Abstract
Background
The ZJU index is an innovative computational method which integrates BMI, FBG, TG, and ALT to AST ratio. It strongly correlates with measures of lipid metabolism and glucose intolerance. No researches have yet explored the relationship between the ZJU index and sarcopenia.
Methods
We analyzed NHANES data from 2011 to 2018, dividing the ZJU index into quartiles. The association was investigated by adjusting for confounders using multivariable linear and logistic regression analysis. Results were visualized through RCS regression and threshold effect analyses. We conducted various subgroup and sensitivity analyses and plotted ROC curves to assess prediction efficacy, with the AUC as the measure of accuracy.
Results
As the ZJU index increases, the prevalence of sarcopenia also rises. Following the control of potential confounders via logistic regression analysis, our research identified a distinct relationship between the ZJU index and sarcopenia, which was statistically significant (P < 0.001), with higher ZJU index values associated with increased risk (OR = 12.40, 95% CI: 8.46–18.17). Interaction analysis suggests that the relationship between the ZJU index and the risk of developing sarcopenia varies significantly between males and females across different ZJU index levels. ROC analysis for the ZJU index shows an AUC of 0.749. Conclusions: The ZJU index significantly correlates with a heightened risk of sarcopenia in Americans, suggesting its potential as a predictive marker for sarcopenia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02373-w.
Keywords: ZJU index, Appendicular lean mass, Sarcopenia, NHANES, Cross-sectional study
Introduction
Sarcopenia is described as a gradual and pervasive condition that primarily affects skeletal muscles, particularly in older adults [1]. Worldwide, the incidence of sarcopenia among the general population is thought to range between 5% and 10% [2]. In recent months, the Global Leadership Initiative in Sarcopenia (GLIS) developed a worldwide standard that addresses the limitations of previous definitions specific to continents and regions. This new definition includes critical components such as muscle mass, strength, and muscle-specific strength, with input from experts across major sarcopenia organizations worldwide [3]. Sarcopenia impairs daily activities, increases the likelihood of falls, and diminishes autonomy. Additionally, it is linked to negative health outcomes, including fractures, osteoporosis, obstructive sleep apnea (OSA), prediabetes, stroke, and cancer [4–10]. The appendicular lean mass (ALM), assessed via the Dual-energy x-ray absorptiometry (DEXA) method, serves as a key indicator in evaluating muscle mass [11]. The ratio of ALM to BMI (ALMBMI) is essential in assessing muscle health. The skeletal muscle system is the foundation of normal functioning in the human body. Thus, obtaining clinically relevant information is essential to the early identification and mitigation of low muscle mass.
The ZJU index is an innovative metabolic parameter which integrates multiple indicators, including triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), BMI, and fasting blood glucose (FBG) [12]. This index offers a comprehensive metabolic health assessment, capturing patterns of metabolic dysfunction and related health risks more effectively than isolated biochemical indicators. Research findings have shown that the ZJU index is associated with lipid regulation, insulin resistance, and obesity, as well as the risks of non-alcoholic fatty liver disease (NAFLD) and obstructive sleep apnea (OSA) syndrome [13–15]. It has been established that the development of sarcopenia shares similar metabolic pathways with NAFLD [16]. Lipid metabolism intermediates and fatty acids are crucial in regulating skeletal muscle mass and function [17]. Abnormal lipid levels could heighten the likelihood of damaging muscle through pathways involving impaired insulin sensitivity, chronic inflammation, and increased oxidative stress [18]. Despite its significance, the relationship between the ZJU index, which provides for lipids, blood sugar levels, and hepatic function, and sarcopenia has not been thoroughly examined, and its connection to overall muscle mass remains uncertain. To fill this gap, we evaluated information from the National Health and Nutrition Examination Survey (NHANES). We hypothesized that elevated ZJU index levels would be associated with reduced muscle mass and an increased occurrence of sarcopenia. This research seeks to assess the potential of the ZJU index as an innovative predictor of muscle mass.
Materials and methods
Data collection and study sample
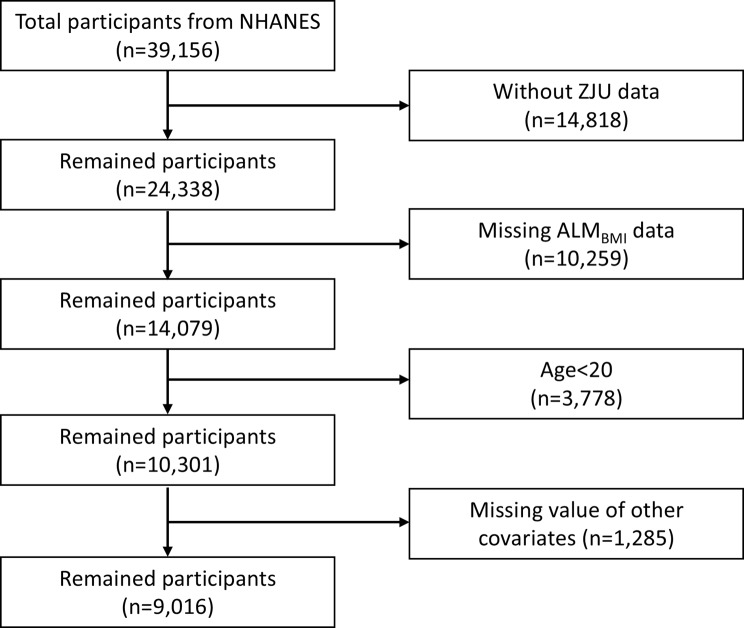
The research evaluated NHANES data covering four survey cycles between 2011 and 2018 to explore the relationship between the ZJU index and the prevalence of sarcopenia. Figure 1 outlines the process of selecting participants. Initially, individuals without ZJU index records (n = 14,818) and those with incomplete ALMBMI data (n = 10,259) were excluded. Furthermore, individuals younger than 20 were excluded from the analysis. In addition, 1,285 individuals were excluded due to missing essential data, which included demographic information (education attainment, marital status, poverty-income ratio), health conditions (hypertension, diabetes, trouble sleeping, cancer), lifestyle factors (current drinker, current smoker, physical activity), and dietary intake (energy, protein, carbohydrate, and fat intake). The resulting dataset comprised 9,016 individuals with full information. All subjects completed evaluations of their health and medical status through health assessments conducted in mobile units and structured home interviews. The ethical application of NHANES involving human subjects was approved by the NCHS Research Ethics Review Board, and informed consent was provided by each participant. We adhered to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines in conducting this cross-sectional study [19].
