Abstract
Background
Improving the microenvironment to augment endogenous regenerative potential has emerged as a fundamental concept for stimulating and expediting periodontal tissue repair and regeneration. Previous studies have demonstrated that TPPU, a soluble epoxide hydrolase inhibitor (sEHi), mediates the suppression of inflammatory bone loss in periodontitis models. However, the underlying mechanisms remain largely elusive.
Methods
In this study, we constructed a human umbilical vein endothelial cell (HUVEC) and periodontal ligament stem cell (PDLSC) coculture system in vitro and tested the anti-inflammatory effect of TPPU under inflammatory conditions. The roles of HIF-1α and Endomucin (EMCN) in the anti-inflammatory effects of TPPU were analyzed. The effects of TPPU on osteogenesis and osteoclastogenesis in cocultured cells were examined. The in vivo periodontitis model further verified the effects of TPPU on inhibiting neutrophil adhesion and inflammation and inhibiting osteoclasts.
Results
Our in vitro experiments demonstrated that TPPU enhances the interaction between mesenchymal stem cells and vascular endothelial cells to enhance anti-inflammatory and osteogenic differentiation effects and revealed a new anti-inflammatory mechanism of TPPU involving the upregulation of EMCN in endothelial cells to prevent lymphocyte recruitment. We also confirmed that TPPU inhibits osteoclast activity. Our in vivo findings showed that TPPU inhibits osteoclast activity and neutrophil adhesion and enhances periodontal tissue repair and regeneration.
Conclusions
TPPU promotes local regeneration in periodontitis by inhibiting inflammation and bone resorption. Thus, targeting soluble epoxide hydrolase represents an endogenous regenerative strategy for periodontitis treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-04054-y.
Keywords: Soluble epoxide hydrolase inhibitor, TPPU, Periodontitis, Tissue regeneration, EMCN
Introduction
Periodontitis is a chronic, destructive, inflammatory disease affecting the soft and hard supporting tissues of teeth due to multifactorial interactions. It currently ranks among the most prevalent oral diseases in humans [1]. A global oral epidemiology survey reported that its prevalence affects up to 50% of the world's population [2]. The initial clinical manifestations of periodontitis include redness and bleeding, progressing to the formation of periodontal pockets and subsequent destruction of alveolar bone. Advanced stages may result in tooth loosening, displacement, and ultimately tooth loss, constituting a primary cause of adult tooth loss [3, 4]. In addition, periodontitis not only compromises the health of periodontal support tissues but is also correlated with various systemic disorders, including cardiovascular diseases, diabetes, rheumatoid arthritis, respiratory disease, adverse pregnancy outcomes, cancer and neurodegenerative disease [5, 6]. Thus, the prevention and treatment of periodontitis have significant implications for human health.
Current treatment modalities for periodontitis include nonsurgical treatments and surgical interventions, which are selected based on disease stage [7]. Despite considerable advancements in periodontal disease management, existing and emerging therapeutic paradigms exhibit limited and variable efficacy. For instance, while nonsurgical treatments can prevent disease onset and progression, they fail to restore damaged or lost periodontal soft and hard tissues [8, 9]. Clinical evidence indicates limited new attachment formation following conventional flap surgery with guided tissue regeneration [10, 11]. However, therapeutics leveraging endogenous regenerative technologies are lacking. Recent regenerative surgical approaches have aimed at reducing microcirculation, attenuating inflammation, inhibiting osteoclast activity, and stimulating endogenous periodontal tissue regeneration [12, 13].
Mesenchymal stem cell (MSC)-based repair and regeneration for periodontitis treatment have emerged as highly promising therapeutic strategies [11, 14]. Although the mechanisms of action underlying stem cell therapies remain unclear, it has been proven that enhancing the effect of transplanted stem cells and their interaction with host vascular endothelial cells can help improve the therapeutic outcomes of stem cells [15]. TPPU, a potent soluble epoxide hydrolase inhibitor (sEHi), has been confirmed to exert cardioprotective, antihypertensive, anti-inflammatory, and neuroprotective analgesic effects by increasing the levels of endogenous epoxyeicosatrienoic acids (EETs)[16]. Preliminary studies have suggested the therapeutic efficacy of TPPU in periodontitis, showing reductions in inflammatory bone loss and inflammatory responses in a mouse model of periodontitis [17, 18]. However, the precise therapeutic mechanism for periodontitis remains elusive. Our research confirmed that TPPU promotes the healing of oral ulcers and the preservation of tooth extraction sockets [19, 20]. Furthermore, we recently established that TPPU enhances the interaction between vascular endothelial cells and dental pulp stem cells, thereby promoting bone repair and regeneration by increasing the number of type H vessels (CD31highEMCNhigh)[21]. Whether these effects also confer benefits for periodontitis treatment remains uncertain and warrants further investigation, particularly with regard to the topical application of TPPU.
Postcapillary venules are major sites of leukocyte recruitment. Endomucin (EMCN), a salivary glycoprotein expressed by postcapillary venules, has been implicated in exerting anti-inflammatory effects by preventing neutrophil adhesion and reducing the infiltration of leukocytes [22]. Treatment of human umbilical vein endothelial cells (HUVECs) with tumor necrosis factor-α (TNF-α) decreased EMCN expression at both the gene and protein levels. In vivo experiments revealed reduced EMCN expression in the ciliary body within 24 h postvitreous injection of TNF-α, and EMCN overexpression significantly mitigated neutrophil accumulation in the ciliary body after TNF-α injection [22]. Our previous investigations indicated that TPPU enhances the interaction between vascular endothelial cells and surrounding stem cells and upregulates HIF-1α and EMCN to promote type H vessel formation [23]. The local expression of EMCN in the periodontium remains unclear, necessitating further exploration into whether topical TPPU administration can enhance EMCN expression to modulate the local periodontal microenvironment. To expedite clinical translation, comprehensive therapeutics for TPPU warrant ongoing development.
Here, we demonstrated that TPPU facilitates the reconstruction and regeneration of periodontal tissues through its multiple biological effects, including enhancing the interaction between vascular endothelial cells and stem cells, inhibiting osteoclast activity and preventing neutrophil adhesion. Our findings provide additional evidence supporting the targeting of soluble epoxide hydrolase (sEH) for the clinical treatment of periodontal disease.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats, aged 8–12 weeks, were provided by the Experimental Animal Center of Dalian Medical University and maintained under standard conditions with a 12 h light‒dark cycle and ad libitum access to food and water. All procedures adhered to the guidelines for laboratory animal care outlined in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Dalian Medical University (No. AEE22006). The work has been reported in line with the ARRIVE guidelines 2.0. The sample size was decided according to the guidelines for sample size calculations of Boston University. If the animal died prematurely, preventing the collection of histological data (no died prematurely in our study).
TPPU treatment
TPPU (HY-101249, MCE, USA) was dissolved in DMSO (Solarbio, China) to prepare a stock solution (10 mg/ml). According to our previous study, 20 μM of TPPU were selected for in vivo experiments [20], 100 μL of TPPU working solution (TPPU stock solution diluted in PBS to 20 μM) was injected into local gingival tissue of rats every 3 days for 2 weeks. The vehicle group received PBS only. For in vitro experiments, TPPU stock solution was added directly to the medium to achieve a final concentration of 10 μM. Inflammatory conditions were created with LPS (1 μg/mL, Sigma‑Aldrich, America).
