Abstract
Background
Bamboo is an important nontimber forestry product worldwide, while growth, development and geographic distribution of bamboo are often affected by abiotic stresses. Fatty acid desaturases have important roles in regulating plant abiotic stress tolerance, especially low-temperature. However, there is no report on genome-wide of FAD genes in bamboo under abiotic stresses.
Results
A toltal of 43 PeFAD genes were identified in moso bamboo genome, which were unevenly located in 17 scaffolds. Phylogenetic analysis indicated that PeFAD genes were divided into 6 groups and ADS/FAD5 group was absence in momo bamboo, and gene structure and histidine-motifs remained highly conserved in each group. The expansion of PeFAD genes was mainly caused by tandem and segmental duplications of SAD/FAB2 group. We also identified 59 types of miRNAs targeting PeFAD genes. RNA-seq data indicated that PeFAD genes were transcribed in various organs/tissues with different degrees, and responded to abiotic stresses and hormone treatments, including cold, salt, drought, SA, ABA, BR, NAA and GA. Co-expression analysis under cold stress showed that PeCBF3 might directly bind the promoter of top cold-responsive PeSLD1 gene that contained LTR cis-element and DRE core element. The qRT-PCR assay also validated the expression pattern of PeSLD1 and its upstream regulatory gene PeCBF3.
Conclusion
In this study, we performed comprehensive genome-wide survey of PeFAD genes in moso bamboo and analyzed their expression patterns in various tissues and organs, and under abiotic stresses and phytohormones treatment. The qRT-PCR assay validated the cold inducibility of PeSLD1 and PeCBF3. This work showed critical roles of PeFAD genes in abiotic stresses responses. This is the first report on genome-wide analysis of PeFAD genes in moso bamboo, which will provide critical gene resources for molecular breeding of stress-toleranct moso bamboo.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-11065-9.
Keywords: Moso bamboo (Phyllostachys edulis), Fatty acid desaturase, Genome-wide identification, Phylogenetic analysis, Expression analysis, Abiotic stresses
Introduction
Bamboo, a perennial evergreen plant of the subfamily Bambooaceae of the Poaceae family, is an extremely important non-wood renewable forestry resources, which also is an important plant for landscaping and has high economical, ecological, and culture values [1, 2]. Most large bamboos, including moso bamboo, are suitable for growing in tropical and subtropical regions, and environment factors such as cold, drought and salt mainly restrict their growth and geographic distribution [3]. Therefore, it is of extreme importance to explore and identify stress-tolerant genes for improving the adaptability of bamboo to abiotic stress through genetic engineering.
Plants have evolved numerous mechanisms to tolerate adverse environments such as low temperature, salt, and drought [4]. Plant fatty acid desaturases (FADs) are responsible for FA desaturations, which catalyze the generation of unsaturated FAs (UFAs) including polyunsaturated FAs (PUFAs) [5]. FA desaturation in membrane lipids can affect the membrane fluidity under abiotic stresses, which contribute to maintaining the structural and functional integrity of cell membranes, and preventing membrane hardening and damage [6]. In addition, PUFAs such as linoleic acid and α-linolenic acid serve as substrates for the biosynthesis of FA-derived signaling molecules including oxylipins and phytohormone JA, in plant responses to abiotic stress [7]. FA desaturation in membrane lipids and PUFA-mediated signaling both play important roles in plant defense against abiotic and biotic stresses [6–9], which are important strategies for surviving adverse environmental conditions.
Nowadays, with the development of society and the advancement of science and technology, reference genomes of more and more plant species have been published [10]. Plant FAD genes play essential roles in response to abiotic stresses [6], and thus genome-wide analysis of FAD genes under various environment factors has been performed in numerous plants, including wheat [11], rice [12], maize [13], soybean [14], rapeseed [15], cotton [16], banana [17], poplar [18], olive [19], and sunflower [20]. Although reference genomes of moso bamboo have been released [1], there is no report on genome-wide analysis of moso bamboo FAD genes (PeFADs) under abiotic stresses. The aim of this study is to comprehensively identify the PeFAD genes at the whole-genome level, and to mine the potential stress-tolerant FAD genes, and then to validate the candidate PeFAD gene using the qRT-PCR assay. This work will provide an important molecular basis for the creation of stress-tolerant materials of moso bamboo.
Results
Identification of PeFAD genes
We totally identified 43 PeFAD genes in moso bamboo genome (Table 1). Gene sequences of PeFAD genes ranged from 553 bp to 21,278 bp in length, and their coding regions varied from 351 bp to 1,386 bp in length. PeFAD protein sequences ranged from 116 aa to 461 aa in length. Predicted on SMART database, soluble PeSADs all had FA_desaturase 2 (PF03405) domain, membrane-bound PeFAD4s all contained TMEM189_B domain (PF10520), and other membrane-bound PeFADs all included FA_desaturase domain (PF00487). Theoretical MWs of PeFAD proteins varied from 12.79 kDa to 51.08 kDa, and their theoretical pI values ranged from 4.57 to 9.71. Predicted on Plant-mPLoc and ProtComp 9.0 databases, PeFAD proteins were mainly localized in chloroplast and endoplasmic reticulum (Supplementary Table 1).
Table 1.
The basic information for PeFAD genes identified in this study
| No. | Gene name | Gene ID | Chromosome ID | Start | End | Strand | Gene length (bp) |
CDS length (bp) |
Protein length (aa) |
Molecular weight |
Theoretical pI |
Histidine mitif 1 |
Histidine motif 2 |
Histidine motif 3 |
CDS types |
Conseved domain (SMART) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PeSAD1 | PH02Gene19500 | hic_scaffold_6 | 76,326,969 | 76,328,496 | - | 1528 | 1233 | 410 | 45375.91 | 8.01 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 2 | PeSAD2 | PH02Gene12257 | hic_scaffold_13 | 45,053,379 | 45,054,600 | + | 1222 | 1080 | 359 | 40816.3 | 5.69 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 3 | PeSAD3 | PH02Gene12258 | hic_scaffold_13 | 45,085,715 | 45,088,532 | + | 2818 | 696 | 231 | 25455.56 | 4.57 | EENRHG | N/A | N/A | Partial | FA_desaturase_2 (PF03405) |
| 4 | PeSAD4 | PH02Gene12259 | hic_scaffold_13 | 45,105,263 | 45,108,251 | + | 2989 | 1233 | 410 | 46141.57 | 6.29 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 5 | PeSAD5 | PH02Gene12260 | hic_scaffold_13 | 45,137,004 | 45,138,246 | + | 1243 | 987 | 328 | 36967.85 | 6.04 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 6 | PeSAD6 | PH02Gene12261 | hic_scaffold_13 | 45,164,684 | 45,167,027 | + | 2344 | 1239 | 412 | 46485.12 | 7.15 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 7 | PeSAD7 | PH02Gene31589 | hic_scaffold_13 | 45,214,613 | 45,215,578 | + | 966 | 498 | 165 | 18814.54 | 5.94 | EENRHG | N/A | N/A | Partial | FA_desaturase_2 (PF03405) |
