Abstract
Background and objective:
Robotic bronchoscopy has demonstrated high navigational success in small peripheral lung nodules but the diagnostic yield is discrepantly lower. Needle based confocal laser endomicroscopy (nCLE) enables real-time microscopic imaging at the needle tip. We aim to assess feasibility, safety and needle repositioning based on real-time nCLE-guidance during robotic bronchoscopy in small peripheral lung nodules.
Methods:
Patients with suspected peripheral lung cancer underwent fluoroscopy and radial EBUS assisted robotic bronchoscopy. After radial EBUS nodule identification, nCLE-imaging of the target area was performed. nCLE-malignancy and airway/lung parenchyma criteria were used to identify the optimal sampling location. In case airway was visualized, repositioning of the biopsy needle was performed. After nCLE tool-in-nodule confirmation, needle passes and biopsies were performed at the same location.
Measurements and main results:
20 patients were included (final diagnosis n=17 (lung) cancer) with a median lung nodule size of 14.5 mm (range 8–28 mm). No complications occurred. In 19/20 patients, good quality nCLE-videos were obtained. In 9 patients (45%), real-time nCLE-imaging revealed inadequate positioning of the needle and repositioning was performed. After repositioning, nCLE-imaging provided tool-in-nodule-confirmation in 19/20 patients. Subsequent ROSE demonstrated representative material in 9/20 patients (45%) and overall diagnostic yield was 80% (16/20). Of the three patients with malignant nCLE-imaging but inadequate pathology, two were diagnosed with malignancy during follow-up.
Conclusion:
Robotic bronchoscopic nCLE-imaging is feasible and safe. nCLE-imaging in small, difficult-to-access lung nodules provided additional real-time feedback on the correct needle positioning with the potential to optimize the sampling location and diagnostic yield.
Keywords: Cytology, Needle based confocal laser endomicroscopy, Peripheral lung nodule, Rapid onsite evaluation, Robotic bronchoscopy, Lung Cancer
Summary at a Glance:
Robotic bronchoscopic nCLE-imaging is feasible, safe and enables real-time visualization of tumor cells at the needle tip. Based on nCLE-guidance, the robotic bronchoscope was repositioned in almost half of patients. Our data show the potential of robotic bronchoscopic nCLE-imaging as a safe, improved strategy to diagnose small difficult-to-access lung nodules.
INTRODUCTION
Lung cancer remains the leading cause of cancer related death worldwide (1). Tissue verification is crucial for the diagnosis and treatment of lung cancer. Consequently, the workload for bronchoscopic nodule analysis is expected to increase significantly.
Over 70% of lung nodules are located in the lung periphery and invisible during bronchoscopy (2). Although several bronchoscopic navigational techniques were developed to target the nodule without tumor visualization, diagnostic yield remains limited and strongly influenced by nodule size, bronchus sign and concentric radial EBUS (r-EBUS) patterns (3–10).
Recently, innovative robotic bronchoscopic platforms (Monarch™® Platform, Ethicon Inc, Redwood City, CA and ION Endoluminal System, Intuitive Surgical Inc, Sunnyvale, CA) were designed with steerable distal ends and optical pattern recognition software to overcome the limitations of conventional navigational bronchoscopy (11). The first multi-center trial on robotic bronchoscopy in peripheral lung nodules reported high navigational success using r-EBUS (96%) but with a lower diagnostic yield (74%) (12).
Although encouraging, the gap between navigational success and diagnostic yield remains suboptimal (13, 14). From a technical perspective, navigational bronchoscopy consists of three steps: navigation to the target nodule, confirmation that biopsy tools are in contact with the nodule and acquisition of adequate tissue (15). Although robotic bronchoscopy demonstrated promising results in the navigation parts, these last steps –confirmation and acquisition- show room for improvement (13).
Therefore, needle based confocal laser endomicroscopy (nCLE) might be a useful adjunct to robotic bronchoscopy. nCLE is a laser-based in-vivo imaging technique (wavelength 488 nm, resolution 3.5 μm and imaging depth up to 70 μm) that, by advancing the flexible CLE-probe past the biopsy needle tip, provides real-time microscopic images at the biopsy needle tip. Previous studies accurately validated nCLE-criteria for lung cancer (enlarged pleomorphic cells, dark clumps and directional streaming) and identified nCLE-criteria for airway/lung parenchyma (conducting elastin fiber bundles, still image, bronchial epithelium and alveoli) (16, 17), allowing confirmation and fine-tuning of the correct needle positioning, as was recently demonstrated in a case-report (18).
In this study, we aim to assess feasibility and safety of nCLE-guided robotic bronchoscopy in patients with small, peripheral lung nodules suspected of lung cancer. Furthermore, we hypothesize that robotic bronchoscopic nCLE-imaging provides real-time feedback on the sampling location and optimal needle positioning to reduce the current near-miss rate.