Cell culture
Murine macrophage RAW264.7 cells and primary pooled human umbilical vein endothelial cells (HUVECs) derived from 10 individual donors were obtained from the American Type Culture Collection (ATCC, USA). RAW264.7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco) following the manufacturer's instructions. HUVECs were cultured in endothelial cell medium (ECM, ScienCell, USA) supplemented with 10% FBS, 1% endothelial cell growth supplement (ECGS, ScienCell), 100 U/mL penicillin, and 100 μg/mL streptomycin (Solarbio).
Periodontal ligament stem cells (PDLSCs) were isolated and cultured as previously described [24]. Briefly, Human third molars from five patients, aged 18 to 24 years, without caries or signs of inflammation, were selected from routinely extracted teeth at the Affiliated Stomatological Hospital of Dalian Medical University (n = 5). Written informed consent was obtained from all donors, and the study received approval from the Ethics Committee of the Affiliated Stomatological Hospital of Dalian Medical University (NO.2022001). The teeth were cleaned with PBS containing 2% penicillin and streptomycin, followed by tissue scraping from the mid-root area. The tissue was cut with scissors and incubated in a mixture of 3 mg/mL type I collagenase (Sigma‑Aldrich) and 4 mg/mL dispase type II collagenase (Sigma‑Aldrich) at 37 °C for 1 h to obtain single cells. PDLSCs were cultured in DMEM/F12 (Gibco) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
Blocking of EMCN by neutralization antibody
To investigate whether TPPU inhibits inflammation via EMCN, an anti-EMCN antibody (RRID: AB_10859306, Abcam, Britain) was added to the medium at a concentration of 10 μg/mL for 3 days with TPPU (10 μM) and LPS (1 μg/mL), followed by RT‒qPCR to assess related gene expression.
Viral infection
The virus used for HIF-1α knockdown was purchased from the GiKai gene (GiKai gene, China), and the sequence details were provided in Additional file 1. The virus was added to the endothelial cell medium at an MOI of 1:20, and the medium was changed after 6 h. GFP-tagged positive cell expression was observed after 72 h. Samples were collected one week after puromycin screening to verify the knockdown efficiency.
Western blotting
HIF-1α was knocked down in HUVECs using a viral vector. Cellular protein was extracted with RIPA buffer (Solarbio), and the protein concentration was measured using a BCA assay kit (Beyotime, China). Gel electrophoresis was performed to detect the protein levels of HIF-1α (RRID: AB_302883, Abcam) and EMCN (RRID: AB_302883, Abcam). Each well was loaded with 15 ng of sample. Following electrophoresis, the proteins were transferred to a PVDF membrane (MCE), blocked with milk for 1 h, and incubated with a HIF-1α antibody for 16 h at 4 °C. After washing with TBST, the membrane was incubated with a secondary antibody (ZSGB-BIO, China) for 1 h. Protein bands were visualized using High-sig ECL Western Blotting (Tanon, China), and the Bio-Rad VersaDoc image system (Bio-Rad, USA) was used for detection. Statistical analysis was performed using ImageJ (National Institutes of Health, USA).
Enzyme-linked immunosorbent assay (ELISA)
The cell culture supernatant was collected and centrifuged at 3000 rpm for 10 min to remove particles and polymers. The cytokines levels of IL-6 and IL-1β were analyzed using an ELISA kit (MeiMian, China), according to the manufacturer's instructions. The ELISA kit was equilibrated at room temperature for 20 min. Standard wells and sample wells were set up, with 50 μL of standard added to each standard well, and 10 μL of sample and 40 μL of sample diluent added to each sample well. Then, 100 μL of horseradish peroxidase (HRP)-labeled detection antibody were added to each well. The reaction wells were sealed with sealing film and incubated at 37 °C for 60 min. The plate was washed five times with washing solution, followed by the addition of 50 μL of substrate solutions A and B to each well. The plate was incubated at 37 °C in the dark for 15 min. Finally, 50 μL of stop solution was added to each well, and the optical density (OD) value was measured at a wavelength of 450 nm using the Bio-Rad VersaDoc image system (Bio-Rad, USA).
Osteogenic differentiation in vitro
HUVECs and PDLSCs were cocultured at a 1:1 ratio, and osteogenic induction was initiated when the cell density reached 90%. The osteogenic induction medium consisted of α-MEM supplemented with 10% FBS, 1% penicillin‒streptomycin, 50 mg/L ascorbate 2-phosphate, 0.1 μM dexamethasone, and 10 mM β-glycerophosphate disodium. After 7 days of osteogenesis, the cells were fixed with 4% paraformaldehyde (PFA) and stained with ALP (Solarbio) according to the manufacturer's instructions.
Osteoclast differentiation in vitro
RAW264.7 cells were plated at a density of 4 × 104 cells/mL with 50 ng/mL recombinant mouse RANKL (ab270070, Abcam) with/without TPPU treatment (10 μΜ). After osteoclast differentiation, the cells were washed and fixed with 4% PFA, followed by staining using a tartrate-resistant acid phosphatase (TRAP) staining kit (TRAP, Sigma‑Aldrich). Mature osteoclasts were identified as TRAP-positive multinucleated cells with three or more nuclei. Osteoclast numbers were determined using a microscope and ImageJ software (National Institutes of Health, USA).
Porphyromonas gingivalis culture
The W83 Porphyromonas gingivalis strain (W83 Pg, ATCC) was cultured and maintained in TSB anaerobic agar medium (Solarbio) supplemented with 36 g/L agar (Solarbio), 500 g/L vitamin K1 (Huayang, China), 5% defibrillated sheep blood (Solarbio) and 0.5 g/L yeast extract (Solarbio) in an anaerobic chamber with 85% N2, 10% H2, and 5% CO2 at 37 °C. Bacteria in the early logarithmic growth phase were used for inoculation procedures. Approximately 2 × 109 colony-forming units of W83 Pg in 100 μL of PBS supplemented with 2% carboxymethyl cellulose (Sigma‒Aldrich) were utilized.
Periodontitis model
The SD rats were randomly divided into no treatment group (Control group, n = 6); PBS treated periodontitis group (TPPU- group, n = 6); TPPU treated periodontitis group (TPPU + group, n = 6). The periodontitis model was induced by silk ligature with Pg infection as previously described to evaluate the therapeutic effect of TPPU. Briefly, a 5–0 silk ligature was tied around the mandible first molar on day 0 after the rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg, VetaKetam, Poland) and xylazine (10 mg/kg, VetaKetam, Poland). Subsequently, the silk ligatures of the rats were inoculated with Pg. After successful establishment of the periodontitis model, the SD rats were randomly divided into two groups. The TPPU + group received local injections of 100 μL of TPPU (20 μM) into the periodontium, while the TPPU- group was injected with PBS every 3 days for 2 weeks. All rats were euthanized with overdose of carbon dioxide (CO2) 4 weeks after TPPU or PBS treatment, and the jaws were isolated for subsequent analysis.
