Abstract
Neutralizing antibodies represent a major host defense mechanism against viral infections. In mammals, passive immunity is provided by neutralizing antibodies passed to the offspring via the placenta or the milk as immunoglobulin G and secreted immunoglobulin A. With the long-term goal of producing virus-resistant livestock, we have generated mice carrying transgenes that encode the light and heavy chains of an antibody that is able to neutralize the neurotropic JHM strain of murine hepatitis virus (MHV-JHM). MHV-JHM causes acute encephalitis and acute and chronic demyelination in susceptible strains of mice and rats. Transgene expression was targeted to the lactating mammary gland by using the ovine β-lactoglobulin promoter. Milk from these transgenic mice contained up to 0.7 mg of recombinant antibody/ml. In vitro analysis of milk derived from different transgenic lines revealed a linear correlation between antibody expression and virus-neutralizing activity, indicating that the recombinant antibody is the major determinant of MHV-JHM neutralization in murine milk. Offspring of transgenic and control mice were challenged with a lethal dose of MHV-JHM. Litters suckling nontransgenic dams succumbed to fatal encephalitis, whereas litters suckling transgenic dams were fully protected against challenge, irrespective of whether they were transgenic. This demonstrates that a single neutralizing antibody expressed in the milk of transgenic mice is sufficient to completely protect suckling offspring against MHV-JHM-induced encephalitis.
Coronaviruses are a group of enveloped viruses with a single-stranded RNA genome of positive polarity (37). They are frequently associated with respiratory and gastrointestinal disorders in both animals and humans. Many coronavirus infections are mild in adult animals, whereas they often cause severe and sometimes lethal diseases in neonates (9, 32). To a large extent, this is due to the immature immune system of the newborn host. Maternal antibodies supplied via the placenta and milk efficiently protect newborn animals against the fatal consequences of acute coronavirus infections during this critical phase (14, 15). Cross-fostering experiments have shown that milk-borne antibodies (immunoglobulin A [IgA] and IgG) are sufficient to completely protect newborn mice against lethal doses of murine hepatitis virus (MHV) (15).
Vaccination against coronavirus infections has been employed with various degrees of success (23, 25, 36). The vaccines are usually highly strain specific (16), but they are also dependent on specific routes of infection and often short-lived. Live-virus vaccines are also associated with the danger of in vivo recombination, leading to novel viruses with increased pathogenicity.
Neutralizing monoclonal antibodies generated in response to coronavirus infections have been isolated in many laboratories (12, 35, 42), and it has been shown that antibodies which inhibit virus entry into susceptible cells in vitro can also effectively prevent acute coronavirus-induced disease in vivo (26, 42). Coronavirus infections cause a high mortality only during a short time period (up to 20 days postpartum in mice), which largely coincides with the suckling period. We and others (3, 39) have therefore reasoned that the recombinant expression of neutralizing antibodies in the milk of transgenic animals may provide an effective strategy to protect animals during this critical phase. To provide a proof of principle, we have generated transgenic mice expressing a highly neutralizing monoclonal antibody directed against the neurotropic MHV strain JHM (MHV-JHM). The recombinant antibody was secreted into the milk at yields of up to 0.7 mg/ml. The biological activity of the milk-borne antibody was demonstrated by virus neutralization assays in vitro, and a linear correlation between antibody expression and neutralization was found. When litters suckling transgenic dams were infected with a lethal dose of MHV-JHM, they were completely protected against virus-induced disease, irrespective of whether the newborn mice were transgenic. These results provide the first example of transgene-mediated lactogenic immunity in vivo.
MATERIALS AND METHODS
DNA cloning.
Monoclonal antibody (MAb) A1 was selected for these studies because it is highly potent with regard to virus neutralization and inhibition of virus-induced cell-to-cell fusion (42). The isolation and cloning of cDNAs encoding the variable regions of MAb A1 have been described previously (21). In brief, mRNA was isolated from the A1 hybridoma cell line and reverse transcribed. The resulting vκ and vγ cDNAs were amplified by PCR, using primers which bind in the framework of the variable regions (21). The variable region-encoding cDNAs were subsequently inserted into expression vectors (Lys30-A1H and Lys17-A1L), providing a signal peptide and human IgG1 constant regions. The chimeric antibody carrying the human constant regions is easily identified against the background of murine antibodies in murine milk by immunological assays. To generate plasmid pBJ41-A1L, the coding region of the chimeric antibody A1 light chain was excised from plasmid Lys17-A1L (21) as a NcoI-EcoRI fragment, blunt ended by treatment with Escherichia coli DNA polymerase I, and inserted into plasmid pBJ41 (39) that had been digested with EcoRV (Fig. 1). To generate the vector pBJ41-A1H, the chimeric antibody A1 heavy chain coding region was excised from plasmid Lys30-A1H (21) as a blunt-ended NcoI-EagI fragment and ligated into expression vector pBJ41 that had been digested with EcoRV (Fig. 1). Expression vector pBJ41 carries 4.3 kb of 5′ flanking region and 1.9 kb of 3′ flanking region derived from the ovine β-lactoglobulin (β-LG) gene. The vector also includes the transcriptional initiation site and polyadenylation signal at the end of exon 7 of the β-LG gene, but it lacks the β-LG translational initiation codon (Fig. 1). The first initiation codon in the chimeric mRNA is therefore provided by the inserted A1L or A1H gene sequences (Fig. 1).
