ABSTRACT
Human immune system (HIS) mice are created by transplanting human immune cells or their progenitor cells into highly immunodeficient recipient mouse hosts, thereby “humanizing” their immune systems. Over past decades, the field of HIS mice has evolved rapidly, as modifications of existing immunodeficient mouse strains have been developed, resulting in increasing levels of human tissue engraftment as humanization is optimized. Current HIS mouse models not only permit elevated levels of human cell engraftment but also demonstrate graft stability. As such, HIS mice are being extensively used to study the human innate and adaptive immune response against microbial infections in vivo. Compared to nonhumanized animal models, which are frequently infected with surrogate or adapted microbes, the HIS mouse models allow the analysis of interactions between human immune cells and bona fide pathogenic microbes, making them a more clinically relevant model. This article reviews the development of HIS mice and covers the different strategies used to humanize mice, as well as discussing the use of HIS mice for studying bacterial infections that cause human disease.
DEVELOPMENT OF IMMUNODEFICIENT MOUSE STRAINS
Immunocompetent mice harbor several layers of immune defense that can promote rejection of cell and tissue xenografts; these include innate mechanisms (mediated by complement, macrophage, and neutrophils) as well as adaptive immune responses (T cell-mediated and antibody-mediated rejection). In addition, resident tissues in the mouse harbor self-renewing stem cells and their differentiated progeny, which can effectively compete with human cells for endogenous tissue resources (physical space, nutrients, growth factors, etc.) that may play a role in sustaining human xenografts. As such, the development of “humanized” mouse models (human immune system [HIS] mice) is closely associated with the history of mutant mouse strains that harbor defects in hematopoietic system development and function. In recent decades, knowledge of the molecular mechanisms that regulate innate and adaptive immunity has led to the development of mouse models with an ever-increasing capacity to engraft human cells and tissues (reviewed in references 1–4). With respect to engraftment of the human hematopoietic system, as the severity of the immune deficiency in the mouse host has increased, the efficiency and durability of human hematopoietic cell “take” have improved remarkably, and importantly, a diverse compartment comprising many unique human immune subsets (including not only lymphocytes but also myeloid cells) has been achieved (Fig. 1).
FIGURE 1.
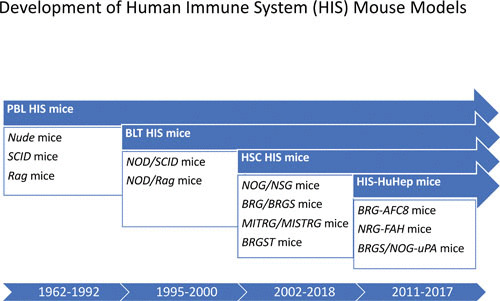
Timeline for development of immunodeficient mouse strains that form the basis for current HIS models. Indicated strains are described in the text.
Mice Deficient in Adaptive Lymphocytes (T and B Cells)
In 1962, a spontaneous mutation in mice causing hair loss (nude) was discovered; nude mice were also remarkable for the absence of a thymus (5). It was later shown that nude mice harbor a mutation in Foxn1, which encodes a transcription factor that is essential for thymic epithelial function; its absence results in a complete block in T cell development (6). The T cell immunodeficiency in nude-bearing mice allowed the earliest studies of patient-derived tumor xenografts (7). Nevertheless, other immune mechanisms were still operative in this strain, as nude mice were not able to support reconstitution of mononuclear cells from human bone marrow even after lethal irradiation (8).
In 1983, a spontaneous mutation was identified in mice that was involved in DNA repair and that severely affected development of B and T cells (9). This mutation was designated scid (for severe combined immune deficiency), and SCID mice were shown to carry a mutation in the gene Prkdc (protein kinase, DNA-activated, catalytic polypeptide), encoding a kinase which plays a critical role in nonhomologous end joining during double-strand-break repair. The B and T cell immunodeficiency in SCID mice arises from the inability of these mice to perform VDJ recombination to generate mature T and B lymphocytes (10); as a result, SCID mice lack both cell-mediated (T cell) and humoral (B cell and antibody) responses. Moreover, the defect in DNA repair that SCID mice manifest is not restricted to the adaptive immune system but is a generalized defect in DNA repair that affects all somatic cells (11).
Curiously, the Prkdc mutation in SCID mice appears to be leaky, meaning that in some SCID mutant mice, a low level of VDJ recombination can be detected, leading to a residual level of B and T cell differentiation (12). As such, a fraction of SCID mice show some immune competence and can elicit immune responses against various types of immunogens. In addition, leaky SCID mice have an increased incidence of thymic lymphoma formation, which unfortunately limits the use of SCID mice for long-term transplantation studies (11). Finally, due to their generalized DNA repair defects, SCID mice are highly sensitive to irradiation, and radiation doses must be carefully titrated for each colony (13).
In order to create a non-leaky SCID model, mouse strains with stable defects in T and B cell development were created by targeted mutation in the VDJ recombinase genes Rag1 and Rag2, which together catalyze double-strand breaks that initiate VDJ recombination but are not involved in general DNA repair (14, 15). Compared with Pkrdc–/– and Foxn1–/– mice, Rag-deficient strains showed similar levels of cell engraftment following injection of human hematopoietic stem cells (HSC) and peripheral blood lymphocytes (PBL), while the formation of thymic lymphomas in Rag-deficient hosts is markedly decreased compared to that in SCID-based models, as Rag–/– mice have normal irradiation sensitivity profiles (16).
Mice with Compromised Macrophage Rejection of Human Xenografts
While nude, SCID, and Rag-deficient hosts demonstrate the importance of adaptive immunity as a mechanism of xenograft rejection, studies comparing SCID mice on different host backgrounds demonstrated that additional nonadaptive immune mechanisms also affected human xenograft take. SCID mice on the nonobese diabetic (NOD) background were superior hosts for human cell transplantation compared with SCID mice on the C57BL/6 background (17). A seminal report published in 2007 illuminated the biology behind these differences (18). Signal regulatory protein α (Sirpa) is an inhibitory transmembrane receptor, mainly expressed on myeloid cells and macrophages. The ligand for Sirpa is the glycoprotein CD47, which is highly expressed by immune cells. Importantly, CD47 interaction with Sirpa delivers an inhibitory signal (“don’t eat me” signal) to Sirpa-expressing macrophages. In NOD mice, the Sirpa allele differs from the one expressed in C57BL/6 mice. Takenaka et al. showed that the SirpaNOD allele could interact with human CD47, whereas SirpaB6 could not (18). As a result, macrophages (and also myeloid cells) in NOD-based mice are more tolerant of human CD47-expressing cells, whereas C57BL/6 macrophages rapidly eliminate human cells via phagocytosis.