Histology, immunohistochemistry and immunofluorescence
The mandibles of SD rats were fixed with 4% PFA for 48 h, followed by decalcification with 10% EDTA and paraffin embedding. Sections with a thickness of 8 μm were stained with tartrate-resistant acid phosphatase (TRAP), and TRAP-positive multinucleated cells (> 3 nuclei) were counted as osteoclasts. Immunohistochemistry was conducted to observe protein expression changes. Primary antibodies, including rat monoclonal anti-EMCN (RRID: AB_10859306, Abcam) and rabbit monoclonal anti-MPO (RRID: AB_2864724, Abcam), were incubated overnight at 4 °C. Horseradish enzyme labeling followed by DAB coloring and hematoxylin for nuclear staining were performed. Images were captured using an inverted microscope (IX71, Olympus, Japan). For immunofluorescence (IF) staining, the sections were incubated with a primary antibody (rabbit polyclonal anti-EMCN, RRID: AB_11090525, Bioss, China) and HIF-1α (RRID: AB_302883, Abcam) overnight at 4 °C, washed in PBS and then incubated with a fluorescent secondary antibody (Abcam) for 1 h at room temperature. Nuclei were counterstained with DAPI, and images were acquired using a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Real-time reverse transcription polymerase chain reaction (RT‒qPCR)
Total RNA was extracted with RNAkey Reagent (SM139-02, SEVEN, China), and reverse transcription and RT‒qPCR were performed with a SevenFast Two Step RT‒qPCR Kit (SRQ-01, SEVEN). Relative mRNA expression was calculated after normalization to the housekeeping gene GAPDH using the 2−ΔΔCt method. The sequences of primers used for RT‒qPCR were listed in Additional file 2.
Radiographic analysis and three-dimensional reconstruction
Representative radiographic images of the rat mandible were captured using micro-CT (InspexiosMX-90CT Plus, Japan) and reconstructed using Mimics 16 software. Analysis was conducted with VGSTUDIO MAX software (Volume Graphics, USA).
Statistical analysis
All the data were presented as the means ± SEM. Normality tests were conducted to confirm the normal distribution of data for parametric statistical analysis. Statistical differences were assessed using one-way ANOVA or t-tests. All statistical analyses were performed with GraphPad Prism 10 (GraphPad Software, USA). A p-value of less than 0.05 was considered statistically significant.
Results
TPPU mitigates LPS-induced inflammation in vitro
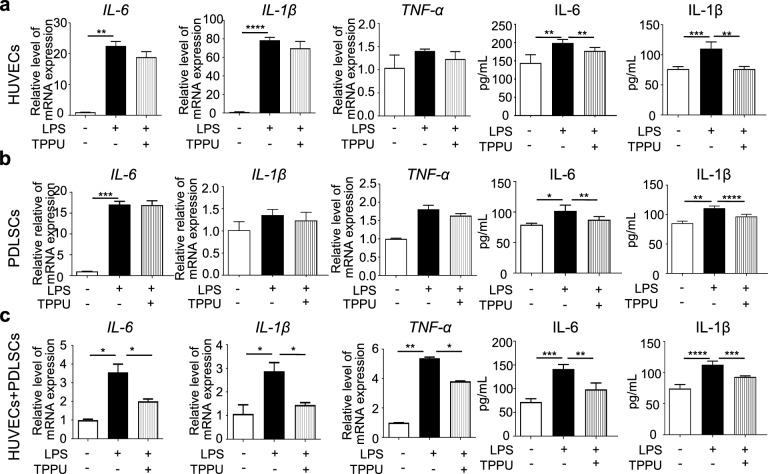
We first examined the anti-inflammatory effects of TPPU on HUVECs and PDLSCs alone and cocultured HUVECs and PDLSCs (cocultured cells) under LPS-induced inflammatory conditions. The expression of IL-6 and IL-1β was significantly upregulated in LPS-stimulated HUVECs alone and cocultured cells (Fig. 1a-c). Notably, gene expression of these inflammatory factors was higher in HUVECs alone than in cocultured cells, suggesting that interactions between vascular endothelial cells and stem cells may enhance resistance to inflammation. TPPU partially reduced IL-6 and IL-1β expression in LPS-stimulated HUVECs and PDLSCs alone, but the reduction was more pronounced in cocultured cells (Fig. 1a-c). ELISA results indicated that TPPU partially decreased the IL-6 and IL-1β protein levels induced by LPS, though the PDLSCs group showed less reduction (Fig. 1a-c). Meanwhile, the level of TNF-α did not change significantly in LPS-treated HUVECs alone and PDLSCs alone, whereas a marked decrease in TNF-α was observed in the TPPU-treated co-culture system. These results suggest that TPPU has the potential to enhance anti-inflammatory effects by promoting cell interactions, providing a basis for its clinical application in the local microenvironment to alleviate inflammation.
Fig. 1.
TPPU reduces LPS-induced inflammation in vitro. a RT‒qPCR analysis showing the expression levels of IL-6, IL-1β, and TNF-α in HUVECs alone, alongside ELISA results demonstrating the levels of IL-6 and IL-1β in HUVECs cultured alone. b RT‒qPCR analysis showing the expression levels of IL-6, IL-1β, and TNF-α in PDLSCs alone, accompanied by ELISA results indicating the levels of IL-6 and IL-1β in PDLSCs alone. c RT‒qPCR analysis showing the expression levels of IL-6, IL-1β, and TNF-α in cocultured HUVECs and PDLSCs, along with ELISA results demonstrating the levels of IL-6 and IL-1β in cocultured cells. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
TPPU restores the LPS-induced reduction in EMCN expression
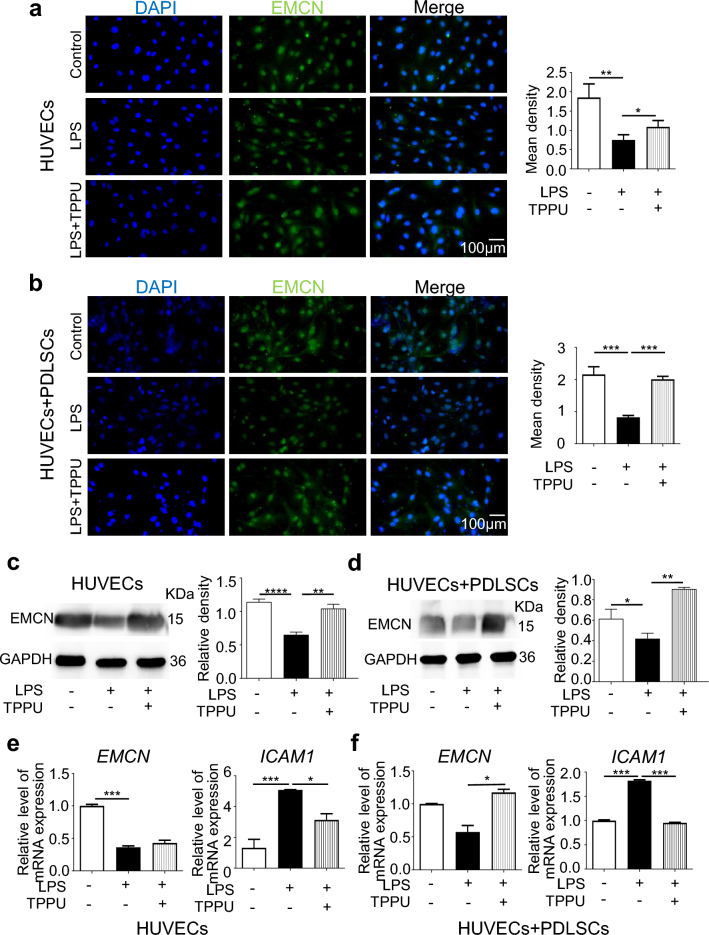
We previously confirmed in vivo and in vitro that TPPU can upregulate EMCN expression and promote type H vessel formation under noninflammatory conditions. Recent studies have shown that EMCN can prevent lymphocyte adhesion in noninflammatory tissues. Next, we examined the effect of TPPU on EMCN expression under inflammatory conditions. IF revealed a significant decrease in EMCN expression in both HUVECs alone and cocultured cells under LPS stimulation, with TPPU treatment restoring EMCN expression, particularly in cocultured cells (Fig. 2a, b). Western Blot analysis confirmed that the EMCN protein levels were reduced under inflammatory conditions, while TPPU treatment significantly increased EMCN protein levels in HUVECs alone and cocultured cells (Fig. 2c, d). RT‒qPCR further confirmed that TPPU partially restored the mRNA expression of EMCN in HUVECs alone and completely restored the mRNA expression of EMCN in cocultured cells under LPS-induced inflammatory conditions (Fig. 2e, f). EMCN mainly exerts its anti-inflammatory effects by preventing neutrophils from binding to intercellular adhesion molecule-1 (ICAM-1) on endothelial cells. Therefore, we examined ICAM-1 expression and found that TPPU reduced LPS-induced ICAM-1 upregulation, especially in cocultured cells (Fig. 2e, f). The results suggested that TPPU upregulated EMCN expression in both HUVECs alone and cocultured cells under LPS treatment, with a more pronounced effect in cocultured cells.
