Abstract
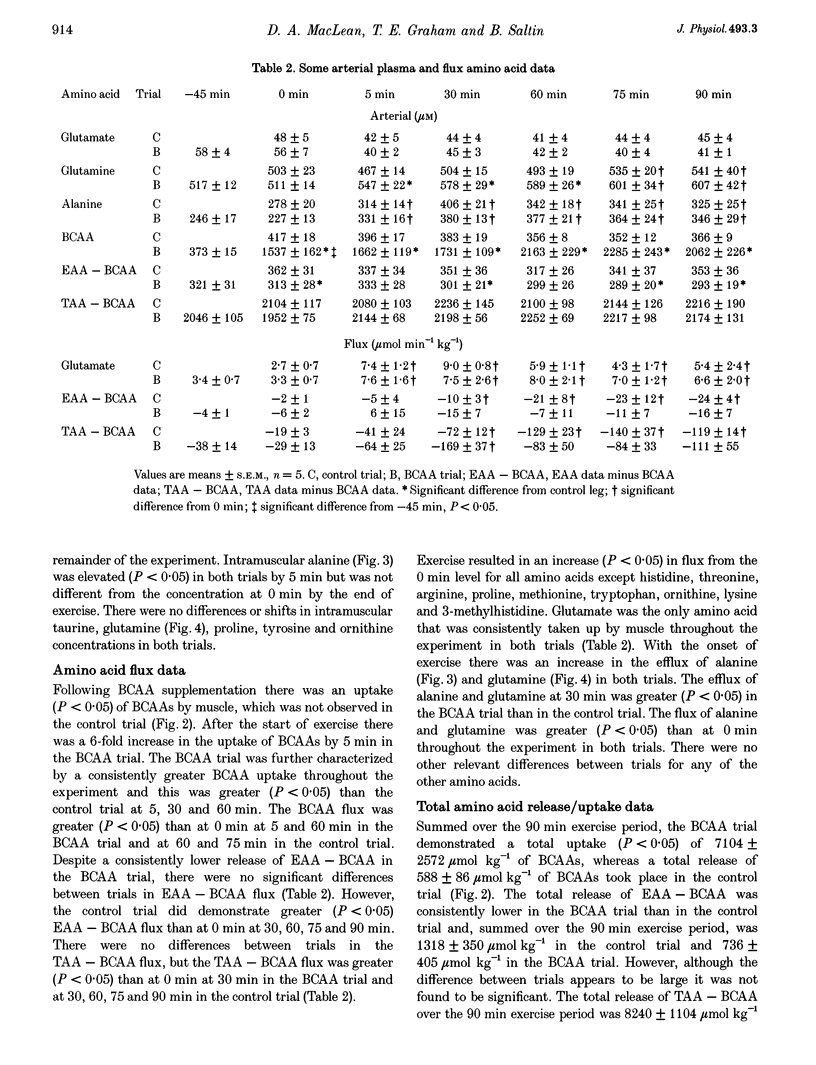
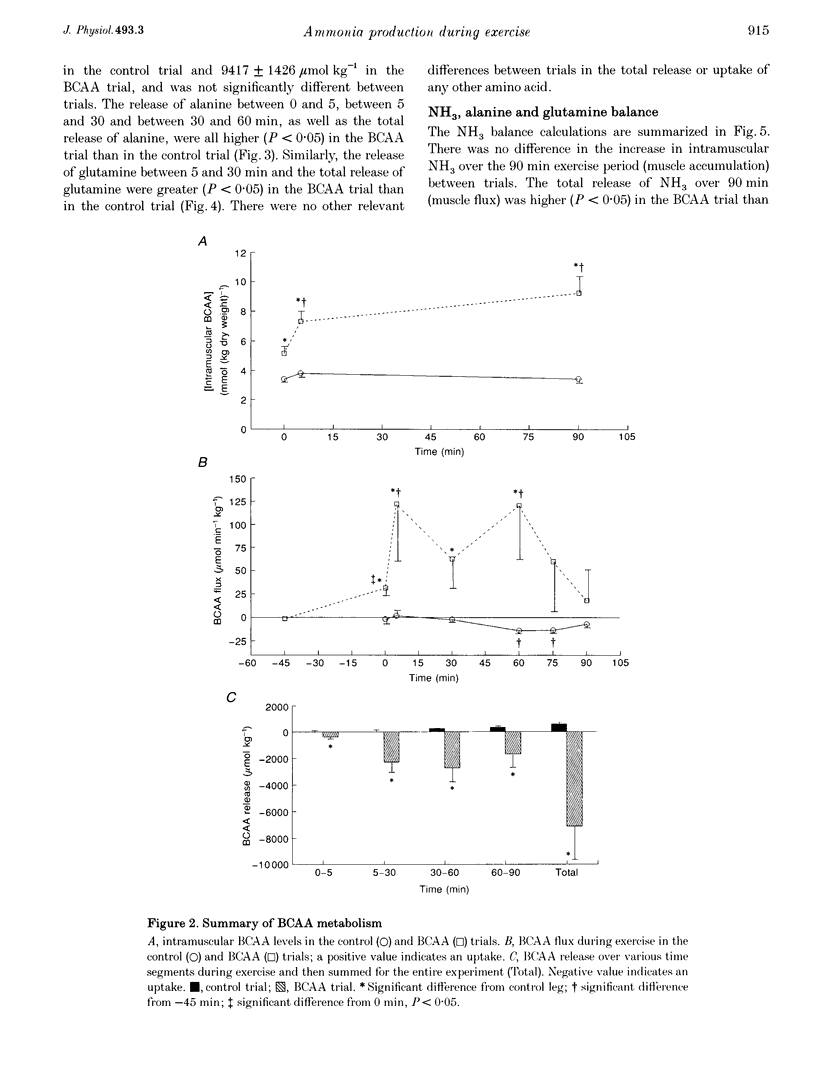
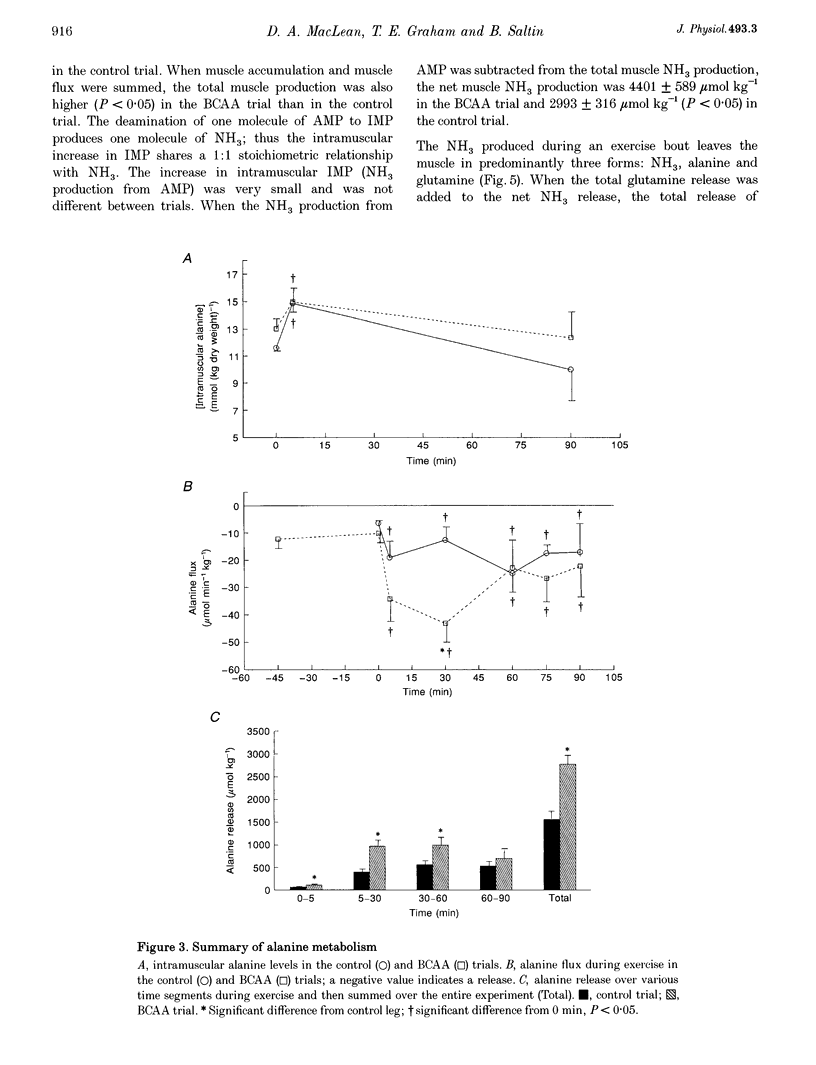
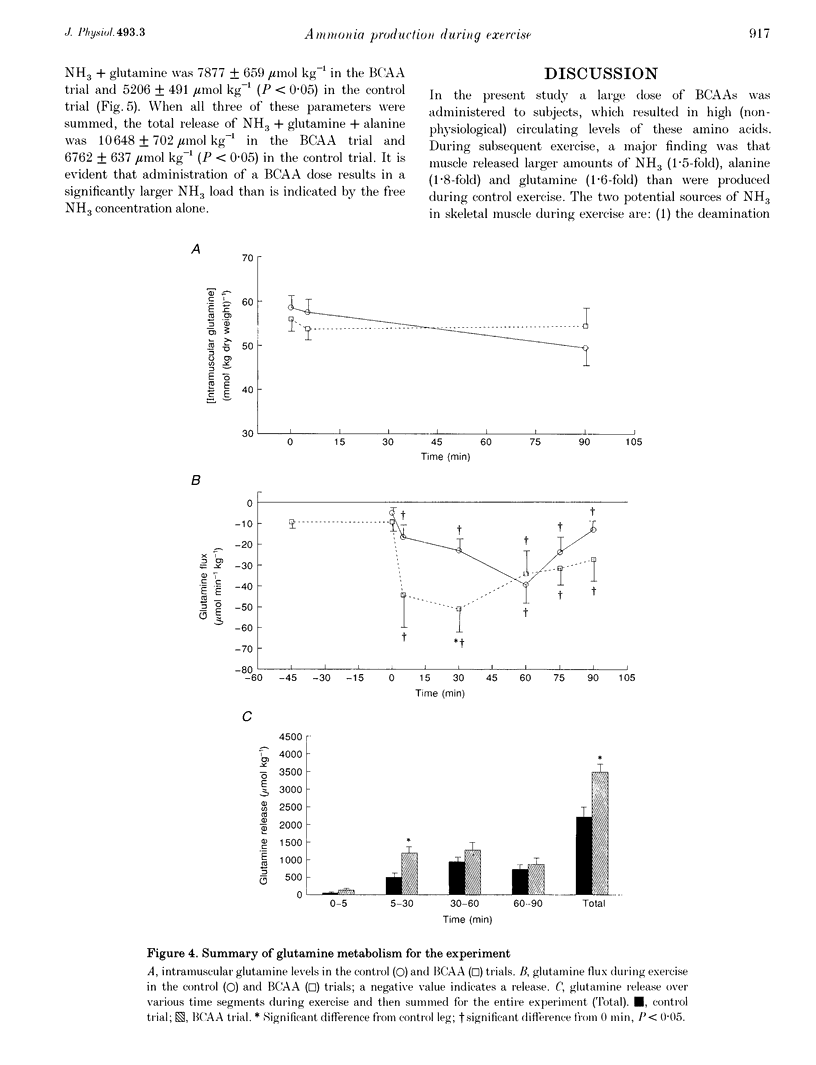
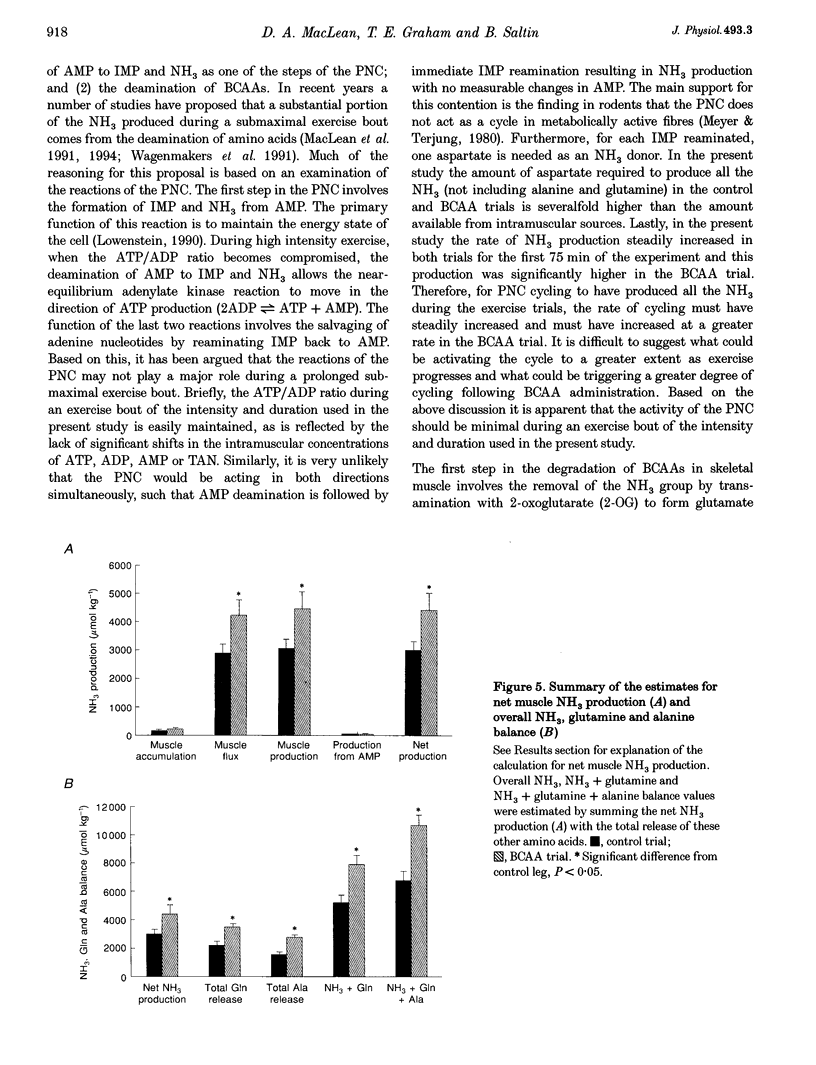
1. This study examined the effects of a large (308 mg kg-1) oral dose of branched-chain amino acids (BCAAs) on muscle amino acid and ammonia (NH3) metabolism during 90 min of dynamic knee extensor exercise (64 +/- 2% of maximum workload). 2. BCAA supplementation resulted in a 4-fold increase in the arterial BCAA level (from 373 to 1537 microM, P < 0.05) and a 1.5-fold increase in the intramuscular BCAA level (from 3.4 +/- 0.2 to 5.2 +/- 0.5 mmol (kg dry weight)-1, P < 0.05) by the onset of exercise. Over the 90 min exercise period, the exercising muscle removed a total of 7104 +/- 2572 mumol kg-1 of BCAAs. In contrast, in the control trial, there was a total release of 588 +/- 86 mumol kg-1 (P < 0.05) of BCAAs. 