Fig. 1.
Flowchart of participant selection
Definition of ZJU index
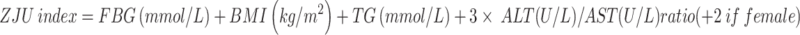 |
12 |
Evaluation of appendicular lean mass and sarcopenia
Between 2011 and 2018, participants received DEXA scans, an extensively acknowledged and verified method for evaluating lean body mass. The ALMBMI was determined by taking the arms’ and legs’ total lean mass (excluding bone mineral content) and dividing it by BMI. Sarcopenia was diagnosed following guidelines from the Foundation for the National Institutes of Health (FNIH), with ALMBMI being less than 0.789 for males and less than 0.512 for females [11].
Covariables
To reduce potential confounding in the analysis of the relationship between the ZJU index and ALMBMI, multivariable adjustment models were employed. The study accounted for various covariates, which included demographic information (education attainment, marital status, PIR), health conditions (hypertension, diabetes, cancer, trouble sleeping), dietary intake (energy, protein, carbohydrate, and fat intake), and lifestyle factors (current drinker, current smoker, physical activity). Participants reported their leisure-time physical activity using the Global Physical Activity Questionnaire (GPAQ). We calculated total physical activity by multiplying the frequency and duration of moderate physical activities (MPA) and vigorous physical activities (VPA), noting that 1 min of VPA is equivalent to 2 min of MPA [20].
Statistical analysis
Details regarding the missing covariates can be found in Supplementary Table (1) To address potential multicollinearity, we calculated the variance inflation factor (VIF) for each predictor, using a threshold of 5. These results are detailed in Supplementary Table (2) Additionally, we used Cook’s distance to identify statistical outliers, with those exceeding 0.005 noted in Supplementary Fig. 1. The results of our analyses are derived from complete case data. ZJU index levels were segmented into four quartiles. Medians and interquartile ranges (IQR) summarized continuous variables, whereas categorical variables were reported as percentages. To assess differences among groups, the Kruskal-Wallis rank-sum test was used for continuous variables, and the Rao-Scott chi-squared test was applied for weighted proportions of categorical variables, providing a comprehensive description of the study population. Multivariable linear and logistic regression analyses were performed to investigate the associations between the ZJU index and ALMBMI or sarcopenia across three progressively adjusted models. Results were reported as regression coefficients (β) and odds ratios (OR), with corresponding 95% confidence intervals (CI). Restricted cubic splines (RCS) were employed to explore potential nonlinear relationships between changes in the ZJU index and ALMBMI, using 4 knots at the 5th, 35th, 65th, and 95th percentiles, with the aim of identifying inflection points. The P-value of less than 0.05 indicated statistical significance. Subgroup analyses examined the relationship between the ZJU index and sarcopenia across different gender and age groups. To minimize the error rate from multiple comparisons, a Bonferroni correction was applied to the p-values. Given that there are four groups, based on gender and age, we adjusted the significance level by dividing 0.05 by four, resulting in a final significance p-value of 0.0125. If a significant interaction were observed, an exploratory analysis would be carried out to identify a potential threshold at which the ZJU index could effectively identify patients who might benefit. The predictive ability of the ZJU index for sarcopenia was assessed through receiver operating characteristic (ROC) curves, with the findings presented as the area under the curve (AUC). To validate this predictive factor, participants were randomly divided into training and validation cohorts at a 7:3 ratio. We evaluated performance using ROC and calibration curves and conducted decision curve analysis (DCA) to determine the net benefit threshold. These analyses are detailed in the Supplementary materials. To reduce the impact of missing variables, we employed multiple imputations with five replications using a chained equations approach, and sensitivity analyses assessed the robustness of results. We used R software (version 4.2) and applied complex sampling weights to ensure the study population’s representativeness.
Results
Characteristics of participants
In this study, 9,016 participants were divided into four categories according to their ZJU index quartiles. Significant differences were identified across the quartiles with respect to age, gender, race, education attainment, marital status, PIR, and various health status. There were noticeable differences by ZJU index groups for BMI, ALT, AST, fasting plasma glucose, and triglycerides. Moreover, Q4 had the highest prevalence of hypertension (37.4%) and diabetes (16.5%), alongside a greater incidence of sleep disturbances (34.5%). Physical activity levels decrease from Q1 to Q4. Dietary intake, including energy, protein, and fats, is also with variations across the groups. Importantly, those in Q4 exhibited the lowest ALMBMI values and the highest rate of sarcopenia (17.0%). Further detailed comparisons between the quartiles can be found in Table 1.
Table 1.