Fig. 2.
TPPU reversed the LPS-induced reduction in EMCN expression. a, b Representative IF image and quantification analysis of EMCN in HUVECs and cocultured HUVECs and PDLSCs. Scale bar = 100 µm. c, d Western blot and quantification analysis for EMCN in HUVECs and cocultured cells. Full-length blots/gels were presented in Additional file 3. e, f RT‒qPCR analysis showing the expression levels of EMCN and ICAM1 in HUVECs and cocultured HUVECs and PDLSCs. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
EMCN inhibition reduces the anti-inflammatory effect of TPPU
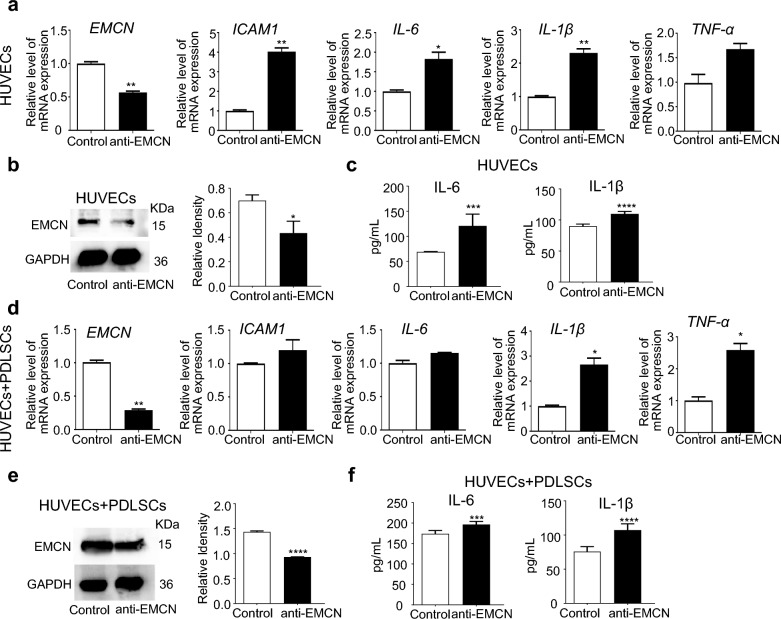
Next, we used EMCN-neutralizing antibodies to detect the effect of TPPU on inflammation. The results demonstrated that the EMCN-neutralizing antibody impaired the anti-inflammatory effect of TPPU in both HUVECs alone and cocultured cells under LPS-induced inflammatory conditions, as indicated by the sustained high levels of ICAM1, IL-6, IL-1β and TNF-α expression (Fig. 3a-f). These findings confirmed that TPPU exerts a novel anti-inflammatory effect through the upregulation of EMCN.
Fig. 3.
EMCN inhibition reduces the anti-inflammatory effect of TPPU. The TPPU treated HUVECs alone and cocultured cells under inflammatory conditions were treated with or without EMCN neutralizing antibody. a RT‒qPCR showing the expression levels of EMCN, ICAM1, IL-6, IL-1β, and TNF-α in HUVECs alone. b Western blot and quantification analysis for EMCN in HUVECs alone. Full-length blots/gels were presented in Additional file 3. c ELISA measurement of cytokine levels (IL-6 and IL-1β) in HUVECs alone. d RT‒qPCR showing the expression levels of EMCN, ICAM1, IL-6, IL-1β, and TNF-α in cocultured HUVECs and PDLSCs. e Western blot and quantification for EMCN in cocultured HUVECs and PDLSCs. Full-length blots/gels were presented in Additional file 3. f ELISA measurement of cytokine levels (IL-6 and IL-1β) in cocultured HUVECs and PDLSCs. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
TPPU suppresses inflammation by enhancing HIF-1α expression
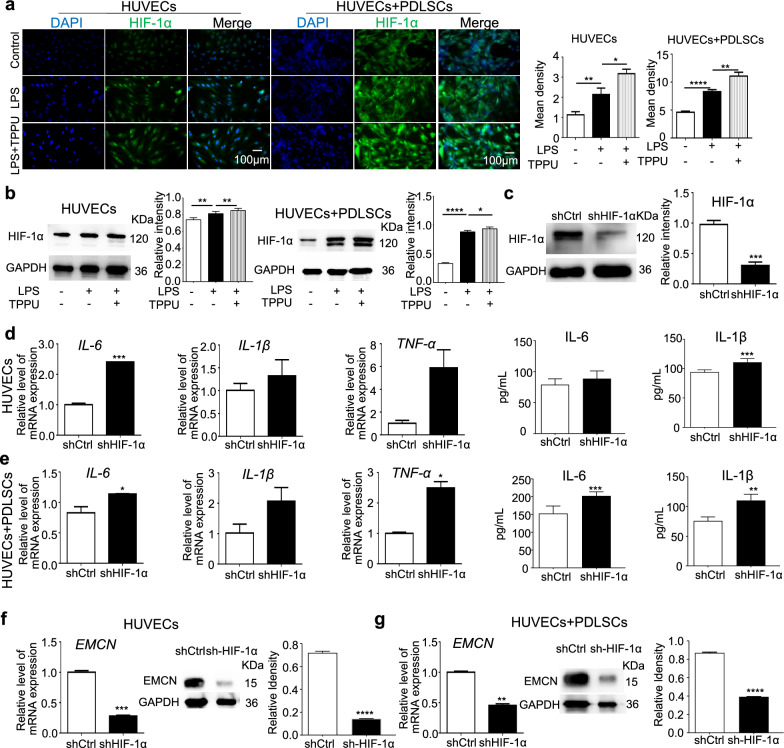
We previously found that TPPU enhances crosstalk between HUVECs and MSCs under noninflammatory conditions to increase the number of type H vessels via the SLIT3-HIF-1α axis for osteogenic-angiogenic coupling. We further investigated the potential of TPPU to alleviate inflammation by regulating HIF-1α. IF staining and Western blot analysis revealed that HIF-1α expression was elevated in LPS-treated HUVECs alone and cocultured cells, and this increase was further amplified by TPPU treatment (Fig. 4a, b). Knocking down HIF-1α in HUVECs abolished the ability of TPPU to reduce IL-6, IL-1β and TNF-α expression in HUVECs alone and cocultured cells under inflammatory conditions (Fig. 4d, e), and also downregulated EMCN expression (Fig. 4f, g). Our results suggested that the anti-inflammatory effects of TPPU and the upregulation of EMCN expression were closely associated with the stabilization of HIF-1α expression in endothelial cells, with biologically relevant functions enriched even at very low fold changes in mRNA levels.