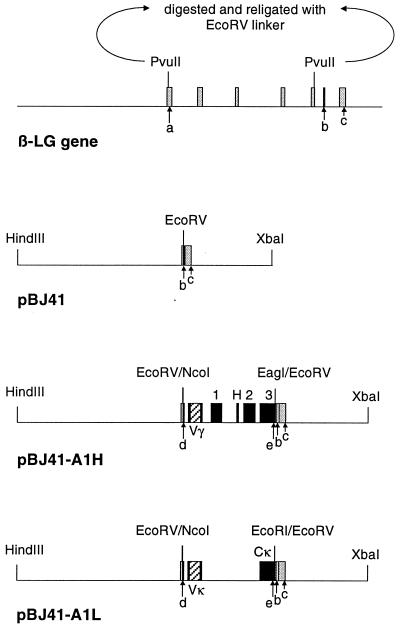
FIG. 1.
Schematic representation of the ovine β-LG gene, the expression vector pBJ41, and the transgene constructs pBJ41-A1H and pBJ41-A1L. Exons of the β-LG gene and the human IgG genes are represented by shaded boxes and filled boxes, respectively. Exons encoding the heavy chain constant region are marked as 1, H, 2, and 3; the exon encoding the light chain constant region is marked as Cκ. The inserted variable regions (Vγ and Vκ) isolated from the A1 mouse hybridoma cell line are represented as hatched boxes. The mammary gland-specific expression vector pBJ41 contains sequences from the first and fifth exons (17 and 8 bp, respectively) and the entire sixth and seventh exons of the β-LG gene. A linker carrying a singular EcoRV site was inserted in between the two PvuII sites in exons 1 and 5. a, b, and c, β-LG translational initiation site, stop codon, and polyadenylation signal, respectively; d and e, IgG translational start and stop codons, respectively. The A1 heavy chain coding region was inserted as a blunt-ended NcoI-EagI fragment into the singular EcoRV site of pBJ41. The A1 light chain coding region was inserted as a blunt-ended NcoI-EcoRI fragment into the same site.
Animals.
Transgenic mice (F1 CBA × C57BL/6) were produced by pronuclear injection at the animal facility of the Roslin Institute as described previously (4, 43). The plasmids pBJ41-A1L and pBJ41-A1H and the genomic β-LG vector pSS1tgXS (1) were all linearized by digestion with SalI-XbaI and microinjected at a 1:1:3 ratio. pSS1tgXS carries the entire β-LG coding region, with 4.3 kb of 5′ flanking region and 1.9 kb of 3′ flanking region. An excess of genomic β-LG vector over the expression vector biases for increased frequency of transgene expression (4). Transgenic mice were identified by PCR and Southern blot analysis of genomic DNA. The pBJ41-A1H transgene was detected by PCR, using the primers pBJup (5′ AGC CTG CCT GTC TCA GCC CT 3′) and A1Hsp (5′-TGC ATG TGA TGG ACA GGC-3′). The primer pair gives rise to a 264-bp product. The pBJ41-A1L transgene was detected by Southern blot analysis of BamHI-digested genomic DNA. The blot was hybridized with a pBJ41-A1L-specific 281-bp PCR product amplified with the primer pair pBJup and A1Lsp (5′-CTA CTA AGG TTT TTG CAT TA-3′), using plasmid DNA as template. Transgene copy number was determined by Southern blot analysis on liver DNA from G1 mice. Aliquots (20 μg) of DNA were digested with BamHI, separated on a 1% agarose gel, and blotted to a nylon membrane. The blot was subsequently probed with a 1.6-kb BamHI-SphI fragment of the β-LG promoter which is present in all three constructs. Eleven transgenic founder mice were generated, eight of which transmitted the transgenes. These eight lines were used for further analysis.
The virus challenge experiments were carried out with pathogen-free C57BL/6 (B6) mice obtained from the National Cancer Institute (Bethesda, Md.). These mice were seronegative for MHV-JHM. Transgenic females were crossed back with B6 males for five generations, and offspring were screened for the presence of the transgenes as described above. For cross-fostering experiments, suckling mice born to B6 females mated with B6 males and transgenic females mated with B6 males were switched within 24 h of delivery.
Protein analysis.
Western blot analyses were done essentially as described previously (20). The milk of transgenic mice (isolated at peak lactation, day 10 postpartum) was diluted 1:5 with water and centrifuged at 14,000 × g for 5 min. Three phases were separated: a layer of fat above an aqueous phase and a pellet. The aqueous phase containing the whey fraction was isolated, and aliquots were mixed with reducing and denaturing sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The electrophoretically separated proteins were transferred to a nitrocellulose membrane by semidry electroblotting. The constant regions of the chimeric A1 antibody were detected by using a rabbit anti-human IgG-horseradish peroxidase (HRP)-linked antiserum (Dianova). The results were quantified by densitometric scanning on a Molecular Dynamics Densitometer.
Cells and viruses.