METHODS
Study design
This is an investigator initiated, proof of principle study and collaboration between Fox Chase Cancer Center (Philadelphia, USA) and Amsterdam University Medical Centers (Amsterdam, the Netherlands). The study was ethically approved at Fox Chase Cancer Center (clinicaltrials.gov NCT04441749) and conducted between July 2020 and July 2021. Patients scheduled for robotic bronchoscopic evaluation of a suspected malignant peripheral lung nodule <30 mm, were eligible for study participation. Patients with an uncorrectable coagulopathy, known allergy to fluorescein, an endobronchially visible tumor, hemodynamic instability, refractory hypoxemia or pregnant and breastfeeding patients were excluded from study participation. All included patients provided written informed consent. Patients underwent r-EBUS and fluoroscopy-assisted robotic bronchoscopy as part of standard care. For study purposes nCLE-imaging was performed. Patient enrollment was conducted in 2 phases, a five patient run-in phase, designed to gain procedural experience, followed by a 20-patient expansion phase. As per protocol, data generated in the run-in phase was not used for final analysis.
Endpoints
The primary endpoints are feasibility (defined as good quality nCLE-videos obtained in >80% of patients) and safety (defined as procedure related adverse advents attributed to the nCLE-imaging and fluorescein administration) of robotic bronchoscopic nCLE-imaging in small, peripheral lung nodules. An exploratory endpoint was the ability of nCLE-imaging to provide tool-in-nodule confirmation and to assess the amount of needle repositionings based on nCLE-guidance. Following previous studies (5, 6, 19–22) pathologic diagnoses were classified into three categories: (1) malignant; (2) non-malignant, including inflammatory, granulomatous, infectious and lymphocyte-predominant patterns; and (3) insufficient material for a classifying diagnosis. In case atypical cells could not be classified further, the samples were considered insufficient. All malignant nodules were considered diagnostic unless surgical resection of the nodule demonstrated false-positive results. Non-malignant nodules were considered diagnostic at one-year follow-up if: (1) the non-malignant diagnosis was confirmed by an alternative sampling method (e.g. surgery or trans-thoracic sampling); (2) follow-up CT-imaging showed regression or resolution of the nodule; (3) nodule remained stable on CT-imaging >1 year follow-up. To validate the nCLE criteria, blinded raters evaluated a set of nCLE-videos twice, including a two week wash-out period.
Study procedure
Robotic bronchoscopy (Monarch™ Platform, Ethicon Inc, Redwood City, CA) was performed under general anesthesia by a single bronchoscopist. nCLE-imaging was performed using the Cellvizio System® and AQ-Flex 19 Confocal Miniprobe (Mauna Kea Technologies, Paris, France) with a resolution of 3.5 μm. Prior to the procedure, the confocal miniprobe was preloaded into the 18G-Flex needle (Broncus Medical Inc, San Jose, USA), positioning the tip of the probe 4 mm past the needle tip using a locking device.
Patients were intubated with an 8.5 endotracheal tube secured about 40 mm from the main carina. Navigation towards the nodule was performed using the Monarch™ Platform navigational software. After positioning of the robotic bronchoscope within close proximity of the nodule, r-EBUS and fluoroscopy were used to confirm nodule localization. The first needle pass was performed at the specified position according to the robotic navigational software and r-EBUS signal.
After the needle had been placed in position, the CLE-probe was advanced past the needle tip. Right before nCLE-imaging, fluorescein (2.5 mg of 10% fluoresceindinatrium) was administered intravenously to enhance imaging of the stromal background and visualization of individual cells. nCLE-imaging was performed under fluoroscopic guidance and nCLE criteria were used to assess the optimal sampling location (16, 17). In case nCLE visualized airway/lung parenchyma, the biopsy needle was repositioned to target the nodule from a different angle (Figure 1). Following nCLE-imaging, the CLE-probe was withdrawn from the needle and transbronchial needle aspiration (TBNA) was performed in the same position using suction. Two smears were prepared from each pass, one air-dried and Diff-Quik stained and one ethanol-fixed and Papanicolaou-stained. Needle rinses were used for cytology cell block preparation. Every nCLE-video was followed by a separate needle pass to have direct comparison between nCLE-imaging and cytology. Rapid onsite evaluation (ROSE) was available and part of clinical decision-making. After TBNA, forceps biopsies were taken at the discretion of the bronchoscopist under fluoroscopic guidance at the specified location according to nCLE-imaging.
Figure 1.
