Abstract
Background
Robot-assisted rehabilitation is considered beneficial for functional recovery in patients with stroke, but the therapeutic effect remains inconclusive. The present study investigated the therapeutic effects of gait training assisted by a user-initiated powered exoskeletal robot (UIPER) in patients in the early stage after stroke. We also characterized patients’ improvement by analyzing chronological changes in clinical measurements together with gait parameters obtained from internal sensors in the exoskeletal robot.
Methods
In this pilot case-controlled study, 17 and 81 patients with stroke onset durations of < 3 months were included in the robot-assisted combined with conventional treatment (RT + CT) group and conventional treatment only (CT) group, respectively. The UIPER, which provides knee flexion and extension support and has hip and knee sensors, was applied to guide gait performance in the RT + CT group. The patients in the RT + CT group received robot-assisted gait training for 40 min/ session, 1 session a day, and 2–3 sessions a week (6 sessions in total). The primary outcome was the proportion of patients reaching the minimum clinically important difference (MCID) in the 5-meter walking speed (5MWS) assessment, and the secondary outcomes were the MCID for the six-minute walking test, the Berg Balance Scale, the Barthel Index, the Fugl-Meyer assessment, and the timed up and go test before, during, and after the interventions. Gait parameters of the hip and knee were evaluated at baseline, midterm, and final sessions.
Results
Gait function improved in both groups after the intervention (both P < 0.05). The primary outcome showed that a greater proportion of patients reached the MCID for the 5MWS in the RT + CT group than in the CT group (70.6 vs. 43.2%, P = 0.040; 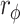 = 0.208). Similarly, in terms of the secondary outcomes, more patients in the RT + CT group reached the MCID for the Barthel index as compared with the CT group (41.2 vs. 17.3%, P = 0.047,
= 0.208). Similarly, in terms of the secondary outcomes, more patients in the RT + CT group reached the MCID for the Barthel index as compared with the CT group (41.2 vs. 17.3%, P = 0.047, 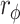 = 0.220). Gait analysis revealed improvements in gait in the RT + CT group, as indicated by increases in the perimeter and area of the hip–knee cyclogram, as well as the range of motion in the hip joint.
= 0.220). Gait analysis revealed improvements in gait in the RT + CT group, as indicated by increases in the perimeter and area of the hip–knee cyclogram, as well as the range of motion in the hip joint.
Conclusions
Gait training assisted by UIPER facilitates the recovery of walking speed and activities of daily living in patients with stroke, and these improvements may be related to improvements in gait parameters. Randomized controlled studies with larger sample sizes are needed to confirm these findings.
Trial registration
This trial was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 202200822B0).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01510-x.
Keywords: Exoskeletal robot, Robot-assisted training, Gait, Stroke, Post-acute care, User-initiated control
Background
The majority of patients with stroke have a clinical presentation of hemiplegia, which can cause a loss of locomotor function [1, 2]. Restoration of walking ability is thus among the most important objectives of rehabilitation, particularly for those in the early stages after stroke. A common therapeutic approach is to correct the patient’s gait pattern, such as hip flexion, for daily activities in patients whose lower limb muscle power has partly recovered [3–5]. However, another challenge emerges during this recovery stage, as patients tend to develop unwanted compensatory movements manifested by exaggerated synergistic patterns [5, 6]. The resulting alternation of hip-knee coordination results in shorter step lengths on the affected side as well as a slower walking speed and unsymmetric gait [7–9]. The mechanical force exerted on the knee assists both extension and flexion of the knee, an approach that could potentially compensate for the inadequate torque in the affected side and thus enhance symmetry of weight-bearing during walking.