Fig. 4.
TPPU suppresses inflammation by enhancing HIF-1α expression. a, b, Expression levels of HIF-1α in HUVECs and cocultured cells across control group, LPS group and LPS + TPPU treatment group. a Representative IF image and quantification analysis of HIF-1α in HUVECs and cocultured HUVECs and PDLSCs. Scale bar = 100 µm. b Western blot and quantification for HIF-1α across different groups. Full-length blots/gels were presented in Additional file 3. c Western blot demonstrating the knockdown of HIF-1α in HUVECs. Full-length blots/gels were presented in Additional file 3. d-g, Knockdown of HIF-1α in HUVECs, examining the levels of cytokines and EMCN in HUVECs and cocultured HUVECs and PDLSCs treated with TPPU under inflammatory conditions. d, e RT‒qPCR and ELISA analyses showing the expression levels of IL-6, IL-1β, and TNF-α in HUVECs and cocultured HUVECs and PDLSCs. f, g, RT‒qPCR, Western blot and quantification for EMCN in HUVECs and cocultured cells across different groups, Full-length blots/gels were presented in Additional file 3. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
Our results from the co-culture system indicated that TPPU treatment activated the Notch signaling pathway (Additional file 4: Fig. S1a). This finding suggested that TPPU may promote Notch signal transduction through interactions between endothelial cells and stem cells. Furthermore, following the targeted knockdown of HIF-1α in endothelial cells, we observed significant inhibition of Notch signaling in the co-culture system (Additional file 4: Fig. S1b). These results imply that TPPU regulated Notch signaling through HIF-1α, which in turn modulated EMCN expression in endothelial cells. Further research is required to elucidate the precise mechanisms involved.
TPPU rescues LPS-impaired osteogenic differentiation and restrains osteoclastogenesis
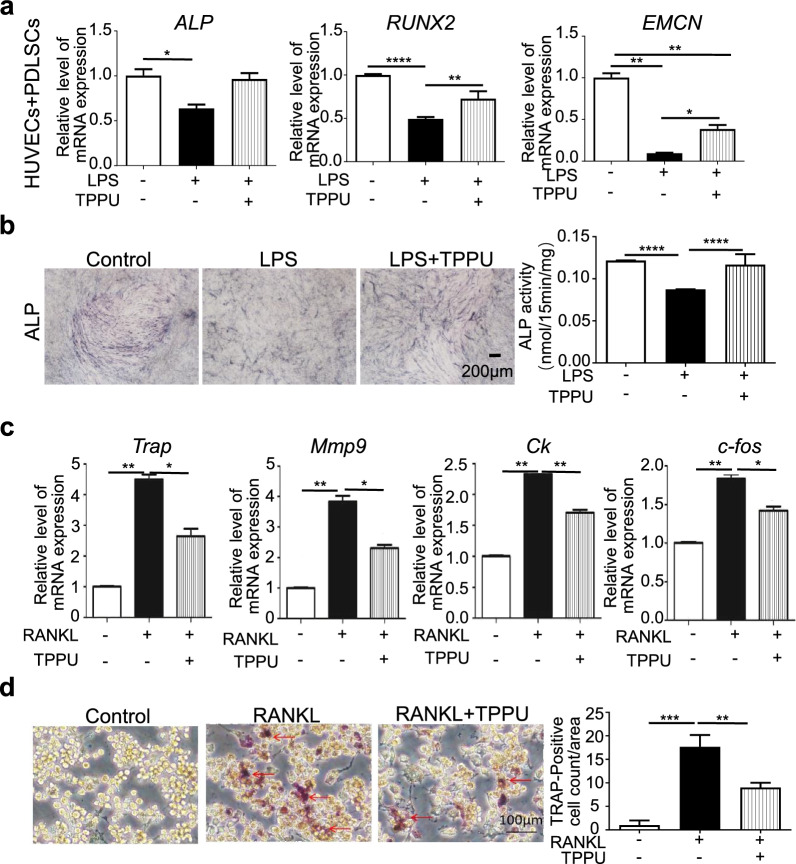
Restoring the osteogenic potential of PDLSCs in the local inflammatory microenvironment is crucial. We have previously demonstrated that TPPU can promote osteogenic differentiation of cocultured cells but has no effect on stem cells alone or on HUVECs alone. Therefore, we investigated the effect of TPPU on the osteogenic differentiation of cocultured cells under LPS-induced inflammatory conditions. After 7 days of osteogenic differentiation in vitro, RT‒qPCR revealed that the expression of the osteogenesis-related factors ALP and RUNX2 was significantly decreased under inflammatory conditions, while TPPU treatment restored the expression of osteogenic genes and EMCN (Fig. 5a). Similar to the RT‒qPCR results, the ALP staining results confirmed that TPPU reversed the LPS-induced decrease in ALP activity (Fig. 5b).
Fig. 5.
TPPU rescues LPS-impaired osteogenic differentiation and inhibits osteoclastogenesis. a RT‒qPCR showing the expression levels of ALP, RUNX2 and EMCN in cocultured HUVECs and PDLSCs after 7 days of osteogenic induction. b Representative image and quantification of ALP. Scale bar = 200 µm. c RT‒qPCR analysis showing the expression levels of Trap, Mmp9, Ck, and c-fos in RAW 264.7 cells subjected to different treatments. d Representative image and quantification analysis of TRAP staining in RAW264.7 cells induced with RANKL. Scale bar = 100 µm, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001
The bacterial products, proinflammatory cytokines secreted by activated immune cells in periodontal tissue, contribute to osteoclast differentiation and activity and thus activate pathological bone loss. Therefore, we tested the effect of TPPU on osteoclastic activity. TPPU downregulated the expression of osteoclast-related genes (Mmp-9, Ck, Trap, and c-fos) induced by RANKL in RAW264.7 cells and reduced the number of TRAP-positive cells compared to those in the RANKL group (Fig. 5c, d).