| 8 | PeSAD8 | PH02Gene31588 | hic_scaffold_13 | 45,220,073 | 45,220,819 | + | 747 | 351 | 116 | 12787.71 | 7.83 | N/A | DEKRHE | N/A | Partial | FA_desaturase_2 (PF03405) |
| 9 | PeSAD9 | PH02Gene31586 | hic_scaffold_13 | 45,250,910 | 45,254,174 | - | 3265 | 1260 | 419 | 47425.09 | 6.45 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 10 | PeSAD10 | PH02Gene31584 | hic_scaffold_13 | 45,303,149 | 45,303,701 | - | 553 | 474 | 157 | 18307.87 | 9.5 | EENRHG | N/A | N/A | Partial | FA_desaturase_2 (PF03405) |
| 11 | PeSAD11 | PH02Gene12263 | hic_scaffold_13 | 45,324,512 | 45,325,670 | + | 1159 | 1011 | 336 | 38142.16 | 5.29 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 12 | PeSAD12 | PH02Gene07862 | hic_scaffold_14 | 75,138,201 | 75,139,990 | + | 1790 | 1137 | 378 | 42764.87 | 7.19 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 13 | PeSAD13 | PH02Gene07237 | hic_scaffold_14 | 80,439,738 | 80,444,570 | - | 4833 | 1200 | 399 | 45254.49 | 6.33 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 14 | PeSAD14 | PH02Gene20845 | hic_scaffold_15 | 29,521,868 | 29,525,430 | + | 3563 | 741 | 246 | 27295.37 | 6.24 | EGKRHY | DERRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 15 | PeSAD15 | PH02Gene15757 | hic_scaffold_15 | 95,578,546 | 95,579,542 | + | 997 | 687 | 228 | 26169.23 | 8.7 | N/A | DEKRHE | N/A | Partial | FA_desaturase_2 (PF03405) |
| 16 | PeSAD16 | PH02Gene05757 | hic_scaffold_16 | 7,947,088 | 7,950,134 | + | 3047 | 1200 | 399 | 45290.61 | 6.43 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 17 | PeSAD17 | PH02Gene40771 | hic_scaffold_17 | 54,111,263 | 54,116,359 | + | 5097 | 1188 | 395 | 45041.32 | 6.48 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 18 | PeSAD18 | PH02Gene36679 | hic_scaffold_20 | 4,886,972 | 4,890,271 | + | 3300 | 1188 | 395 | 44710.03 | 6.42 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 19 | PeSAD19 | PH02Gene46704 | hic_scaffold_21 | 53,781,875 | 53,784,182 | + | 2308 | 1287 | 428 | 46657.29 | 7.22 | EENRHG | DERRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 20 | PeSAD20 | PH02Gene14299 | hic_scaffold_23 | 48,366,094 | 48,370,535 | + | 4442 | 1185 | 394 | 44956.12 | 7.13 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 21 | PeSAD21 | PH02Gene44797 | hic_scaffold_23 | 73,114,501 | 73,115,835 | + | 1335 | 1218 | 405 | 44938.25 | 8.43 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 22 | PeSAD22 | PH02Gene41206 | hic_scaffold_23 | 73,123,140 | 73,125,194 | + | 2055 | 1236 | 411 | 46134.48 | 7.19 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 23 | PeSAD23 | PH02Gene41208 | hic_scaffold_23 | 73,151,331 | 73,152,665 | - | 1335 | 1218 | 405 | 44968.27 | 8.43 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 24 | PeSAD24 | PH02Gene28642 | hic_scaffold_24 | 26,003,718 | 26,009,357 | - | 5640 | 1182 | 393 | 44880.99 | 8.22 | EENRHG | DEKRHE | N/A | Complete | FA_desaturase_2 (PF03405) |
| 25 | PeSAD25 | PH02Gene24080 | hic_scaffold_2757 | 81,719 | 82,673 | - | 955 | 477 | 158 | 17749.3 | 5.83 | N/A | DEKCHG | N/A | Partial | FA_desaturase_2 (PF03405) |
| 26 | PeFAD2.1 | PH02Gene22841 | hic_scaffold_3 | 88,491,036 | 88,496,439 | - | 5404 | 1167 | 388 | 44307.1 | 8.45 | HECGHH | HRRHH | HVAHH | Complete | FA_desaturase (PF00487) |
| 27 | PeFAD2.2 | PH02Gene16801 | hic_scaffold_17 | 16,604,581 | 16,609,362 | + | 4782 | 1161 | 386 | 44077.92 | 8.63 | HECGHH | HRRHH | HVAHH | Complete | FA_desaturase (PF00487) |
| 28 | PeFAD6 | PH02Gene20531 | hic_scaffold_13 | 64,242,752 | 64,248,094 | + | 5343 | 1329 | 442 | 50969.26 | 9.18 | HDCAH | HDRHH | HVPHH | Complete | FA_desaturase (PF00487) |
| 29 | PeFAD3.1 | PH02Gene44248 | hic_scaffold_2 | 28,553,924 | 28,575,201 | - | 21,278 | 1194 | 397 | 45371.84 | 8.4 | HDCGH | HRIHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 30 | PeFAD3.2 | PH02Gene44250 | hic_scaffold_2 | 28,609,938 | 28,630,686 | - | 20,749 | 1194 | 397 | 45325.92 | 8.58 | HDCGH | HRIHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 31 | PeFAD3.3 | PH02Gene21566 | hic_scaffold_13 | 349,596 | 353,537 | - | 3942 | 1176 | 391 | 44943.39 | 8.93 | HDCGH | HRTHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 32 | PeFAD7.1 | PH02Gene00068 | hic_scaffold_15 | 51,724,798 | 51,728,190 | + | 3393 | 1347 | 448 | 50207.64 | 8.97 | HDCGH | HRTHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 33 | PeFAD7.2 | PH02Gene23551 | hic_scaffold_21 | 82,848,094 | 82,850,856 | + | 2763 | 1218 | 405 | 45608.43 | 8.99 | HDCGH | HRTHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 34 | PeFAD8.2 | PH02Gene16646 | hic_scaffold_4 | 380,222 | 383,958 | - | 3737 | 1275 | 424 | 48000.08 | 8.86 | HDCGH | HRTHH | HVIHH | Complete | FA_desaturase (PF00487) |
| 35 | PeFAD8.1 | PH02Gene13663 | hic_scaffold_2 | 28,512,939 | 28,523,496 | - | 10,558 | 450 | 149 | 17299.8 | 9.71 | N/A | N/A | HVIHH | Partial | FA_desaturase (PF00487) |
| 36 | PeFAD4.1 | PH02Gene39556 | hic_scaffold_18 | 19,787,694 | 19,789,046 | + | 1353 | 876 | 291 | 31284.38 | 9.09 | FQGHHR | HSWAH | HAAHH | Complete | TMEM189_B_dmain (PF10520) |
| 37 | PeFAD4.2 | PH02Gene24139 | hic_scaffold_22 | 48,180,260 | 48,181,155 | - | 896 | 615 | 204 | 21878.7 | 6.36 | N/A | N/A | HAAHH | Partial | TMEM189_B_dmain (PF10520) |
| 38 | PeFAD4.3 | PH02Gene39558 | hic_scaffold_18 | 19,834,563 | 19,835,264 | + | 702 | 513 | 170 | 18359.85 | 4.92 | FQGHHR | N/A | N/A | Partial | TMEM189_B_dmain (PF10520) |
| 39 | PeDES1.1 | PH02Gene40570 | hic_scaffold_3 | 74,351,567 | 74,357,270 | + | 5704 | 987 | 328 | 37967.77 | 8.79 | HELSH | HLEHH | HNEHH | Complete | FA_desaturase (PF00487) |
| 40 | PeDES1.2 | PH02Gene16913 | hic_scaffold_17 | 29,332,269 | 29,335,436 | - | 3168 | 981 | 326 | 37593.39 | 8.5 | HELSH | HLEHH | HNEHH | Complete | FA_desaturase (PF00487) |
| 41 | PeDES1.3 | PH02Gene02088 | hic_scaffold_24 | 51,356,905 | 51,360,358 | + | 3454 | 987 | 328 | 37783.66 | 8.77 | HELSH | HLEHH | HNEHH | Complete | FA_desaturase (PF00487) |
| 42 | PeSLD1 | PH02Gene30788 | hic_scaffold_3 | 33,818,104 | 33,820,342 | - | 2239 | 1386 | 461 | 51077.84 | 8.53 | HDSGHH | HNTHH | QIEHH | Complete | FA_desaturase (PF00487) |
| 43 | PeSLD2 | PH02Gene31223 | hic_scaffold_19 | 5,531,353 | 5,532,717 | - | 1365 | 1248 | 415 | 46608.5 | 8.87 | HDSGHH | HNTHH | QIEHH | Complete | FA_desaturase (PF00487) |
Phylogenetic relationship analysis
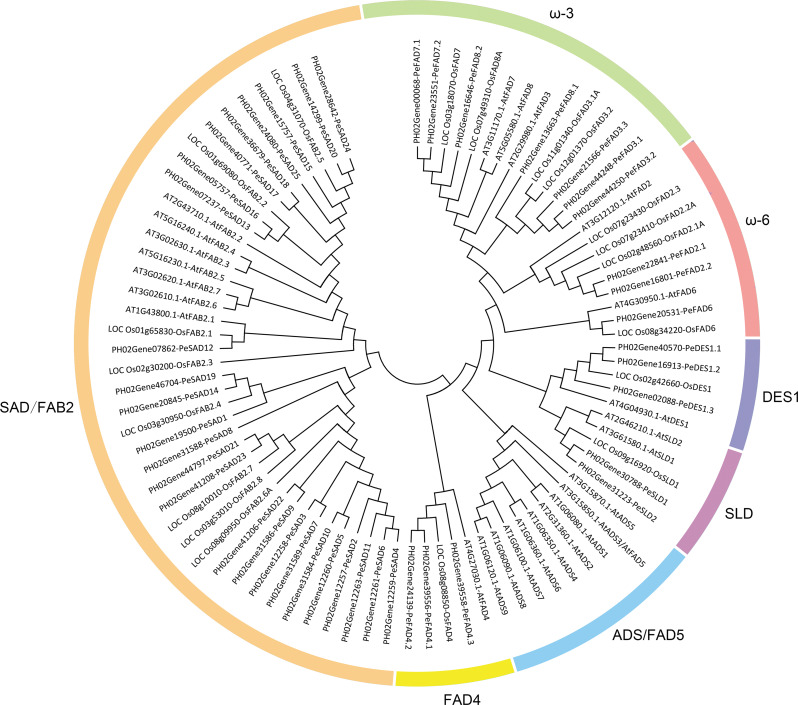
In total, 87 FAD proteins from P. edulis, O. sativa and A. thaliana, were utilized to produce the phylogenetic tree. The evolutionary relationship analysis indicated that these 87 FAD proteins were classified into seven groups: SAD/FAB2, DES1, SLD, ADS/FAD5, FAD4, ω-6 and ω-3 (Fig. 1). As shown in Table 2, ADS/FAD5 group only existed in A. thaliana, while it was absence both in P. edulis, O. sativa. The remaining six groups all existed in these three species. In moso bamboo, soluble SAD/FAB2 group contained 25 members, DES1, FAD4 and ω-6 groups both had 3, SLD group harbored 2, ω-3 group contained 7.