It is hypothesized that, as patients’ ambulatory ability gradually improve along the course of recovery, rehabilitation robots will progressively reduce the magnitude of assistance over time. Over recent decades, lower-limb exoskeletal robots have offered a novel approach for facilitating gait recovery in patients with stroke. Exoskeletal robots typically provide assistance for overground walking through advanced engineering using user-initiated, robot-initiated, and adaptive control with adjustable personalized parameters, thus offering precise robot actuation to augment the movement of the lower limbs during both the stance and swing phases of the gait cycle [10–13]. However, because motor capacity varies widely among patients with stroke, different motor recovery stages of post-stroke gait are supposed to benefit from specific type of rehabilitation robots, each of which was designed to address distinct phases of motor recovery [14–16]. Treadmill-based systems such as the Lokomat, a device with hip and knee actuations, ankle support, and body suspension, are suited for early-stage stroke patients who have minimal or no hip activation. Other hip-knee-ankle systems, such as the full-body, trunk-supported Ekso or the bioelectrically controlled Hybrid Assistive Limb (HAL), can facilitate weight-bearing, ground contact, and hip extension to trigger exoskeletal knee flexion. In later stages of motor recovery, a user-initiated control mechanism is essential for improving patient-driven gait, particularly for helping patients to perform knee flexion followed by hip activation. It is important to note that robots with this functionality is not yet widely applied clinically.
For this purpose, the Keeogo, a user-initiated powered exoskeletal robot (UIPER), is primarily designed to assist the patient with mobility impairments with a higher motor function stage for enhancing their locomotive abilities in communities and daily activities, such as walking, stair climbing, and transitioning [10]. Based on this design concept, the robot has knee motors but no hip motors and uses sensors in the hip and knee joints to detect and interpret the user’s intended movement, and then applies complementary torque through knee motors to assist performing stance and swing phases in gait. Although, the device has shown its potential to benefit motor control, postural stability, and movement kinetics in patients with stroke [17], its treatment effects and related gait parameters remain unclear.
Gait parameters during walking play a crucial role in determining the optimal settings for the application of UIPER on patients with stroke. Parameters such as walking speed [18] and gait symmetry [19, 20] are commonly used for assessing gait performance in the patients with stroke. The cyclogram, a measurement of inter-joint coordination during walking obtained from the data recorded by cameras or wearable sensers [21], provides a continuous, quantitively, and visual depiction of how two joints coordinate throughout a gait cycle [22]. For example, the cyclogram of normal gait obtained from a healthy participant shows smooth and consistent patterns, while that in patients with stroke would reflect deviations in joint coordination. In addition, range of motion, joint symmetry, gait similarity between both affected and unaffected sides, and the length of the gait trajectory can also be analyzed from the cyclogram. Therefore, analyzing kinematic parameters from sensors to objectively measure changes in gait performance can help understand how robot-assisted gait training facilitates post-stroke gait.
In this pilot study, we explored the therapeutic effects of the exoskeletal robot-assisted gait training (ERGT) in patients with stroke who demonstrated a relative higher levels of gait function at the early recovery stage, qualifying them for the UIPER category. A key innovation of our study was not only evaluating the therapeutic outcome, but also analyzing the alterations of gait parameters using data recorded by the internal sensors of the exoskeletal robot for characterizing how the robot enhance the gait. This process represents a state-of-the-art approach for objectively guiding and refining robot-assisted gait training throughout the rehabilitation in patients with stroke. Furthermore, this information offers critical insights for optimizing the selection of lower-limb robotic exoskeletons tailored to the specific needs of individual stroke patients for enhancing therapeutic outcomes and personalization of treatment.
Materials and methods
Study design
The present study applied a case-control study design using retrospective cohort data, in which the outcomes of ERGT were compared between the RT + CT and CT groups.
Participants
The present study recruited patients in the early stage after stroke and who were enrolled in the Taiwan postacute care (PAC) program, which entails daily sessions of physical therapy (emphasizing movement, balance, and walking), occupational therapy (targeting daily activities), and speech and language therapy (addressing communication and swallowing abilities) over a span of up to 12 weeks. The PAC guidelines in Taiwan, issued by the National Health Insurance Administration (NHIA), outline the parameters for enrollment in PAC programs, emphasizing the necessity for patients to exhibit high rehabilitation potential, to be typically within 30 days of stroke onset, and to possess a modified Rankin score (mRS) ranging from 2 to 4 [23].
