Abstract
Background
Leptospirosis poses a diagnostic challenge owing to its wide array of symptoms, ranging from asymptomatic cases and febrile syndromes to severe disease with a high mortality rate. Risk factors are associated with exposure and the immune response, particularly in immunosuppressed patients.
Case presentation
A clinical case involving a 49-year-old patient with a history of splenectomy and no immunization schedule. The patient presented to the emergency room with non-specific symptoms, primarily myalgias, arthralgias, and emesis, initially suggestive of a viral infection. However, there was a rapid progression to hypoxemic respiratory failure, requiring invasive ventilatory support. Given the immune status due to spleen absence, antibiotic treatment with meropenem and linezolid was promptly initiated, to mitigate the risk of post-splenectomy sepsis. During antibiotic administration, the patient experienced febrile episodes, accompanied by chills, myalgias, and emesis, which gradually decreased in both duration and intensity. Ultimately, the patient exhibited satisfactory progress, successfully underwent extubation, and completed a 7-day antibiotic course. Final reports confirmed positive IgM for Leptospira.
Conclusion
Leptospirosis is a global zoonotic disease, displaying a diverse array of manifestations; recognized as a potential cause of undifferentiated fever, often confused with other prevalent tropical infections. The imperative to consider this diagnosis extends beyond the general population to encompass individuals in states of altered immunity. Recognizing and addressing leptospirosis in at-risk populations is crucial, as it can significantly impact the prompt initiation of treatment and, consequently, influence associated mortality rates.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09854-4.
Keywords: Jarisch-Herxheimer reaction, Pulmonary leptospirosis, Splenectomy, Immune system, Case report
Background
Leptospirosis is a zoonosis caused by a bacteria of the genus Leptospira. The reported incidence ranges from 0.10 to 975 cases per 100,000 people annually [1]. It presents a diagnostic challenge due to the variety of symptoms, as it is the most common febrile syndrome with pulmonary and hematologic involvement. The most severe form being related to hemorrhage, kidney failure and jaundice, known as Weil syndrome; and without underestimating the less common but possible neurological, cardiovascular, and hepatic manifestations. Individuals at heightened risk include those with compromised immune responses or those exposed to contaminated water sources. Despite this, the literature provides limited evidence concerning the infection’s manifestations beyond its natural history and even less evidence in immunocompromised groups and of immune-mediated reactions, such as Jarisch-Herxheimer reaction (JHR), which is characterized by an acute inflammatory response secondary to the massive production and release of cytokines, as a result of the rupture and removal of spirochetes from the bloodstream, typically occurring after the administration of antibiotic treatment [2, 3]. We present a clinical case belonging to a special population, asplenic patients, in which the interactions of the immune response to common bacteria and even less to spirochetes remain unclear.
Case presentation
A 49-year-old male patient with medical history of hypothyroidism and splenectomy secondary to pancreatic cyst resection, without immunization schedule, presented with a 20-day history of myalgias in the lower limbs, arthralgias in the knees, which migrated to the ankles and feet, accompanied by nausea and vomiting. He denied fever, as well as gastrointestinal, respiratory or urinary symptoms. 25 days before the onset of symptoms, he traveled to a city at sea level and swam in a natural river. On arrival, his vital signs showed blood pressure of 117/72 mmHg, heart rate of 77 beats per minute, respiratory rate of 22 breaths per minute, oxygen saturation of 91% and body temperature of 37 ºC. On initial physical examination he was alert and cooperative, upon inspection he had dry mucous membranes, on auscultation were found diminished breath sounds at the bases of the lungs, abdominal palpation was normal and there was reproduction of pain in the legs on palpation. The rest of the physical exam in the emergency room was unremarkable.