Fig. 1.
Phylogenetic tree of FAD proteins among P. edulis, O. sativa and A. thaliana
Table 2.
The total number of FAD genes within each group in P. edulis, O. sativa and A. thaliana
| Category | P. edulis | O. sativa | A. thaliana |
|---|---|---|---|
| Total | 43 | 19 | 25 |
| Total Soluble FADs | 25 | 8 | 7 |
| SAD/FAB2 | 25 | 8 | 7 |
| Total Membrane FADs | 18 | 11 | 18 |
| DES1 | 3 | 1 | 1 |
| SLD | 2 | 1 | 2 |
| ADS/FAD5 | 0 | 0 | 9 |
| FAD4 | 3 | 1 | 1 |
| ω-6 | 3 | 4 | 2 |
| ω-3 | 7 | 4 | 3 |
Analysis of gene structure and conserved motifs
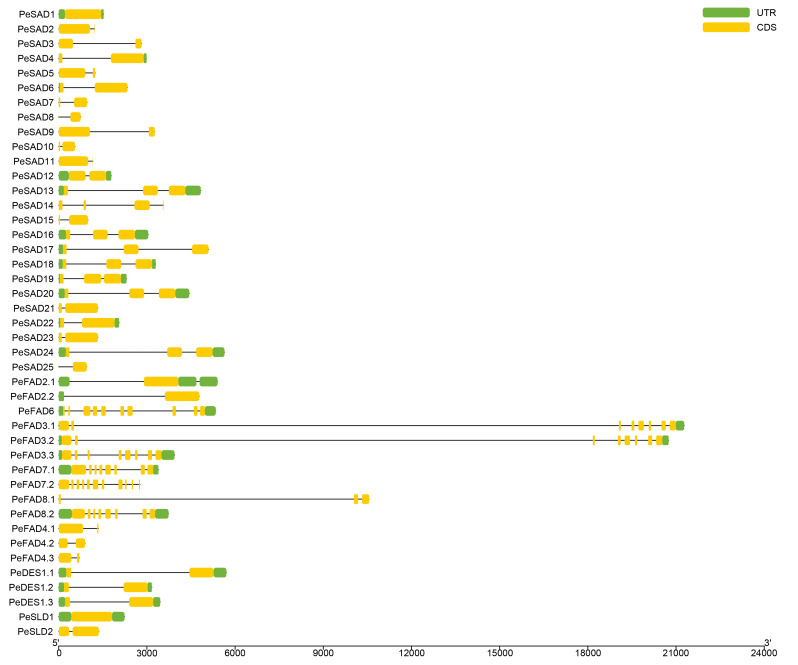
To analyze gene structures of PeFAD genes, their exon-intron organizations were visualized on TBtools program based on the GFF3 file of reference genome (Fig. 2). Among 43 PeFAD genes, 2 had no intron, i.e., PeSAD1 and PeSLD1. The remaining 41 PeFAD genes harbored 1 (e.g., PeSAD2, PeFAD2.1 and PeFAD4.1) to 9 (PeFAD6 and PeFAD7.2) introns. As shown in Table 1, 19 soluble PeFADs contained 2 conserved histidine-motifs, whereas 15 membrane-bound PeFADs had 3 conserved histidine-motifs, which play vital roles in maintaining the catalytic activity of desaturases. The remaining soluble PeFADs (PeSAD3, PeSAD7, PeSAD8, PeSAD10, PeSAD15 and PeSAD25) and membrane-bound PeFADs (PeFAD8.1, PeFAD4.2 and PeFAD4.3) all harbored 1 histidine-boxes, which belong to partial genes and should have no catalytic activity.
Fig. 2.
Gene structure of PeFAD genes in P. edulis
Chromosome location and gene duplication analysis
In total, 43 PeFADs were unevenly located in 17 hic_scaffolds, in which each hic_scaffold had 1–12 PeFADs (Table 1). The hic_scaffold_13 harbored 12, hic_scaffold_23 had 4, hic_scaffold_15, hic_scaffold_17, hic_scaffold_2 and hic_scaffold_3 all contained 3, hic_scaffold_14, hic_scaffold_18, hic_scaffold_21 and hic_scaffold_24 all included 2, and hic_scaffold_16, hic_scaffold_19, hic_scaffold_20, hic_scaffold_22, hic_scaffold_4, hic_scaffold_6 and hic_scaffold_2757 all possessed 1. Gene duplication analysis indicated that 46 duplicated gene pairs were identified in 43 PeFAD genes (Table 3), in which 34 gene pairs (73.91%; composed of PeSADs, PeFAD2s, PeFAD3s, PeFAD7s, PeDES1s and PeSLD1s) were segmental duplication type and the rest 12 (26.09%; consisting of PeSADs and PeFAD3s) were tandem duplication type. Of 34 segmental duplications, 26 (76.47%) belonged to PeSADs, whereas among 12 tandem duplications, 11 (91.67%) were PeSADs. To examine the selection pressures for duplicated gene pairs, the Ka and Ks values were computed (Table 3). For 46 duplicated gene pairs, only 1 contained a Ka/Ks ratio of more than 1, and the lest all had the Ka/Ks ratios with less than 1, revealing that there mainly occurred purifying selection in their evolution process.
Table 3.
Gene duplication types and Ka/Ks analysis for duplicated gene pairs of PeFADs
| No. | Gene_1 | Gene_2 | Ka | Ks | Ka/Ks | Duplication type |
|---|---|---|---|---|---|---|
| 1 | PeSAD5 | PeSAD2 | 0.1219 | 0.1579 | 0.7719 | Tandem duplication |
| 2 | PeSAD5 | PeSAD6 | 0.1170 | 0.1303 | 0.8980 | Tandem duplication |
| 3 | PeSAD5 | PeSAD4 | 0.1321 | 0.1765 | 0.7485 | Tandem duplication |
| 4 | PeSAD5 | PeSAD11 | 0.1148 | 0.1874 | 0.6124 | Tandem duplication |
| 5 | PeSAD9 | PeSAD5 | 0.1751 | 0.2581 | 0.6787 | Tandem duplication |
| 6 | PeSAD9 | PeSAD2 | 0.0713 | 0.1537 | 0.4638 | Tandem duplication |
| 7 | PeSAD9 | PeSAD6 | 0.0610 | 0.1629 | 0.3749 | Tandem duplication |
| 8 | PeSAD9 | PeSAD4 | 0.0579 | 0.1555 | 0.3722 | Tandem duplication |
| 9 | PeSAD9 | PeSAD11 | 0.0600 | 0.1822 | 0.3293 | Tandem duplication |
| 10 | PeSAD13 | PeSAD16 | 0.0204 | 0.1219 | 0.1677 | Segmental duplication |
| 11 | PeSAD14 | PeSAD19 | 0.1150 | 0.2381 | 0.4832 | Segmental duplication |
| 12 | PeSAD17 | PeSAD18 | 0.0395 | 0.2908 | 0.1358 | Segmental duplication |
| 13 | PeSAD17 | PeSAD24 | 0.0825 | 0.8448 | 0.0977 | Segmental duplication |
| 14 | PeSAD17 | PeSAD20 | 0.0921 | 0.8861 | 0.1039 | Segmental duplication |
| 15 | PeSAD18 | PeSAD20 | 0.0948 | 0.8397 | 0.1130 | Segmental duplication |
| 16 | PeSAD18 | PeSAD24 | 0.0880 | 0.7487 | 0.1175 | Segmental duplication |
| 17 | PeSAD20 | PeSAD24 | 0.0169 | 0.1198 | 0.1409 | Segmental duplication |
| 18 | PeSAD21 | PeSAD22 | 0.1231 | 0.2220 | 0.5543 | Tandem duplication |
| 19 | PeSAD21 | PeSAD9 | 0.1190 | 0.2188 | 0.5436 | Segmental duplication |