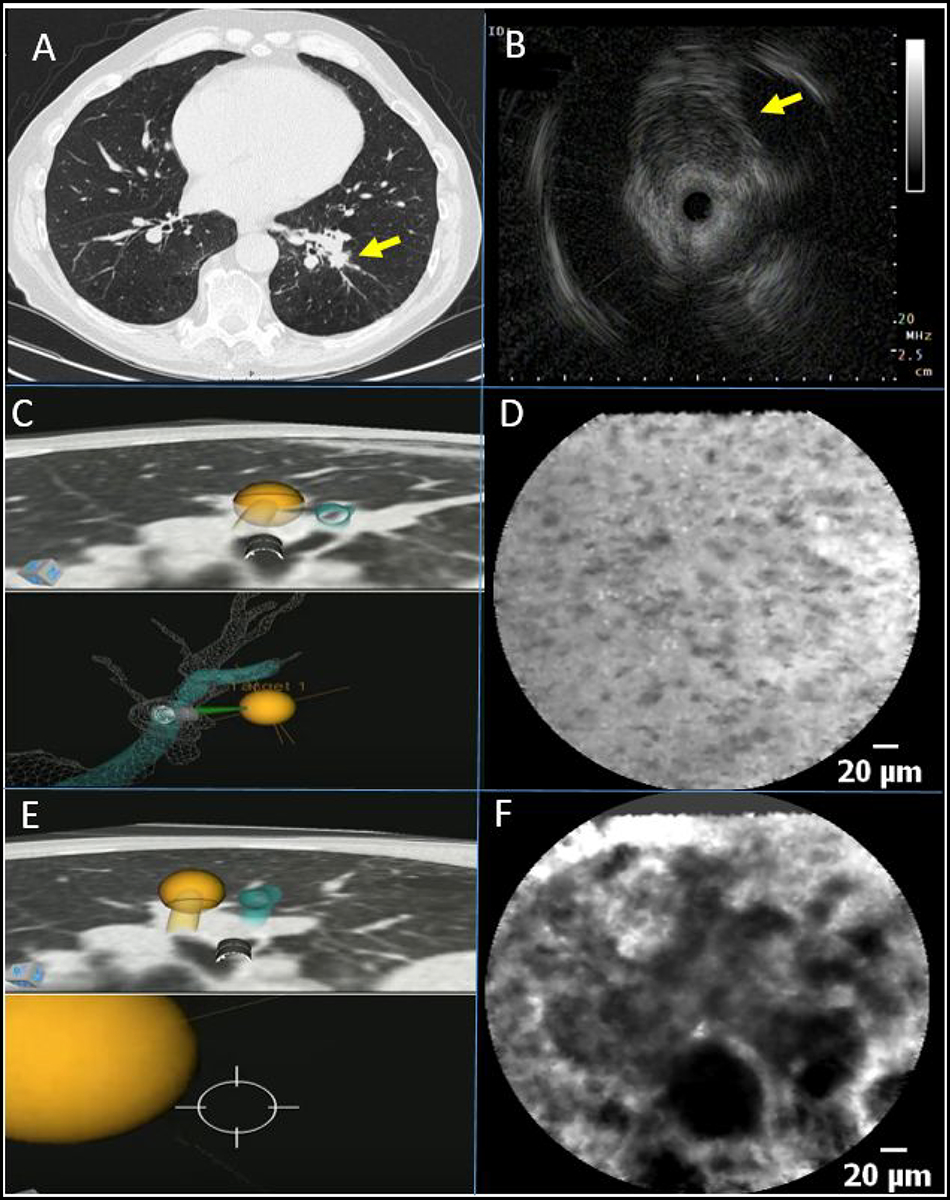
A) Chest CT-scan with a 14 mm sized lesion in the left lower lobe (arrow) and B) radial endobronchial ultrasound image demonstrating an eccentric lesion (arrow). C) During the first needle pass, the lesion was punctured in line with the virtual target according to the robotic bronchoscopic navigation software. D) Corresponding nCLE-imaging shows bronchial epithelium cells, indicating the biopsy needle is not positioned within the lesion. E) During the second needle pass, the robotic bronchoscope was repositioned and the lesion was punctured just posterior to the virtual target according to the robotic bronchoscopic navigation software. F) Corresponding nCLE-imaging shows enlarged pleomorphic cells and dark clumps, indicating the needle is in the right position for tissue sampling. This patient was diagnosed with primary lung adenocarcinoma.
Statistical analysis
The statistical analysis was performed using the standard definitions and software from the SPSS statistical package V.28.0 (IBM Corporation). Continuous, normally distributed data were reported as the mean ± SD. Non-normally distributed data were presented as the median (interquartile range). The accuracy, sensitivity, specificity, positive predictive value and negative predictive value of the validation sessions were calculated using the scores of five blinded raters in both validation sessions. Two-sided 95% confidence intervals were reported for results of interest. Fleiss’ multi-rater (23) was calculated using MATLAB R2020b (MathWorks, Natick, Massachusetts, USA) to estimate the inter-observer agreement (IOA). The Intra-observer reliability (IOR) was calculated by comparing the results of the first and second validation session using Cohen’s κ. The IOA and IOR were interpreted according to the Landis-Koch interpretation system: poor <0.2, fair 0.21–0.4, moderate 0.41–0.6, substantial 0.61–0.8 and excellent 0.81–1 (24).