The patients were divided into two groups: the RT + CT group and the CT group. The patients in the RT + CT group received ERGT and conventional rehabilitation, while those in the CT group only received conventional rehabilitation. The inclusion criteria for the participants in the RT + CT group were as follows: (1) aged 20 years or older and able to maintain a seated or standing position for at least 1 h with support; (3) a BBS score > 30 (mobility aids are required for scores 31 to 35); (4) a hip muscle power score ≥ 3; (5) a knee muscle power score ≥ 2; (6) a MAS score of the upper and lower limbs < 3; and (7) a Fugl-Meyer assessment score of lower extremity > 18. The exclusion criteria were as follows: (1) an inability to stand and walk even with the support of the lower limb exoskeletal robot; (2) vulnerable individuals prone to injuries; (3) nonnative speakers; (4) patients with conditions such as aphasia, moderate to severe cognitive impairment (e.g., a Mini-Mental State Examination (MMSE) score < 18 or moderate to severe dementia), or mental disorders (e.g., delirium) that would hinder their ability to cooperate with the experiment; (5) severe musculoskeletal disorders of the lower limbs (e.g., fractures, tendon ruptures) that would render them unable to withstand external forces; (6) skin disorders (e.g., pressure sores, wounds, cellulitis) that would prevent the use of the lower limb exoskeletal robot for rehabilitation; (7) complete paralysis of the lower limbs or unhealed ulcers; (8) unilateral or bilateral deep vein thrombosis of the lower limbs or amputations; (9) severe hypertension not managed to a resting blood pressure within 180/100 mmHg under medical control; (10) severe heart diseases, including a history of hospitalization for myocardial infarction or cardiac surgery within the past 3 months, congestive heart failure, severe and unstable arrhythmias, hypertrophic cardiomyopathy, severe aortic valve stenosis, angina, or respiratory difficulties during rest or daily activities; (11) joint diseases of the hip or knee joints limiting sufficient active range of motion (AROM) for walking, sitting, or squatting: a hip joint AROM < 60° or a knee joint AROM < 90° or a knee joint flexion contracture > 30°; (12) spasticity of the lower limbs, with a modified Ashworth Scale (MAS) score greater than 3; (13) severe pain when standing, with a pain scale score (numerical rating scale, NRS) greater than 6; (14) known severe osteoporosis, with a bone density more than 2.5 standard deviations below the average value for young adults and with one or more osteoporotic fractures; and (15) a history of epileptic seizures.
Patients in the CT group were included according to: (1) the case data which is from the PAC database of Taoyuang Chang Gung Memorial Hospital from March 2014 to January 2024; (2) the patient’s medical records were reviewed to ensure their functional clinical assessments met the inclusion criteria for the CT + RT group, except for the Fugl-Meyer assessment for the lower extremities, as this measurement was not included in the database. The exclusion criteria for the CT group were as follows: (1) incomplete demographic or functional assessment data; (2) older or younger than the participants in the RT + CT group, age greater than 71 years or less than 34 years; (3) not meeting the inclusion criteria to use the exoskeletal robot; and (4) a MMSE score < 18; (5) an mRS score ≦ 2; and (6) bilaterally affected lower limbs. To minimize selection bias, we used the exclusion criteria for screening the CT group data, an approach that could avoid subjective selection of data.
Experimental set-up and intervention
For the RT + CT group, UIPER-assisted ERGT was administered as the intervention. We modified the dosage of ERGT from the previous study that conducted 1 training session a day for five consecutive days [24]. A 1–2 day inter-intervention-interval in the training protocol was designed for avoiding fatigue and reduce the effectiveness of the training in the patient caused by a high frequency of treatment. The training was 40 min per session, 1 session a day, 2–3 sessions a week, and 6 sessions in total. The setting parameters for the force of assistance provided by the knee actuators were determined according to the participant’s motor ability for each training session to ensure proper gait support. The patients in the RT + CT group also received regular conventional rehabilitation in the PAC program, which included at least 50 min of occupational therapy, 50 min of physical therapy per day for 5 days a week, and may also receive speech therapy according to clinical requirements. As mentioned, the patients in the CT group received regular conventional occupational, physical training, and speech therapy. The regular conventional occupational, physical, and speech therapies consists of facilitation techniques, balance training, ambulation training, upper limb functions, fine motor functions, cognition training, swallowing training, speech comprehensive and expression, and etc.