TPPU elevates local EMCN expression and reduces neutrophil infiltration
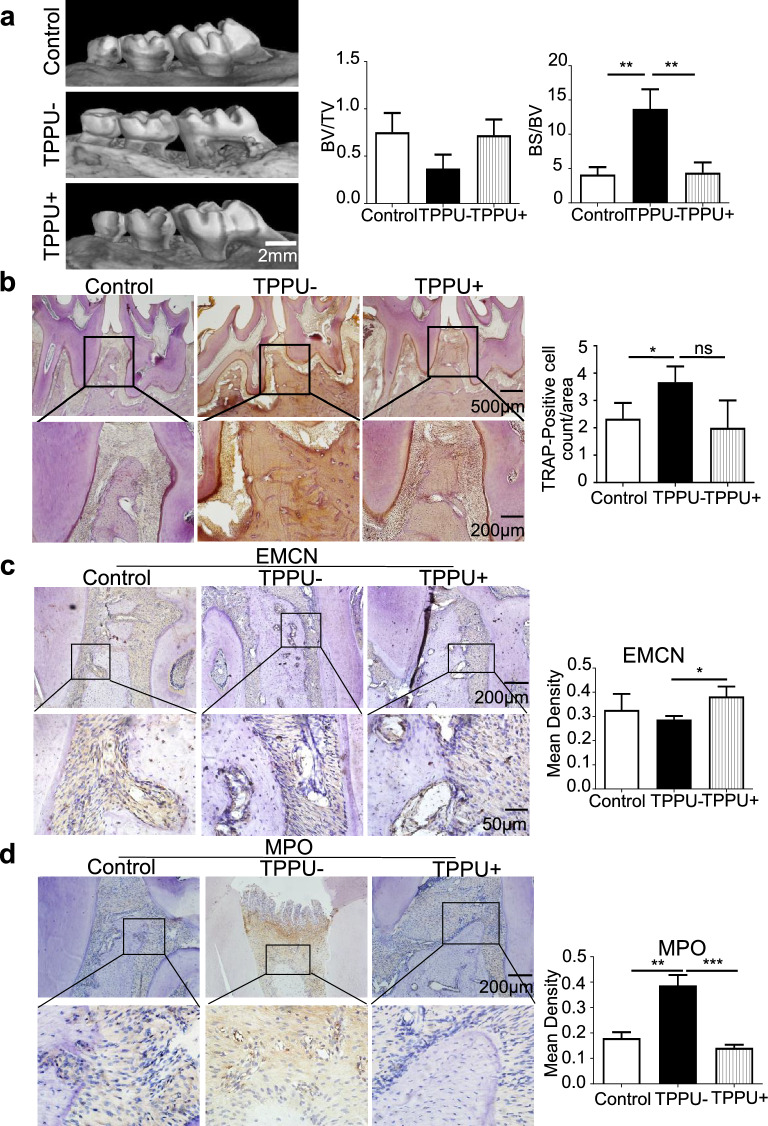
Finally, we tested the therapeutic effect of TPPU in a periodontitis model in SD rats. Micro-CT revealed less periodontal bone resorption in rats treated with local TPPU injection than in those treated with PBS. Quantification of the BV/TV (bone volume fraction) and BS/BV (bone surface area–bone volume ratio) ratio revealed superior bone conditions in the TPPU + group (Fig. 6a). TRAP staining revealed fewer TRAP + cells in the TPPU + group (Fig. 6b). Immunohistochemical results revealed more EMCN-positive cells in the TPPU-treated group (Fig. 6c). Furthermore, there were fewer MPO + cells in the periodontal region in the TPPU + group than in the TPPU- group (Fig. 6d). These in vivo results confirmed that TPPU increased EMCN expression, reduced osteoclastic activity and inhibited neutrophil adhesion, which contributed to the repair and regeneration of periodontitis.
Fig. 6.
TPPU restrains inflammatory bone loss and osteoclastogenesis. a Representative micro-CT images and BV/TV (bone volume fraction) and BS/BV (bone surface area–bone volume ratio) analyses in the control, TPPU- and TPPU + groups; scale bar = 2 mm. b Representative image of TPAR staining and quantification of TRAP-positive cells in control, TPPU-, and TPPU + groups; scale bar = 500 μm (upper panel); scale bar = 200 μm (lower panel). c Representative image and quantitative analysis of immunohistochemical staining showing the expression of EMCN in control, TPPU-, and TPPU + groups; scale bar = 200 μm (upper panel); scale bar = 50 μm (lower panel). d Representative image and quantitative analysis of immunohistochemical staining showing MPO + cells in the control, TPPU-, and TPPU + groups; scale bar = 200 μm (upper panel); scale bar = 50 μm (lower panel). * P < 0.05, ** P < 0.01, *** P < 0.001
Discussion
Periodontitis is a chronic infectious disease characterized by the destruction of periodontal tissue. It ranks among the three most prevalent oral diseases globally and is a leading cause of tooth loss. With the aging global population and inadequate oral health care, periodontal disease-induced tooth loss has emerged as a widespread concern. Consequently, the prevention and treatment of periodontal disease represent critical medical and social issues. Periodontitis involves local inflammation, immunity, and destruction of periodontal tissue, presenting a significant challenge in achieving periodontal tissue regeneration [25]. In recent years, there has been increasing interest in improving the microenvironment through physical, chemical, and tissue engineering techniques to stimulate local endogenous regeneration potential [26, 27].
Due to the complexity of the periodontium, conventional mechanical and anti-infective treatments, as well as guided tissue regeneration and tissue engineering technologies for lost periodontal tissue, have shown limited and variable outcomes. Approaches based on endogenous regenerative technologies, which use stem cells, biologically active proteins, growth factors, and biomaterial scaffolds, provide a new strategy for stimulating and accelerating periodontal tissue repair and regeneration. Stem cell-based tissue engineering has achieved promising results in creating an osteogenic and odontogenic microenvironment for periodontal regeneration [28, 29]. However, translating stem cell therapies into clinical practice faces challenges such as production, storage, maintaining optimal cell viability, stem cell immunogenicity, ethical concerns, and safety. As an alternative, cell-free therapies aim to promote local tissue repair by facilitating cell mobilization and homing. Biomaterial scaffolds and growth factors have been developed to accelerate and enhance periodontal bone regeneration by modulating the inflammatory microenvironment, promoting the endogenous activity of stem cells, suppressing pro-inflammatory cytokines, and ensuring an adequate blood supply [30, 31]. The role of cytokines and growth factors in tissue repair and regeneration depends on their design, mode of administration, and controlled release [32].
This study focuses on cell-free therapy to promote local endogenous periodontal regeneration. Compared with other treatments for endogenous periodontal regeneration, TPPU not only exhibits anti-inflammatory and pro-angiogenic effects but also achieves these effects by inhibiting soluble epoxide hydrolase, which targets fatty acid metabolism and exerts multiple biological effects to improve the local microenvironment of the periodontium. Its anti-inflammatory action is primarily mediated by enhancing the inhibitory effect of EMCN on local neutrophil adhesion [21], as the adhesion and migration of neutrophils are key mechanisms in the occurrence and progression of periodontitis [33]. Additionally, as an important marker of type H blood vessels, EMCN expression indicates that enhancing local H-type blood vessel osteogenic coupling is beneficial for bone tissue repair and regeneration. Our in vitro experiments confirmed that TPPU not only promotes the interaction between stem cells and endothelial cells to stimulate osteogenesis and angiogenesis but also enhances its anti-inflammatory effect. Furthermore, we established that TPPU's inhibition of local osteoclast activity can help reduce bone loss. The localized drug effect also demonstrates the feasibility of clinical translation, providing potential avenues for the future development of drugs targeting fatty acid metabolism pathways to improve the periodontal microenvironment for the treatment of periodontitis.