RESULTS
Patient and nodule characteristics
Robotic bronchoscopic nCLE-imaging was performed in 25 patients, including five patients of the run-in phase who were excluded from further analysis. Patient characteristics of the remaining twenty patients are displayed in Table 1 (n=17 malignant, n=2 non-malignant, n=1 indeterminate). The median nodule size was 14.5 mm (range 8–28 mm). The mean distance to the pleura was 17 mm (range 0–54 mm). In twelve patients (60%) a bronchus sign was visible on pre-procedural CT-scan.
Table 1.
Baseline characteristics of patients with peripheral lung nodules undergoing robotic bronchoscopic nCLE imaging
| Variable | Value |
|---|---|
| Total patients | 20 |
| Age (mean, SD) | 63.5 ±11 |
| Sex | |
| Male | 10 |
| Female | 10 |
| Final diagnosis | |
| Malignant | 17 |
| NSCLC Adenocarcinoma | 8 |
| Carcinoid | 4 |
| Melanoma | 2 |
| NSCLC squamous cell carcinoma | 1 |
| Renal cell carcinoma | 1 |
| Thyroid carcinoma | 1 |
| Non-malignant | 2 |
| Lymphoplastmatic infiltrate | 1 |
| Benign granulomatous reaction | 1 |
| Indeterminate | 1 |
| Lesion size (median largest diameter in mm, range) | 14.5 (8–28) |
| < 20 mm | 16 |
| > 20 mm | 4 |
| Lesion localization | |
| Right upper lobe | 4 |
| Right middle lobe | 3 |
| Right lower lobe | 2 |
| Left upper lobe | 6 |
| Left lower lobe | 5 |
| Bronchus sign on CT-scan | |
| Present | 12 |
| Absent | 8 |
| Bronchoscopic radial EBUS signal | |
| Eccentric | 13 |
| Concentric | 6 |
| Absent | 1 |
Procedure characteristics
No adverse events related to the bronchoscopic procedure or nCLE-imaging/fluorescein administration occurred. Following robotic navigation, successful navigation was confirmed with r-EBUS in 19/20 patients (95%) and in one patient with a subsolid nodule, no r-EBUS signal was obtained. Eccentric r-EBUS images were obtained in 13/20 patients (65%) and concentric r-EBUS images in 6/20 patients (30%). In 11/20 patients (55%) the nodule was visible using fluoroscopy. In 19/20 patients (95%) good quality nCLE-images were obtained with a total of 54 nCLE-videos (1–6 nCLE videos per patient). TBNA was performed in all patients, followed by forceps biopsies in 13 patients (Figure 2). Per patient on average 8 tissue samples (TBNA and/or biopsies) were taken. The average procedure time, from scope insertion until removal was 52 minutes.
Figure 2: Flow of study conduct of robotic bronchoscopic nCLE-imaging.
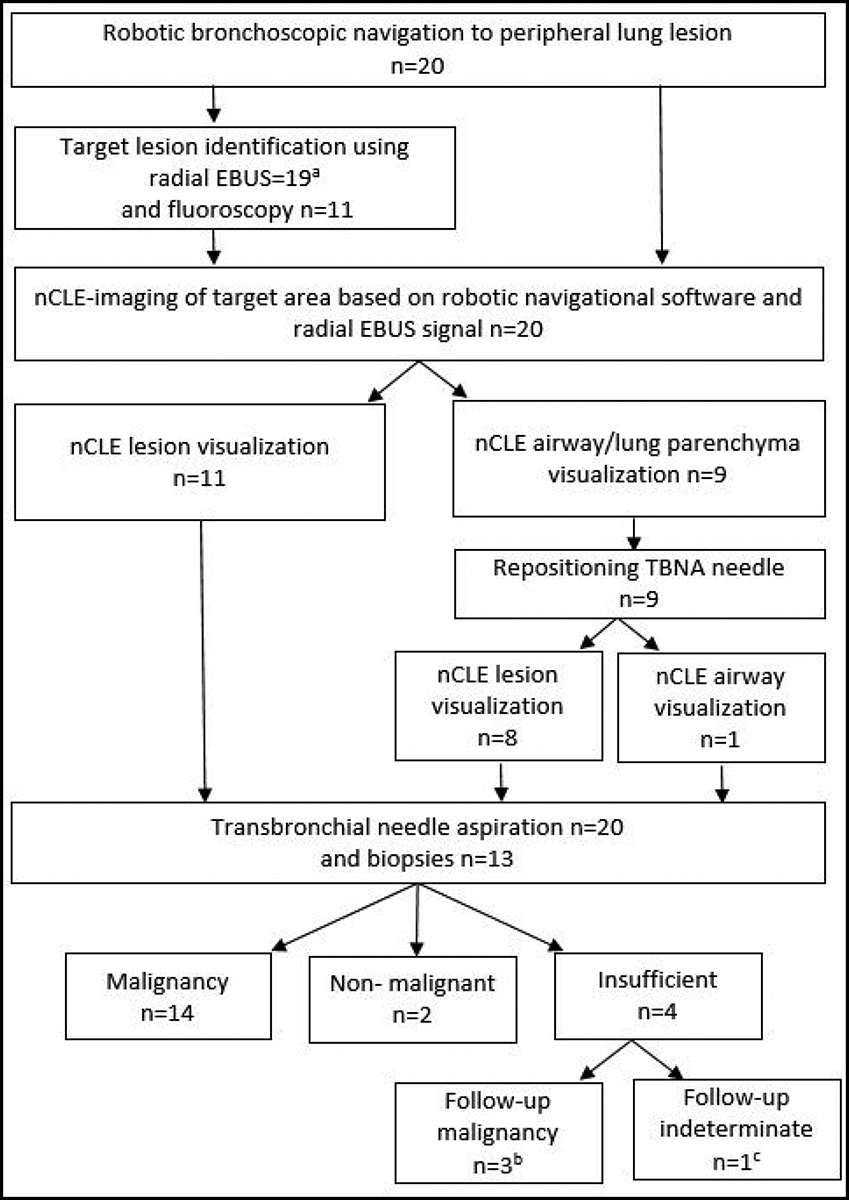
a In 13 patients an eccentric r-EBUS signal was obtained and in 6 patients a concentric r-EBUS signal. In one patient with a sub-solid lesion, r-EBUS was unable to detect the lesion.