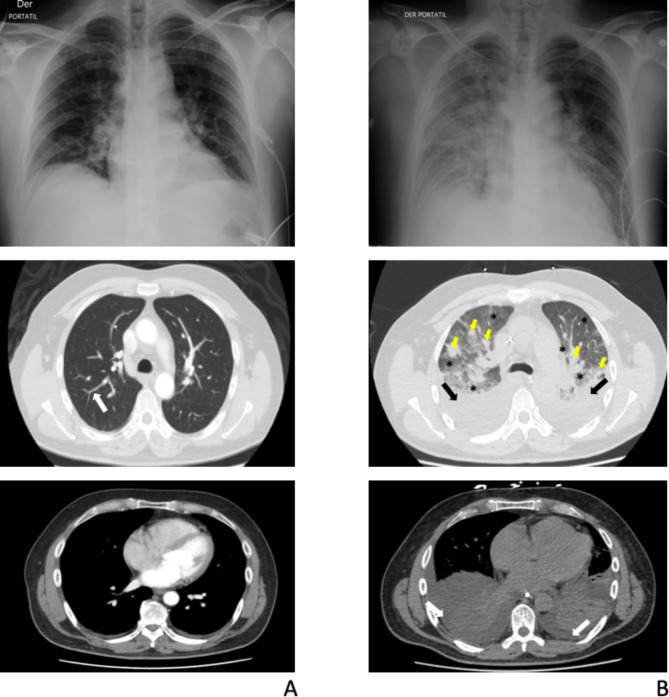
Initial laboratory tests revealed a complete blood count without leukocytosis but with neutrophilia, an elevated reactive C protein (RCP) level of 87.6 mg/L, and abnormal renal function parameters (creatinine 1.4 mg/dL and blood urea nitrogen [BUN] 28 mg/dL), complementary laboratory tests are described in Supplementary information Tables 1 and 2. Initially, a chest X-ray and a computed tomography (CT) scan of the chest were performed (see Fig. 1A). Given the likely prodromal symptoms of a viral infection, he received intravenous fluids. Twelve hours after admission, his condition worsened with hypotension unresponsive to fluids and desaturation with a non-rebreather mask at 20 L/min. Consequently, the patient was admitted to the intensive care unit (ICU) for vasopressor support and high-flow nasal cannula (HFNC) due to acute hypoxemic respiratory failure. A diagnostic multiplex polymerase chain reaction (mPCR) for respiratory viruses was performed on a nasopharyngeal swab sample and the result was negative (Supplementary Information Table 3). Therefore, empirical treatment with meropenem and linezolid was initiated, considering the patient’s asplenic status without immunization and risk to post-splenectomy sepsis. Following antibiotic administration, the patient experienced febrile episodes accompanied by chills, myalgias, and vomiting, lasting two hours and gradually diminishing in both duration and intensity. After 36 h of HFNC support, there was worsening hypoxemia and an increase in pulmonary infiltrates, as described in Fig. 1B, so orotracheal intubation and mechanical ventilation were initiated immediately. With the proposed syndromic diagnosis and among the differential diagnoses, which included pneumonia, pulmonary embolism, pulmonary hemorrhage, cardiac and non-cardiac pulmonary edema; additional examinations were therefore carried out to clarify the diagnosis. Bronchoscopy with bronchoalveolar lavage (BAL) was performed, with normal macroscopic findings, yielding negative cultures for aerobic and anaerobic microorganisms after 72 h of incubation, according to institution´s microbiology area protocols and also a negative pneumonia mPCR of BAL results (Supplementary Information Table 4). Heart failure and pulmonary embolism were ruled out by transthoracic echocardiogram and computed tomography pulmonary angiogram. On the third day of mechanical ventilation, oxygenation and radiologic findings improved, leading to a successful extubation. Forthy-eight hours after extubation and seven days after meropenem treatment, laboratory results revealed high levels of Leptospira IgM (micro enzyme-linked immunosorbent assay technic), suggesting acute leptospirosis with pulmonary involvement associated with the Jarisch-Herxherimer reaction.
Fig. 1.