DBT (delayed brain tumor) cells (24) were cultivated at 37°C in minimum essential medium (Life Technologies) supplemented with 10% fetal bovine serum (Sigma), nonessential amino acids, glutamine, and antibiotics. The MHV-JHM strain used for the in vitro experiments was described previously (35). Virus neutralization was quantified in syncytium focus reduction assays. MHV-JHM infections lead to extensive cell-to-cell fusion without inducing cytophathic effects in the first 24 h postinfection. However, syncytium formation (as is the case with plaque formation) indicates the presence of an infection center and is therefore equivalent to plaque formation in its diagnostic value. In keeping with the literature, the number of infectious centers is referred to as PFU. Defatted and diluted milk samples were further diluted in phosphate-buffered saline. Aliquots (500 μl) of a 10−4 dilution of MHV-JHM, equivalent to about 70 PFU of virus, were mixed with 500 μl of different dilutions of milk samples isolated from transgenic lines or nontransgenic control mice and incubated for 1 h at 37°C. Subsequently, the virus-antibody mixture was added to confluent DBT cells for 1 h at 37°C. The virus-containing supernatant was then removed. The cells were washed twice with phosphate-buffered saline, and fresh medium was added. Foci of syncytium formation were counted after the infected cells were incubated for 16 h at 37°C. The virus strain used for the in vivo studies was grown and titers were determined as described previously (31). To determine the protective efficacy of breast milk-expressed antibody, 10-day-old mice were challenged by intranasal or intracerebral inoculation, as described previously (31). Intranasal challenge was done by inoculation of 2 × 104 to 4 ×104 PFU of MHV-JHM. Intracerebral challenge was done by inoculation of 7 × 102 or 7 × 103 PFU of MHV-JHM (corresponding to 200 and 2,000 50% lethal doses, respectively). Mice were monitored daily for mortality and morbidity.
RESULTS
MHV-JHM infections can be effectively prevented in vitro and in vivo by neutralizing antibodies, CD4+ T cells, and CD8+ T cells (10, 40, 42). The majority of neutralizing antibodies are directed against the viral surface (S) glycoprotein. MAb A1 (42), which binds to the S1 subunit of the MHV-JHM S protein, is one of the most potent antibodies with regard to virus neutralization and the inhibition of virus-induced cell-to-cell fusion. We have isolated the variable regions of MAb A1 and transferred them into different eukaryotic expression vectors. The recombinantly expressed version of MAb A1 displayed the same biological activity as the parental MAb secreted from the A1 hybridoma cell line (21, 22). In the studies described here, the MAb A1 variable regions were linked to human constant regions of the IgG isotype to facilitate their identification against the background of murine antibodies. The resulting chimeric open reading frames were inserted into a mammary gland-specific expression cassette based on the ovine β-LG gene (Fig. 1). After confirming expression of the antibody genes in cell culture (data not shown), the two expression vectors (pBJ41-A1L and pBJ41-A1H) were used for the generation of transgenic animals.
In all, eight lines of transgenic mice were generated. The expression vectors pBJ41-A1L and pBJ41-A1H were microinjected together with the β-LG expression construct pSS1tgXS (1) at a ratio of 1:1:3. This vector is expressed in a copy number-dependent manner in transgenic animals (43) and has been shown to increase the expression of colinked transgenes (4). The molecular basis of this “transgene rescue effect” is not known, but it is assumed that the β-LG construct is able to generate independent chromatin domains which escape the silencing effect of neighboring chromatin structures (4).
Defatted milk of transgenic mothers was analyzed for the expression of recombinant antibody by Western blotting. To do this, the human constant regions of the MHV-neutralizing antibody were detected by using an HRP-linked rabbit anti-human IgG antiserum. Of the eight transgenic lines transmitting all three transgenes, five expressed the heavy and the light chains of the recombinant antibody. In two transgenic lines, only light chain protein but no heavy chain protein was detectable (Table 1). The seven lines which expressed the light chain gene expressed the genomic β-LG construct as well. The light chain was always expressed in excess of the heavy chain (Table 1 and Fig. 2) and is also readily detected in a Coomassie blue-stained protein gel (data not shown). The transgene copy number was estimated in seven of the eight lines obtained (Table 1) and varied from 1 to 10 copies (heavy chain) and 4 to 15 copies (light chain). The transgenes encoding the antibody light chain and the heavy chain were microinjected into fertilized oocytes at equimolar levels. However, the transgene copy numbers seem to indicate that consistently more copies of the light than the heavy chain construct were incorporated into the host genome. The reason for this is unknown, but it may be due to a leaky expression of the transgenes during early developmental stages which, in turn, leads to a cytotoxic overproduction of heavy chain protein and the subsequent loss of embryos. No correlation between the transgene copy numbers and the levels of antibody expression could be detected (Table 1). We interpret this to indicate that the site of transgene integration had a dominant effect on gene expression. Although expression of the light chain protein was consistently higher than expression of the heavy chain protein, this did not directly correlate with transgene copy number. Additionally, no correlation could be detected between the levels of β-LG protein expression and antibody expression (Table 1).
TABLE 1.