b In three patients malignancy was diagnosed during the follow-up period based on surgical resection of the tumor (n=2) and CT-guided transthoracic punctures (n=1). c In one patient no final diagnosis was established after 1 year follow-up period.
Final diagnoses
Robotic bronchoscopic TBNA and biopsies established a diagnosis in 16/20 patients (Table 2). Of the four patients in which pathology deemed insufficient, three patients were diagnosed with malignancy in the follow-up period based on surgical resection of the tumor (n=2) and CT-guided transthoracic punctures (n=1). In one patient no definitive diagnosis was established as no clinical indication for additional tissue acquisition occurred and the nodule remained stable on CT-imaging after 1 year follow-up.
Table 2.
Nodule detection on nCLE-imaging compared to the diagnostic adequacy of ROSE, TBNA cytology, biopsy histology and final diagnosis
| Patient number | Radial EBUS Signal | Repositioning | nCLE-imaging | TBNA ROSE | TBNA cytology | Forceps biopsy histology | Final diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | Eccentric | Yes | + | − | − | + | Lymphoplasmatic infiltrate |
| 2 | Eccentric | Yes | + | + | + | + | Thyroid carcinoma |
| 3 | Concentric | No | + | + | + | + | Adenocarcinoma |
| 4 | Eccentric | No | + | + | + | n/a | Adenocarcinoma |
| 5 | Eccentric | Yes | + | − | + | n/a | Squamous cell carcinoma |
| 6 | Eccentric | Yes | + | − | − | + | Renal cell carcinoma |
| 7 | Eccentric | Yes | + | − | + | n/a | Carcinoid |
| 8 | Concentric | Yes | + | − | − | − | Adenocarcinoma |
| 9 | Concentric | No | + | − | − | + | Epithelioid granuloma |
| 10 | Eccentric | No | + | − | + | + | Adenocarcinoma |
| 11 | Eccentric | No | + | − | + | + | Melanoma |
| 12 | Concentric | No | + | + | + | + | Adenocarcinoma |
| 13 | Concentric | No | + | + | − | + | Adenocarcinoma |
| 14a | Absent | Yes | − | − | − | n/a | Adenocarcinoma |
| 15 | Eccentric | Yes | + | − | − | − | Carcinoid |
| 16 | Eccentric | No | + | + | + | n/a | Carcinoid |
| 17 | Eccentric | Yes | + | + | + | − | Adenocarcinoma |
| 18 | Eccentric | No | + | + | + | n/a | Carcinoid |
| 19 | Concentric | No | + | + | + | n/a | Melanoma |
| 20 | Eccentric | No | + | − | − | − | Indeterminateb |
| Adequacy for lesion detection and diagnosis: | 19/20=95% | 9/20=45% | 12/20=60% | 9/13=69% | |||
| TBNA+biopsies=16/20=80% | |||||||
nCLE nodule detection: += nCLE criteria compatible with malignancy, granuloma or inflammation − = absence of nCLE malignancy, granuloma or inflammation criteria. ROSE/TBNA/biopsies; + =tissue specimen shows signs of a malignant or non-malignant diagnosis, − =bronchus epithelium/blood contamination without signs of malignancy or an alternative diagnosis.
In one patient with a subsolid nodule, no r-EBUS signal was obtained and nCLE-imaging was unable to detect the nodule.
In one patient no final diagnosis was established and the nodule remained stable at 12months follow-up. nCLE= needle based confocal laser endomicroscopy; TBNA= transbronchial needle aspiration; ROSE= rapid onsite evaluation.