EETs exhibit various biological functions, including promoting angiogenesis and possessing anti-inflammatory properties, as well as inhibiting osteoclastogenesis and adipogenesis [33, 34]. In this study, TPPU was employed to increase local EET levels. In vivo experiments demonstrated that TPPU enhances CD31 expression in periodontal tissue and promotes alveolar bone regeneration, suggesting that increasing endogenous EETs via sEHi can enhance microcirculation. Furthermore, we propose that TPPU may facilitate the reconstruction and regeneration of periodontal tissues by suppressing inflammation and osteoclastogenesis.
Inflammatory conditions disrupt the periodontal microenvironment and contribute to the progression and outcome of periodontitis. For instance, local inflammation-induced vasculitis and increased blood vessel permeability facilitate the infiltration of inflammatory cells, immune cells, and inflammatory factors into periodontal connective tissue, triggering cascade immunity and resulting in periodontal tissue destruction [35, 36]. Mice infected with Aggregatibacter actinomycetemcomitans exhibited increased sEH expression, which was mitigated by TPPU treatment, leading to reduced sEH expression and several proinflammatory cytokines in gingival tissue [22]. Previous studies have demonstrated the anti-inflammatory effects of EETs, which can prevent activated leukocytes from adhering to blood vessel walls [37]. Exogenous EETs downregulate the expression of cell adhesion molecules (CAMs), including vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, in endothelial cells. These effects are mediated by the inhibition of the transcription factors NF-κB and IκB kinase [36].
In the environment of periodontal inflammation, communication disorders among cells can also affect bone regeneration. Our study showed that sEHi effectively suppresses the expression of HUVEC-related inflammatory factors and promotes periodontal regeneration. This effect is primarily attributed to the ability of TPPU to increase EMCN expression while simultaneously reducing endothelial cell adhesion molecules. Additionally, communication between osteoblasts and endothelial cells plays a crucial role in bone remodeling. Studies have indicated that under inflammatory conditions, the STAT1 transcription factor can upregulate CXCL9 expression in osteoblasts, which competitively binds with VEGF to obstruct endothelial cell and osteoblast VEGF signal transduction, thereby impairing angiogenesis and osteogenesis [37]. Further research into the interaction between EETs and endothelial cells, as well as osteoblasts, will elucidate the mechanisms underlying the actions of EETs.
Local microcirculation is crucial for ensuring the delivery of oxygen and nutrients essential for tissue regeneration, as well as for the absorption of necrotic substances and the control of local infections [38]. Research has demonstrated that capillaries in the epiphysis exhibit elevated expression of CD31 and EMCN (type H vessels), while those in the bone marrow cavity display lower levels of these markers (referred to as type L vessels). Type H capillaries are characterized by relatively high expression of osteogenesis-related factors, facilitating the coupling of angiogenesis and osteogenesis [39]. Our previous research verified that TPPU can promote type H vessel-coupled angiogenesis and osteogenesis, leading to enhanced long bone growth and skull defect regeneration [21]. However, further investigations are needed to determine whether EETs play a role in mediating the coupling between periodontal tissue regeneration and type H angiogenesis.
Limitations of the study
This study has several limitations. First, while we demonstrated the anti-inflammatory effect of TPPU through EMCN, we did not validate the effect of TPPU on the inhibition of neutrophil adhesion in vitro, nor did we examine the impact of EMCN antibody neutralization on this effect. Second, both our prior study and the present investigation demonstrated that TPPU upregulates EMCN, which is closely associated with HIF-1α; however, the detailed regulatory mechanisms require further exploration. Finally, this study did not provide information on the optimal dosage of TPPU or the assessment of systemic versus local administration for potential clinical applications.
Conclusions
We present evidence demonstrating that TPPU exerts anti-inflammatory effects and impaired endothelial cell adhesion by upregulating EMCN, consequently reducing osteoclast activity. Mechanistically, the ability of TPPU to enhance HIF-1α expression is pivotal. Targeted inhibition of sEH alleviates periodontitis and promotes the repair and regeneration of periodontal tissue, representing a promising therapeutic strategy for the treatment of periodontal disease.
Supplementary Information
Additional file 3. Uncropped full-length gels and blot
Additional file 4. Supplementary figures and figure legends
Acknowledgements
The authors declare that they have not used Artificial Intelligence in this study.
Abbreviations
- BS/BV
Bone Surface Area–Bone Volume Ratio
- BV/TV
Bone volume fraction;
- EMCN
Endomucin
- HUVECs
Human umbilical vein endothelial cells
- ICAM-1
Intercellular adhesion molecule-1
- IF
Immunofluorescence
- MSC
Mesenchymal stem cell
- PDLSCs
Periodontal ligament stem cells
- Pg
Porphyromonas gingivalis
- RT-qPCR
Real-time quantification reverse transcription polymerase chain reaction
- SD
Sprague Dawley
- sEH
Epoxyeicosatrienoic acids
- EETs
Soluble epoxide hydrolase
- sEHi
Soluble epoxide hydrolase inhibitor
- TRAP
Tartrate-resistant acid phosphatase
- TNF-α
Tumor necrosis factor-α
- VCAM-1
Vascular cell adhesion molecule-1
Author contributions
All the authors contributed to the study conception and design. F.W. and J.Z. designed and directed the entire study; J.L. and N.K. conducted and analyzed the cellular experiments; X.S. and L.K. carried out the animal and histological experiments; W.C. directed the animal experiments; and X.Y. and Y.Z. were included in the data compilation. X.S., N.K., L.K., and W.C. verified the underlying data. F.W. and J.L. drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81771032 to FW) and the Basic Scientific Research Project of the Educational Department of Liaoning Province (LJKFZ20220249 to FW).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
The animal care and experimental protocols were approved by the Animal Care and Use Committee of Dalian Medical University (Project title: The effects of KDM2B/EZH2 protein complex on inflammatory immune regulation and tissue regeneration in rodent periodontitis model; Approval No. AEE22006; Date of approval: 2022-06-15). The Ethics Committee of the Affiliated Stomatological Hospital of Dalian Medical University approved the isolation of PDLSCs from the teeth (Project title: Research on tissue engineering regeneration of dental stem cells; Approval No. 2022001; Date of approval: 2022-01-07). All participants gave their written informed consent.
Consent for publication
All authors confirm their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juanjuan Li and Ni Kou have contributed equally to this work.
Contributor Information
Jie Zhao, Email: zhaoj@dmu.edu.cn.
Fu Wang, Email: fuwang@dmu.edu.cn.
References
- 1.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol. 2000;2012(60):15–39. 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 2.FDI World Dental Federation. Periodontal health and disease: Apractical guide to reduce the global burden of periodontal disease. 2018.