Transgene copy number and expressiona
| Transgenic line | pSS1tgXS
|
pBJ41-A1H
|
pBJ41-A1L
|
|||
|---|---|---|---|---|---|---|
| No. of copies | Level of β-LG expressionb | No. of copies | Level of expression and concn (μg/ml)c | No. of copies | Level of expression and concn (μg/ml) | |
| HEP3 | 16 | ++++ | 2 | (+) (<20) | 5 | + (40) |
| HEP10 | 20 | ++++ | 2 | (+) (<20) | 7 | + (400) |
| HEP17 | NDd | ND | ND | − | ND | (+) (300) |
| HEP20 | 12 | − | 1 | − | 6 | − |
| HEP30 | 6 | +++++ | 1 | (+) (70) | 4 | + (800) |
| HEP36 | 10 | ND | 2 | − | 4 | (+) (150) |
| HEP38 | 30 | ++ | 10 | (+) (<20) | 15 | + (650) |
| HEP50 | 2 | ++ | 2 | + (500) | 10 | ++ (2,900) |
Transgene copy number was determined as described in Materials and Methods. Relative levels of transgene expression were determined by Western blotting and Coomassie brilliant blue protein staining after polyacrylamide gel electrophoresis.
+, transgene expression could be detected by Western blotting and protein staining. The number of plus signs indicates the relative level of expression. (+), transgene expression could be detected by Western blotting but not by protein staining; −, transgene expression could not be detected by Western blotting.
The concentration of heavy and light chain protein as quantified by densitometric scanning of Western blots (as described in Materials and Methods).
ND, not determined.
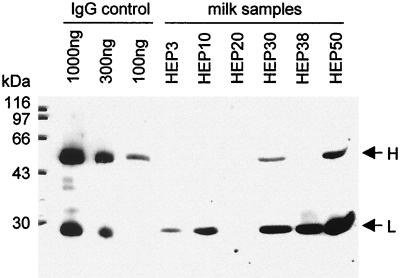
FIG. 2.
Western blot analysis of milk samples. Aliquots of defatted milk samples (corresponding to 1 μl of milk) isolated from mice of six transgenic lines were separated by 15% polyacrylamide gel electrophoresis alongside a human IgG standard (1,000, 300, and 100 ng of IgG) and blotted onto nitrocellulose. The human IgG portion of the chimeric recombinant antibody was detected by using an HRP-linked rabbit anti-human IgG antiserum. The blot was developed using a chemiluminescent detection system (Pierce). The positions of the antibody chains and the sizes of the molecular marker proteins are indicated.
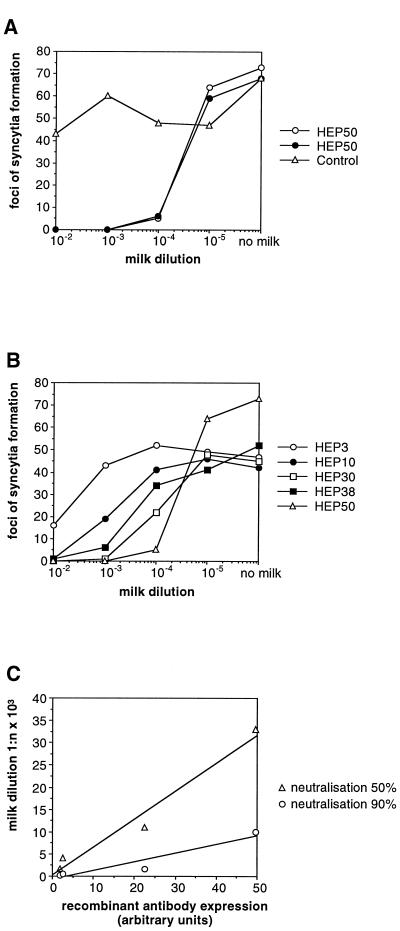
The concentrations of the heavy and light chain proteins were quantified by densitometry in comparison to IgG standards (Beriglobin; pooled human immunoglobulin; Behringwerke). Maximum levels of 0.5 and 2.9 mg/ml, respectively, of heavy and light chain proteins were obtained in transgenic line HEP50. The heavy chain, which comprises 69% of the total molecular weight of an IgG molecule, can only be secreted from cells as part of a complete antibody. In contrast, the light chain protein can be secreted individually. Therefore the 0.5 mg of heavy chain protein per ml measured in the milk of transgenic line HEP50 corresponds to a total recombinant IgG concentration of 0.7 mg/ml. The defatted milk of transgenic line HEP50 was subsequently analyzed in a virus neutralization assay. A 10−4 dilution of defatted milk reduced MHV-JHM infectivity by 90% (Fig. 3A), which corresponds to 125 pg of IgG being required to neutralize 1 PFU of MHV-JHM. This is consistent with results we have obtained in cell culture, where 100 pg of IgG was required to neutralize 1 PFU of MHV-JHM (21). Milk isolated from nontransgenic mice did not show any neutralizing effect against the MHV-JHM infection (Fig. 3A). This confirms the fact that the breeding colony of mice was seronegative for MHV-JHM and also that the recombinant antibody is the decisive virus-neutralizing factor in milk. Defatted milk samples from four other transgenic lines were also analyzed by neutralization assays. All of the samples neutralized MHV infectivity, albeit with different levels of efficacy (Fig. 3B). The dilutions at which MHV-JHM infectivity was reduced by 90 and 50% were correlated with expression of the heavy chain (which is the limiting factor for antibody formation) (Fig. 3C). The linear correlation observed confirms that the concentration of the recombinant antibody in the milk of these transgenic animals is the critical factor determining neutralization activity.
FIG. 3.