Chest X-rays and chest computed tomography (CT) scans. A Initial radiologic studies. Fibro atelectatic tracts compromising the posterior segments of bilateral inferior lobes (white arrow). B. Control radiologic studies (taken after respiratory deterioration). Bilateral basal consolidations involving all segments of the lower lobes (black arrows), associated with posterior pleural effusions (white arrows). Extensive patchy opacities in the periphery of both lung fields (yellow arrows). Ground glass pattern in the remaining lung fields (black asterisks)
Discussion and conclusions
Leptospirosis is a zoonotic disease caused by a spirochete of the genus Leptospira. Although it is widespread worldwide, it is more common in tropical regions. Transmission occurs via the urine of infected animals or contaminated water. Therefore, at-risk groups are associated with resource-limited settings and individuals who perform activities in contaminated environments, such as farmers, sewage workers, military personnel, participants in water sports and natural water recreation [4].
Epidemiologic and demographic data have been collected by several groups, with the International Leptospirosis Society (ILS) and the Leptospirosis Epidemiological Reference Group (LERG) among the major stakeholders. The reported incidence varies widely, ranging from 0.10 to 975 cases per 100,000 people annually, with mortality rates from 10 to 70%, contingent upon the severity of the infection [1].
The incubation period of leptospirosis is approximately 2 to 30 days and the clinical presentation is diverse, ranging from asymptomatic to a self-limiting febrile and, in severe instances, multi-organ failure. The symptoms are heterogeneous and mainly include fever, headache, myalgia, arthralgia, nausea, vomiting, abdominal pain, among others. Severe forms of the disease can lead to bleeding, meningitis, respiratory failure, kidney and liver failure; the most fatal form is characterized by kidney failure, jaundice and bleeding and is known as Weil syndrome. Leptospira particularly targets the liver, lungs and kidneys. In hepatocytes, it induces hepatocellular damage and disrupts intercellular junctions [5, 6]; in the lungs, immunoglobulin and complement deposits have been detected in the alveolar membrane, particularly in cases with severe hemorrhagic manifestations [7], and in the kidneys, where Leptospira tends to concentrate in reservoirs, characteristic tubular changes and interstitial nephritis are found [8, 9].
The immune response to these spirochetes remains incompletely understood, influenced by various factors, such as bacteria’s virulence factors and a wide spectrum of variations in innate and adaptive immune responses, which would explain the heterogeneity of clinical presentation and severity. Regarding the innate immune response, Leptospira is recognized by innate receptors of phagocytic cells, which are subsequently associated with Toll-like receptors (TLR) and Nod-like receptors (NLR). This recognition has a probable role in the early control of infection, eventually leading to the production of tumor necrosis factor alpha (TNFα), which, together with the strong activation of neutrophils and thus the pro-inflammatory response, are the main contributors to the development of the systemic inflammatory response and had been found elevated among other cytokines in severe forms of the disease. With respect to macrophages, although they have been recognized as the effector mechanism in the phagocytic control of Leptospira, they can be inactivated or induced to apoptosis by the same bacteria, or the inflammasome and perpetrator cytokines (IL-1β, IL-18), which will facilitate survival and proliferation of the bacteria. Few recent articles have found controversial data about Leptospira preventing macrophage death, mainly in animal models, founding that in specific serovars of Leptospira, there was no induction of pyroptosis or cell death; however, it is still a hypothesis, but it would be an interesting point of research [2]. In the adaptive immune system, T lymphocytes and inflammatory cytokines (IL-1β, IL-2, IL-6, IL-12, TNFα) play a dual role. Initially responsible for eliminating the bacteria, but also for their uncontrolled production, culminating in a cytokine storm with subsequent immune paralysis leading to septic shock and multiple organ dysfunction; all in combination with inhibition of the protective T-response added to inflammatory overproduction. High levels of these inflammatory proteins have been found mainly in immunological phases of the most severe forms of the infection, and can be related as markers of severity. Finally, the humoral response involves the production of IgM and IgG antibodies towards the third day, and IgA from the fifth day to the ninth month of infection [4, 10].