| 20 | PeSAD21 | PeSAD5 | 0.2383 | 0.3513 | 0.6783 | Segmental duplication |
| 21 | PeSAD21 | PeSAD2 | 0.1397 | 0.2778 | 0.5027 | Segmental duplication |
| 22 | PeSAD21 | PeSAD6 | 0.1483 | 0.2754 | 0.5383 | Segmental duplication |
| 23 | PeSAD21 | PeSAD4 | 0.1530 | 0.3029 | 0.5050 | Segmental duplication |
| 24 | PeSAD21 | PeSAD11 | 0.1417 | 0.2971 | 0.4768 | Segmental duplication |
| 25 | PeSAD22 | PeSAD9 | 0.0737 | 0.1444 | 0.5106 | Segmental duplication |
| 26 | PeSAD22 | PeSAD5 | 0.2245 | 0.3072 | 0.7309 | Segmental duplication |
| 27 | PeSAD22 | PeSAD2 | 0.1218 | 0.2054 | 0.5933 | Segmental duplication |
| 28 | PeSAD22 | PeSAD6 | 0.1161 | 0.2030 | 0.5721 | Segmental duplication |
| 29 | PeSAD22 | PeSAD4 | 0.1124 | 0.2146 | 0.5239 | Segmental duplication |
| 30 | PeSAD22 | PeSAD11 | 0.1129 | 0.2305 | 0.4899 | Segmental duplication |
| 31 | PeSAD23 | PeSAD22 | 0.1244 | 0.2220 | 0.5603 | Tandem duplication |
| 32 | PeSAD23 | PeSAD9 | 0.1190 | 0.2188 | 0.5436 | Segmental duplication |
| 33 | PeSAD23 | PeSAD5 | 0.2383 | 0.3513 | 0.6783 | Segmental duplication |
| 34 | PeSAD23 | PeSAD2 | 0.1397 | 0.2778 | 0.5027 | Segmental duplication |
| 35 | PeSAD23 | PeSAD6 | 0.1496 | 0.2754 | 0.5433 | Segmental duplication |
| 36 | PeSAD23 | PeSAD4 | 0.1544 | 0.3029 | 0.5095 | Segmental duplication |
| 37 | PeSAD23 | PeSAD11 | 0.1417 | 0.2971 | 0.4768 | Segmental duplication |
| 38 | PeFAD2.1 | PeFAD2.2 | 0.0116 | 0.0872 | 0.1335 | Segmental duplication |
| 39 | PeFAD3.1 | PeFAD3.2 | 0.0196 | 0.0124 | 1.5804 | Tandem duplication |
| 40 | PeFAD3.1 | PeFAD3.3 | 0.0548 | 0.1697 | 0.3227 | Segmental duplication |
| 41 | PeFAD3.2 | PeFAD3.3 | 0.0687 | 0.1683 | 0.4082 | Segmental duplication |
| 42 | PeFAD7.1 | PeFAD7.2 | 0.0426 | 0.1950 | 0.2184 | Segmental duplication |
| 43 | PeDES1.1 | PeDES1.2 | 0.0175 | 0.0939 | 0.1864 | Segmental duplication |
| 44 | PeDES1.1 | PeDES1.3 | 0.0465 | 0.6322 | 0.0735 | Segmental duplication |
| 45 | PeDES1.2 | PeDES1.3 | 0.0446 | 0.6555 | 0.0681 | Segmental duplication |
| 46 | PeSLD1 | PeSLD2 | 0.0371 | 0.1967 | 0.1887 | Segmental duplication |
Prediction of miRNAs targeting PeFAD genes
The miRNAs play important roles in gene post-transcription regulation, and thus the miRNAs targeting PeFAD genes were predicted. As shown in Supplementary Table 2, there existed a total of 59 types of miRNAs for targeting 22 PeFAD genes, and their regulation types all belonged to the cleavage effect. There were 37 miRNAs, which all targeted PeFAD3.3 gene, and 6 miRNAs all for PeFAD4.1 gene, whereas PeFAD8.2, PeFAD2.2, PeFAD2.1, PeFAD7.2, PeSAD18 and PeSAD10 all had 2 miRNAs, and the remaining 14 PeFAD genes all contained only 1 miRNA. This showed that these identified miRNAs might participate in gene post-transcription regulations of corresponding PeFAD genes.
Expression of PeFAD genes in different organs/tissues
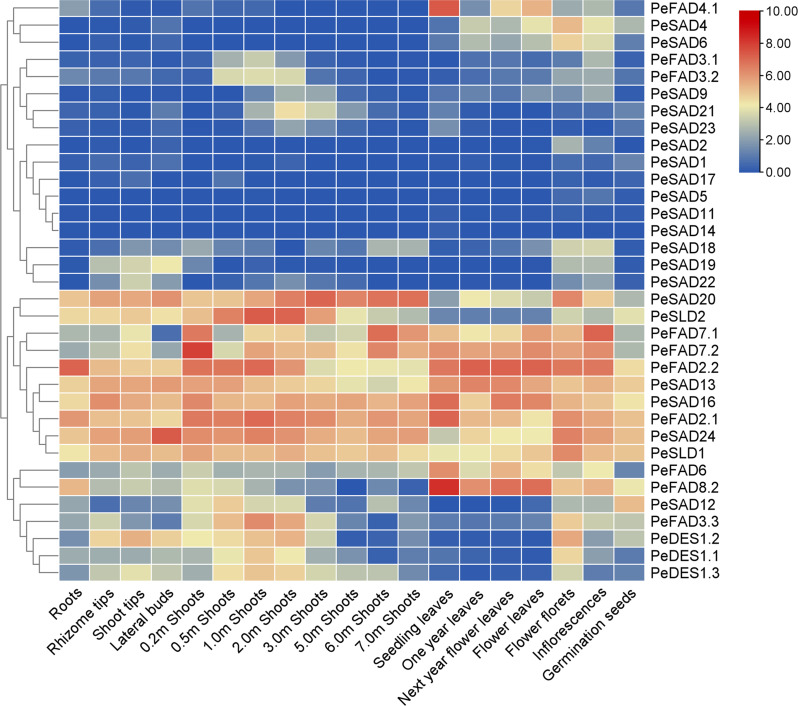
To detect the potential biological function of 34 full-length PeFAD genes with complete histidine-boxes, we analyzed their expression patterns in 19 different tissues/organs based on published RNA-seq data (Supplementary Table 3), including roots, lateral buds, rhizome tips, shoot tips, 0.2–7.0 m shoots, inflorescences, seedling leaves, one-year leaves, next-year flower leaves, flower leaves, flower florets, and germination seeds. In total, transcriptomic data of these 34 PeFADs all were obtained (Fig. 3; Supplementary Table 4), in which 4 (PeSAD17, PeSAD5, PeSAD11 and PeSAD14) almost had no expression in all tissues (their TPM values all were < 1), and the remaining 30 PeFADs were expressed in various tissues/organs with the different levels. The TPM values of PeSAD1 were > 1 only in germination seeds (1.42), and PeSAD2 was only in flower florets and inflorescences. PeFAD3.1, PeFAD3.2 and PeSAD21 both had higher expression levels in 0.5–3.0 m shoots than other organs/tissues. PeSAD4 and PeSAD6 both were expressed in flower florets with higher levels than other organs/tissues. PeFAD2.2, PeSAD16, PeSAD13, PeFAD2.1, PeSAD24, and PeSLD1 all had constitutively high expression with the TPM values of 8.13-162.72. PeFAD3.3, PeDES1.2, PeDES1.3 and PeDES1.1 contained high expression levels in rhizome tips, shoot tips, 0.2–6.0 m shoots and flower florets. This result showed that PeFAD genes might have important roles in growth and development of moso bamboo.
Fig. 3.