By extensive backcrossing of the scid and Rag mutations on the NOD background, novel immunodeficient mouse strains harboring the congenic SirpaNOD allele were developed that showed enhanced levels of human immune cell engraftment following transfer of PBL or CD34+ HSC. Due to these properties, NOD/SCID and NOD/Rag mouse models rapidly became the gold standard for studies investigating in vivo human HSC biology in humanized mice (16, 17). Nevertheless, the immune subsets that differentiated from injected human HSC in NOD/SCID mice consisted mostly of transitional B cells, whereas T cell development was still largely underrepresented. As such, these first-generation NOD/SCID strains were not adequate models to study human innate and adaptive immune responses in vivo.
Mice with Deficiencies in Endogenous Lymphocyte Precursors
Engrafted human HSC generate hematopoietic precursor cells that must take up residence in primary lymphoid organs of the mouse (bone marrow and thymus), expand, and differentiate into mature human lymphocytes before exiting to seed peripheral lymphoid organs for immune responses. As mentioned above, human hematopoietic precursors will in many cases have to compete with endogenous mouse hematolymphoid precursors for niches that provide resources for survival and growth. Immunodeficient mice described thus far have defects in mature B and T cells, but the mutations (Rag and Prkdc) do not perturb early lymphoid precursor development or homeostasis, which depends on cytokines and other growth factors.
The common cytokine receptor γ chain (γc, encoded at the locus Il2rg) is a receptor subunit shared by interleukin 2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 receptors. IL2RG is mutated in human X-linked SCID, a disease characterized by an absence of T cells and NK cells (19). Il2rg–/– mice have multiple defects in immune development, with few mature B or T cells and a complete absence of NK cells (20). Importantly, these immune phenotypes have been traced to the role of γc as a survival and expansion factor for early lymphoid precursors. As such, Il2rg–/– mice have severely depleted NK, B, and T cell precursors in the thymus and bone marrow (21).
The development of Il2rg–/– mice spawned a new series of immunodeficient mouse models that incorporated mutations in this shared cytokine receptor (22–25). These include NOD/SCID/Il2rg (NSG or NOG), NOD/Rag/Il2rg (NRG), BALB/c Rag/Il2rg (BRG), and BALB/c Rag2/Il2rg/SirpaNOD (BRGS) mice. Quite unexpectedly, mice with these different Il2rg-based immunodeficiencies showed robust development of human T cells after HSC engraftment that was achieved in situ in the residual mouse thymus (22–25). Human thymopoiesis that was achieved in NSG or BRG mice injected with human CD34+ HSC was remarkably similar to that observed in human thymus, and mature T cells that developed in NSG- and BRG-based HIS mice appeared diverse in their T cell-receptor repertoire and in their capacity to be stimulated via the T cell receptor (26, 27), suggesting that they had undergone a normal process of differentiation. These “second-generation” Il2rg-based HIS models (NSG, NOG, and BRGS) rapidly became the models of choice for studies of human immune responses, as both cell-mediated (T cell) and humoral (B cell) immunity could be elicited following infection or immunization (reviewed in references 1–4). The basis for the improved human thymopoiesis in Il2rg-based HIS mice is not fully understood, but it could relate to the absence of competition with mouse lymphoid precursors and/or to the absence of NK cell-mediated xenograft rejection.
Mice with Immune Deficiencies but Intact Innate Lymphoid Cell Function
Innate lymphoid cells (ILC) are a recently identified group of hematopoietic effector cells with lymphoid morphology yet lacking rearranged antigen-specific receptors. ILC include cytotoxic NK cells and diverse helper ILC subsets (ILC1, ILC2, and ILC3) that mirror cytotoxic CD8+ T lymphocytes and Th1/2/17 helper CD4 T lymphocyte subsets, respectively (reviewed in references 28 and 29). While NK cells rely on γc signals delivered through IL-2 and IL-15 for their development and maturation, helper ILC require IL-7 signals for differentiation and survival. As such, Il2rg–/– mice have a deficiency in mouse NK cells (as indicated above) but also lack all helper ILC subsets. ILC play diverse roles in immune defense, but one subset of ILC3, called lymphoid tissue inducer cells, is active during the fetal period and promotes the formation of secondary lymphoid tissues (SLT), such as peripheral lymph nodes (LN) and Peyer’s patches (30). As such, all HIS mouse models based on Il2rg deficiency (BRGS, NSG, NOG, and NRG) fail to generate SLT. The absence of SLT in BRGS/NSG/NOG/NRG HIS mice likely impacts immune performance in these models, since SLT are known to orchestrate and promote effective T and B cell responses.
Very recently, a solution to the SLT deficiency in Il2rg-based HIS mouse models was reported (31). Thymic stroma-derived lymphopoietin (TSLP) is an IL-7-like protein that binds the IL-7 receptor but does not require γc for its function (32, 33). TSLP supplementation was able to rescue lymphoid tissue inducer cell function in Il2rg–/– mice and restored LN development in BRGS-based HIS mice (31). This novel BRGS-TSLP (BRGST) HIS mouse model should find many applications aimed at dissecting the role of LN in human immune and infection-related pathologies.
Human Cytokine and Growth Factor Replacement Mice
Despite high and sustainable human hematopoietic cell engraftment in BRG/NSG/NOG/NRG-based HIS mice, the overall composition of human immune subsets remains biased, with prominent T and B lymphocyte development. In contrast, innate lymphocytes (NK cells and ILC), myeloid lineages (neutrophils, eosinophils, and basophils), and monocytes/macrophages are underrepresented in most HIS models (reviewed in references 1–4). Part of the reason for this unbalanced hematopoiesis relates to the fact that several mouse cytokines (including macrophage colony-stimulating factor [M-CSF], granulocyte-macrophage CSF [GM-CSF], IL-3, thrombopoietin, IL-15, and to a lesser extent IL-7) trigger only partially or fail to trigger their corresponding receptors on human hematopoietic target cells. Several studies have reported that injection of human recombinant cytokines, hydrodynamic injection of plasmids expressing human cytokines, or “replacement” of coding exons for mouse cytokines with human counterparts can alleviate this issue and result in enhanced production of human innate lymphocytes (IL-2 and IL-15), dendritic cells (DC) (Flt3L, GM-CSF, and IL-4), and/or macrophages (M-CSF, GM-CSF, and IL-3) (34–39) (Fig. 2).
FIGURE 2.
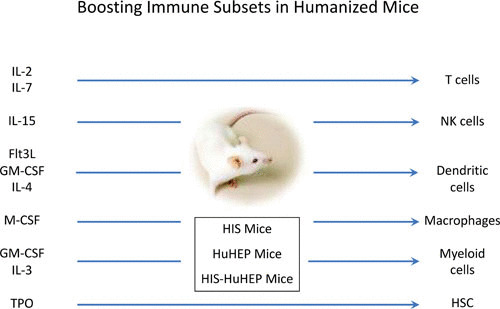
Boosting immune subsets in humanized mice. Cytokines and growth factor supplementation (left) in HIS mice can promote the expansion, differentiation, and function of selected hematopoietic lineages (right). TPO, thrombopoietin.
