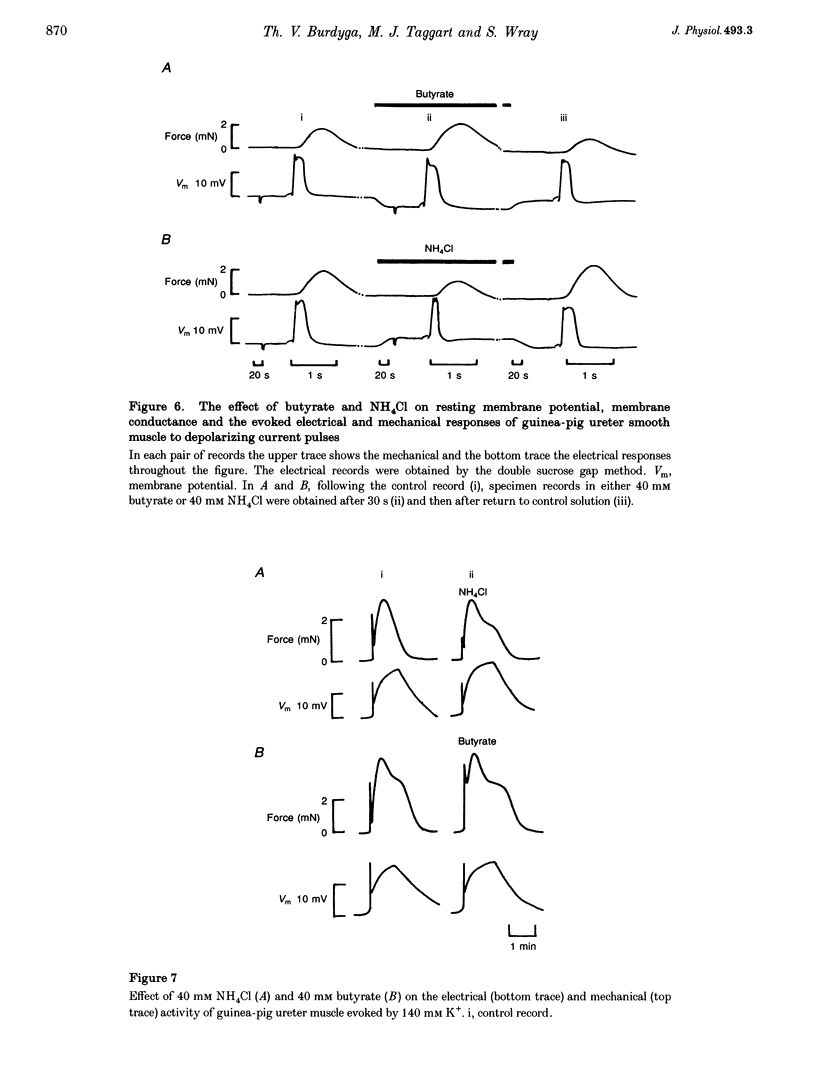
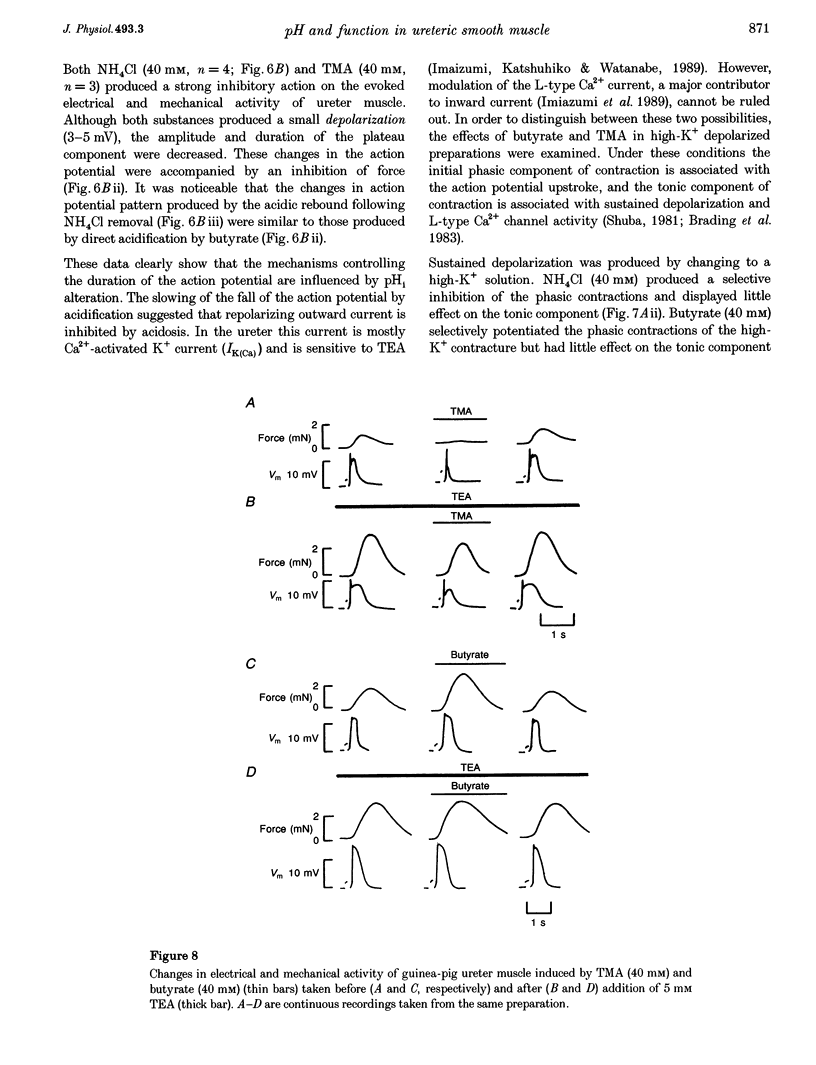
1. We have altered intracellular (pHi) and extracellular pH (pHo) in the smooth muscle of guinea-pig ureter and determined the effects on evoked phasic contractions. In order to investigate the mechanisms underlying the effects of pH alteration, intracellular Ca2+ ([Ca2+]i), pHi, electrical activity and force were measured. 2. Intracellular acidification, produced by the weak acid butyrate, application of CO2 at constant pHo or removal of weak bases, greatly increased phasic contractions. Alkalinization with weak bases or by removal of CO2 inhibited contractions. The results were similar whether Hepes or CO2-HCO3-buffered the solutions. 3. Phasic contractions were preceded by intracellular Ca2+ transients in the ureter. Acidification of the cytoplasm led to an increase in the amplitude of the Ca2+ transient, and alkalinization decreased its magnitude. 4. In the ureter the action potential leads to Ca2+ influx, therefore electrophysiological recordings of its configuration were made during alteration of pHi. Acidification led to the action potential duration and amplitude being increased, whereas alkalinization shortened the action potential and reduced its amplitude. 5. As the effects of acidification on the action potential resembled the effects of blocking of K+ channels, we investigated whether pHi alteration was able to alter tension when K+ channels were blocked by tetraethylammonium. Acidification was unable to potentiate force under these conditions nor did alkalinization decrease force. 6. External pH over the range 6.8-8.0 had little or no effect on pHi, phasic contractions and [Ca2+]i. Tonic contractions were enhanced, however, when pHo was increased. 7. These data suggest that pHi alteration in the guinea-pig ureter modulates the action potential, probably by alteration of K+ currents. Subsequent changes in [Ca2+]i and contraction then occur. A potentiating effect of acidic pH on force is not common in muscle, but may be a characteristic of the smooth muscle of the urinary tract. Changes of pHo had little effect on phasic force or pHi, but modulated tonic contractions. The possible physiological significance of these results is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Regulation of intracellular pH in the smooth muscle of guinea-pig ureter: Na+ dependence. J Physiol. 