Expression patterns of PeFAD genes in 19 different tissues/organs
Expression of PeFAD genes in response to abiotic stresses and hormone treatments
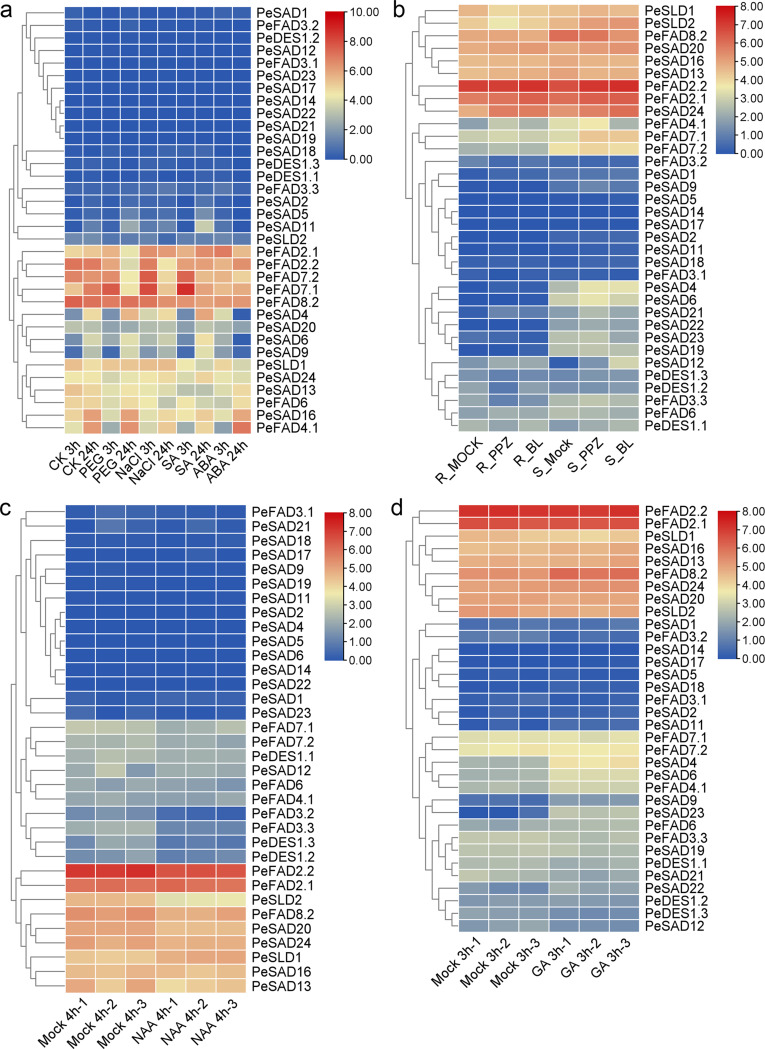
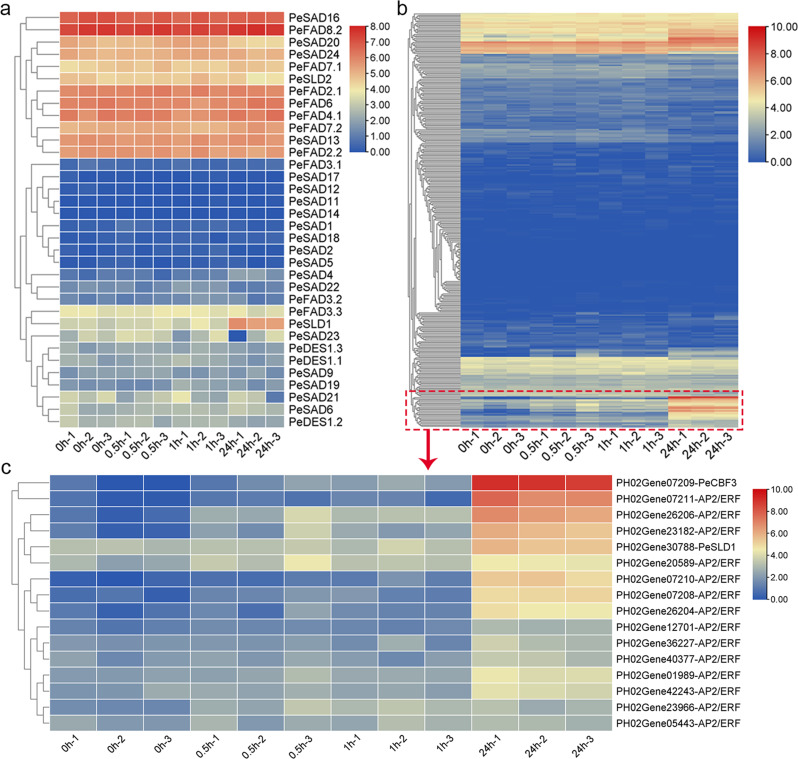
To analyze important roles of 34 full-length PeFAD genes in plant stress responses, and thus we detected their expression patterns in response to drought, salt, cold, SA, ABA, BR, NAA and GA treatments using published transcriptome data (Supplementary Tables 5–9). Under PEG treatment (Fig. 4a), there existed 2 up-regulated PeFAD DEGs (PeFAD7.1 and PeSAD6) and 6 down-regulated DEGs at 3 h, whereas 2 up-regulated DEGs (PeSAD5 and PeSAD11) and 4 down-regulated DEGs were obtained at 24 h. Under NaCl treatment (Fig. 4a), we detected 6 up-regulated DEGs (PeSAD11, PeFAD7.1, PeSAD4, PeSAD9, PeFAD2.1, PeSAD6) and 1 down-regulated DEGs at 3 h, and there were 1 up-regulated DEGs (PeFAD2.1) and 7 down-regulated DEGs at 24 h. Under SA treatment (Fig. 4a), 1 up-regulated DEGs (PeFAD7.1) and 6 down-regulated DEGs were observed at 3 h, whereas there existed 4 up-regulated DEGs (PeSAD11, PeSAD5, PeFAD2.1, and PeSAD4) and 6 down-regulated DEGs at 24 h. Under ABA treatment (Fig. 4a), we detected 5 up-regulated DEGs (PeSAD4, PeSAD9, PeFAD2.1, PeFAD3.3, and PeSAD6) and 6 down-regulated DEGs at 3 h, whereas there were 7 DEGs at 24 h, which all were down-regulated. Under the treatment of PPZ with/without BR (Fig. 4b), a total of 5 up-regulated DEGs and 3 down-regulated DEGs (PeDES1.2, PeFAD3.2, and PeFAD3.3) for PPZ-responsive genes in shoot and root parts, whereas there were 2 up-regulated DEGs (PeSAD12 and PeDES1.2) and 1 down-regulated DEGs for BR-responsive genes in these two parts. There existed a total of 4 NAA-responsive PeFAD DEGs (PeSLD2, PeDES1.3, PeFAD3.3 and PeFAD3.2), whose expression levels all were decreased upon NAA treatment (Fig. 4c). In response to GA treatment, there only existed 4 up-regulated PeDAD DEGs (PeSAD23, PeSAD9, PeSAD4 and PeSAD6) (Fig. 4d). Under cold treatment (Fig. 5a), there existed only 2 cold-responsive DEGs (both up-regulated), PeSLD1 and PeSAD4, in which expression levels and change-folds of PeSLD1 both were higher than PeSAD4. This result suggested that PeSLD1 might play a more important role in cold response and tolerance.
Fig. 4.
Expression patterns of PeFAD genes under the treatment of salt and drought stresses, and plant hormones. a, PEG, NaCl, SA and ABA; b, PPZ and BL (BR); c, NAA; d, GA
Fig. 5.
Expression patterns of PeFAD genes under cold stress (a) and co-expression of top cold-responsive PeSLD1 and potential upstream regulatory AP2-ERFs (b and c)
Validation of cold-responsive PeSLD1 gene and analysis of its upstream regulatory gene
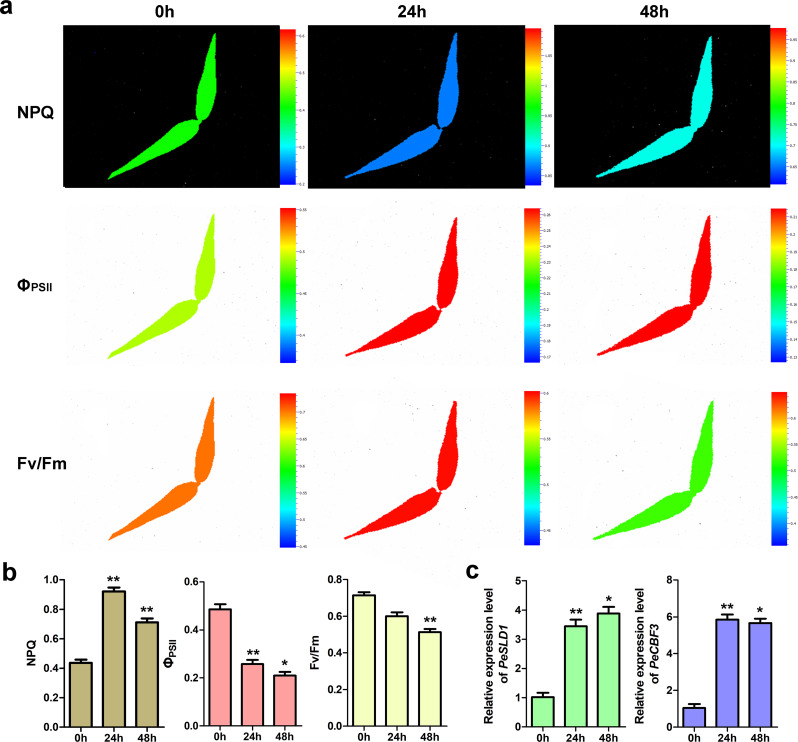
Top cold-responsive gene PeSLD1 was selected to perform the qRT-PCR assay for validating the accuracy of transcriptome results. Results showed that the expression levels of PeSLD1 were significantly up-regulated after cold treatment (Fig. 6c). cis-Element analysis of PeSLD1 promoter showed that there existed low-temperature responsiveness (LTR)-element and DRE core element in its promoter. Plant AP2/ERF proteins can bind to DNA sequences of DRE core element, and are also involved in regulating plant cold tolerance, such as CBF1, CBF2 and CBF3 [21]. To mine potential AP2/ERF family member that regulates PeSLD1 expression, we performed co-expression analysis of PeSLD1 with all AP2/ERF family genes under cold stress in moso bamboo (Fig. 5b; Supplementary Table 10). Results showed that there were a cold-responsive cluster, in which PeSLD1 clustered with 15 AP2/ERF genes. PeCBF3 is the top cold-responsive gene among these 15 AP2/ERF candidates (Fig. 5c). The qRT-PCR assay also validated the cold inducibility of PeCBF3 (Fig. 6c), suggesting that PeCBF3 might directly target PeSLD1 promoter. The regulatory effect of upstream gene PeCBF3 on PeSLD1 is worth being confirmed with protein-DNA interaction experiments. Additionally, the difference of chlorophyll fluorescence parameters of moso bamboo leaves after cold stress compared with the control were analyzed by plant phenotype imager. Results indicated that chlorophyll fluorescence parameters, NPQ, ΦPSII and Fv/Fm varied after cold treatment (Fig. 6a, b), suggesting the potential application of this device in screening of cold-tolerant germplasm materials of bamboo.