The user-initiated powered exoskeletal robot (UIPER)
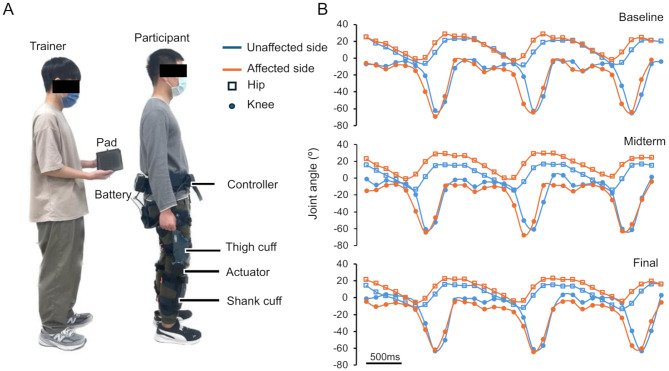
The UIPER (Keeogo, Wistron Medical Technology, Taiwan) was used exclusively by participants in the RT + CT group. The device provides power assistance for knee torque and consists of bilateral actuators in the knee joint module that assist the left and right knees during specific phases of walking (Fig. 1A). The right and left hips and knees are equipped with gyroscopic sensors, accelerometers, and angle sensors. We recorded these signals during walking with a sampling period of 120 ms, and the kinematic data of the joint angles were further analyzed (Fig. 1B).
Fig. 1.
Exoskeletal robot and kinematic signals obtained from the joint angle sensors for the hip and knee. (A) Exoskeletal robot used for gait training. The pad is used to adjust the knee joint strength assistance parameters and signal recording. During the gait data recording session, the trainer holds the pad and connects it to the exoskeletal robot via a cable, walking together with the patient. (B) Three gait cycles of hip and knee joint angles during walking before an intervention session, recorded without power support (free mode). Kinematic data of bilateral hip and knee joints (colored orange on the affected side, blue on the unaffected side) as a function of the walking time. The data were obtained from a sample patient at three time points during the course of ERGT: the baseline, midterm, and final assessments
Kinematics analysis
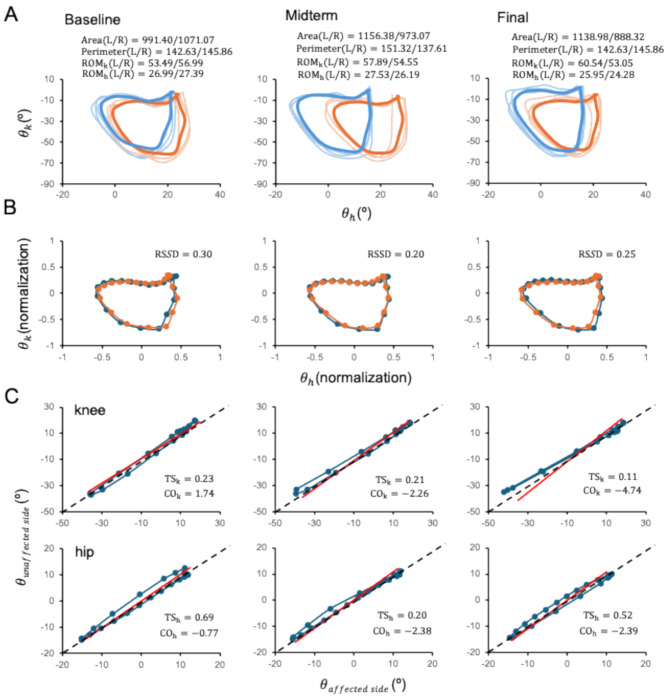
For the kinematics analysis, we specifically analyzed the inter-joint coordination [21] between the hip and knee joints as well as between the joints on the paralyzed and healthy sides. A cyclogram, also called an angle-angle diagram [22], is then used to represent the functional relationship between joint pairs during a full gait cycle. The cyclogram is constructed by plotting two angles, such as the alteration of the hip and knee joint angles, on the X-Y plane, and it can yield several types of clinically relevant parameters, such as the range of motion of the joints, the cyclogram area, and the cyclogram perimeter, as their utilities have been supported in orthopedic [25, 26] and neurologic conditions [27, 28].
The angle variations at the hip and knee joints in the sagittal plane (Fig. 1B) were used to construct the hip-knee cyclograms for the affected and unaffected sides (Fig. 2A). Various parameters were then used to quantify the cyclograms as follows:
Fig. 2.