Weighted characteristics of the study population based on ZJU quartiles
| Characteristic | ZJU index groups | p-value | |||
|---|---|---|---|---|---|
| Q1 Weighted N = 112,347,294 Unweighted n = 2,2541 |
Q2 Weighted N = 115,108,515 Unweighted n = 2,2541 |
Q3 Weighted N = 108,715,831 Unweighted n = 2,2541 |
Q4 Weighted N = 103,476,318 Unweighted n = 2,2541 |
||
| Age (years) | 33 (25, 45) | 40 (30, 50) | 42 (32, 51) | 42 (32, 50) | < 0.001 2 |
| Gender | < 0.001 3 | ||||
| Male | 37.7% | 54.8% | 58.8% | 49.3% | |
| Female | 62.3% | 45.2% | 41.2% | 50.7% | |
| Race | < 0.001 3 | ||||
| Mexican American | 5.2% | 8.3% | 12.6% | 14.4% | |
| Other Hispanic | 5.7% | 6.0% | 8.6% | 7.4% | |
| Non-Hispanic White | 67.4% | 65.7% | 60.5% | 59.0% | |
| Non-Hispanic Black | 9.8% | 9.7% | 10.0% | 13.3% | |
| Other Race -Including Multi-Racial | 12.0% | 10.2% | 8.3% | 5.9% | |
| Education attainment | < 0.001 3 | ||||
| ˂High school | 10.0% | 10.1% | 14.0% | 13.8% | |
| High school | 18.1% | 20.3% | 23.7% | 25.0% | |
| College | 71.9% | 69.6% | 62.3% | 61.3% | |
| Marital status | < 0.001 3 | ||||
| Married or living with a partner | 53.6% | 65.2% | 65.5% | 65.8% | |
| Divorced, separated, or widowed | 10.9% | 13.1% | 15.4% | 13.9% | |
| Never married | 35.5% | 21.7% | 19.1% | 20.3% | |
| PIR groups | < 0.001 3 | ||||
| ≤ 1.3 | 24.5% | 19.4% | 22.7% | 26.1% | |
| 1.3–3.5 | 32.1% | 33.0% | 35.3% | 39.1% | |
| ˃ 3.5 | 43.5% | 47.6% | 42.0% | 34.8% | |
| Hypertension | < 0.001 3 | ||||
| Yes | 8.8% | 17.5% | 27.2% | 37.4% | |
| No | 91.2% | 82.5% | 72.8% | 62.6% | |
| Diabetes | < 0.001 3 | ||||
| Yes | 1.0% | 1.8% | 4.3% | 16.5% | |
| No | 98.2% | 96.9% | 93.8% | 80.4% | |
| Borderline | 0.7% | 1.3% | 1.8% | 3.1% | |
| Cancer | 0.4693 | ||||
| Yes | 4.8% | 4.7% | 4.3% | 5.7% | |
| No | 95.2% | 95.3% | 95.7% | 94.3% | |
| Trouble sleeping | < 0.001 3 | ||||
| Yes | 22.9% | 23.8% | 26.2% | 34.5% | |
| No | 77.1% | 76.2% | 73.8% | 65.5% | |
| Current drinker | < 0.001 3 | ||||
| Yes | 33.7% | 33.0% | 29.5% | 20.7% | |
| No | 66.3% | 67.0% | 70.5% | 79.3% | |
| Current smoker | 0.1563 | ||||
| Yes | 38.7% | 40.3% | 42.9% | 42.6% | |
| No | 61.3% | 59.7% | 57.1% | 57.4% | |
| PA (min/week) | 180 (0, 422) | 120 (0, 420) | 60 (0, 300) | 0 (0, 240) | < 0.001 2 |
| Energy intake(kcal) | 2,005 (1,520, 2,697) | 2,134 (1,589, 2,762) | 2,147 (1,623, 2,781) | 2,120 (1,568, 2,863) | 0.004 2 |
| Protein intake (g) | 75 (52, 101) | 81 (57, 108) | 82 (60, 113) | 80 (57, 108) | < 0.001 2 |
| Carbohydrate intake (g) | 242 (178, 324) | 244 (175, 324) | 242 (175, 330) | 247 (178, 336) | 0.6802 |
| Fat intake (g) | 74 (52, 106) | 82 (57, 111) | 82 (55, 115) | 82 (57, 117) | < 0.001 2 |
| BMI (kg/m2) | 22 (21, 23) | 26 (25, 27) | 30 (29, 32) | 37 (34, 40) | < 0.001 2 |
| ALT(U/L) | 16 (13, 21) | 20 (15, 26) | 24 (18, 33) | 27 (19, 39) | < 0.001 2 |
| AST(U/L) | 21 (18, 25) | 22 (18, 27) | 23 (19, 27) | 23 (19, 29) | < 0.001 2 |
| Fasting plasma glucose (mmol/L) | 4.77 (4.49, 5.05) | 4.94 (4.66, 5.33) | 5.11 (4.83, 5.55) | 5.44 (5.00, 6.33) | < 0.001 2 |
| Triglycerides (mmol/L) | 0.88 (0.64, 1.22) | 1.22 (0.85, 1.75) | 1.59 (1.06, 2.30) | 1.92 (1.27, 2.97) | < 0.001 2 |
| ZJU index | 30 (29, 32) | 36 (34, 37) | 41 (39, 42) | 49 (46, 53) | < 0.001 2 |
| ALM BMI | 0.81 (0.70, 1.02) | 0.85 (0.67, 1.03) | 0.84 (0.64, 0.96) | 0.71 (0.59, 0.88) | < 0.001 2 |
| sarcopenia | < 0.001 3 | ||||
| Yes | 0.9% | 3.3% | 7.9% | 17.0% | |
| No | 99.1% | 96.7% | 92.1% | 83.0% | |
1Median (IQR); %
2Kruskal-Wallis rank-sum test for complex survey samples
3chi-squared test with Rao & Scott’s second-order correction
Relationship between ZJU index and skeletal muscle mass
Table 2 illustrates the association between the ZJU index, segmented into quartiles, and the outcome variable across three progressively adjusted models. In the completely adjusted model (Model 3), the ZJU index shows a strong association with the outcome variable. Q1 serves as the reference group. In comparison to Q1, Q4 exhibits the strongest association, with a beta of -0.13 (95% CI: -0.14, -0.13). All quartiles have p-values < 0.001, indicating significant differences across groups. The trend analysis reveals a dose-response relationship (p for trend < 0.001), where higher ZJU quartiles are progressively linked to a stronger negative effect on the outcome variable.
Table 2.