Fig. 6.
The impact of cold stress on photosynthesis of moso bamboo leaves (a, b), and the qRT-PCR expression level of top cold-responsive gene PeSLD1 and its potential upstream regulatory gene PeCBF3, (c). Standard images of the Fv/Fm, ΦPSII and NPQ from cold-treated moso bamboo leaves at 0, 24 and 48 h. A false color scale is used for each parameter. The values represent the average ± SD of 3 biological replicates. Fv/Fm, maximum quantum yield of PSII; ΦPSII, effective quantum yield of PSII; NPQ, non-photochemical quenching. * p < 0.05 and ** p < 0.01 compared with samples at 0 h
Discussion
Bamboo is an extremely important non-wood renewable forestry resource with high economical, ecological, and culture values. Nowadays, numerous environmental factors such as low temperature, drought, and salinity severely restricted bamboo growth, development, and geographic distribution. Plant FAD genes play important roles in defense against abiotic stresses by regulating the FA desaturation in cell membrane, and FA-mediated signaling transduction. Although reference genomes of various bamboo species have been sequenced, the information of comprehensive analysis of bamboo FAD genes upon abiotic stresses at whole-genome level is limited. In this study, we performed a systemically genome-wide analysis of moso bamoo PeFAD genes under abiotic stresses and phytohormone treatments, and identified substantial abiotic stress-responsive PeFAD genes, which is of great importance for creating stress-tolerant bamboo cultivar using genetic engineering.
A total of 43 PeFAD genes were identified in moso bamboo genome. There existed 46 duplicated gene pairs, which contained 34 segmental duplications and 12 tandem duplications. Gene duplication analysis showed that diversity and expansion of PeFAD genes mainly resulted from segmental duplications (76.47%, 26/34) and tandem duplications (91.67%, 11/12) of PeSAD group. Gene expansion of chia FAD genes also mainly resulted from gene duplications of ShSAD group, but it all belonged to tandem duplication type [22]. Moreover, the Ka/Ks ratios of duplicated gene pairs of PeFADs were less than 1 except for PeFAD3.1/PeFAD3.2, revealing that they mainly subjected to negative selection in evolution process, which was similar with those of banana [17], poplar [18], and sunflower [20].
Phylogenetic relationship analysis revealed that ADS/FAD5 gene group was absent in moso bamboo and rice, but Arabidopsis contained this group. This phenomenon also occurred in banana, barley, wheat, and maize, while there also existed ADS/FAD5 group in wheat [11], soybean [14], cotton [16], rapeseed [15], sunflower [20], chia and perilla [22], poplar [18], and camelina [23]. These reports and this study obviously both supported the hypothesis that monocots might lose ADS/FAD5 group and dicots retained it after separation from the ancestral species, while the functional evolution mechanism need to further be studied and revealed.
Substantial studies have indicated that FAD genes play critical roles in plant response to various stresses such as cold, salinity and drought [6]. For example, ADS2 gene was found to play important roles in chilling and freezing tolerance in Arabidopsis by modulating the FA composition of organelle membrane [24]. PpSFD mutants for Physcomitrium patens SLD gene exhibited a cold-sensitive phenotype, and PpSFD over-expression in atads2 mutant could functionally complemented its phenotype, suggesting its important roles in cold tolerance [25]. Tomato SlSLD gene also had important roles in plant chilling resistance [26]. Here, we found two cold-responsive genes, PeSLD1 and PeSAD6, in moso bamboo, and cold inducibility of PeSLD1 was also validated by the qRT-PCR assay, which thus PeSLD1 had great potential in improving cold tolerance in bamboo. In addition, transgenic soybeans over-expressing GmFAD3A possessed the tolerance to drought and salinity stresses, while soybean plants of GmFAD3 silencing were sensitive to drought and salinity stresses [27]. Arabidopsis FAD2 gene were involved in plant ER stress [28], and loss-of-function mutant of FAD2 was sensitive to salt stress [29]. In this study, two PeFAD DEGs (PeFAD7.1 and PeSAD6) were also found to be significantly up-regulated both in salt and drought stresses, showing their potential roles in abiotic stress tolerance. Heterogeneous expression of flax LuSAD1 and LuSAD2 also enhanced seedling cold and drought tolerance in rapeseed [30].
Plant FADs have been reported to catalyze the UFA production [6], and UFAs such as linoleic acid and linolenic acid are precursors for the biosynthesis of FA-derived signal molecules, including JA, oxylipin, and sphingolipid [25, 31]. JA signaling pathway often occur crosstalk with other phytochrome pathways for regulation of the trade-offs in growth and defense [32, 33]. In this study, there both existed 2 common upregulated PeFAD DEGs (PeFAD2.1 and PeSAD4) under SA and ABA treatments. In tomato, SlFAD2-4 and SlFAD2-7 were also upregulated by SA, and their expression is independent of JA synthesis [34]. In poplar, PtFAB2.3, PtFAB2.4 and PtFAB2.5 were also upregulated under ABA treatments [18]. PeDES1.2 was up-regulated under BR treatment, and down-regulated under BR-inhibitor PPZ treatment. Four up-regulated DEGs were found in response upon GA treatment, respectively, while there only existed 4 down-regulated DEGs under NAA treatments. In rice, most OsFAD genes were responsive to GA treatment [12]. In Arabidopsis, auxin and cytokinin treatments upregulated ectopic FAD3 expression in roots only during vegetative growth [35]. Therefore, plant FAD genes play important roles in regulating the synthesis and transduction of plant signal molecules as well as plant defense.
Previous studies indicated that various types of TFs directly targeted plant FAD genes. For example, banana MaABI5-like directly targeted MaFAD3-1, MaFAD3-4, MaFAD3-5, MaFAD6-2, MaFAD6-3 and thus could increase plant cold tolerance [36]. The three most highly expressed SAD genes in Arabidopsis seeds, FAB2, AAD1, and AAD5, were directly activated by the WRI1 TF [37]. In this study, PeCBF3 binding site (GCCGAC, DRE core) and LTR element was predicted in promoter of top cold-responsive PeSLD1. Co-expression and qRT-PCR assay both validated their strong cold inducibility. This result suggests that the expression of PeSLD1 gene might be directly regulated by PeCBF3, which thus deserves further study.
The miRNAs serve as small endogenous RNAs that alter gene-expression at posttranscriptional level, which exist widely in organism including plants and animals. miRNAs participate in regulating plant growth, development, and defense [38]. In banana, 12 MaFAD genes were predicted to be regulated by 30 miRNAs [17]. In peanut, 20 AhFAD genes were predicted to be targeted by 19 miRNAs [39]. This study found that 59 types of miRNAs were predicted to target 22 PeFAD genes. These PeAD genes might be directly regulated by the corresponding predicted miRNAs, which need to be validated in the further study.
For the characterization of plant cold tolerance, it is of quite significance to apply a rapid high-throughput, and non-invasive detection procedure instead of time-consuming and labor-intensive detection means. In this study, we for the first time detected the potential of plant phenotype imager in checking the low temperature response in moso bamboo seedling. Under cold stress, values of three chlorophyll fluorescence parameters, NPQ, ΦPSII, and Fv/Fm, all altered significantly. There also were similar reports in Arabidopsis and oats [40, 41]. This result suggested that plant phenotype imager had a great potential in measuring cold response of bamboos with an easy and non-invasive method at a large scale.
Conclusion
Here, a systemically whole-genome-wide analysis of PeFAD genes were performed in moso bamboo. A total of 43 PeFAD genes were identified in moso bamboo, which were mapped in 17 scaffolds. Evolutionary relationship analysis revealed that PeFAD genes were classified into 6 groups and there was no ADS/FAD5 group in momo bamboo, and each group remained highly conserved in gene exon-intron organization and protein histidine-motifs. Gene duplication of SAD/FAB2 group mainly resulted in diversity and expansion of PeFAD genes. Top cold-responsive gene contained LTR cis-element and DRE core element in its promoter. Co-expression analysis and qRT-PCR assay confirmed that PeCBF3 might directly target PeSLD1. In total, 59 types of miRNAs were predicted to target PeFAD genes. Transcriptome data analysis implied that PeFAD genes were differentially expressed in 19 tissues/organs as well as were responsive to abiotic stresses and various phytohormones. This study will provide important references for further functional studies of stress-responsive PeFAD genes.