Analysis of kinematic data at the three assessment time points using the data in Fig. 1(B). (A) Cyclograms illustrating the inter-joint coordination in a gait cycle, from which the area, perimeter, and range of motion (ROM) of the knee (ROMk) and hip (ROMh) can be computed for the gait parameters. The orange and blue lines indicate the data obtained from the affected (R) and unaffected (L) sides, respectively; the thinner line represents the individual gait cycles, the thicker line represents the averaged gait cycle, 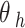 is the range of motion of the hip joint, and
is the range of motion of the hip joint, and 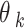 is the range of motion of the knee joint. (B) Gait similarity analysis of gait cycles on the affected and unaffected sides based on the cyclograms. RSSD, the square root of the sum of squared deviations. (C) Symmetry analysis of the knee (top) and hip (bottom) joints in a gait cycle. TS, trend symmetry; CO, cyclogram orientation
is the range of motion of the knee joint. (B) Gait similarity analysis of gait cycles on the affected and unaffected sides based on the cyclograms. RSSD, the square root of the sum of squared deviations. (C) Symmetry analysis of the knee (top) and hip (bottom) joints in a gait cycle. TS, trend symmetry; CO, cyclogram orientation
-
The square root of the sum of squared deviations (RSSD)(1) obtained by the SSD after uniform scaling and translation of the cyclogram centroids to the origin was used to analyze the similarity between the bilateral hip-knee cyclogram j and cyclogram k. A value of 0 indicates identity in the patterns, and larger values indicate greater dissimilarity (Fig. 2B).
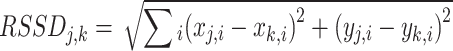
1 where the symbols
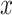 and
and 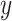 represent the scaled and transformed hip- and knee- angles, respectively at the same point
represent the scaled and transformed hip- and knee- angles, respectively at the same point 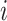 .
. -
The symmetry of the joints corresponds to the trend symmetry (TS) [29, 30]. The TS quantifies the similarity between the angular trends of the right and left legs across gait cycles for both the hip and knee joints and is calculated using the following equation:
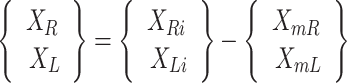
2 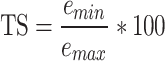
3 where the symbols R and L represent the right and left sides, respectively. The variable ‘m’ denotes the mean value calculated from ‘i’ data points within a gait parameter waveform, where ‘i’ represents each individual data point. Additionally, ‘emin’ and ‘emax’ represent the minimum and maximum eigenvectors, respectively. For the TS, a value of 0 indicates perfect symmetry, and a higher value (3) indicates a higher level of asymmetry. Notably, this parameter remains unaffected by waveform shifts or magnitude differences between the two waveforms (Fig. 2C).
-
Cyclogram orientation (CO) represents perfect interlimb symmetry based on the TS of the left joint angle–right joint angle (Fig. 2C). Smaller CO values (4) indicate a greater degree of inter joint symmetry and is calculated as follows:

4 where e1 and e2 denote the elements of the eigenvectors.
-
Cyclogram perimeter (
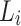 ): The length of the trajectory reflects the average joint velocity and the distance covered by the hip and knee joints. As such, the perimeter can be considered a measure of the coordination between the hip and knee joints during a gait cycle (Fig. 2A) and is calculated as follows:
): The length of the trajectory reflects the average joint velocity and the distance covered by the hip and knee joints. As such, the perimeter can be considered a measure of the coordination between the hip and knee joints during a gait cycle (Fig. 2A) and is calculated as follows: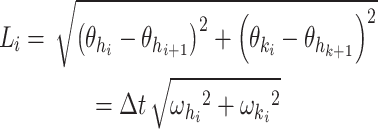
5 Where
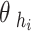 and
and 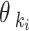 represent the hip and knee joint angles, respectively, at point i. The variables
represent the hip and knee joint angles, respectively, at point i. The variables 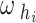 and
and 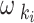 represent the average angular velocities of the hip and knee joints, respectively, within a specific time interval
represent the average angular velocities of the hip and knee joints, respectively, within a specific time interval 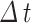 .
. -
Cyclogram area: The area of the closed trajectory corresponds to the simultaneous angular variation occurring at the two joints of interest during the gait cycle. A larger area typically signifies a greater conjoint range of angular movements experienced at a specific joint pair throughout a complete gait cycle (Fig. 2A) and is calculated as follows:
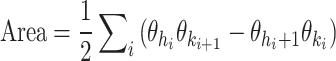
6
Clinical assessments
The therapeutic effect of the intervention was assessed with outcomes to measure changes in performance before and after the intervention, noted as the baseline and final assessments, respectively. In the RT + CT group, an additional assessment was performed at the midterm of the training program. The assessments were performed by the medically licensed personnel and were performed when the patient was not wearing any exoskeletal robot. The final assessments were conducted within 2 days after the 6th treatment, and the midterm assessments were performed after the 3rd treatment.