To our knowledge, this is the first case report of Jarisch Herxheimer reaction in an asplenic patient with leptospirosis. We have only found one case report of a patient with leptospirosis, asplenia and other risk factors, in whom this reaction did not occur [3]. However, it is globally recognized that this patient group is at a higher risk of developing serious infections, particularly those caused by encapsulated bacteria, a condition commonly referred as post-splenectomy sepsis. Physiologically, the absence of the spleen is associated with the loss of antigenic, opsonizing, and phagocytic presentation activity, allowing hematogenous dissemination accompanied by a significant inflammatory response. In the presented case of asplenia, this would explain the failure to effectively clear Leptospira, the severe inflammatory reaction in its clinical presentation, and despite testing positive for IgM, the immunological alteration is also associated with a delayed and altered production of immunoglobulins, with weak functionality that does not endure over time. This underscores the importance of vaccination in asplenic patients, due to the considerable limitation in the production of natural antibodies. Furthermore, since this disease is not vaccine preventable, not only in this group of special patients but also in overall population, general prevention recommendations such as those generated by the Center for Disease Control and Prevention (CDC), are to avoid contact with contaminated water and to wear protective clothing and footwear during activities in natural waters. There is no scientific evidence that other preventive measures such as antibiotic prophylaxis reduce the incidence of symptomatic infection.
On the other hand, it is worth highlighting within the diagnosis of the case and its manifestations, the well-recognized reaction of JHR; which is characterized by an acute inflammatory response secondary to the massive production and release of cytokines, as a result of the rupture and removal of spirochetes from the bloodstream, typically occurring after the administration of antibiotic treatment. Although extensively studied in other infections such as syphilis and Lyme disease, its characterization in leptospirosis is not fully understood, and it is believed to be related to a lower bacteremic load [11, 12]. Despite this, it is interesting to note that in our clinical case, symptoms consistent with this reaction were observed, almost immediately following the administration of the beta-lactam antibiotic. Furthermore, the duration of these episodes decreased over time, with the first lasting approximately two hours, followed by a maximum of 30 min until complete disappearance, coinciding with clinical improvement. Additionally, as previously mentioned, the absence of a spleen and its implications likely contributed to increased inflammatory production, and could therefore also be related to a more noticeable and exaggerated JHR.
For the diagnosis of leptospirosis, detection of the microorganism in body fluids or tissues, isolation in cultures, or detection of specific antibodies is necessary. In many cases, including ours, antibody detection (IgM) is the only method available. But although the standard diagnostic test is the microscopic agglutination test (MAT), it is admitted that under limited conditions and recognizing its limitations, having a round clinical scenario, risk factors and time of exposure, other methods can be used as diagnostic tools.
Regarding the treatment of leptospirosis, early initiation of antibiotics can be the cornerstone to prevent progression to severe disease, in addition to providing organ-specific life support when necessary. Leptospira is susceptible to various antibiotic classes, including beta-lactams, macrolides, tetracyclines, and fluoroquinolones. However, beta-lactams are the antimicrobial group of choice, particularly penicillin, ampicillin, ceftriaxone, or cefotaxime, this is based on clinical experience, which is known to be not very robust. The choice depends on the disease and considerations of pharmacokinetic and pharmacodynamic variables found in the clinical scenario, recognizing the non-inferiority of one class in relation to another, and specifically talking about non-inferiority of ceftriaxone to penicillin, both proposed as ideal intravenous management.
On the other hand, given the nature of the described pathophysiology, the use of immune therapy such as the use of corticosteroids and plasma exchange has been investigated; it seems to have a special impact on survival, on the early course of severe disease with hemorrhagic pulmonary involvement, in conjunction with organic support and antibiotic therapy; however, as mentioned above, the lack of high quality studies does not allow recommendations for its routine use. The risk of adverse events (e.g. nosocomial infections) and other indications associated with its use, must be taken into account [13, 14].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Fundación Clínica Shaio.