Diagnostic yield
Overall diagnostic yield of the TBNA and biopsies combined was 80% (16/20). TBNA cytology alone established a diagnosis in 12/20 patients (60%) compared to 9/13 patients (69%) with forceps biopsies (Table 2).
nCLE guidance
After puncturing the nodule at the specified location according to the robotic software and r-EBUS signal, in 11/20 patients (55%), nCLE-imaging of the first needle pass revealed immediate nodule detection (n=10 malignant, n=1 granuloma) (Figure 2). After TBNA, corresponding ROSE confirmed representative material in 7/11 patients (Table 2).
In 9/20 patients (45%) nCLE-imaging of the first needle pass revealed airway/lung parenchyma visualization (Figure 2), indicating a near-miss. In all 9 patients, adjustments were made to target the nodule from a different angle, resulting in nCLE nodule detection in 8/9 patients (n=7 malignant, n=1 inflammation). Of the 9 patients in which repositioning was performed, 8/9 patients had an eccentric or absent radial EBUS signal. Corresponding ROSE confirmed malignancy in two patients (Table 2).
nCLE vs ROSE
nCLE-imaging provided tool-in-nodule confirmation in 19/20 patients (95%) whereas corresponding ROSE confirmed representative material in 9/20 patients (45%). When extracting this data on a per-puncture level, a total of 52 individual needle passes were performed with corresponding nCLE-imaging and ROSE feedback. In 32/52 needle passes (62%), nCLE-imaging and ROSE corresponded (n=13 nodule and n=19 airway/lung parenchyma). In 20/53 needle passes (38%), a discrepancy between nCLE-imaging and ROSE was found. In all 20 needle passes, nCLE-imaging provided tool-in-nodule confirmation while ROSE showed non-representative material with blood (n=15) and bronchial epithelium (n=5). No needle passes with nCLE airway/lung parenchyma visualization but representative ROSE occurred.
nCLE nodule characterization
In 19/20 (95%) patients, nCLE-imaging provided tool-in-nodule confirmation (n=17 malignant, n=2 non-malignant). In two patients with a non-malignant diagnosis, nCLE-imaging revealed granulomatous structures and increased densities of small homogenous cells (16, 25). Of the 3 patients with malignant nCLE-imaging but inadequate TBNA and biopsies, two patients were diagnosed with malignancy during follow-up (Table 2). In one patient, nCLE only visualized airway, indicating the needle never reached the nodule. nCLE-imaging revealed a ‘loss of contact’ signal in one patient with necrotic adenocarcinoma (16, 17). In another patient, inflammation surrounding the tumor was also visualized with nCLE as an increased density of small homogeneous cells (16).
nCLE malignancy and airway/lung parenchyma discrimination
Of the 54 obtained nCLE videos, 6 nCLE videos were used for training purposes and 26 videos (n=15 airway/lung parenchyma, n=11 malignancy) for validation purposes (32/54, 59%). Five blinded raters distinguished nCLE-videos of malignancy from airway/lung parenchyma with an overall sensitivity and specificity of 91.9% respectively (Appendix 1). The IOA (κ= 0.80 (95% CI 0.71–0.81)) and IOR (κ= 0.80 ±0.05) between the five raters were substantial (Appendix 1).
DISCUSSION
In this prospective trial, we demonstrate for the first time that robotic bronchoscopic nCLE-imaging is feasible and safe. Importantly, in small, difficult-to-access lung nodules nCLE-guidance provided important feedback on the needle positioning to avoid a near-miss in almost halve of patients. Outcomes of this study show the potential of nCLE-guided robotic bronchoscopy as an improved strategy to diagnose small, peripheral lung nodules in a minimally invasive fashion.
To the best of our knowledge, this is the first study to use nCLE criteria prospectively for real-time feedback on the optimal needle positioning during robotic bronchoscopic procedures (16, 17). Remarkably, when the nodule was punctured at the specified location according to navigational software and r-EBUS signal, nCLE-imaging showed airway and lung parenchyma in 9/20 patients (45%). As the Monarch™ Platform relies on a pre-procedural CT-scan for navigation, this is probably attributed to CT-to-body divergence. Based on our initial results, most added value of nCLE-imaging in peripheral lung nodules analysis is probably found in patient with an eccentric r-EBUS signal.
After repositioning of the robotic bronchoscope, nCLE provided tool-in-nodule confirmation in almost all patients (19/20, 95%). Surprisingly, after nCLE nodule detection, ROSE confirmed adequate tissue sampling with representative cytology in only half of the patients. To what extent the observed combination of near-miss and suboptimal ROSE feedback play a role in the described discrepancy between navigational success and diagnostic yield (26), needs further evaluation
To date, limited data are available on other techniques for tool-in-nodule confirmation during robotic bronchoscopy. A technique that demonstrated promising results during conventional bronchoscopy is cone-beam CT-imaging (CBCT) (9, 22). CBCT guided shape sensing robotic bronchoscopy (Ion Endoluminal system) resulted in an unclearly defined diagnostic yield of 86% in nodules <2cm (27). In 9/50 patients (15%) repositioning of the biopsy needle was indicated. How nCLE compares to CBCT for tool-in-nodule confirmation during robotic bronchoscopy is currently under evaluation (Clinicaltrial.gov NCT05231278).