Neutralization assay of milk samples. (A) Dilutions of milk samples isolated from mice of the transgenic line HEP50 and from a nontransgenic control mouse were tested in a virus neutralization assay. The number of syncytia obtained with different milk dilutions and in the absence of milk is indicated. Each line represents the results of one experiment. (B) Dilutions of milk samples isolated from mice of the transgenic lines HEP3, HEP10, HEP30, HEP38, and HEP50 were tested in a virus neutralization assay. The number of syncytia obtained with the different dilutions and in the absence of milk is indicated. Each line represents the results of one experiment. (C) Correlation of neutralization activity with antibody expression in milk samples from mice of transgenic lines HEP10, HEP30, HEP38, and HEP50. The milk dilutions at which MHV-JHM-induced plaque formation is inhibited by 50 and 90% were determined by virus neutralization assays and correlated with expression of the recombinantly expressed MAb A1 heavy chain protein as determined by densitometric scanning of Western blots (arbitrary units).
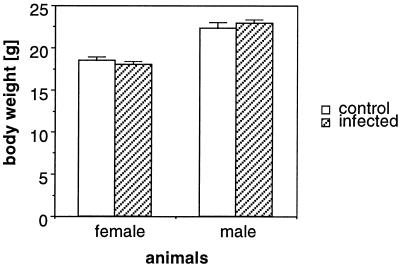
Finally, the ability of recombinant antibody secreted in breast milk to protect suckling mice in vivo was determined. Transgenic mice and their nontransgenic littermates were infected intranasally with virulent MHV-JHM. Under these conditions (31), 100% of naive mice succumb to acute, fatal encephalitis by 5 to 7 days postinoculation. However, as shown in Table 2, 23 of 23 suckling mice nursed by transgenic dams did not develop acute encephalitis, whereas 6 of 6 suckling mice nursed by transgene-negative animals died by 7 days postinfection (Table 2). Infected mice nursed by transgenic dams also grew at the same rate as uninfected mice nursed by transgenic dams (Fig. 4). In cross-fostering experiments, we showed that antibody delivery in the breast milk was critical to this protection. When naive B6 suckling mice were nursed by transgenic dams, they were also fully protected (n = 20) from acute encephalitis (Table 2). Mice were subsequently monitored for signs of late onset disease caused by chronic demyelination (31). However, no indicative symptoms could be detected for as many as 60 days postinoculation, indicating that sufficient antibody was transferred to the suckling offspring to control the initial inoculum (31). In other experiments, we showed that the inoculated mice also did not transmit MHV-JHM to seronegative contacts.
TABLE 2.
MHV-specific antibody in breast milk protects mice from acute encephalitisa
| Dams (litters) | Offspring (no.) | % Mortality |
|---|---|---|
| HEP50[+] (3) | HEP50[+] × B6 (23) | 0 |
| HEP50[−] (1) | HEP50[−] × B6 (6) | 100 |
| HEP50[+] (3) | B6 × B6 (20) | 0 |
| HEP36[+] (2) | HEP36[+] × B6 (8) | 100 |
Transgenic mice are designated HEP50[+] and HEP36[+]. B6 mice are nontransgenic control mice. Nontransgenic littermates of HEP50[+] mice are designated HEP50[−]. Offspring from transgenic mice (HEP50[+] and HEP36[+] females mated with B6 males), nontransgenic mice (HEP50[−] females mated with B6 males) and naive B6 mice were challenged intranasally with MHV-JHM as described in Materials and Methods. Mortality was monitored in the indicated number of litters of suckling mice nursed by transgenic (HEP50[+], HEP36[+]) or nontransgenic (HEP50[−]) dams.
FIG. 4.
Weight gain in infected and control animals. MHV-JHM-infected mice (n = 23, 12 females and 11 males) nursed by HEP50-positive transgenic dams and noninfected control mice (n = 20, 11 females and 9 males) were weighed at 44 days of age (34 days postinfection). Mean values and standard deviations are shown.
Mice from another transgenic line (HEP36), which expresses only minute amounts of recombinant antibody A1 (Table 1), were challenged intranasally with MHV-JHM. None of the offspring of HEP36 dams were protected against the virus challenge (Table 2). This suggests that transgenesis per se does not influence the ability of mice to protect their offspring against MHV-JHM infection.
The protection exerted by the recombinant antibody supplied in milk could be local (inhibiting virus entry via the oronasal tract) or systemic (preventing infection throughout the animal after uptake of the antibody through the intestinal mucosa). Antibodies can be taken up as intact proteins through the gut via specific receptors up to 19 days postpartum in mice (27). To determine whether the protection is systemic, offspring suckling HEP50 dams were challenged by intracerebral inoculation of 700 PFU (n = 5) or 7,000 PFU (n = 4) of MHV-JHM (corresponding to 50% lethal doses of 200 and 2,000, respectively). All mice survived the challenge without sign of disease. This suggests that the recombinant antibody provides a strong systemic protection, although additional local effects may also occur. Moreover, these results suggest that the chimeric, recombinant antibody carrying human constant regions is successfully transported across the gut epithelium and to and across the blood-brain barrier. These results also indicate that the recombinant antibody is able to exert its protective activity in the absence of other milk components (for example, complement) that may have specifically or nonspecifically bound to it.