Materials and methods
Sequence retrieval and structural analysis
To identify the PeFAD genes, we downloaded the HMM files of FAD domain (PF03405, PF00487 and PF10520) from Pfam database (http://Pfam.sanger.ac.uk/), and then we performed the hmmsearch operation (e-value 10e − 5; http://hmmer.org) against moso bamboo genome (http://gigadb.org/dataset/view/id/100498) to obtain PeFAD proteins. Meanwhile, we also perform BlastP to obtain the PeFAD proteins using Arabidopsis FAD (AtFAD) protein sequences as queries. All candidates were checked using SMART database ((http://smart.embl-heidelberg.de/) and SUPERFAMILY 2 database (https://beta.supfam.org/), and non-FADs all were removed. Based on chromosome locations and homology with AtFAD genes [15], we determined the names of PeFAD genes. We predicted the subcellular location of PeFAD proteins using ProtComp 9.0 (http://www.softberry.com/) and Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) websites. We calculated their theoretical isoelectric points (pI) and molecular weights using the ProtParam tool (https://web.expasy.org/protparam/).
Phylogenetic relationship analysis
The multiple alignment of the of PeFAD, OsFAD and AtFAD protein sequences [15], were performed using MAFFT7 with the default parameters, and then we generated the phylogenetic tree on MEGA7 software with neighbor-joining (NJ) method using the 1000 bootstrap replicates and p-distance model.
Analysis of gene structure and conserved motifs
We analyzed the PeFAD gene structures based on GFF3 file of reference genome using TBtools-II [42]. We also analyzed the conserved histidine motifs of PeFAD proteins based on the multiple alignment result of PeFAD proteins, and OsFAD and AtFAD proteins.
Chromosome location, gene duplication and selection pressure analysis
We analyzed the chromosome location information of PeFAD genes using GFF3 file of reference genome on TBtools-II. Duplicated gene pairs of PeFAD genes were identified as previously described [43]. We calculated the non-synonymous substitution (Ka) and synonymous substitution (Ks) values of the duplicated gene pairs using simple Ka/Ks Calculator on TBtools-II, and then we determined the selection mode based on the Ka/Ks ratio.
Analysis of cis-acting elements in PeFAD promoter
We obtained the 1,500 bp sequences upstream of initiation codon of PeFAD gene from the reference genome, and then predicted the cis-regulatory elements on PlantCARE websites (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
MicroRNA (miRNA) target predictions in PeFAD genes
We predicted the potential miRNAs targeting PeFAD genes based on all of miRNAs in database, using psRNATarget website (https://www.zhaolab.org/psRNATarget/) with the expected value of 3 and other default parameters.
Expression of PeFAD genes in different organs and under abiotic stresses
Expression levels of full-length PeFAD genes in different organs/tissues (Supplementary Table 3) as well as under abiotic stresses and phytohormone treatments including cold (NCBI accession no., GSE130314), drought, salt, SA and ABA (GSE169067), BR (GSE123529), NAA (GSE100172) and GA (GSE104596), were analyzed using published RNA-seq data, and then visualized in a heat map using TBtools-II, in which PeFAD genes were determined as differentially expressed genes (DEGs) if log2FC of gene expression values were ≥ 1 or ≤-1 and the FPKM or TPM values were also ≥ 1.
Expression of PeFAD genes in response to cold stress
The cold treatment (4 °C) of 2-months-old seedlings of moso bamboo were performed as previously described [45], the seedling leaves were sampled at 0 h, 24 h and 48 h under cold stress, and they were all immediately frozen in liquid nitrogen and stored in -80 °C. The top cold-responsive PeSLD1 gene and its potential upstream regulatory gene PeCBF3 were selected to perform the further qRT-PCR to validate their response to cold stress.
Chlorophyll fluorescence imaging
The fluorescence parameters (Fv/Fm, NPQ and ΦPSII) and chlorophyll fluorescence images of the chia seedling leaves at 0 h, 24 h and 48 h after cold treatment were analyzed using the plant phenotype imager (device no. 20A00005; a chlorophyll fluorescence imaging system FluorCam7.0; Photon Systems Instruments, Brno, Czech Republic) as previously described [44].
RNA extraction and qRT-PCR analysis
Moso bamboo (P. edulis) materials were collected from the botanical garden of bamboo at Leshan Normal University (E103°68′; N29°59′). Total RNA was extracted from the moso bamboo seedling leaves under cold stress by the RNAsimple Total RNA Kit (DP419, Tiangen, Beijing) and then the first-strand total cDNA was synthesized using one µg of total RNA by the PrimeScript Reagent Kit with gDNA Eraser (Takara Dalian, China). The qRT-PCR experiment was performed using the TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Dalian, China) on CFX96 Real-time PCR System (Bio-Rad, USA) with 3 replicates as described on our previous report [45]. The primers for the selected PeSLD1 and PeCBF3 genes and the internal control gene PeUBQ [3] were shown in Supplementary Table 11.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Y.X. designed the study. C.F., Q.F., S.W., F.W., N.J., and R.Z. performed the experiments. C.F. and Y.X. analyzed the data. Y.X. undertook the use and maintenance of plant phenotype imager. C.F. and Y.X. wrote and revised the manuscript.
Funding
This work was supported by the Science and technology program of Leshan Normal Univerisity (2021SSDJS003), National Key Research and Development Program of China (2022YFD1901405), National Natural Science Foundation of China (32001441), Opening Foundation of Key Laboratory of Sichuan Province for Bamboo Pests Control and Resource Development (ZLKF202306), and Science and Technology Program of Leshan Normal University (2022SSDJ005, KYPY2023-0006, XJR17005, LZD010).
Data availability
The genome sequences, protein sequences and gene annotation files of P. edulis were downloaded in GigaDB (http://gigadb.org/dataset/view/id/100498). RNA raw data (Supplementary Table 3) for 19 different organs/tissues (GSE90517, GSE104951, GSE121216, PRJNA842835, and PRJNA217219), and abiotic stresses and hormones treatments (GSE130314, GSE169067, GSE104596, GSE100172, and GSE123529) in P. edulis was downloaded in NCBI database (https://www.ncbi.nlm.nih.gov/).
Declarations
Ethics approval and consent to participate
The moso bamboo (P. edulis) materials used in qRT-PCR analysis were taken from the botanical garden of bamboo at Key Laboratory of Sichuan Province for Bamboo Pests Control and Resource Development, Leshan Normal University, Leshan, Sichuan. The collection of plant material complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao H, Gao Z, Wang L, Wang J, Wang S, Fei B, Chen C, Shi C, Liu X, Zhang H, Lou Y, Chen L, Sun H, Zhou X, Wang S, Zhang C, Xu H, Li L, Yang Y, Wei Y, Yang W, Gao Q, Yang H, Zhao S, Jiang Z. Chromosome-level reference genome and alternative splicing atlas of moso bamboo (Phyllostachys edulis). Gigascience. 2018;7(10):giy115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Wu C, Hu X, Gao H, Wang Y, Luo H, Cai S, Li G, Zheng Y, Lin C, Zhu Q. Transcriptome profiling reveals the crucial biological pathways involved in cold response in moso bamboo (Phyllostachys edulis). Tree Physiol. 2020;40(4):538–56. [DOI] [PubMed] [Google Scholar]
- 3.Hu X, Liang J, Wang W, Cai C, Ye S, Wang N, Han F, Wu Y, Zhu Q. Comprehensive genome-wide analysis of the DREB gene family in moso bamboo (Phyllostachys edulis): evidence for the role of PeDREB28 in plant abiotic stress response. Plant J. 2023;116(5):1248–70. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Zhu J, Gong Z, Zhu JK. Abiotic stress responses in plants. Nat Rev Genet. 2022;23(2):104–19. [DOI] [PubMed] [Google Scholar]
- 5.He M, Qin CX, Wang X, Ding NZ. Plant unsaturated fatty acids: biosynthesis and regulation. Front Plant Sci. 2020;11:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao R, Zou Y, Guo X, Li H, Lu H. Fatty acid desaturases (FADs) modulate multiple lipid metabolism pathways to improve plant resistance. Mol Biol Rep. 2022;49(10):9997–10011. [DOI] [PubMed] [Google Scholar]
- 7.Hou Q, Ufer G, Bartels D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016;39(5):1029–48. [DOI] [PubMed] [Google Scholar]
- 8.Lim GH, Singhal R, Kachroo A, Kachroo P. Fatty acid- and lipid-mediated signaling in plant defense. Annu Rev Phytopathol. 2017;55:505–36. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Huang Y, Liu C, Chen K, Li M. Functions and interaction of plant lipid signalling under abiotic stresses. Plant Biol (Stuttg). 2023;25(3):361–78. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Shang L, Zhu QH, Fan L, Guo L. Twenty years of plant genome sequencing: achievements and challenges. Trends Plant Sci. 2022;27(4):391–401. [DOI] [PubMed] [Google Scholar]