Remarkable, however, is that the above-mentioned study –like in the present study- implies that tool-in-nodule confirmation does not guarantee adequate tissue acquisition (27). Although nCLE-imaging provided tool-in-nodule confirmation in 19/20 patients, in three patients no adequate tissue was obtained for diagnosis. Thus, although robotic bronchoscopic nCLE-imaging shows good results in the navigation and confirmation parts of the procedure, the acquisition part remains challenging. Whether more extensive sampling, including cryobiopsies (28), could increase the diagnostic yield should be evaluated. Nevertheless, when taking into account the significant smaller nodule size (median 14.5 mm) and frequent eccentric r-EBUS pattern (65%), a diagnostic yield of 80% seems to be an improvement (12, 21).
This proof-of-principle study has several limitations, including the single-center design and limited sample size with high prevalence of lung cancer. Although it was not an inclusion criterion, virtually all nodules were detectable with r-EBUS. Strengths of this study are the small and difficult-to-access nodules that were targeted. Also, the strict definition of (non-) diagnostic samples required convincing evidence of malignancy.
For future use of the nCLE technique, we advise taking biopsies after nCLE tool-in-lesion confirmation to prevent obscured image quality. To assess the effect of nCLE-imaging on diagnostic yield and cost-effectiveness, prospective multi-center studies are needed. It could be that robotic bronchoscopy with advanced techniques for tool-in-nodule confirmation (nCLE/CBCT), is only desirable and cost-effective in a specific, yet to be determined subset of patients (e.g. absence of bronchus sign, eccentric r-EBUS image). Also, identification of benign nCLE criteria will be of great importance. Interestingly, first studies report nCLE-imaging with fluorescent molecular tracers (29–32). In a desirable future concept, immediate bronchoscopic tumor ablation could be performed after bronchoscopic nodule detection and malignancy confirmation (33).
In conclusion, robotic bronchoscopic nCLE-imaging in peripheral lung nodules is feasible and safe. nCLE-imaging provided real-time guidance on the biopsy needle positioning to optimize the sampling area. Whether additional nCLE-guidance results in an improved diagnostic yield should be evaluated in a multi-center prospective trial.
Supplementary Material
Acknowledgements:
We thank the patients who participated in this study. We thank Pieta Wijsman, Kirsten Kalverda, Peter Bonta, Jan-Erik Freund and Rob Hoogvorst for their time and effort in rating the nCLE videos.
Conflict of Interest:
This study was financially and materially supported by Mauna Kea Technologies and Johnson & Johnson Enterprise Innovation. This work is part of the MedPhot research program 00770372 with project number 00770384, which is (partly) financed by the Dutch Research Council (NWO).
CJM reports consulting fees from Auris Health and Johnson and Johnson as well as research support from Mauna Kea Technologies and Johnson and Johnson. TK, PB and JTA report research support from Mauna Kea Technologies.
Footnotes
This work was presented in part during at Annual European Respiratory Society (ERS) Congress 2021 and the Annual ERS Congress 2022.
Human Ethics Approval Declaration: This human participant study was performed in accordance with the Declaration of Helsinki and approved by the Medical Ethical Committee of the Fox Chase Cancer Center (approval number 20–1006). All participants provided written informed consent to participate in this study.
Clinical Trial Registration: NCT04441749 at clinicaltrials.gov
Data Availability Statement:
All data relevant to the study are included in the article. Needle-based confocal laser endomicroscopy video data and source data of the statistical analysis are available upon reasonable request.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187(8):848–54. [DOI] [PubMed] [Google Scholar]
- 3.Minezawa T, Okamura T, Yatsuya H, Yamamoto N, Morikawa S, Yamaguchi T, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seijo LM, de Torres JP, Lozano MD, Bastarrika G, Alcaide AB, Lacunza MM, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a Bronchus sign on CT imaging: results from a prospective study. Chest. 2010;138(6):1316–21. [DOI] [PubMed] [Google Scholar]
- 5.Chen A, Chenna P, Loiselle A, Massoni J, Mayse M, Misselhorn D. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc. 2014;11(4):578–82. [DOI] [PubMed] [Google Scholar]
- 6.Folch EE, Pritchett MA, Nead MA, Bowling MR, Murgu SD, Krimsky WS, et al. Electromagnetic Navigation Bronchoscopy for Peripheral Pulmonary Lesions: One-Year Results of the Prospective, Multicenter NAVIGATE Study. J Thorac Oncol. 2019;14(3):445–58. [DOI] [PubMed] [Google Scholar]
- 7.Ali MS, Sethi J, Taneja A, Musani A, Maldonado F. Computed Tomography Bronchus Sign and the Diagnostic Yield of Guided Bronchoscopy for Peripheral Pulmonary Lesions. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2018;15(8):978–87. [DOI] [PubMed] [Google Scholar]