DISCUSSION
Milk-borne antibodies have been shown to be an efficient means of preventing infectious disease in mammals (5, 6, 8, 11, 17, 18). Synthesis of maternal neutralizing antibodies can be induced by vaccines or natural infections. In some instances (e.g., if vaccination is ineffective or if infection with live pathogens results in high mortality), alternative routes have to be employed to provide passive immunization to mammalian offspring. Purified neutralizing MAbs can be injected into pregnant or lactating host animals (5) or directly administered to neonates (2, 38). In order to generate milk which contains antibodies of therapeutic value, animals which are not the natural host (preferably ruminants, which yield large amounts of milk) can be immunized with live pathogens (7, 17).
We have analyzed whether expressing a neutralizing antibody as a recombinant protein in the milk of transgenic animals may provide an alternative to these procedures. This transgenic approach is particularly useful if (i) no useful vaccines are available, (ii) neutralizing antibodies are a major component of the host's defense against the pathogen in question, and (iii) the period during which the infection is life-threatening is limited. The use of transgenic animals offers the additional advantage that the most potent antibodies can be selected by in vitro analyses before transgenes are established from the respective hybridoma cell lines. Moreover, no live pathogens have to be introduced into an animal colony. Coronaviruses, which cause diseases of economic importance in animals and humans, fulfill all of the above-mentioned criteria. Therefore, transgenic animals that express neutralizing antibodies in the milk may, in the long term, provide a strategy to protect animals during the suckling period. The experiments described here demonstrate that this approach is practicable. However, antibody isotypes mediating lactogenic immunity vary between different species (27). There are also profound differences in the intestinal uptake of immunoglobulins (27). Transgene-mediated strategies manipulating lactogenic immunity in livestock will therefore have to be adapted to the requirements of individual species in terms of immunoglobulin isotype and expression strategy. In that respect, the transgenic mouse-MHV model provides an excellent opportunity to determine the critical factors for successful immune protection through in vivo challenge experiments.
Two features distinguish transgene-mediated lactogenic immunity from the natural process of passive immunization by maternal antibodies. In the natural situation, newborn animals are provided with a polyclonal mixture of antibodies against a particular pathogen. The transgenic dams described here only provide a single, albeit highly neutralizing, antibody to their offspring. As has been shown previously, the β-LG-based expression system is able to supply immunoglobulin at a high concentration throughout the entire lactation period (39). In mammals, the total immunoglobulin concentration in colostrum ranges between 5 mg/ml (rat) and 250 mg/ml (cow). Lower immunoglobulin levels, ranging from 1 mg/ml (human) up to 10 mg/ml (pig), are found in milk (27, 41). Therefore, the concentration of recombinantly expressed neutralizing antibody of 0.7 mg/ml exceeds the concentration of any single monospecific antibody present in milk by some orders of magnitude. Nevertheless, there appeared to be no selection of virus escape mutants, which could overcome the immune protection and lead to overt disease within the time frame of the experiment. This indicates that production of a single MHV-JHM neutralizing antibody in milk is sufficient to provide full protection during the suckling period.
Milk proteins and their derivatives have been shown to have antibacterial and antiviral effects (29, 34, 44). In our experimental model, the neutralizing effect of the mouse milk samples was dependent on the concentration of the recombinant antibody. This suggests that no other natural components of milk provide a protective effect against MHV-JHM infections.
The levels of antibody production and secretion into milk are critical factors for the establishment of transgene-mediated virus resistance. The concentration of 0.7 mg of mature IgG per ml is at the lower end of expression levels that have been previously reported for other recombinant antibodies expressed in the milk of transgenic mice (0.4 mg/ml [28], 0.8 mg/ml [30], 4 mg/ml [13], 5 mg/ml [3], and 6 mg/ml [39]). Nevertheless, complete protection of litters suckling transgenic dams could be obtained at these expression levels. The comparably low expression level is at least in part due to the failure to produce equimolar amounts of heavy and light chain protein (compare Table 1 and Fig. 2). This confirms our previous experiences with cell culture (21) and data published by others (33, 44). The consistent excess of light chain protein over heavy chain protein is due to the cytotoxicity of unpaired heavy chains (19), which leads to the death of cells in which the heavy chain is overexpressed. Additionally, we found that the MAb A1 heavy chain transgene was present at a lower copy number than the MAb A1 light chain construct in all strains of mice analyzed. This is consistent with the findings of Sola and colleagues (39), who used a β-LG-based expression system to express a recombinant antibody that neutralizes the porcine coronavirus transmissible gastroenteritis virus in the milk of transgenic mice. In contrast, when an expression system based on the whey acidic protein (WAP) promoter was used to express IgG-encoding genes, most of the transgenic lines generated carried an excess of heavy chain expression constructs (3). One possible explanation is that the β-LG promoter is active at an early stage of development and causes the loss of embryos in which the (cytotoxic) heavy chain is overexpressed. Transgenic animals in which the two antibody chains are expressed in equimolar amounts can be generated by microinjection (33) but may require the screening of a larger number of transgenic lines.
In conclusion, we have demonstrated that high levels of a virus-neutralizing antibody can be generated in the milk of transgenic mice and that full protection against virus-induced disease can be accomplished in newborn animals via this route. Thus, in the murine system, we have established a proof of principle, and the application of this technology to generate virus-resistant animals can now be pursued. Additionally, it now seems feasible that ruminants could be modified by transgenic methodology to produce milk containing neutralizing antibodies directed against pathogens responsible for major human infant diseases.