Combinations of several human cytokines can have dramatic additive effects, leading to almost complete humanization of the mouse bone marrow. For example, MITRG and MISTRG mice, in which human M-CSF-, IL-3-, GM-CSF-, and thrombopoietin-coding exons are inserted as knock-in alleles on the BRG background, have multilineage human myeloid and monocyte/macrophage development, and strong innate immune responses can be elicited (40). The boost in human myelopoiesis, however, comes at a price, as the expressed human cytokines do not stimulate mouse hematopoietic precursors, resulting in defects in mouse hematopoietic progenitors and phagocytic cells. Subsequent reconstitution of human HSC in these mice shows an increase in human monocytes and macrophages but a decrease in mouse life span due to anemia caused by enhanced phagocytosis of mouse red blood cells (RBC) by human macrophages (40).
APPROACHES TO GENERATE HIS MICE
Mouse models have added considerably to our understanding of pathogenesis and have contributed to the development of numerous prophylactic and therapeutic medications for these devastating diseases. It is generally accepted that animal models (mice as well as other species) will continue to advance our knowledge in this area. Still, the human and mouse immune systems began to diverge roughly 65 million years ago, and since then, significant differences in the structure and function of immune receptors, soluble factors, and signaling pathways have occurred during evolution (reviewed in reference 41). Certain pathogens exhibit unique tropism for humans but not for mice, while many pathogens display distinctly different disease progression and severity in human and mouse models. Such differences can limit the value of mice as preclinical models for certain human infectious diseases. Humanized mice can bypass some of these limitations, and in this regard, accurate modeling of human-specific pathogenesis in vivo has been a driving force in the development of improved HIS mouse models. Several humanization strategies for creating HIS mice are available and are described in detail below. As immune responses to infections result from key encounters with specific cell types within the hematopoietic system (antigen-presenting cells, T cell subsets, etc.), putting in place the appropriate HIS mouse model should be carefully considered.
Creating HIS Mice following Engraftment of Human CD34+ HSC
In order to recapitulate the entire developmental range of human hematopoietic elements in HIS mice, transfer of human CD34+ HSC is required. The sources of human CD34+ HSC may include fetal liver, cord blood, and adult bone marrow-derived cells. These multipotent, self-renewing progenitors are commonly injected into sublethally irradiated BRG/NSG/NOG/NRG recipients (newborn or adult mice) via intracardiac or intrahepatic injection. After a period of 8 to 14 weeks, human hematopoietic cells can be detected in tissues and in the circulation. Both adult and newborn mice allow persistent multilineage human hematopoietic engraftment (lasting up to 1 year), but use of newborn pups as hosts is preferred, as T cell development in pups appears to be more robust than that in adults.
The main advantage of the HSC-derived HIS mouse model is its simplicity; only a single injection of CD34+ HSC into an appropriately conditioned (irradiated) host is required. Since the human T cells develop in the context of the mouse thymus, they are “educated” (tolerant) to mouse tissues but remain reactive to foreign antigens. Still, some deficiencies in human immune responses are apparent in HSC-derived HIS mice. While both B and T cells develop, the dynamics are different, with B cells arising after 6 weeks, while T cells require around 12 weeks to emerge from the thymus. As such, T/B cooperation is suboptimal. In this type of HIS model, T cells are selected on the mouse major histocompatibility complex in the thymus, which may explain the delayed T-cell-developmental kinetics. Along these lines, BRGS/NSG/NOG strains that express human HLA-A2, DR2, and DR4 transgenes have been developed and show improved generation of CD4+ and CD8+ T cells, more rapid T cell emergence, and higher levels of antigen-specific T cell responses (42, 43; Di Santo, manuscript in preparation).
As detailed below, CD34+ HSC HIS mice have been used to study many different types of infectious agents that cause human disease, including viral (HIV, Epstein-Barr virus, cytomegalovirus, and Dengue virus), bacterial (Salmonella enterica serovar Typhi, Mycobacterium tuberculosis, Mycobacterium bovis bacille Calmette-Guérin [BCG], Staphylococcus aureus, and others), and parasitic (Plasmodium falciparum and Leishmania major) infections (reviewed in references 1–4 and 44) (Fig. 3). For example, HIV infection in HSC-based HIS mice leads to preferential depletion of CD4+ T cells and activation of CD8+ T cells, similar to that found in primo-infected patients (reviewed in references 45–47). HIV-infected HIS mice can be treated with highly active antiretroviral therapy (HAART) to suppress HIV replication with the formation of latent viral reservoirs; interruption of HAART leads to a viral rebound in HIS mice similar to that observed in clinics with HAART-treated HIV patients. These studies show the utility of HIS-based mouse models for understanding HIV pathogenesis and for establishing novel therapeutic approaches for eliminating viral reservoirs (HIV “cure”).
FIGURE 3.
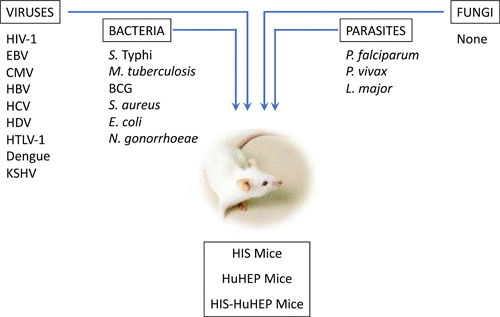
Studying human pathogens in humanized mice. A variety of human pathogens, including viruses, bacteria, and parasites, have been analyzed in HIS mouse models. EBV, Epstein-Barr virus; CMV, cytomegalovirus; HTLV-1, human T cell leukemia virus type 1; KSHV, Kaposi’s sarcoma-associated herpesvirus.
Improving the quality and breadth of human innate and adaptive immune responses in HIS mice remains a constant challenge. Despite the abundance of mature B and T cells, HIS mice show poor antibody responses, in part due to lack of SLT, as noted above. B cells in immunized or infected HIS mice fail to demonstrate appreciable levels of somatic hypermutation in their Ig genes, suggesting that germinal center reactions are suboptimal (reviewed in references 2, 4, and 44)). Providing additional B cell factors (IL-6, BAFF, CXCL13, etc.) (31, 48, 49) in combination and in the context of an appropriate SLT (31) may allow this issue to be resolved.