- 3.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–90. 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 4.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 5.Falcao A, Bullón P. A review of the influence of periodontal treatment in systemic diseases. Periodontol. 2000;2019(79):117–28. 10.1111/prd.12249. [DOI] [PubMed] [Google Scholar]
- 6.Kalhan AC, Wong ML, Allen F, Gao X. Periodontal disease and systemic health: An update for medical practitioners. Ann Acad Med Singap. 2022;51:567–74. 10.47102/annals-acadmedsg.2021503. [PubMed] [Google Scholar]
- 7.Dentino A, Lee S, Mailhot J, Hefti AF. Principles of periodontology. Periodontol. 2000;2013(61):16–53. 10.1111/j.1600-0757.2011.00397.x. [DOI] [PubMed] [Google Scholar]
- 8.Pretzl B, Eickholz P, Saure D, Pfefferle T, Zeidler A, Dannewitz B. Endodontic status and retention of molars in periodontally treated patients: results after 10 or more years of supportive periodontal therapy. J Clin Periodontol. 2016;43:1116–23. 10.1111/jcpe.12621. [DOI] [PubMed] [Google Scholar]
- 9.Salvi GE, Mischler DC, Schmidlin K, Matuliene G, Pjetursson BE, Brägger U, et al. Risk factors associated with the longevity of multirooted teeth. Long-term outcomes after active and supportive periodontal therapy. J Clin Periodontol. 2014;41:701–7. 10.1111/jcpe.12266. [DOI] [PubMed] [Google Scholar]
- 10.Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006;2:Cd001724. 10.1002/14651858.CD001724. [DOI] [PubMed] [Google Scholar]
- 11.Nuñez J, Vignoletti F, Caffesse RG, Sanz M. Cellular therapy in periodontal regeneration. Periodontol. 2000;2019(79):107–16. 10.1111/prd.12250. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Chen H, Zhao X, Ding T, Wang Y, Chen Z, et al. Advances of exosomes in periodontitis treatment. J Transl Med. 2022;20:279. 10.1186/s12967-022-03487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro FV, Mehta JJ, Monteiro MF, Moore J, Casati MZ, Nibali L. Minimal invasiveness in nonsurgical periodontal therapy. Periodontol. 2000;2023(91):7–19. 10.1111/prd.12476. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Chen J, Tian W. Strategies of cell and cell-free therapies for periodontal regeneration: the state of the art. Stem Cell Res Ther. 2022;13:536. 10.1186/s13287-022-03225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schäfer R, Schwab M, Siegel G, von Ameln-Mayerhofer A, Buadze M, Lourhmati A, et al. Modulating endothelial adhesion and migration impacts stem cell therapies efficacy. EBioMedicine. 2020;60: 102987. 10.1016/j.ebiom.2020.102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011;63:597–609. 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Trindade-da-Silva CA, Bettaieb A, Napimoga MH, Lee KSS, Inceoglu B, Ueira-Vieira C, et al. Soluble epoxide hydrolase pharmacological inhibition decreases alveolar bone loss by modulating host inflammatory response, RANK-related signaling, endoplasmic reticulum stress, and apoptosis. J Pharmacol Exp Ther. 2017;361:408–16. 10.1124/jpet.116.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napimoga MH, Rocha EP, Trindade-da-Silva CA, Demasi APD, Martinez EF, Macedo CG, et al. Soluble epoxide hydrolase inhibitor promotes immunomodulation to inhibit bone resorption. J Periodontal Res. 2018;53:743–9. 10.1111/jre.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Wen Z, Lou Y, Chen J, Gao L, Li X, et al. Soluble epoxide hydrolase inhibitor promotes the healing of oral ulcers. Clinics (Sao Paulo). 2023;78: 100208. 10.1016/j.clinsp.2023.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang H, Chen W, Chen L, Huo X, Wang F. TPPU inhibits inflammation-induced excessive autophagy to restore the osteogenic differentiation potential of stem cells and improves alveolar ridge preservation. Sci Rep. 2023;13:1574. 10.1038/s41598-023-28710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L, Chen W, Li L, Li J, Kongling W, Zhang Y, et al. Targeting soluble epoxide hydrolase promotes osteogenic-angiogenic coupling via activating SLIT3/HIF-1α signalling pathway. Cell Prolif. 2023. 10.1111/cpr.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahr A, Alcaide P, Yang J, Jones A, Gregory M, et al. Endomucin prevents leukocyte-endothelial cell adhesion and has a critical role under resting and inflammatory conditions. Nat Commun. 2016;7:10363. 10.1038/ncomms10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 24.Alves L, Machado V, Botelho J, Mendes JJ, Cabral JMS, da Silva CL, et al. Enhanced proliferative and osteogenic potential of periodontal ligament stromal cells. Biomedicines. 2023;11(5):1352. 10.3390/biomedicines11051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slots J. Periodontitis: facts, fallacies and the future. Periodontol. 2000;2017(75):7–23. 10.1111/prd.12221. [DOI] [PubMed] [Google Scholar]
- 26.Nasiri K, Masoumi SM, Amini S, Goudarzi M, Tafreshi SM, Bagheri A, et al. Recent advances in metal nanoparticles to treat periodontitis. J Nanobiotechnology. 2023;21:283. 10.1186/s12951-023-02042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–9. 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, et al. Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol. 2006;77(6):1003–7. 10.1902/jop.2006.050341. [DOI] [PubMed] [Google Scholar]
- 29.Yu N, Bronckers AL, Oortgiesen DA, Yan X, Jansen JA, Yang F, et al. Periodontal cell implantation contributes to the regeneration of the periodontium in an indirect way. Tissue Eng Part A. 2015;21(1–2):166–73. 10.1089/ten.tea.2014.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Yang L, Hou Y, Zhang Z, Chen M, Wang M, et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–27. 10.1016/j.bioactmat.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ming P, Liu Y, Yu P, Jiang X, Yuan L, Cai S, et al. A Biomimetic Se-nHA/PC composite microsphere with synergistic immunomodulatory and osteogenic ability to activate bone regeneration in periodontitis. Small. 2024;20(9):e2305490. 10.1002/smll.202305490. [DOI] [PubMed] [Google Scholar]
- 32.Chen FM, Shelton RM, Jin Y, Chapple ILC. Localized delivery of growth factors for periodontal tissue regeneration: role, strategies, and perspectives. Med Res Rev. 2009;29(3):472–513. 10.1002/med.20144. [DOI] [PubMed] [Google Scholar]
- 33.Hajishengallis G. New developments in neutrophil biology and periodontitis. Periodontol. 2020;82(1):78–92. 10.1111/prd.12313. [DOI] [PubMed] [Google Scholar]
- 34.Guan H, Zhao L, Cao H, Chen A, Xiao J. Epoxyeicosanoids suppress osteoclastogenesis and prevent ovariectomy-induced bone loss. Faseb j. 2015;29:1092–101. 10.1096/fj.14-262055. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol. 2000;2020(84):14–34. 10.1111/prd.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, et al. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Huang B, Wang W, Li Q, Wang Z, Yan B, Zhang Z, et al. Osteoblasts secrete Cxcl9 to regulate angiogenesis in bone. Nat Commun. 2016;7:13885. 10.1038/ncomms13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 39.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3. Uncropped full-length gels and blot
Additional file 4. Supplementary figures and figure legends
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.