ACKNOWLEDGMENTS
This work was supported by the EC Bridge Program (ERBSC1*CT000684), the DFG (Si 357/1–1), the National Institutes of Health (NS 40438), and the National Multiple Sclerosis Society.
We acknowledge the technical assistance of Claire Miller, Monika Lechermaier, Angelien Heister, and Atiye Toksoy.
REFERENCES
- 1.Ali S, Clark A J. Characterization of the gene encoding ovine beta-lactoglobulin. Similarity to the genes for retinol binding protein and other secretory proteins. J Mol Biol. 1988;199:415–426. doi: 10.1016/0022-2836(88)90614-6. [DOI] [PubMed] [Google Scholar]
- 2.Arrowood J M, Mead J R, Mahrt J L, Sterling C R. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect Immun. 1989;57:2283–2288. doi: 10.1128/iai.57.8.2283-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castilla J, Pintado B, Sola I, Sanchez-Morgado J M, Enjuanes L. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat Biotechnol. 1998;16:349–354. doi: 10.1038/nbt0498-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark A J, Cowper A, Wallace R, Wright G, Simons J P. Rescuing transgene expression by co-integration. Bio/Technology. 1992;10:1450–1454. doi: 10.1038/nbt1192-1450. [DOI] [PubMed] [Google Scholar]
- 5.Duchet-Suchaux M, Menanteau P, van Zijderveld F G. Passive protection of suckling infant mice against F41-positive enterotoxigenic Escherichia coli strains by intravenous inoculation of the dams with monoclonal antibodies against F41. Infect Immun. 1992;60:2828–2834. doi: 10.1128/iai.60.7.2828-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebina T, Ohta M, Kanamaru Y, Yamamoto Osumi Y, Baba K. Passive immunizations of suckling mice and infants with bovine colostrum containing antibodies to human rotavirus. J Med Virol. 1992;38:117–123. doi: 10.1002/jmv.1890380209. [DOI] [PubMed] [Google Scholar]
- 7.Ebina T. Prophylaxis of rotavirus gastroenteritis using immunoglobulin. Arch Virol Suppl. 1996;12:217–223. doi: 10.1007/978-3-7091-6553-9_23. [DOI] [PubMed] [Google Scholar]
- 8.Englund J, Glezen W P, Piedra P A. Maternal immunization against viral disease. Vaccine. 1998;16:1456–1463. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 9.Enjuanes L, van der Zeijst B A M. Molecular basis of transmissible gastroenteritis virus epidemiology. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 337–376. [Google Scholar]
- 10.Flory E, Stühler A, Wege H, Siddell S, Wege H. Recombinant vaccinia viruses which express MHV-JHM proteins: protective immune response and the influence of vaccination on coronavirus-induced encephalomyelitis. Adv Exp Med Biol. 1993;342:401–406. doi: 10.1007/978-1-4615-2996-5_63. [DOI] [PubMed] [Google Scholar]
- 11.Fried M, Nosten F, Brockman A, Brabin B J, Duffy P E. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher T M, Parker S E, Buchmeier M J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990;64:731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavin W G, Pollock D, Fell P, Yelton D, Cammuso C, Harrington M, Lewis-Williams J, Midura P, Oliver A, Smith T E, Wilburn B, Echelard Y, Meade H. Expression of the antibody hBR96–2 in the milk of transgenic mice and production of hBR96–2 transgenic goats. Theriogenology. 1997;47:214. [Google Scholar]
- 14.Gustafsson E, Blomqvist G, Bellman A, Holmdahl R, Mattsson A, Mattsson R. Maternal antibodies protect immunoglobulin deficient neonatal mice from mouse hepatitis virus (MHV)-associated wasting syndrome. Am J Reprod Immunol. 1996;36:33–39. doi: 10.1111/j.1600-0897.1996.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homberger F R. Maternally-derived passive immunity to enterotropic mouse hepatitis virus. Arch Virol. 1992;122:133–141. doi: 10.1007/BF01321123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homberger F R, Barthold S W, Smith A L. Duration and strain-specificity of immunity to enterotropic mouse hepatitis virus. Lab Anim Sci. 1992;42:347–351. [PubMed] [Google Scholar]
- 17.Jenkins C M, O'Brien C, Trout J, Guidry A, Fayer R. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine. 1999;17:2453–2460. doi: 10.1016/s0264-410x(98)00369-7. [DOI] [PubMed] [Google Scholar]
- 18.Kohl S, Loo L S. The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J Infect Dis. 1984;149:38–42. doi: 10.1093/infdis/149.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Köhler G. Immunoglobulin chain loss in hybridoma lines. Proc Natl Acad Sci USA. 1980;77:2197–2199. doi: 10.1073/pnas.77.4.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb A F, Maile J, Heister A, Siddell S G. Characterization of functional domains in the human coronavirus HCV 229E receptor. J Gen Virol. 1996;77:2515–2521. doi: 10.1099/0022-1317-77-10-2515. [DOI] [PubMed] [Google Scholar]
- 21.Kolb A F, Siddell S G. Expression of a recombinant monoclonal antibody from a bicistronic mRNA. Hybridoma. 1997;16:421–426. doi: 10.1089/hyb.1997.16.421. [DOI] [PubMed] [Google Scholar]