1994 Sep 1;479(Pt 2):301–316. doi: 10.1113/jphysiol.1994.sp020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C., Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol. 1993 Jul;466:1–8. [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Peces R., LaPointe M. S., Ye M., Daugirdas J. T. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 1):C932–C943. doi: 10.1152/ajpcell.1993.264.4.C932. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burdyga T. V., Scripnyuk Z. D. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J Physiol. 1983 Jan;334:79–89. doi: 10.1113/jphysiol.1983.sp014481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga ThV, Magura I. S. The effects of local anaesthetics on the electrical and mechanical activity of the guinea-pig ureter. Br J Pharmacol. 1986 Jul;88(3):523–530. doi: 10.1111/j.1476-5381.1986.tb10232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Fry C. H., Shuttleworth K. E. Effects of acid-base changes on human ureteric smooth muscle contractility. Br J Urol. 1990 Sep;66(3):257–264. doi: 10.1111/j.1464-410x.1990.tb14923.x. [DOI] [PubMed] [Google Scholar]
- Eldrup J., Thorup J., Nielsen S. L., Hald T., Hainau B. Permeability and ultrastructure of human bladder epithelium. Br J Urol. 1983 Oct;55(5):488–492. doi: 10.1111/j.1464-410x.1983.tb03354.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H., Gallegos C. R., Montgomery B. S. The actions of extracellular H+ on the electrophysiological properties of isolated human detrusor smooth muscle cells. J Physiol. 1994 Oct 1;480(Pt 1):71–80. doi: 10.1113/jphysiol.1994.sp020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H., Poole-Wilson P. A. Effects of acid-base changes on excitation--contraction coupling in guinea-pig and rabbit cardiac ventricular muscle. J Physiol. 