Abbreviations
- CRP
C-reactive protein
- ICU
Intensive care unit
- HFNC
High-flow nasal cannula
- mPCR
Multiplex polymerase chain reaction
- ILS
International Leptospirosis Society
- LERG
Leptospirosis Epidemiological Reference Group
- TLRs
Toll-like receptors
- NLRs
Nod-like receptors
- TNFα
tumor necrosis factor-alpha
- JHR
Jarisch-Herxheimer reaction
- MAT
Microscopic agglutination test
- CDC
Center for Disease Control and Prevention
Author contributions
MZE: conceptualization, formal analysis, methodology, project administration, supervision, visualization, prepared Fig. 1, writing original draft, writing review and editing. MPG: conceptualization, formal analysis, methodology, project administration, supervision, visualization, prepared Fig. 1, writing original draft, writing review, editing and funding acquisition. JTP: visualization, writing original draft, writing review and editing. PSH: prepared Fig. 1, writing original draft, writing review and editing.
Funding
All authors received funding from Fundación Clínica Shaio, the publishment and funding of this study was approved by Fundación Clínica Shaio DIB code DIB 24 − 16.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
We obtained institutional authorization from the Research and Ethics Committee of Fundación Clínica Shaio, to use the information and carry out this project.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonhomme D, Hernandez-Trejo V, Papadopoulos S, Pigache R, Fanton d’Andon M, Outlioua A, Boneca IG, Werts C. Leptospira interrogans Prevents Macrophage Cell Death and Pyroptotic IL-1β Release through Its Atypical Lipopolysaccharide. J Immunol. 2023;210(4):459–474. 10.4049/jimmunol.2200584. PMID: 36602965. [DOI] [PubMed]
- 3.García J, Cervera E, Atilano D, et al. Leptospirosis in an asplenic patient -case report. BMC Infect Dis. 2020;20:186. 10.1186/s12879-020-4869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyahara S, Saito M, Kanemaru T, Villanueva SY, Gloriani NG, Yoshida S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int J Exp Pathol. 2014;95(4):271–81. 10.1111/iep.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merien F, Truccolo J, Rougier Y, Baranton G, Perolat P. In vivo apoptosis of hepatocytes in guinea pigs infected with Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol Lett. 1998;169(1):95–102. 10.1111/j.1574-6968.1998.tb13304.x. [DOI] [PubMed] [Google Scholar]
- 7.Nally JE, Chantranuwat C, Wu XY, Fishbein MC, Pereira MM, Da Silva JJ, Blanco DR, Lovett MA. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am J Pathol. 2004;164(3):1115–27. 10.1016/S0002-9440(10)63198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herath NJ, Kularatne SA, Weerakoon KG, Wazil A, Subasinghe N, Ratnatunga NV. Long term outcome of acute kidney injury due to leptospirosis? A longitudinal study in Sri Lanka. BMC Res Notes. 2014;7:398. 10.1186/1756-0500-7-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CW, Hung CC, Wu MS, Tian YC, Chang CT, Pan MJ, Vandewalle A. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 2006;69(5):815–22. 10.1038/sj.ki.5000119. [DOI] [PubMed] [Google Scholar]
- 10.Ramírez R, Agudelo P, Acevedo L. Inmunología De La leptospirosis. Rev CES Med. 2019;33(3):192–200. [Google Scholar]
- 11.Guerrier G, D’Ortenzio E. The Jarisch-Herxheimer reaction in leptospirosis: a systematic review. PLoS ONE 8(3): e59266. 10.1371/journal.pone.0059266 [DOI] [PMC free article] [PubMed]
- 12.Cagliero J, Villanueva SYAM, Matsui M. Leptospirosis Pathophysiology: into the storm of cytokines. Front Cell Infect Microbiol 8:204. 10.3389/fcimb.2018.00204 [DOI] [PMC free article] [PubMed]
- 13.Petakh P P, Behzadi P, Oksenych V, Kamyshnyi O. Current treatment options for leptospirosis: a mini-review. Front Microbiol. 2024;15(1403765). 10.3389/fmicb.2024.1403765. [DOI] [PMC free article] [PubMed]
- 14.Rodrigo C, et al. High dose corticosteroids in severe leptospirosis: a systematic review. Trans Royal Soc Trop Med Hygiene vol. 2014;108(12):743–50. 10.1093/trstmh/tru148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.