The association between ZJU and ALMBMI
| Characteristic | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI1 | p-value | Beta | 95% CI1 | p-value | Beta | 95% CI1 | p-value | |
| ZJU index (quartile) | |||||||||
| Q1 | reference | reference | reference | ||||||
| Q2 | -0.02 | -0.03, -0.01 | < 0.001 | -0.06 | -0.06, -0.05 | < 0.001 | -0.06 | -0.06, -0.05 | < 0.001 |
| Q3 | -0.07 | -0.08, -0.06 | < 0.001 | -0.10 | -0.10, -0.09 | < 0.001 | -0.09 | -0.10, -0.09 | < 0.001 |
| Q4 | -0.13 | -0.14, -0.12 | < 0.001 | -0.14 | -0.15, -0.13 | < 0.001 | -0.13 | -0.14, -0.13 | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||||
1CI = Confidence Interval
Model 1: no covariates were adjusted
Model 2: adjusted for Age, Gender, Race, Education attainment, Marital status, and PIR
Model 3: adjusted for Age, Gender, Race, Education attainment, Marital status, PIR, Hypertension, Diabetes, Trouble sleeping, Cancer, Current drinker, Current smoker, PA (min/week), Protein intake (g), Carbohydrate intake (g), and Fat intake (g)
Table 3 presents the relationship between the ZJU index and sarcopenia. Utilizing Q1 as the reference, Q4 shows the strongest association, with an OR of 12.40 (95% CI: 8.46, 18.17), indicating individuals in Q4 have about 12.4 times higher odds of having sarcopenia compared to those in Q1. Q3 has an OR of 4.96 (95% CI: 3.37, 7.32), and Q2 has an OR of 2.73 (95% CI: 1.82, 4.11). All quartiles have p-values < 0.001, showing significant differences between groups. The p-value for the trend is < 0.001, confirming a dose-response relationship, where higher ZJU quartiles are associated with increasing odds of sarcopenia.
Table 3.
The association between ZJU and Sarcopenia
| Characteristic | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR1 | 95% CI1 | p-value | OR1 | 95% CI1 | p-value | OR1 | 95% CI1 | p-value | |
| ZJU index (quartile) | |||||||||
| Q1 | reference | reference | reference | ||||||
| Q2 | 3.32 | 2.23, 4.96 | < 0.001 | 2.77 | 1.84, 4.16 | < 0.001 | 2.73 | 1.82, 4.11 | < 0.001 |
| Q3 | 7.02 | 4.82, 10.24 | < 0.001 | 5.17 | 3.52, 7.61 | < 0.001 | 4.96 | 3.37, 7.32 | < 0.001 |
| Q4 | 15.90 | 11.04, 22.90 | < 0.001 | 13.08 | 9.00, 19.02 | < 0.001 | 12.40 | 8.46, 18.17 | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||||
1OR = Odds Ratio, CI = Confidence Interval
Model 1: no covariates were adjusted
Model 2: adjusted for Age, Gender, Race, Education attainment, Marital status, and PIR
Model 3: adjusted for Age, Gender, Race, Education attainment, Marital status, PIR, Hypertension, Diabetes, Cancer, Trouble sleeping, Current drinker, Current smoker, PA (min/week), Protein intake (g), Carbohydrate intake (g), and Fat intake (g)
Nonlinear relationship between the ZJU index and sarcopenia
The findings examined the nonlinear relationships among the ZJU index, ALMBMI, and the likelihood of sarcopenia, as depicted in Figs. 2 and 3. The RCS analysis in Fig. 2 demonstrated a significant nonlinear relationship between the ZJU index and ALMBMI, with a p-value of < 0.001 for both the overall association and nonlinearity. The analysis pinpointed a turning point at a ZJU index of 44. Below this value, each standard deviation increase in the ZJU index was associated with a decrease of 0.14 in ALMBMI (β = -0.14, 95% CI: -0.16 to -0.12, p < 0.001). Above an index of 44, with each standard deviation increase in ZJU associated with a decrease of 0.14 (β = -0.14, 95% CI: -0.19 to -0.10, p < 0.001) (Table 4). Similarly, Fig. 3 illustrates the nonlinear association between the ZJU index and the risk of sarcopenia. An inflection point was found at a ZJU index of 33. Below this value, the relationship was not significant (OR: 1.08, 95% CI: 0.91 to 1.28, p = 0.40). However, above a ZJU index of 33, each unit increase in the ZJU index was associated with an 8% increase in the risk of sarcopenia (OR: 1.08, 95% CI: 1.07 to 1.09, p < 0.001) (Table 5), indicating a significant rise in risk as the ZJU index exceeds this threshold.
Fig. 2.
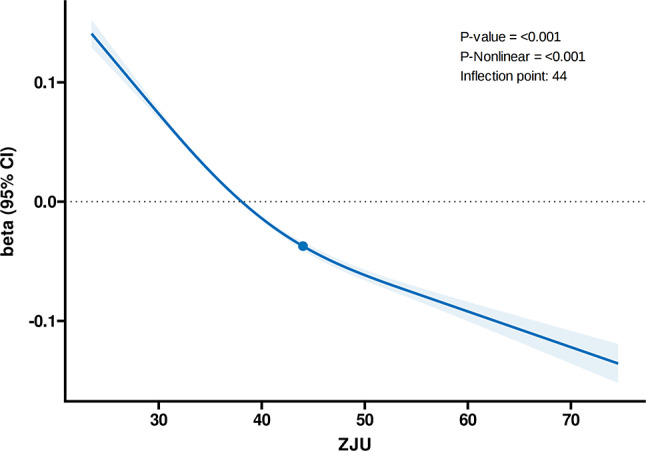
Association between ZJU index and ALMBMI with the RCS function. Model with 4 knots located at 5th, 35th, 65th and 95th percentiles
Fig. 3.
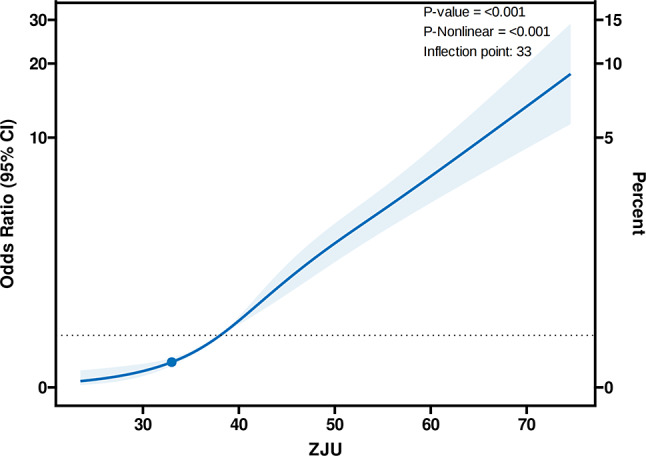
Association between ZJU index and sarcopenia with the RCS function. Model with 4 knots located at 5th, 35th, 65th and 95th percentiles
Table 4.