Primary outcome
Five-meter walking speed (5MWS)
The 5MWS test, which is the time it takes for a patient with stroke to walk 5 m at a comfortable high speed (in m/s) on a flat surface compared to that of other persons, has been commonly used for assessing longitudinal changes in walking speed after stroke. The minimum clinically important difference (MCID) for the 5MWS is 0.16 m/s [31].
Secondary outcomes
Barthel index (BI)
The BI is used in clinics to assess independence in daily activities and ranges from 0 (completely dependent) to 100 (independent) [32]. The MCID for patients that fit the criteria for the PAC program is 16.18 for ischemic stroke and 5.02 for hemorrhagic stroke [33].
Berg balance test (BBS)
The BBS is an assessment tool with high reliability that can be used to measure static and dynamic balance in patients with stroke [34]. The MCID for the BBS is 12.5 [35].
Six-minute walking test (6MWT)
The 6WMT is used to evaluate gait function and endurance [36], in which a higher score (distance walked) reflects better gait endurance. The MCID at 2–6 months after stroke onset is 44 m in patients with an initial gait speed < 0.40 m/s, and 71 m in patients with an initial gait speed ≥ 0.40 m/s [37].
Fugl–Meyer assessment of the upper extremity (FMA-UE)
The FMA-UE is used to measure motor recovery in the shoulder, elbow, forearm, wrist, and hand after stroke. The FMA-UE total score ranges from 0 to 66, and each item uses a three-point ordinal scale (0, cannot perform; 1, perform partially; and 2, perform completely). The MCID for the FMA-UE is 9 [38].
Fugl–Meyer assessment of sensory (FMA-sensory)
The FMA-sensory is used to evaluate the sensory function of patients with stroke, with a maximum score of 44 for sensory function (Appendix).
Fugl-Meyer assessment of the lower extremity (FMA-LE)
The FMA-LE is used to identify motor recovery of paretic lower limbs in patients after stroke [39].
Timed up and go test (TUG)
The TUG is a timed test used to assess lower limb mobility in patients after stroke; it involves standing up, walking for 3 m, turning around, walking back to the starting spot, and sitting down [40].
Statistics
The statistical analysis was conducted by SPSS version 26 (IBM, US). The demographic and clinical properties of the patients were analyzed to evaluate the degree of homogeneity between the RT + CT and CT groups; the Wilcoxon rank-sum test was used for days after stroke onset, the Fisher’s exact test was used for sex, and the X2 test was used for the mRS. Independent t tests were used for age, MMSE, BBS, BI, 5MWS, FMA-UE, and 6MWT.
To analyze the clinical outcome assessments, within-group comparisons were conducted using paired t tests, and between-group comparisons were conducted using independent t tests. For the proportion of patients who reached the MCID, the Fisher’s exact test or X2 test was used for the BI, BBS and FMA-UE, 5MWS, and 6MWT. The gait kinematic parameters for each patient were the average across gait steps. To analyze the changes in the clinical assessments and gait kinematic parameters across different sessions in the RT + CT group, one-way repeated-measures ANOVA with Bonferroni correction as post-hoc test was applied. An alpha error level of 0.05 was chosen.
Effect sizes were calculated by Cohen’s index (d) for t tests and were rated as small (< 0.5), medium (0.5 to 0.8) or large (> 0.8); the phi coefficient (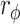 ) was calculated for the chi-squared test and was rated as no or very weak (> 0), weak (> 0.05) moderate (> 0.1); strong (> 0.15), or very strong (> 0.25). The data and error bars in the figures are presented as the mean ± SD.
) was calculated for the chi-squared test and was rated as no or very weak (> 0), weak (> 0.05) moderate (> 0.1); strong (> 0.15), or very strong (> 0.25). The data and error bars in the figures are presented as the mean ± SD.