Creating HIS Mice following Engraftment of Human Fetal Liver, Fetal Thymus, and CD34+ HSC (BLT Mice)
Mucosal tissues (including the gut, lung, skin, and urinary and reproductive tracts), are portals of entry for pathogens. At these sites, strategically placed sentinel cells (epithelial cells, antigen-presenting cells, and macrophages) are targets of infection and/or capture infected cells to initiate immune responses. Innate and adaptive lymphocytes are abundant in mucosal surfaces and include B cells, T cells, NK cells, and various ILC subsets (28, 29). Some viral infections (HIV-1) rely on active replication within the mucosal immune system and form latent reservoirs in this tissue under HAART. Having the capacity to model this aspect of HIV replication in HIS mice may lead to new approaches to target mucosal immunity to a variety of human pathogens.
Mucosal immune system development is essentially absent in HSC-based HIS mice; the reasons for this are not clear but may relate to poor induction of gut-specific homing receptors that guide lymphocytes into these sites (50). In contrast, another technique to generate HIS mice using coengraftment of fetal liver and thymus fragments under kidney capsules of NOD/SCID mice followed by CD34+ HSC injection (called BLT mice, for “bone marrow, liver, thymus”) results in abundant T cell reconstitution of mucosal tissues (51). The implanted fetal thymus and liver fragment in BLT mice provide autologous thymic epithelium to facilitate HLA-restricted thymocyte development. Moreover, the use of the NOD/SCID strain, in which peripheral lymph node and Peyer’s patch anlagen exist, allows the reconstitution of SLT (52). As such, BLT mice show strong antigen-specific HLA-restricted T cell responses.
The robust mucosal engraftment of human T cells in BLT mice has allowed several important studies on HIV transmission mechanisms (saliva and breast milk) and for prophylactic prevention strategies (reviewed in references 45–47). Still, the BLT HIS model has several limitations, including the need for access to fetal tissues and special technical and surgical skills to engineer these mice. Moreover, BLT HIS mice have been reported to have a shorter life span than other HIS models, possibly related to the development of a xenograft-versus-host disease (xeno-GVHD) mediated by human T cells (53). Nevertheless, BLT mice represent an important model for studying T cell immunity against a variety of human pathogens.
Creating HIS Mice Following Engraftment of Human PBL
One of the earliest versions of HIS mice involved transfer of PBL to SCID mice (54). This SCID-PBL HIS model was widely used prior to the advent of Il2rg-based immunodeficient hosts and has the advantage that large cohorts of HIS mice can be generated in a very short time frame (weeks). In this model, small numbers of adult PBL (5 to 10 million cells) are injected into NOD/SCID recipients; irradiation is not necessary, although it can accelerate the kinetics of humanization. While normal PBL contain several hematopoietic lineages (B cells, T cells, DC, NK cells, neutrophils, etc.), the predominant cell types that expand in this context are mature T cells (both CD4+ and CD8+) (55). Over a period of 2 months, T cell expansion occurs, generating a large population of activated and memory T cells that can be studied in the context of infection or immunization (56). Adoptive transfer of antigen-primed DC into SCID-PBL HIS mice can also be used to study recall responses to previous vaccines or immunogens (57); these approaches have utility and can be used to monitor pathogen exposure in individuals.
The T cell expansion in this model is driven largely by the sensing of xenodeterminants in the mouse (primarily major histocompatibility complex molecules) by mature human T cells. This process bears some similarity to GVHDs that are T cell mediated and occur in humans following tissue or cell transplantation, and as such, SCID-PBL HIS mice have been used as a model to study some of the immune mechanisms that operate in human GVHD (58). Still, the intensity of xeno-GVHD in this model prevents long-term studies, and the resultant systemic inflammatory reaction makes interpretation of results difficult. Modifications of BRG/NSG/NOG mice to eliminate expression of murine class I and class II molecules allows prolonged survival of HIS-PBL mice (58, 59) and may open new avenues of research.
A special type of HIS-PBL mice can be generated to study human erythrocyte biology. Human RBC develop poorly in almost all HIS models due to the inability of mouse erythropoietin to trigger human erythrocytic precursor cells and removal by mouse macrophages (60, 61). In contrast, adoptive transfer of human circulating RBC to macrophage-depleted immunodeficient mice can establish a short-lived pool of human RBC that can then be studied as target for infection by malaria parasites, including P. falciparum (62). This RBC transfer approach in HIS mice can be additionally performed in other HIS contexts (CD34+ HSC HIS, BLT HIS, etc.) to create more complex systems for studies involving immunity to malaria parasites.
Creating “Dual” HIS Mice Harboring Additional Nonhematopoietic Tissues
The HIS models described above provide a means to study a multitude of pathogens that directly target human hematopoietic cells. However, several major human diseases result from infection of nonhematopoietic target cells with tissue tropism requirements that are not met by the analogous murine tissue. For example, hepatitis B and C viruses (HBV and HCV) infect human (and some primate) hepatocytes but not mouse hepatocytes (63). In order to create a mouse model for studying human hepatitis virus infection and pathology, genetic engineering of immunodeficient mice was performed to allow humanization of the mouse liver. Several models, including albumin promoter-driven urokinase plasminogen activator (64, 65), albumin-driven thymidine kinase (66), fumarylacetoacetate hydrolase deficiency (Fah–/–) (67, 68), and inducible activation of hepatocyte-restricted death-signaling pathways (AFC8) (69), resulted in strong selective pressure against mouse hepatocytes, thereby creating a niche for engraftment of human hepatocytes in mice. By combining these human hepatocyte (HuHEP) mice with existing HIS models, doubly humanized mice bearing both human immune systems and human hepatocytes have been obtained (reviewed in reference 70). HIS-HuHEP mice have been shown to be susceptible to infection by HBV and HCV as well as P. falciparum sporozoites, and human immune responses against HBV have been demonstrated that restrict viral replication and spread (70–72). The HIS-HuHEP model is just one example of how multitissue-humanized mice can be generated to address particular aspects of human infections and to provide valuable insights into the role of human immunity in disease progression. As other human tissues can be engrafted in immunodeficient hosts (skin, gut, muscle, fat, etc.), one can envisage ever-more-complex humanized mouse models that can recapitulate the multitissue nature of human disease (following infection but also involving inflammation, autoimmunity, and metabolic stress).
USING HUMAN IMMUNE SYSTEM MICE TO UNDERSTAND THE BIOLOGY OF BACTERIAL INFECTIONS
In this final section, we present some examples of how HIS mouse models can provide an opportunity to study bacterial infections that cause human disease (Fig. 3). Although these studies are somewhat limited in number, they provide evidence for the utility of HIS mice and also suggest avenues for improvements of these models.