Effect of standardized ZJU index level on ALMBMI: adjusted coefficients from segmented linear regression analysis
| Characteristic | Adjusted Beta per SD | 95% CI1 | p-value |
|---|---|---|---|
| ZJU index (< 44) | -0.14 | -0.16, -0.12 | < 0.001 |
| ZJU index (≥ 44) | -0.14 | -0.19, -0.10 | < 0.001 |
Table 5.
Effect of ZJU index level on Sarcopenia: adjusted coefficients from segmented linear regression analysis
| Characteristic | Adjusted OR | 95% CI1 | p-value |
|---|---|---|---|
| ZJU index (< 33) | 1.08 | 0.91, 1.28 | 0.40 |
| ZJU index (≥ 33) | 1.08 | 1.07, 1.09 | < 0.001 |
Subgroup analysis
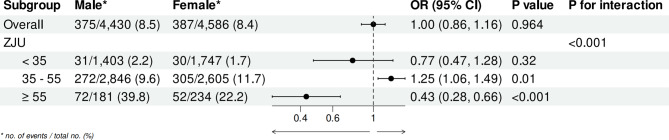
The stability of the link involving the ZJU index and sarcopenia underwent evaluation via subgroup analysis, as specified in Table 6. Significant associations were found across age and gender subgroups. Based on the Bonferroni-corrected p Value, we found that the interaction P-value for gender is 0.011, indicating significant differences in effect between genders. Specifically, males showed a slightly stronger association (OR: 1.12, 95% CI: 1.10–1.13) compared to females (OR: 1.08, 95% CI: 1.07–1.10), with a significant interaction.
Table 6.
Subgroup analysis of the association between ZJU and Sarcopenia
| Subgroup | N | Adjusted OR (95% CI)1 | P value | P for interaction |
|---|---|---|---|---|
| Age groups | 0.303 | |||
| ˂ 40 | 4600 | 1.11 (1.09–1.13) | < 0.001 | |
| ≥ 40 | 4416 | 1.09 (1.08–1.11) | < 0.001 | |
| Gender | 0.011 | |||
| Male | 4430 | 1.12 (1.10–1.13) | < 0.001 | |
| Female | 4586 | 1.08 (1.07–1.10) | < 0.001 |
1 adjusted for Race, Education attainment, Marital status, PIR, Hypertension, Diabetes, Trouble sleeping, Cancer, Current drinker, Current smoker, PA (min/week), Carbohydrate intake (g), Protein intake (g), and Fat intake (g)
Subgroup analysis revealed a significant interaction between gender and the association of the ZJU index with sarcopenia, prompting further investigation. Figure 4 depicts a spline curve showing the adjusted ORs for the risk of sarcopenia, comparing females with males, modeled using RCS with knots at the 5th, 35th, 65th, and 95th percentiles of the ZJU index. The graph demonstrates a notable peak in the OR at a ZJU index of around 40. As the ZJU index continues to rise beyond this point, the OR gradually decreases, indicating a lower relative risk for females at higher ZJU index values.
Fig. 4.
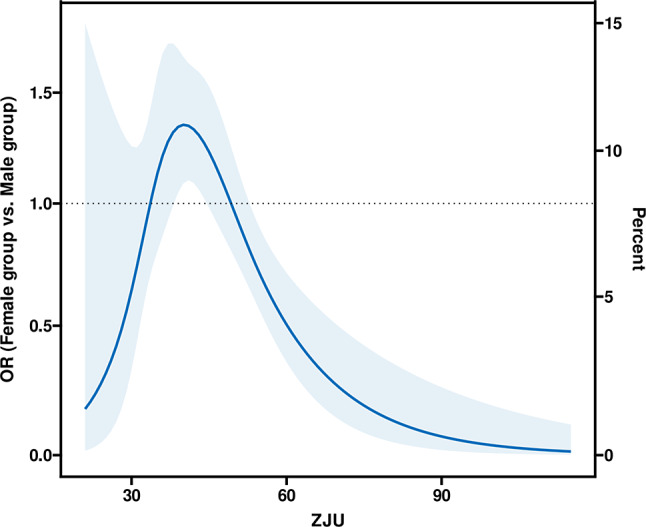
Spline figures plotted adjusted ORs with females compared with males for the risk of sarcopenia. A model with 4 knots at 5th, 35th, 65th, and 95th percentiles
As presented in Fig. 5. The overall adjusted OR for sarcopenia in females compared to males was 1.00 (95% CI: 0.86–1.16), with a p-value of 0.964, indicating no substantial statistical distinction between genders in the overall population. However, when stratified by ZJU index values, the analysis revealed more specific findings. For individuals with a ZJU index below 35, the prevalence of sarcopenia was 2.1% in males and 1.7% in females, with a p-value of 0.32, indicating the absence of statistically significant gender difference in this range. In the ZJU index range of 35–55, the prevalence of sarcopenia was similar between males (9.8%) and females (11.7%), with an adjusted OR of 1.25 (95% CI: 1.06–1.49) and a p-value of 0.01, indicating a markedly different risk of sarcopenia in females and males. Moreover, for individuals with a ZJU index of 55 or higher, males had a much higher prevalence of sarcopenia (39.8%) compared to females (22.2%), with an adjusted OR of 0.43 (95% CI: 0.28–0.66) and a p-value of < 0.001, indicating a significantly lower risk in females. The p-value for interaction (< 0.001) indicates a significant overall difference in how the ZJU index levels relate to sarcopenia risk between males and females.
Fig. 5.
Adjusted ORs for sarcopenia across ZJU index subgroups, stratified by gender
.