3. The total release of NH3 over the 90 min exercise period was 2889 +/- 317 mumol kg-1 (P < 0.05) in the control trial and 4223 +/- 552 mumol kg-1 (P < 0.05) in the BCAA trial. Similarly, the total release of alanine and glutamine was 1557 +/- 153 and 2213 +/- 270 mumol kg-1, respectively, for the control trial and 2771 +/- 178 and 3476 +/- 217 mumol kg-1, respectively, for the BCAA trial. 4. The lactate release and arterial lactate values were all consistently lower in the BCAA trial than in the control trial. The net production of lactate (intramuscular shifts + total release) was lower (P < 0.05) in the BCAA trial (49.9 +/- 11.4 mmol kg-1) than in the control trial (64.0 +/- 11.7 mmol kg-1). 5. It is concluded that: (1) the administration of BCAAs can greatly increase their concentration in plasma and subsequently their uptake by muscle during exercise, and (2) long-term exercise following BCAA administration results in significantly greater muscle NH3, alanine and glutamine production, as well as lower lactate production, than is observed during exercise without BCAA supplementation. These data strongly suggest that BCAAs are an important source of NH3 during submaximal exercise and that their contribution to NH3, alanine and glutamine production can be significantly altered by changes in BCAA availability.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangsbo J., Graham T. E., Kiens B., Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Glickman M. G., Jacob R., Sherwin R. S., DeFronzo R. A. Removal of infused amino acids by splanchnic and leg tissues in humans. Am J Physiol. 1986 Apr;250(4 Pt 1):E407–E413. doi: 10.1152/ajpendo.1986.250.4.E407. [DOI] [PubMed] [Google Scholar]
- Hagg S. A., Morse E. L., Adibi S. A. Effect of exercise on rates of oxidation, turnover, and plasma clearance of leucine in human subjects. Am J Physiol. 1982 Jun;242(6):E407–E410. doi: 10.1152/ajpendo.1982.242.6.E407. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Kasperek G. J., Dohm G. L., Snider R. D. Activation of branched-chain keto acid dehydrogenase by exercise. Am J Physiol. 1985 Feb;248(2 Pt 2):R166–R171. doi: 10.1152/ajpregu.1985.248.2.R166. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- Paxton R., Harris R. A. Regulation of branched-chain alpha-ketoacid dehydrogenase kinase. Arch Biochem Biophys. 1984 May 15;231(1):48–57. doi: 10.1016/0003-9861(84)90361-8. [DOI] [PubMed] [Google Scholar]
- Saltin B., Henriksson J., Nygaard E., Andersen P., Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- Sellevold O. F., Jynge P., Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J Mol Cell Cardiol. 1986 May;18(5):517–527. doi: 10.1016/s0022-2828(86)80917-8. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Beckers E. J., Brouns F., Kuipers H., Soeters P. B., van der Vusse G. J., Saris W. H. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am J Physiol. 1991 Jun;260(6 Pt 1):E883–E890. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Brookes J. H., Coakley J. H., Reilly T., Edwards R. H. Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur J Appl Physiol Occup Physiol. 1989;59(3):159–167. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Wolfe M. H., Royle G. T., Nadel E. R. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):458–466. doi: 10.1152/jappl.1982.52.2.458. [DOI] [PubMed] [Google Scholar]