Infection of HIS Mice with S. Typhi
Typhoid fever is a life-threatening human disease caused by S. Typhi. Because of the lack of effective vaccine and the emergence of multidrug-resistant S. Typhi strains, this pathogen presents a serious potential threat to global health. The understanding of S. Typhi pathogenesis has been impeded by the lack of clinically relevant animal models, as this bacterium exclusively infects humans and does not cause any obvious disease in most laboratory strains of mice. Interestingly, intravenous or intraperitoneal S. Typhi infection of CD34+ HSC-engrafted HIS mice showed cardinal clinical features of human typhoid fever, including fever, increased inflammatory cytokines, neurological signs (meningitis), and high mortality (73–75). Increased bacterial burdens in the livers and spleens of HIS mice suggest replication of S. Typhi. Importantly, infection of nonhumanized BRG and NSG host strains showed no disease, demonstrating an obligate role for human hematopoietic cells in this process.
These results show the utility of HIS mice as a novel and valuable tool to investigate the pathological mechanisms of S. Typhi infection and to characterize the immune responses of typhoid fever. Still, there appears to be some variability in the severity, kinetics, and clinical symptoms of typhoid fever reported from three independent published studies with HIS mice (73–75). This may reflect differences in the immune reactivity of the different human HSC donors or other experimental variables (such as mouse genetic background and infection routes). Moreover, the natural route of human S. Typhi infection is via the digestive tract, whereas studies using HIS mice used intravenous or intraperitoneal routes of infection (76).
Infection of HIS Mice with Mycobacteria
M. tuberculosis infection results in more than 1.4 million deaths per year and is the primary cause of death for HIV-infected patients. Reports of multi-drug-resistant strains of M. tuberculosis are on the rise, adding urgency to the need to find new therapeutic approaches to restrain or eliminate persistent M. tuberculosis infection. While mouse models of M. tuberculosis infection have largely contributed to our understanding of host-pathogen interactions, the inability of M. tuberculosis-infected mice to develop latent infections characterized by the formation of organized granulomas (macrophage cores ringed by lymphocytes) has impeded research on this disease (77, 78).
Advances in the development of macrophage and myeloid cell development in HIS mice offered hope that new relevant animal models of M. tuberculosis infection might be on the horizon. This appears to be the case, as CD34+ HSC-based HIS mice were recently found to form granuloma-type structures in liver and lung after intravenous injection of M. tuberculosis or BCG vaccine (79). As was the case for S. Typhi infection, granuloma formation was not observed in nonreconstituted NSG mice, implying a specific role for human hematopoietic cells in this process.
Latent M. tuberculosis infection was not observed in this system, but rather, HIS mice harbored increased numbers of mycobacteria in several organs compared to nonhumanized NSG mice; this result was apparently mediated by human CD4+ T cells (79). Enhanced macrophage reconstitution in HIS mice following supplementation of human M-CSF resulted in better control of BCG infection (34). Progressive infection was also observed in BLT-based HIS mice after intranasal M. tuberculosis infection (80) and in NSG-HLA-A2 HIS mice after intravenous BCG administration (81). These studies suggest that improved HLA-restricted adaptive T cell responses are not sufficient to contain the active M. tuberculosis infection and may even promote infection. Finally, a report of HIV-1–M. tuberculosis coinfection using BLT-based HIS mice found that CD4+ T cell depletion and CD8+ T cell activation following HIV-1 infection exacerbated pulmonary M. tuberculosis infection, resulting in more severe lung pathology and increased mycobacterial dissemination (82). The similar findings of enhanced M. tuberculosis infection in the context of these two different T cell activation systems in HIS mice suggest a common cellular mechanism that operates to control mycobacterial burden in vivo.
Infection of HIS Mice with Staphylococcus aureus
S. aureus is a commensal organism with minor representation within human skin and nasopharynx microbiotas that is normally well controlled by the immune system. However, S. aureus is also a dangerous pathogen that can cause life-threatening skin infection, pneumonia, peritonitis, endocarditis, and frequently fatal septicemia. A more troubling fact is that the incidence of methicillin-resistant S. aureus is increasing, and vaccines targeting this infection have had little preclinical success (83–85).
Several recent studies described S. aureus infection in CD34+ HSC-based HIS mice (86–88). Comparing to uninfected NSG mice or immunocompetent mice, the presence of human immune cells in HIS mice increased the susceptibility to S. aureus infection irrespective of the inoculation routes, suggesting that virulence factors of S. aureus can appropriately target human cells. Boosting of human myeloid cells in BLT HIS mice using human IL-3 and GM-CSF transgenic hosts enhanced S. aureus infection, with a higher bacterial burden in the lung after intranasal infection (86). This increased susceptibility may be explained by the preferential targeting of S. aureus to human macrophages via a PVL-C5aR receptor interaction. Hence, these HIS models can be used to better understand human-specific virulent factors that S. aureus uses to establish infection and eventually evade immunity.
Using HIS Mice To Study Commensal Microbiota and Their Products
A diverse and resilient microbiota trains the immune system and is thought to play an important role in the prevention of autoimmunity (reviewed in reference 89). Perturbations of microbial communities following antibiotic treatment can have a profound impact on the composition and function of gut immune cells. If these changes occur at critical time windows in human development, long-lasting consequences of these events may eventually occur, leading to alterations in organ (brain) function and increased risk of developing disease (autoimmunity).
In order to study a role for commensal communities in human lymphocyte development, CD34+ HSC-based HIS mice (on the NSG background) were treated with an antibiotic cocktail to reduce the microbiota diversity. Antibiotic treatment lead to increased numbers of effector T cells and development of anti-nuclear autoantibodies (90). Notably, reduced numbers of IL-10-producing macrophage were observed in the guts of antibiotic-treated HIS mice.
Adequate integrity of the intestinal barrier is required to prevent bacterial translocation of commensal microorganisms that can provoke system inflammation. Early in the course of HIV infection, the intestinal barrier is disrupted, in part, by excessive inflammation caused by virus replication in gut T cells and the subsequent immune responses to this infection. On the other hand, dysbiosis caused by shifts in commensal communities may also impact pathogen reservoirs, allowing their activation. These two aspects of mucosal homeostasis have been explored using HIV infection in HIS mice (91, 92).
The study of the impact of intestinal barrier disruption used CD34+ HSC-based HIS mice (on the BRG background) and treatment with dextran sodium sulfate (DSS), which causes lysis of intestinal epithelial cells and promotes bacterial translocation (91). Elevated levels of lipopolysaccharide (LPS) are generated after DSS treatment and are rapidly eliminated by tissue macrophages. However, in HIV-infected HIS mice, macrophage clearance of LPS is compromised, resulting in accentuated T cell activation, which fuels viral replication and T cell loss (91). The inflammation following HIV infection apparently creates a feed-forward loop that comprises mucosal T cell homeostasis at multiple levels.