ROC curve
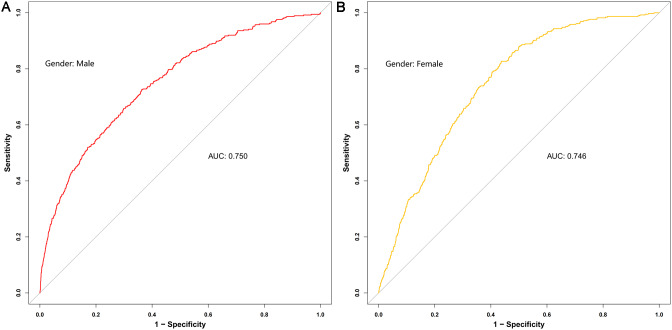
The ZJU index is a composite index that incorporates several factors. We plotted the ROC curves for these indicators against sarcopenia individually. Figure 6 displays these findings. The areas under the ROC curve, listed from highest to lowest, are the ZJU index (AUC = 0.749), fasting plasma glucose (AUC = 0.621), TG (AUC = 0.620), ALT (AUC = 0.590) and AST (AUC = 0.533). Figure 7 display the ROC curves comparing the ZJU index with sarcopenia across different sexes. The analysis revealed an AUC value of 0.750 (95% CI: 0.724–0.776) for males in this study, whereas the AUC value for females was 0.746 (95% CI: 0.724–0.768). The sensitivity and specificity were 82.4% and 87.9%, as well as 50.1% and 50.0%, respectively. The ideal cut-off values were 37.82 and 36.93.
Fig. 6.
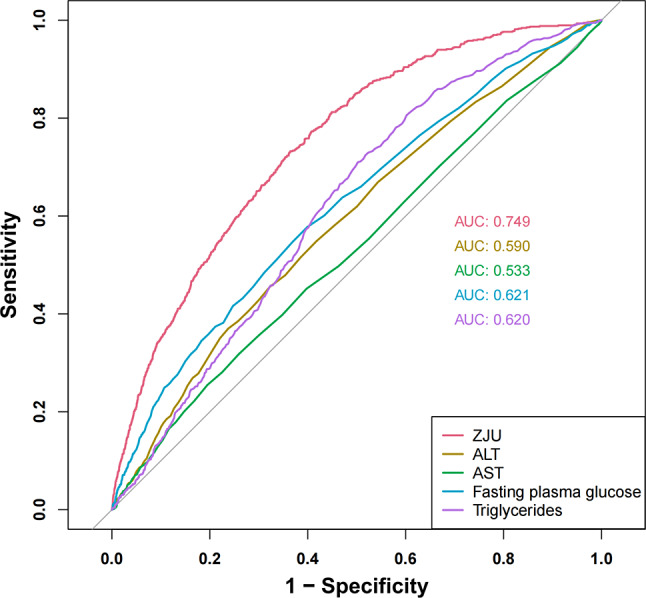
Identification of ROC curves for sarcopenia using various markers
Fig. 7.
Identification of ROC curves sarcopenia using the ZJU index in males (A) and females (B)
Discussion
Sarcopenia is described as a widespread and advancing condition of the skeletal muscles, marked by a rapid decline in both muscle mass and function. While it is commonly linked to the aging process, it is not exclusive to this process and is observed across various species, including humans [21]. This condition poses a major public health challenge, leading to considerable healthcare expenses and adding to the yearly disease impact [22]. Maintaining myofiber homeostasis involves balancing protein synthesis and degradation, ensuring adequate energy production through proper nutrition and metabolism, securing oxygen and nutrient supply through vascular channels, and maintaining proper nerve function for muscle contraction. In sarcopenia, these processes are disrupted alongside age-related changes in the local extracellular matrix and systemic variations in hormones and immune responses [21]. Recent research emphasizes that changes in fat metabolism and the accumulation of fat within muscle tissues are fundamental to sarcopenia and aging [23]. This results in muscle insulin resistance, the accumulation of ceramides [24], oxidative stress, and the formation of lipid droplets within cells. These alterations disrupted mitochondrial energy production, leading to metabolic imbalances in the muscles and reduced muscle performance [25]. Early screening and intervention for individuals at high risk of sarcopenia are crucial in reducing risk factors and managing the condition effectively. Clinically, Sarcopenia can be assessed through different techniques, including DEXA, bioelectrical impedance analysis (BIA), and computed tomography (CT) [26]. Each technique has distinct assessment standards and effectively evaluates muscle mass. Despite the effectiveness of techniques like BIA, DEXA, and CT in assessing sarcopenia, their practical application is restricted by the time required, the expense of the tests, the necessity for patient cooperation, and the potential risks from radiation exposure. Consequently, there is a need to identify cost-effective, accessible, and reliable biomarkers for the prompt identification of metabolic disorders and predicting the risk of sarcopenia.
Although no direct research has specifically explored the connection between sarcopenia and the ZJU index, a wealth of metabolic studies has uncovered links between sarcopenia and a range of contributing factors. Multiple meta-analyses have demonstrated a positive correlation between elevated TG levels and the onset of sarcopenia. In contrast, high levels of high-density lipoprotein cholesterol (HDL-C) in older adults are significantly linked to an increased risk of muscle strength decline and the development of sarcopenia [27–29]. Furthermore, various studies conducted in China and Korea with middle-aged and older adults have revealed an inverse relationship between the TG/HDL-C ratio and sarcopenia [30–32], and this index is negatively associated with grip strength [33]. Sarcopenia correlates with diminished glucose disposal at skeletal muscle sites [34]. During the euglycemic hyperinsulinemic clamp experiments, skeletal muscle is responsible for roughly 80% of glucose uptake [35]. It acts as a protective buffer against hyperglycemia following a glucose intake, notably during the post-prandial phase under normal physiological conditions [36]. The preservation of skeletal muscle mass is crucial for preventing the onset of prediabetes and its progression to type 2 diabetes since robust, insulin-sensitive skeletal muscle plays a vital role in managing glucose disposal [37]. The loss of muscle mass can lead to insulin resistance, a significant risk factor for NAFLD [38]. Several common pathogenetic mechanisms, such as insulin resistance, hormonal imbalances, systemic inflammation, dysregulation of myostatin and adiponectin, nutritional deficiencies, and physical inactivity, link sarcopenia and NAFLD. These shared factors suggest a bidirectional relationship between sarcopenia and NAFLD [39]. Developed initially to identify NAFLD [12], the ZJU index has also proven highly effective in predicting NAFLD in overweight women in Western countries, according to cohort studies [40]. While these risk factors are closely associated with sarcopenia, no study has explored the relationship between the ZJU index level and sarcopenia.