- 22.Kolb A F, Lechermaier M, Heister A, Toksoy A, Siddell S G. Isolation and recombinant expression of an MHV-JHM neutralising monoclonal antibody. Adv Exp Med Biol. 1998;440:657–664. doi: 10.1007/978-1-4615-5331-1_85. [DOI] [PubMed] [Google Scholar]
- 23.Koo M, Bendahmane M, Lettieri G A, Paoletti A D, Lane T E, Fitchen J H, Buchmeier M J, Beachy R N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci USA. 1999;96:7774–7779. doi: 10.1073/pnas.96.14.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumanishi T. Brain tumors induced with Rous sarcoma virus, Schmidt-Ruppin strain. I. Induction of brain tumors in adult mice with Rous chicken sarcoma cells. Jpn J Exp Med. 1967;37:461–474. [PubMed] [Google Scholar]
- 25.Lamarre A, Lecomte J, Talbot P J. Antiidiotypic vaccination against murine coronavirus infection. J Immunol. 1991;147:4256–4262. [PubMed] [Google Scholar]
- 26.Lamarre A, Talbot P J. Protection from lethal coronavirus infection by immunoglobulin fragments. J Immunol. 1995;154:3975–3984. [PubMed] [Google Scholar]
- 27.Larson B L. Immunolobulins of the mammary secretions. In: Fox P F, editor. Advanced dairy chemistry. 1. Proteins. London, United Kingdom: Elsevier; 1992. pp. 231–254. [Google Scholar]
- 28.Limonta J, Pedraza A, Rodriguez A, Freyre F M, Barral A M, Castro F O, Lleonart R, Gracia C A, Gavilondo J V, de la Fuente J. Production of active anti-CD6 mouse/human chimeric antibodies in the milk of transgenic mice. Immunotechnology. 1995;1:107–113. doi: 10.1016/1380-2933(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 29.Neurath A R, Jiang S, Strick N, Lin K, Li Y Y, Debnath A K. Bovine beta-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- 30.Newton D L, Pollock D, DiTullio P, Echelard Y, Harvey M, Wilburn B, Williams J, Hoogenboom H R, Raus J C, Meade H M, Rybak S M. Antitransferrin receptor antibody-RNase fusion protein expressed in the mammary gland of transgenic mice. J Immunol Methods. 1999;231:159–167. doi: 10.1016/s0022-1759(99)00154-4. [DOI] [PubMed] [Google Scholar]
- 31.Perlman S, Schelper R, Bolger E, Ries D. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb Pathog. 1987;2:185–194. doi: 10.1016/0882-4010(87)90020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickel K, Müller M A, ter Meulen V. Analysis of age-dependent resistance to murine coronavirus JHM infection in mice. Infect Immun. 1981;34:648–654. doi: 10.1128/iai.34.3.648-654.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock D P, Kutzko J P, Birck W E, Williams J L, Echelard Y, Meade H M. Transgenic milk as a method for the production of recombinant antibodies. J Immunol Methods. 1999;231:147–157. doi: 10.1016/S0022-1759(99)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu J, Hendrixson D R, Baker E N, Murphy T F, St. Geme J W, Plaut A G. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Routledge E, Stauber R, Pfleiderer M, Siddell S G. Analysis of murine coronavirus surface glycoprotein functions by using monoclonal antibodies. J Virol. 1991;65:254–262. doi: 10.1128/jvi.65.1.254-262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saif L, Wheeler M B. WAPing gastroenteristis with transgenic antibodies. Nat Biotechnol. 1998;16:334–335. doi: 10.1038/nbt0498-334. [DOI] [PubMed] [Google Scholar]
- 37.Siddell S G. The Coronaviridae: an introduction. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 1–10. [Google Scholar]
- 38.Smith I K, Lida J. Passive protection of piglets by monoclonal antibodies against experimental infection with Actinobacillus pleuropneumoniae. Res Vet Sci. 1990;49:144–150. [PubMed] [Google Scholar]
- 39.Sola I, Castilla J, Pintado B, Sanchez-Morgado J M, Whitelaw C B, Clark A J, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J Virol. 1998;72:3762–3772. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stohlman S A, Bergmann C C R, van der Veen C, Hinton D R. Mouse hepatitis virus-specific cytotoxic T lymphocytes protect from lethal infection without eliminating virus from the central nervous system. J Virol. 1995;69:684–694. doi: 10.1128/jvi.69.2.684-694.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telemo E, Hanson L A. Antibodies in milk. J Mamm Gland Biol Neopl. 1996;1:243–250. doi: 10.1007/BF02018077. [DOI] [PubMed] [Google Scholar]
- 42.Wege H, Dörries R, Wege H. Hybridoma antibodies to the murine coronavirus JHM: characterization of epitopes on the peplomer protein E2. J Gen Virol. 1984;65:1913–1941. doi: 10.1099/0022-1317-65-11-1931. [DOI] [PubMed] [Google Scholar]
- 43.Whitelaw C B, Harris S, McClenaghan M, Simons J P, Clark A J. Position-independent expression of the ovine beta-lactoglobulin gene in transgenic mice. Biochem J. 1992;286:31–39. doi: 10.1042/bj2860031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yolken R H, Peterson J A, Vonderfecht S L, Fouts E T, Midthun K, Newburg D S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Investig. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]