A study of Neisseria gonorrhoeae infection in HIV-infected HIS mice showed the impact of pathogen coinfection at mucosal surfaces (92). CD34+ HSC-based HIS mice (on the NSG background) were infected by HIV-1 Bal, and subsequently, N. gonorrhoeae was administered intravaginally. While systemic HIV levels were unchanged in the presence of vaginal N. gonorrhoeae, the mucosal shedding of HIV-1 was increased in N. gonorrhoeae-infected HIS mice. Although the mechanisms behind this observation remain unclear, this report suggests specific interactions between N. gonorrhoeae and HIV-1 in mucosal sites that can now be dissected using HIS mice.
Using HIS Mice To Study Sepsis
Sepsis is the leading cause of death in critically ill patients and represents a systemic inflammatory response to severe bacterial infection. Sepsis can be modeled experimentally using cecal ligation and puncture (CLP), where leakage of bacterial contents provokes a systemic septic shock syndrome in mice. One report of CLP using CD34+ HSC-based HIS mice demonstrated induced human cytokine responses and lymphocyte apoptosis (93). Severe impairment of human hematopoiesis was observed following CLP-induced sepsis or LPS administration, providing evidence for cross-tissue signaling between the gut and the bone marrow (94). Using BLT-based HIS mice, a small interfering RNA targeting high-mobility group protein 1 in human macrophages and DC reduced the cytokine storm and lymphocyte apoptosis and could rescue HIS mice from CLP-induced mortality (95). Finally, a recent study used HIS mice to model neonatal Escherichia coli sepsis and its subsequent immune response (96).
CONCLUDING REMARKS
HIS mice have substantially evolved since their conception almost 6 decades ago and now represent robust models to study human immune development and function. HIS mice can also be used to model a diverse set of human pathologies, especially those caused by pathogenic microorganisms. Advances in gene editing technologies have revolutionized our capacity to modify cellular genomes and provide a means to further refine and optimize HIS mouse models. The ability to multiplex cellular compartments in humanized mice will provide more relevant models that recapitulate the complexity of human tissues. The reliability of HIS mouse models, in terms of quality and reproducibility, suggests that these unique tools can form the basis for preclinical platforms dedicated to drug testing and therapeutic screening.
Contributor Information
Yan Li, Innate Immunity Unit, Immunology Department, Institut Pasteur, Paris, France; Inserm U1223, Paris, France.
James P. Di Santo, Innate Immunity Unit, Immunology Department, Institut Pasteur, Paris, France Inserm U1223, Paris, France.
Pascale Cossart, Institut Pasteur, Paris, France.
Craig R. Roy, Yale University School of Medicine, New Haven, Connecticut
Philippe Sansonetti, Institut Pasteur, Paris, France.
REFERENCES
- 1.Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat Rev Immunol 7:118–130 10.1038/nri2017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12:786–798 10.1038/nri3311. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito R, Takahashi T, Katano I, Ito M. 2012. Current advances in humanized mouse models. Cell Mol Immunol 9:208–214 10.1038/cmi.2012.2. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz MG. 2007. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26:537–541 10.1016/j.immuni.2007.05.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Isaacson JHC, Cattanach BM. 1962. Two new ‘hairless’ mutants—sha and Hfh11. Mouse News Lett 27:31. [Google Scholar]
- 6.Schorpp M, Hofmann M, Dear TN, Boehm T. 1997. Characterization of mouse and human nude genes. Immunogenetics 46:509–515 10.1007/s002510050312. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Fogh J, Fogh JM, Orfeo T. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59:221–226 10.1093/jnci/59.1.221. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Ganick DJ, Sarnwick RD, Shahidi NT, Manning DD. 1980. Inability of intravenously injected monocellular suspensions of human bone marrow to establish in the nude mouse. Int Arch Allergy Appl Immunol 62:330–333 10.1159/000232530. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Bosma GC, Custer RP, Bosma MJ. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527–530 10.1038/301527a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Malynn BA, Blackwell TK, Fulop GM, Rathbun GA, Furley AJ, Ferrier P, Heinke LB, Phillips RA, Yancopoulos GD, Alt FW. 1988. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell 54:453–460 10.1016/0092-8674(88)90066-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Fulop GM, Phillips RA. 1990. The scid mutation in mice causes a general defect in DNA repair. Nature 347:479–482 10.1038/347479a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Greiner DL, Hesselton RA, Shultz LD. 1998. SCID mouse models of human stem cell engraftment. Stem Cells 16:166–177 10.1002/stem.160166. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Biedermann KA, Sun JR, Giaccia AJ, Tosto LM, Brown JM. 1991. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci USA 88:1394–1397 10.1073/pnas.88.4.1394. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877 10.1016/0092-8674(92)90030-G. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Shinkai Y, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855–867 10.1016/0092-8674(92)90029-C. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Shultz LD, Lang PA, Christianson SW, Gott B, Lyons B, Umeda S, Leiter E, Hesselton R, Wagar EJ, Leif JH, Kollet O, Lapidot T, Greiner DL. 2000. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol 164:2496–2507 10.4049/jimmunol.164.5.2496. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Shultz LD, et al. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154:180–191. [PubMed] [PubMed] [Google Scholar]
- 18.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8:1313–1323 10.1038/ni1527. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. 1993. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73:147–157 10.1016/0092-8674(93)90167-O. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Di Santo JP, Müller W, Guy-Grand D, Fischer A, Rajewsky K. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA 92:377–381 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colucci F, Guy-Grand D, Wilson A, Turner M, Schweighoffer E, Tybulewicz VLJ, Di Santo JP. 2000. A new look at Syk in αβ and γδ T cell development using chimeric mice with a low competitive hematopoietic environment. J Immunol 164:5140–5145 10.4049/jimmunol.164.10.5140. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. 2002. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182 10.1182/blood-2001-12-0207. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104–107 10.1126/science.1093933. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γchainnull mice. Blood 106:1565–1573 10.1182/blood-2005-02-0516. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick-Marchand H, de Geus SJ, Pouw SM, Böhne M, Voordouw A, Weijer K, Di Santo JP, Spits H. 2011. Functional CD47/signal regulatory protein alpha (SIRPα) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci USA 108:13224–13229 10.1073/pnas.1101398108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Plet A, Weijer K, Jacques Y, Spits H, Di Santo JP. 2011. Autonomous and extrinsic regulation of thymopoiesis in human immune system (HIS) mice. Eur J Immunol 41:2883–2893 10.1002/eji.201141586. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Marodon G, Desjardins D, Mercey L, Baillou C, Parent P, Manuel M, Caux C, Bellier B, Pasqual N, Klatzmann D. 2009. High diversity of the immune repertoire in humanized NOD.SCID.γc–/– mice. Eur J Immunol 39:2136–2145 10.1002/eji.200939480. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. 2013. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149 10.1038/nri3365. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Spits H, Di Santo JP. 2011. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12:21–27 10.1038/ni.1962. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5:64–73 10.1038/ni1022. [PubMed] [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Masse-Ranson G, Garcia Z, Bruel T, Kök A, Strick-Marchand H, Jouvion G, Serafini N, Lim AI, Dusseaux M, Hieu T, Bourgade F, Toubert A, Finke D, Schwartz O, Bousso P, Mouquet H, Di Santo JP. 2018. A human immune system mouse model with robust lymph node development. Nat Methods 15:623–630 10.1038/s41592-018-0071-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 32.Verstraete K, van Schie L, Vyncke L, Bloch Y, Tavernier J, Pauwels E, Peelman F, Savvides SN. 2014. Structural basis of the proinflammatory signaling complex mediated by TSLP. Nat Struct Mol Biol 21:375–382 10.1038/nsmb.2794. [PubMed] [DOI] [PubMed] [Google Scholar]