The study identified a negative association between the ZJU index and ALMBMI. It seems that a rise in the ZJU index could increase the risk of sarcopenia. Additional analyses employing RCS and threshold effects uncovered a curvilinear relationship. Suitable turning points were determined at ZJU index values of 44 and 33. It is important to note that when the ZJU index exceeded 33, each unit increase in the ZJU index resulted in an 8% increase in the risk of developing sarcopenia. Subgroup analysis indicated that the association between the ZJU index and sarcopenia varied significantly by gender. Spline curve analysis also revealed distinct risk patterns across different ZJU index values, especially between genders at higher index levels. ROC curves demonstrate that the ZJU index possesses high identification power and effective screening capability for detecting sarcopenia in both males and females. Therefore, assessing the ZJU index value to identify individuals at an early risk of sarcopenia is beneficial, as it enables the timely initiation of preventive measures and treatments before the disease develops. Blood biochemistry tests, commonly performed in clinical environments, make the ZJU index an accessible and cost-effective option for broad application. Future research should include more extensive, multicenter cohort studies to establish its efficacy in diagnosing sarcopenia further. Additionally, the molecular mechanisms associated with these findings need more in-depth investigation.
Advantages and limitations
The primary advantage of our study lies in its emphasis on the nonlinear relationship between the ZJU index and sarcopenia. Subgroup analysis highlighted significant interactions between gender and the ZJU index’s correlation with sarcopenia, prompting further investigation. Utilizing RCS, our spline curve analysis also identified distinct risk patterns across different ZJU index values, particularly notable between genders at higher levels. Moreover, our findings are credible and representative due to the extensive sample size and meticulous adjustment for covariates. However, there are several limitations to this study. Firstly, it is a cross-sectional observational study and does not establish causality between the ZJU index and sarcopenia. Secondly, the inability to access complete data that constitutes the ZJU index from other mainstream databases prevents the validation of its efficacy using other publicly available databases. This issue awaits further research in larger medical centers in the future. Additionally, although many confounding factors were adjusted based on clinical practice and previous studies, the impact of unknown variables on our findings cannot be entirely dismissed.
Conclusions
The results suggest a positive association with the increased prevalence of sarcopenia, indicating that the ZJU index could serve as a novel predictor of sarcopenia. Clinicians could promote self-regulation through health education and targeted interventions, guiding patients to maintain their body weight, care about blood glucose, control blood lipids, and examine liver function to lower the risk of sarcopenia. Further validation of these findings will require additional prospective studies, randomized controlled trials, and more research to understand the underlying mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the staff at the National Center for Health Statistics, part of the Centers for Disease Control, for their valuable work in designing, collecting, and compiling the NHANES data, as well as for creating and maintaining the public database.
Abbreviations
- GLIS
Global Leadership Initiative in Sarcopenia
- OSA
Obstructive sleep apnea
- ALM
Appendicular lean mass
- DEXA
Dual-energy x-ray absorptiometry
- BMI
Body mass index
- TG
Triglycerides
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- FBG
Fasting Blood Glucose
- NAFLD
Non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- ALMBMI
Appendicular lean mass to body mass index ratio
- PA
Physical activity
- FNIH
Foundation for the National Institutes of Health
- PIR
Poverty-income ratio
- RCS
Restricted cubic splines
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- HDL-C
High-density lipoprotein cholesterol
Author contributions
J.-Q.H. collected the data. J.-W.Z. and M-R X analyzed and interpreted. J.-Q.H and S.-Y.H. wrote the main manuscript text. J.-Q.H., Z.-X.Z., and R.W. designed the study. M.-J.W. and W.Z. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Research Projects of the Department of Science and Technology of Sichuan Province, China, under Grant NO.2022YFS0164, 2023YFS0176.
Data availability
The NHANES data used in this study are publicly available and can be accessed through the following link: https://www.cdc.gov/nchs/nhanes. Additionally, the datasets analyzed in this study can be obtained from the corresponding author upon reasonable request.
Declarations
Institutional review board
The study followed the ethical guidelines outlined in the Declaration of Helsinki and was approved by the National Center for Health Statistics Institutional Review Board.
Informed consent
All participants provided informed consent before enrollment.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis, and treatment: consensus is growing. Age Ageing. 2022;51(10). [DOI] [PMC free article] [PubMed]
- 2.Golabi P, Gerber L, Paik JM, Deshpande R, de Avila L, Younossi ZM. Contribution of Sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Reports: Innov Hepatol. 2020;2(6):100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk B, Cawthon PM, Arai H, Ávila-Funes JA, Barazzoni R, Bhasin S et al. The conceptual definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing. 2024;53(3). [DOI] [PMC free article] [PubMed]
- 4.Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou SK, Papadimitriou K, Voulgaridou G, Georgaki E, Tsotidou E, Zantidou O et al. Exercise and Nutrition Impact on osteoporosis and Sarcopenia-the incidence of Osteosarcopenia: a narrative review. Nutrients. 2021;13(12). [DOI] [PMC free article] [PubMed]
- 6.Xiang S, Li Y, Li Y, Zhang J, Pan W, Lu Y, et al. Increased Dietary Niacin Intake improves muscle strength, Quality, and glucose homeostasis in adults over 40 years of age. J Nutr Health Aging. 2023;27(9):709–18. [DOI] [PubMed] [Google Scholar]
- 7.Qiao YS, Chai YH, Gong HJ, Zhuldyz Z, Stehouwer CDA, Zhou JB, et al. The Association between Diabetes Mellitus and Risk of Sarcopenia: accumulated evidences from Observational studies. Front Endocrinol. 2021;12:782391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao X, Niu R, Lu W, Zeng X, Sun X, Liu C. Obstructive sleep apnea (OSA) is associated with increased risk of early-onset sarcopenia and sarcopenic obesity: Results from NHANES 2015–2018. International journal of obesity (2005). 2024;48(6):891-9. [DOI] [PubMed]