- 11.Hajiahmadi Z, Abedi A, Wei H, Sun W, Ruan H, Zhuge Q, Movahedi A. Identification, evolution, expression, and docking studies of fatty acid desaturase genes in wheat (Triticum aestivum L). BMC Genomics. 2020;21(1):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen EZ, Yang C, Tong J, Li H, Wang T, Chen L. Genome-wide analysis of fatty acid desaturase genes in rice (Oryza sativa L). Sci Rep. 2019;9(1):19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Wei J, He L, Zhang Y, Zhao Y, Xu X, Wei Y, Ge S, Ding D, Liu M, Gao S, Xu J. Identification of fatty acid desaturases in maize and their differential responses to low and high temperature. Genes (Basel). 2019;10(6):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Xia P, Yu H, Li W, Chai W, Liang Z. Based on the whole genome clarified the evolution and expression process of fatty acid desaturase genes in three soybeans. Int J Biol Macromol. 2021;182:1966–80. [DOI] [PubMed] [Google Scholar]
- 15.Xue Y, Chen B, Wang R, Win AN, Li J, Chai Y. Genome-wide survey and characterization of fatty acid desaturase gene family in Brassica napus and its parental species. Appl Biochem Biotechnol. 2018;184(2):582–98. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Dong Y, Liu W, He Q, Daud MK, Chen J, Zhu S. Genome-wide identification of membrane-bound fatty acid desaturase genes in Gossypium hirsutum and their expressions during abiotic stress. Sci Rep. 2017;7:45711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng C, Liu F, Sun X, Wang B, Liu J, Ni X, Hu C, Deng G, Tong Z, Zhang Y, Lü P. Genome-wide identification of FAD gene family and their contributions to the temperature stresses and mutualistic and parasitic fungi colonization responses in banana. Int J Biol Macromol. 2022;204:661–76. [DOI] [PubMed] [Google Scholar]
- 18.Wei H, Movahedi A, Xu S, Zhang Y, Liu G, Aghaei-Dargiri S, Ghaderi Zefrehei M, Zhu S, Yu C, Chen Y, Zhong F, Zhang J. Genome-wide characterization and expression analysis of fatty acid desaturase gene family in poplar. Int J Mol Sci. 2022;23(19):11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu E, Gao S, Hu W, Zhang C, Liu D, Shen G, Zhu S. Genome-wide identification and functional differentiation of fatty acid desaturase genes in Olea europaea L. Plants (Basel). 2022;11(11):1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Liu A, Najeeb U, Zhou W, Liu H, Yan G, Gill RA, Yun X, Bai Q, Xu L. Genome-wide investigation and expression analysis of membrane-bound fatty acid desaturase genes under different biotic and abiotic stresses in sunflower (Helianthus annuus L). Int J Biol Macromol. 2021;175:188–98. [DOI] [PubMed] [Google Scholar]
- 21.Kidokoro S, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022;27(9):922–35. [DOI] [PubMed] [Google Scholar]
- 22.Xue Y, Wu F, Chen R, Wang X, Tseke Inkabanga A, Huang L, Qin S, Zhang M, Chai Y. Genome-wide analysis of fatty acid desaturase genes in Chia (Salvia hispanica) reveals their crucial roles in cold response and seed oil formation. Plant Physiol Biochem. 2023;199:107737. [DOI] [PubMed] [Google Scholar]
- 23.Raboanatahiry N, Yin Y, Chen K, He J, Yu L, Li M. In silico analysis of fatty acid desaturases structures in Camelina sativa, and functional evaluation of CsaFAD7 and CsaFAD8 on seed oil formation and seed morphology. Int J Mol Sci. 2021;22(19):10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M, Thelen JJ. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:1430–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resemann HC, Herrfurth C, Feussner K, Hornung E, Ostendorf AK, Gömann J, Mittag J, van Gessel N, Vries J, Ludwig-Müller J, Markham J, Reski R, Feussner I. Convergence of sphingolipid desaturation across over 500 million years of plant evolution. Nat Plants. 2021;7(2):219–32. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Zeng L, Fu X, Mei X, Cheng S, Liao Y, Deng R, Xu X, Jiang Y, Duan X, Baldermann S, Yang Z. The sphingolipid biosynthetic enzyme Sphingolipid delta8 desaturase is important for chilling resistance of tomato. Sci Rep. 2016;6:38742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh AK, Raina SK, Kumar M, Aher L, Ratnaparkhe MB, Rane J, Kachroo A. Modulation of GmFAD3 expression alters abiotic stress responses in soybean. Plant Mol Biol. 2022;110(1–2):199–218. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen VC, Nakamura Y, Kanehara K. Membrane lipid polyunsaturation mediated by FATTY ACID DESATURASE 2 (FAD2) is involved in endoplasmic reticulum stress tolerance in Arabidopsis thaliana. Plant J. 2019;99(3):478–93. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Liu H, Sun J, Li B, Zhu Q, Chen S, Zhang H. Arabidopsis fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth. PLoS ONE. 2012;7(1):e30355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Shao Y, Yang X, Zhang C, Guo Y, Liu Z, Chen M. Heterogeneous expression of stearoyl-acyl carrier protein desaturase genes SAD1 and SAD2 from Linum usitatissimum enhances seed oleic acid accumulation and seedling cold and drought tolerance in Brassica napus. J Integr Agr. 2024;23(6):1864–78. [Google Scholar]
- 31.Okazaki Y, Saito K. Roles of lipids as signaling molecules and mitigators during stress response in plants. Plant J. 2014;79(4):584–96. [DOI] [PubMed] [Google Scholar]
- 32.Liu H, Timko MP. Jasmonic acid signaling and molecular crosstalk with other phytohormones. Int J Mol Sci. 2021;22(6):2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Xu M, Cai X, Han Z, Si J, Chen D. Jasmonate signaling pathway modulates plant defense, growth, and their trade-offs. Int J Mol Sci. 2022;23(7):3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MW, Padilla CS, Gupta C, Galla A, Pereira A, Li J, Goggin FL. The FATTY ACID DESATURASE2 family in tomato contributes to primary metabolism and stress responses. Plant Physiol. 2020;182(2):1083–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda O, Watanabe C, Iba K. Hormonal regulation of tissue-specific ectopic expression of an Arabidopsis endoplasmic reticulum-type omega-3 fatty acid desaturase (FAD3) gene. Planta. 2001;213(6):833–40. [DOI] [PubMed] [Google Scholar]
- 36.Song Z, Lai X, Chen H, Wang L, Pang X, Hao Y, Lu W, Chen W, Zhu X, Li X. Role of MaABI5-like in abscisic acid-induced cold tolerance of ‘Fenjiao’ banana fruit. Hortic Res. 2022;9:uhac130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazaz S, Barthole G, Domergue F, Ettaki H, To A, Vasselon D, De Vos D, Belcram K, Lepiniec L, Baud S. Differential activation of partially redundant ∆9 stearoyl-ACP desaturase genes is critical for omega-9 monounsaturated fatty acid biosynthesis during seed development in Arabidopsis. Plant Cell. 2020;32(11):3613–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Castillo-González C, Yu B, Zhang X. The functions of plant small RNAs in development and in stress responses. Plant J. 2017;90(4):654–70. [DOI] [PubMed] [Google Scholar]
- 39.Gai W, Sun H, Hu Y, Liu C, Zhang Y, Gai S, Yuan Y. Genome-wide identification of membrane-bound fatty acid desaturase genes in three peanut species and their expression in Arachis hypogaea during drought stress. Genes. 2022;13(10):1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizza F, Pagani D, Stanca AM, Cattivelli L. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breeding. 2001;120(5):389–96. [Google Scholar]
- 41.Mishra A, Heyer AG, Mishra KB. Chlorophyll fluorescence emission can screen cold tolerance of cold acclimated Arabidopsis thaliana accessions. Plant Methods. 2014;10(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, Xia R. TBtools-II: a one for all, all for one bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–42. [DOI] [PubMed] [Google Scholar]
- 43.Xue Y, Zhang C, Shan R, Li X, Tseke Inkabanga A, Li L, Jiang H, Chai Y. Genome-wide identification and expression analysis of nsLTP gene family in rapeseed (Brassica napus) reveals their critical roles in biotic and abiotic stress responses. Int J Mol Sci. 2022;23(15):8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Jiang C, Ren J, Dong J, Shi X, Zhao X, Wang X, Wang J, Zhong C, Zhao S, Liu X, Gao S, Yu H. An advanced lipid metabolism system revealed by transcriptomic and lipidomic analyses plays a central role in peanut cold tolerance. Front Plant Sci. 2020;11:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue Y, Chen B, Win AN, Fu C, Lian J, Liu X, Wang R, Zhang X, Chai Y. Omega-3 fatty acid desaturase gene family from two ω-3 sources, Salvia hispanica and Perilla frutescens: Cloning, characterization and expression. PLoS ONE. 2018;13(1):e0191432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequences, protein sequences and gene annotation files of P. edulis were downloaded in GigaDB (http://gigadb.org/dataset/view/id/100498). RNA raw data (Supplementary Table 3) for 19 different organs/tissues (GSE90517, GSE104951, GSE121216, PRJNA842835, and PRJNA217219), and abiotic stresses and hormones treatments (GSE130314, GSE169067, GSE104596, GSE100172, and GSE123529) in P. edulis was downloaded in NCBI database (https://www.ncbi.nlm.nih.gov/).