1981;313:141–160. doi: 10.1113/jphysiol.1981.sp013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994 Apr;103(4):647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston T. G., Palfrey E. L., Raimbach S. J., Fry C. H. The effects of pH changes on human and ferret detrusor muscle function. J Physiol. 1991 Jan;432:1–21. doi: 10.1113/jphysiol.1991.sp018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P. Effect of the Ca(2+)-ATPase inhibitor, cyclopiazonic acid, on electromechanical coupling in the guinea-pig ureter. Br J Pharmacol. 1995 Jan;114(1):127–137. doi: 10.1111/j.1476-5381.1995.tb14916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Parratt J., Taggart M., Wray S. Abolition of contractions in the myometrium by acidification in vitro. Lancet. 1994 Sep 10;344(8924):717–718. doi: 10.1016/s0140-6736(94)92209-8. [DOI] [PubMed] [Google Scholar]
- Pitts R. F., Ayer J. L., Schiess W. A., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. III. THE REABSORPTION AND EXCRETION OF BICARBONATE. J Clin Invest. 1949 Jan;28(1):35–44. doi: 10.1172/JCI102050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz I., Thévenod F., Dehlinger-Kremer M. Modulation of intracellular free Ca2+ concentration by IP3-sensitive and IP3-insensitive nonmitochondrial Ca2+ pools. Cell Calcium. 1989 Jul;10(5):325–336. doi: 10.1016/0143-4160(89)90058-4. [DOI] [PubMed] [Google Scholar]
- Shuba M. F. The effect of sodium-free and potassium-free solutions, ionic current inhibitors and ouabain on electrophysiological properties of smooth muscle of guinea-pig ureter. J Physiol. 1977 Jan;264(3):837–851. doi: 10.1113/jphysiol.1977.sp011697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurway N. C., Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol. 1987 Dec;393:57–71. doi: 10.1113/jphysiol.1987.sp016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart M., Wray S. Simultaneous measurement of intracellular pH and contraction in uterine smooth muscle. Pflugers Arch. 1993 Jun;423(5-6):527–529. doi: 10.1007/BF00374951. [DOI] [PubMed] [Google Scholar]
- Wray S., Duggins K., Iles R., Nyman L., Osman V. The effects of metabolic inhibition and acidification on force production in the rat uterus. Exp Physiol. 1992 Mar;77(2):307–319. doi: 10.1113/expphysiol.1992.sp003590. [DOI] [PubMed] [Google Scholar]