- 9.Xu J, Han X, Chen Q, Cai M, Tian J, Yan Z, et al. Association between Sarcopenia and prediabetes among non-elderly US adults. J Endocrinol Investig. 2023;46(9):1815–24. [DOI] [PubMed] [Google Scholar]
- 10.Cai X, Hu J, Wang M, Wen W, Wang J, Yang W, et al. Association between the Sarcopenia index and the risk of stroke in elderly patients with hypertension: a cohort study. Aging. 2023;15(6):2005–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(5):547 – 58. [DOI] [PMC free article] [PubMed]
- 12.Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci Rep. 2015;5:16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Nie G, Yan F, Zhou N, Zhang M, Peng W. The ZJU index is associated with the risk of obstructive sleep apnea syndrome in Chinese middle-aged and older people: a cross-sectional study. Lipids Health Dis. 2023;22(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H, Liu B, Luo X, Shen X, Sun J, Zhang A. Non-alcoholic fatty liver disease risk prediction model and health management strategies for older Chinese adults: a cross-sectional study. Lipids Health Dis. 2023;22(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai KZ, Chu CC, Huang WC, Sui X, Lavie CJ, Lin GM. Prediction of various insulin resistance indices for the risk of hypertension among military young adults: the CHIEF cohort study, 2014–2020. Cardiovasc Diabetol. 2024;23(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harring M, Golabi P, Paik JM, Shah D, Racila A, Cable R, et al. Sarcopenia among patients with nonalcoholic fatty liver Disease (NAFLD) is Associated with Advanced Fibrosis. Clinical gastroenterology and hepatology: the official clinical practice. J Am Gastroenterological Association. 2023;21(11):2876–e885. [DOI] [PubMed] [Google Scholar]
- 17.Lipina C, Hundal HS. Lipid modulation of skeletal muscle mass and function. J cachexia Sarcopenia Muscle. 2017;8(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao JQ, Zhuang ZX, Hu SY, Zhang YJ, Zhang JW, He FJ, et al. The association between non–high–density lipoprotein cholesterol to high–density lipoprotein cholesterol ratio (NHHR) and low muscle mass in adults aged 20–59: a population-based study in the United States. Lipids Health Dis. 2024;23(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei L, Li J, Wang W, Yu Y, Pu B, Peng Y, et al. The associations of weekend warrior and regularly active physical activity with abdominal and general adiposity in US adults. Obes (Silver Spring Md). 2024;32(4):822–33. [DOI] [PubMed] [Google Scholar]
- 21.Sayer AA, Cooper R, Arai H, Cawthon PM, Ntsama Essomba MJ, Fielding RA, et al. Sarcopenia Nat Reviews Disease Primers. 2024;10(1):68. [DOI] [PubMed] [Google Scholar]
- 22.Kirk B, Mooney K, Vogrin S, Jackson M, Duque G, Khaiyat O, et al. Leucine-enriched whey protein supplementation, resistance-based exercise, and cardiometabolic health in older adults: a randomized controlled trial. J cachexia Sarcopenia Muscle. 2021;12(6):2022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos International: J Established as Result Cooperation between Eur Foundation Osteoporos Natl Osteoporos Foundation USA. 2017;28(10):2781–90. [DOI] [PubMed]
- 24.Al Saedi A, Debruin DA, Hayes A, Hamrick M. Lipid metabolism in Sarcopenia. Bone. 2022;164:116539. [DOI] [PubMed] [Google Scholar]
- 25.Kadoguchi T, Shimada K, Miyazaki T, Kitamura K, Kunimoto M, Aikawa T, et al. Promotion of oxidative stress is associated with mitochondrial dysfunction and muscle atrophy in aging mice. Geriatr Gerontol Int. 2020;20(1):78–84. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Jentoft AJ, Sayer AA, Sarcopenia. Lancet (London England). 2019;393(10191):2636–46. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Oh C, No J. Associations between Sarcopenia and metabolic risk factors: a systematic review and Meta-analysis. J Obes Metabolic Syndrome. 2018;27(3):175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi B, Dong X, Yan M, Zhao Z, Liu R, Li S, et al. Dyslipidemia is associated with sarcopenia of the elderly: a meta-analysis. BMC Geriatr. 2024;24(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua N, Qin C, Wu F, Wang A, Chen J, Zhang Q. High-density lipoprotein cholesterol level and risk of muscle strength decline and sarcopenia in older adults. Clinical nutrition (Edinburgh. Scotland). 2024;43(10):2289–95. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Zhong S, Sun Z. Association between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia among elderly patients with diabetes: a secondary data analysis of the China Health and Retirement Longitudinal Study. BMJ open. 2023;13(8):e075311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Chen M, Fang D. Relationship between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia occurrence rate in community-dwelling Chinese adults. Lipids Health Dis. 2020;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung TH, Kwon YJ, Shim JY, Lee YJ. Association between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia in elderly Korean males: The Korean National Health and Nutrition Examination Survey. Clinica chimica acta; international journal of clinical chemistry. 2016;463:165-8. [DOI] [PubMed]
- 33.Huang Y, Liao J, Liu Y. Triglyceride to high-density lipoprotein cholesterol ratio was negatively associated with relative grip strength in older adults: a cross-sectional study of the NHANES database. Front Public Health. 2023;11:1222636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2019;12:1057–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Hu T, Shen Y, Wang Y, Bao Y, Ma X. Association of skeletal muscle mass and its change with diabetes occurrence: a population-based cohort study. Diabetol Metab Syndr. 2023;15(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–903. [DOI] [PubMed] [Google Scholar]
- 37.Lisco G, Disoteo OE, De Tullio A, De Geronimo V, Giagulli VA, Monzani F et al. Sarcopenia and Diabetes: a detrimental Liaison of advancing age. Nutrients. 2023;16(1). [DOI] [PMC free article] [PubMed]
- 38.Kim JA, Choi KM. Sarcopenia and fatty liver disease. Hep Intl. 2019;13(6):674–87. [DOI] [PubMed] [Google Scholar]
- 39.Joo SK, Kim W. Interaction between Sarcopenia and nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29(Suppl):S68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu CP, Ali H, Rachakonda VP, Oczypok EA, DeLany JP, Kershaw EE. The ZJU index is a powerful surrogate marker for NAFLD in severely obese north American women. PLoS ONE. 2019;14(11):e0224942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NHANES data used in this study are publicly available and can be accessed through the following link: https://www.cdc.gov/nchs/nhanes. Additionally, the datasets analyzed in this study can be obtained from the corresponding author upon reasonable request.