- 33.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 192:659–670 10.1084/jem.192.5.659. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Chen Q, Zheng D, Yin L, Chionh YH, Wong LH, Tan SQ, Tan TC, Chan JK, Alonso S, Dedon PC, Lim B, Chen J. 2013. Induction of functional human macrophages from bone marrow promonocytes by M-CSF in humanized mice. J Immunol 191:3192–3199 10.4049/jimmunol.1300742. [PubMed] [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Mention JJ, Court N, Masse-Ranson G, Toubert A, Spits H, Legrand N, Corcuff E, Strick-Marchand H, Di Santo JP. 2016. A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. Eur J Immunol 46:1291–1299 10.1002/eji.201546132. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. 2009. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 206:25–34 10.1084/jem.20082013. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, He F, Kwang J, Chan JK, Chen J. 2012. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J Immunol 189:5223–5229 10.4049/jimmunol.1201789. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Auerbach W, Eynon EE, Stevens S, Manz MG, Flavell RA. 2011. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci USA 108:2390–2395 10.1073/pnas.1019682108. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Strick-Marchand H, Lim AI, Ren J, Masse-Ranson G, Dan Li, Jouvion G, Rogge L, Lucas S, Bin Li, Di Santo JP. 2017. Regulatory T cells control toxicity in a humanized model of IL-2 therapy. Nat Commun 8:1762 10.1038/s41467-017-01570-9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. 2014. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 32:364–372 10.1038/nbt.2858. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mestas J, Hughes CC. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738 10.4049/jimmunol.172.5.2731. [PubMed] [DOI] [PubMed] [Google Scholar]
- 42.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. 2010. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc Natl Acad Sci USA 107:13022–13027 10.1073/pnas.1000475107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki M, Takahashi T, Katano I, Ito R, Ito M, Harigae H, Ishii N, Sugamura K. 2012. Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/γcnull mouse. Int Immunol 24:243–252 10.1093/intimm/dxs045. [PubMed] [DOI] [PubMed] [Google Scholar]
- 44.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu Rev Pathol 12:187–215 10.1146/annurev-pathol-052016-100332. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Victor Garcia J. 2016. Humanized mice for HIV and AIDS research. Curr Opin Virol 19:56–64 10.1016/j.coviro.2016.06.010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masse-Ranson G, Mouquet H, Di Santo JP. 2018. Humanized mouse models to study pathophysiology and treatment of HIV infection. Curr Opin HIV AIDS 13:143–151 10.1097/COH.0000000000000440. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Denton PW, García JV. 2011. Humanized mouse models of HIV infection. AIDS Rev 13:135–148. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Borsotti C, Schickel JN, Zhu S, Strowig T, Eynon EE, Frleta D, Gurer C, Murphy AJ, Yancopoulos GD, Meffre E, Manz MG, Flavell RA. 2017. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood 129:959–969 10.1182/blood-2016-04-709584. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang J, Zhang B, Kelly M, Peterson JN, Barbee J, Freed BM, Di Santo JP, Matsuda JL, Torres RM, Pelanda R. 2017. Replacing mouse BAFF with human BAFF does not improve B-cell maturation in hematopoietic humanized mice. Blood Adv 1:2729–2741 10.1182/bloodadvances.2017010090. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cimbro R, Vassena L, Arthos J, Cicala C, Kehrl JH, Park C, Sereti I, Lederman MM, Fauci AS, Lusso P. 2012. IL-7 induces expression and activation of integrin α4β7 promoting naive T-cell homing to the intestinal mucosa. Blood 120:2610–2619 10.1182/blood-2012-06-434779. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12:1316–1322 10.1038/nm1431. [PubMed] [DOI] [PubMed] [Google Scholar]
- 52.Denton PW, Nochi T, Lim A, Krisko JF, Martinez-Torres F, Choudhary SK, Wahl A, Olesen R, Zou W, Di Santo JP, Margolis DM, Garcia JV. 2012. IL-2 receptor γ-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol 5:555–566 10.1038/mi.2012.31. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenblatt MB, Vbranac V, Tivey T, Tsang K, Tager AM, Aliprantis AO. 2012. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS One 7:e44664 CORRECTION PLoS One 8:10.1371/annotation/e413f2a1-5767-4c82-9e27-dd556155f124 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. 1988. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 335:256–259 10.1038/335256a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. 1994. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med 180:1817–1827 10.1084/jem.180.5.1817. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali N, Flutter B, Sanchez Rodriguez R, Sharif-Paghaleh E, Barber LD, Lombardi G, Nestle FO. 2012. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One 7:e44219 10.1371/journal.pone.0044219. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harui A, Kiertscher SM, Roth MD. 2011. Reconstitution of huPBL-NSG mice with donor-matched dendritic cells enables antigen-specific T-cell activation. J Neuroimmune Pharmacol 6:148–157 10.1007/s11481-010-9223-x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, Laning J, Fodor W, Foreman O, Burzenski L, Chase TH, Gott B, Rossini AA, Bortell R, Shultz LD, Greiner DL. 2009. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol 157:104–118 10.1111/j.1365-2249.2009.03933.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Büchner SM, Sliva K, Bonig H, Völker I, Waibler Z, Kirberg J, Schnierle BS. 2013. Delayed onset of graft-versus-host disease in immunodeficent human leucocyte antigen-DQ8 transgenic, murine major histocompatibility complex class II-deficient mice repopulated by human peripheral blood mononuclear cells. Clin Exp Immunol 173:355–364 10.1111/cei.12121. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amaladoss A, Chen Q, Liu M, Dummler SK, Dao M, Suresh S, Chen J, Preiser PR. 2015. De novo generated human red blood cells in humanized mice support Plasmodium falciparum infection. PLoS One 10:e0129825 10.1371/journal.pone.0129825. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Z, Van Rooijen N, Yang YG. 2011. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood 118:5938–5946 10.1182/blood-2010-11-321414. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Q, Amaladoss A, Ye W, Liu M, Dummler S, Kong F, Wong LH, Loo HL, Loh E, Tan SQ, Tan TC, Chang KT, Dao M, Suresh S, Preiser PR, Chen J. 2014. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc Natl Acad Sci USA 111:1479–1484 10.1073/pnas.1323318111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allweiss L, Dandri M. 2016. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J Hepatol 64(Suppl):S17–S31 10.1016/j.jhep.2016.02.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. 2001. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 33:981–988 10.1053/jhep.2001.23314. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. 2001. Hepatitis C virus replication in mice with chimeric human livers. Nat Med 7:927–933 10.1038/90968. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, Suemizu H. 2011. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem Biophys Res Commun 405:405–410 10.1016/j.bbrc.2011.01.042. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. 2007. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol 25:903–910 10.1038/nbt1326. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bissig KD, Le TT, Woods NB, Verma IM. 2007. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci USA 104:20507–20511 10.1073/pnas.0710528105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. 2011. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 140:1334–1344 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kremsdorf D, Strick-Marchand H. 2017. Modeling hepatitis virus infections and treatment strategies in humanized mice. Curr Opin Virol 25:119–125 10.1016/j.coviro.2017.07.029. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Kaushansky A, Mikolajczak SA, Vignali M, Kappe SH. 2014. Of men in mice: the success and promise of humanized mouse models for human malaria parasite infections. Cell Microbiol 16:602–611 10.1111/cmi.12277. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dusseaux M, Masse-Ranson G, Darche S, Ahodantin J, Li Y, Fiquet O, Beaumont E, Moreau P, Riviere L, Neuveut C, Soussan P, Roingeard P, Kremsdorf D, Di Santo JP, Strick-Marchand H. 2017. Viral load affects the immune response to HBV in mice with humanized immune system and liver. Gastroenterology 153:1647–1661.e9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, Cookson BT, Karlinsey JE, Kinkel TL, Porwollik S, Canals R, Cummings LA, Fang FC. 2010. Humanized nonobese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci USA 107:15589–15594 10.1073/pnas.1005566107. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Firoz Mian M, Pek EA, Chenoweth MJ, Ashkar AA. 2011. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol Immunol 8:83–87 10.1038/cmi.2010.52. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galán JE. 2010. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe 8:369–376 10.1016/j.chom.2010.09.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mian MF, Pek EA, Chenoweth MJ, Coombes BK, Ashkar AA. 2011. Humanized mice for Salmonella typhi infection: new tools for an old problem. Virulence 2:248–252 10.4161/viru.2.3.16133. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Hunter RL, Jagannath C, Actor JK. 2007. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 87:267–278 10.1016/j.tube.2006.11.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Harper J, Skerry C, Davis SL, Tasneen R, Weir M, Kramnik I, Bishai WR, Pomper MG, Nuermberger EL, Jain SK. 2012. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis 205:595–602 10.1093/infdis/jir786. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heuts F, Gavier-Widén D, Carow B, Juarez J, Wigzell H, Rottenberg ME. 2013. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci USA 110:6482–6487 10.1073/pnas.1219985110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, Sutjita P, Johnston RK, Estes DM, Hunter RL, Actor JK, Cirillo JD, Endsley JJ. 2013. A humanized mouse model of tuberculosis. PLoS One 8:e63331 10.1371/journal.pone.0063331. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. 2013. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol 14:53 10.1186/1471-2172-14-53. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nusbaum RJ, Calderon VE, Huante MB, Sutjita P, Vijayakumar S, Lancaster KL, Hunter RL, Actor JK, Cirillo JD, Aronson J, Gelman BB, Lisinicchia JG, Valbuena G, Endsley JJ. 2016. Pulmonary tuberculosis in humanized mice infected with HIV-1. Sci Rep 6:21522 10.1038/srep21522. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S, Emerging Infections Program–Active Bacterial Core Surveillance MRSA Surveillance Investigators. 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 10.1001/jama.298.15.1763. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Schaumburg F, Köck R, Mellmann A, Richter L, Hasenberg F, Kriegeskorte A, Friedrich AW, Gatermann S, Peters G, von Eiff C, Becker K, study group. 2012. Population dynamics among methicillin-resistant Staphylococcus aureus isolates in Germany during a 6-year period. J Clin Microbiol 50:3186–3192 10.1128/JCM.01174-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prince A, Wang H, Kitur K, Parker D. 2017. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J Infect Dis 215:1386–1395. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knop J, Hanses F, Leist T, Archin NM, Buchholz S, Gläsner J, Gessner A, Wege AK. 2015. Staphylococcus aureus infection in humanized mice: a new model to study pathogenicity associated with human immune response. J Infect Dis 212:435–444 10.1093/infdis/jiv073. [PubMed] [DOI] [PubMed] [Google Scholar]
- 88.Tseng CW, Biancotti JC, Berg BL, Gate D, Kolar SL, Müller S, Rodriguez MD, Rezai-Zadeh K, Fan X, Beenhouwer DO, Town T, Liu GY. 2015. Increased susceptibility of humanized NSG mice to Panton-Valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog 11:e1005292 10.1371/journal.ppat.1005292. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141 10.1016/j.cell.2014.03.011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gülden E, Vudattu NK, Deng S, Preston-Hurlburt P, Mamula M, Reed JC, Mohandas S, Herold BC, Torres R, Vieira SM, Lim B, Herazo-Maya JD, Kriegel M, Goodman AL, Cotsapas C, Herold KC. 2017. Microbiota control immune regulation in humanized mice. JCI Insight 2:e91709 10.1172/jci.insight.91709. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hofer U, Schlaepfer E, Baenziger S, Nischang M, Regenass S, Schwendener R, Kempf W, Nadal D, Speck RF. 2010. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog 6:e1000867 10.1371/journal.ppat.1000867. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu SX, Leontyev D, Kaul R, Gray-Owen SD. 2018. Neisseria gonorrhoeae co-infection exacerbates vaginal HIV shedding without affecting systemic viral loads in human CD34+ engrafted mice. PLoS One 13:e0191672 10.1371/journal.pone.0191672. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. 2009. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol 86:219–227 10.1189/jlb.1008615. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Skirecki T, Kawiak J, Machaj E, Pojda Z, Wasilewska D, Czubak J, Hoser G. 2015. Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther 6:142 10.1186/s13287-015-0135-9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye C, Choi JG, Abraham S, Wu H, Diaz D, Terreros D, Shankar P, Manjunath N. 2012. Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model. Proc Natl Acad Sci USA 109:21052–21057 10.1073/pnas.1216195109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlieckau F, Schulz D, Fill Malfertheiner S, Entleutner K, Seelbach-Goebel B, Ernst W. 2018. A novel model to study neonatal Escherichia coli sepsis and the effect of treatment on the human immune system using humanized mice. Am J Reprod Immunol 80:e12859 10.1111/aji.12859. [PubMed] [DOI] [PubMed] [Google Scholar]