- 8.Shinagawa N, Yamazaki K, Onodera Y, Asahina H, Kikuchi E, Asano F, et al. Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest. 2007;131(2):549–53. [DOI] [PubMed] [Google Scholar]
- 9.Verhoeven RLJ, Fütterer JJ, Hoefsloot W, van der Heijden E. Cone-Beam CT Image Guidance With and Without Electromagnetic Navigation Bronchoscopy for Biopsy of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol. 2021;28(1):60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett MA. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J Bronchology Interv Pulmonol. 2021;28(2):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent AJ, Byrnes KA, Chang SH. State of the Art: Robotic Bronchoscopy. Semin Thorac Cardiovasc Surg. 2020;32(4):1030–5. [DOI] [PubMed] [Google Scholar]
- 12.Chen AC, Pastis NJ Jr., Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2021;159(2):845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer T, Manley CJ, Annema JT. Robotic Bronchoscopy for Diagnosing Peripheral Lung Lesions: Are We There Yet? Chest. 2021;160(3):e326–e7. [DOI] [PubMed] [Google Scholar]
- 14.van der Heijden E, Verhoeven RLJ. Robotic Assisted Bronchoscopy: The Ultimate Solution for Peripheral Pulmonary Nodules? Respiration. 2022:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casal RF. Cone Beam CT-Guided Bronchoscopy: Here to Stay? J Bronchology Interv Pulmonol. 2018;25(4):255–6. [DOI] [PubMed] [Google Scholar]
- 16.Kramer T, Wijmans L, de Bruin M, van Leeuwen T, Radonic T, Bonta P, et al. Bronchoscopic needle-based confocal laser endomicroscopy (nCLE) as a real-time detection tool for peripheral lung cancer. Thorax. 2022;77(4):370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijmans L, Yared J, de Bruin DM, Meijer SL, Baas P, Bonta PI, et al. Needle-based confocal laser endomicroscopy for real-time diagnosing and staging of lung cancer. Eur Respir J. 2019;53(6). [DOI] [PubMed] [Google Scholar]
- 18.Manley C, Kramer T, Kumar R, Gong Y, Ehya H, Bonta PI, et al. Robotic Navigational Bronchoscopy Combined with Needle-Based Confocal Laser Endomicroscopy: Case Report of a Novel Approach to Diagnose Small Lung Nodules. Respiration. 2022;101(5):494–9. [DOI] [PubMed] [Google Scholar]
- 19.Kalchiem-Dekel O, Connolly JG, Lin IH, Husta BC, Adusumilli PS, Beattie JA, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaddha U, Kovacs SP, Manley C, Hogarth DK, Cumbo-Nacheli G, Bhavani SV, et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: results from the initial multicenter experience. BMC Pulm Med. 2019;19(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchett MA, Schampaert S, de Groot JAH, Schirmer CC, van der Bom I. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol. 2018;25(4):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–82. [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 25.Kramer T, Wijmans L, De Bruin M, Bonta P, Annema J. Needle based confocal laser endomicroscopy (nCLE) for the real-time diagnosis of mediastinal lymph nodes involved in sarcoidosis. European Respiratory Journal. 2019;54(suppl 63):PA3399. [Google Scholar]
- 26.Chen AC, Pastis NJ Jr., Mahajan AK, Khandhar SJ, Simoff MJ, Machuzak MS, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benn BS, Romero AO, Lum M, Krishna G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung. 2021;199(2):177–86. [DOI] [PubMed] [Google Scholar]
- 28.Sryma PB, Mittal S, Madan NK, Tiwari P, Hadda V, Mohan A, et al. Efficacy of Radial Endobronchial Ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL’s): A systematic review and meta-analysis. Pulmonology. 2021. [DOI] [PubMed] [Google Scholar]
- 29.Karstensen JG, Klausen PH, Saftoiu A, Vilmann P. Molecular confocal laser endomicroscopy: a novel technique for in vivo cellular characterization of gastrointestinal lesions. World J Gastroenterol. 2014;20(24):7794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guisier F, Bohn P, Patout M, Piton N, Farah I, Vera P, et al. In- and ex-vivo molecular imaging of apoptosis to assess sensitivity of non-small cell lung cancer to EGFR inhibitors using probe-based confocal laser endomicroscopy. PLoS One. 2017;12(7):e0180576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy GT, Azari FS, Bernstein E, Nadeem B, Chang A, Segil A, et al. Targeted detection of cancer cells during biopsy allows real-time diagnosis of pulmonary nodules. Eur J Nucl Med Mol Imaging. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy GT, Azari FS, Bernstein E, Marfatia I, Din A, Kucharczuk JC, et al. Targeted Intraoperative Molecular Imaging for Localizing Nonpalpable Tumors and Quantifying Resection Margin Distances. JAMA Surg. 2021;156(11):1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer T, Annema JT. Advanced bronchoscopic techniques for the diagnosis and treatment of peripheral lung cancer. Lung Cancer. 2021;161:152–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article. Needle-based confocal laser endomicroscopy video data and source data of the statistical analysis are available upon reasonable request.