Results
Participant characteristics
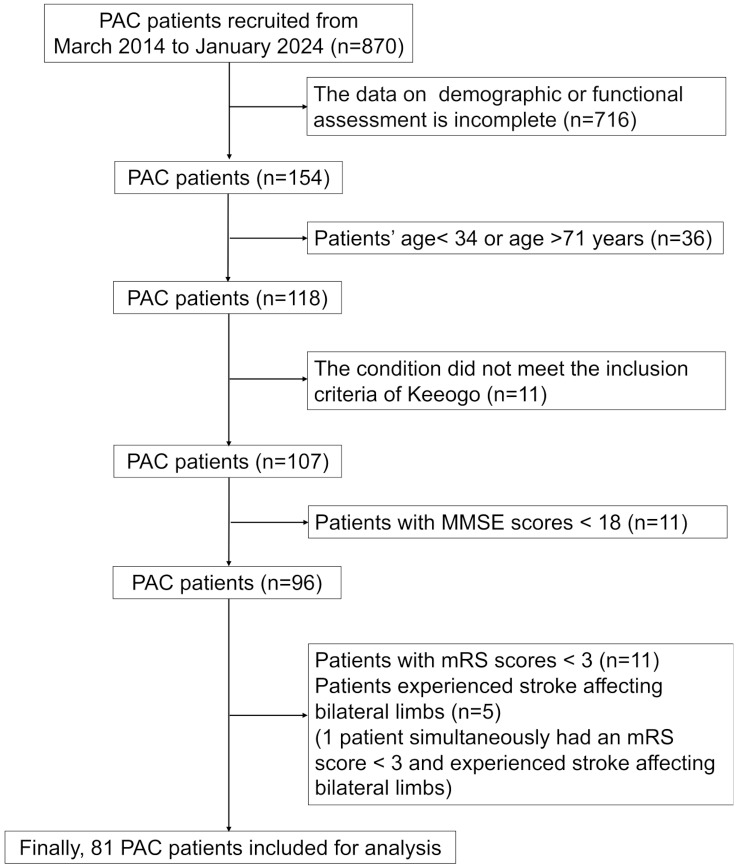
Seventeen patients with stroke were included in the RT + CT group, and in the CT group, we included 81 participants from the PAC dataset (Fig. 3). The characteristics of the participants in the RT + CT and CT groups are shown in Table 1. The two groups did not differ in their anthropometric parameters, including age and sex, or in their functional condition parameters, including BI, mRS, BBS, 5MWS, 6MWT, and FMA-UE. The RT + CT group (35.1 ± 13.2 days) had a longer stroke onset period than did the CT group (14.2 ± 8.8 days) (p < 0.001, effect size r = 0.551). All participants in the RT + CT group successfully completed the full six training sessions, and no adverse events were reported.
Fig. 3.
Flow diagram of the inclusion of patients in the CT group from the PAC database
Table 1.
Comparison of the demographic and baseline functional data between the RT + CT and CT groups
| Variable | All | Group | P value | |
|---|---|---|---|---|
| RT + CT | CT | |||
| Patient number (n) | 98 | 17 | 81 | |
| Age (years) | 57.0 ± 9.5 | 56.4 ± 11.4 | 57.1 ± 9.1 | 0.794 |
| Sex (F/M) | 26/72 | 6/11 | 20/61 | 0.547 |
| MMSE | 26.6 ± 3.4 | 28.0 ± 3.3 | 26.4 ± 3.3 | 0.066 |
| Days after stroke onset | 17.8 ± 12.5 | 35.1 ± 13.2 | 14.2 ± 8.8 | < 0.001*** |
| mRS (3/4) | 60/38 | 8/9 | 52/29 | 0.187 |
| BI | 56.8 ± 10.0 | 59.1 ± 5.9 | 56.3 ± 10.6 | 0.292 |
| BBS | 44.4 ± 6.5 | 44.6 ± 8.7 | 44.4 ± 6.0 | 0.890 |
| 5MWS (s) | 11.3 ± 5.8 | 12.1 ± 5.9 | 11.1 ± 5.8 | 0.535 |
| 6MWT (m) | 241.5 ± 112.8 | 252.6 ± 105.8 | 239.2 ± 114.7 | 0.657 |
| FMA-UE | 46.7 ± 16.6 | 49.6 ± 16.1 | 46.1 ± 16.7 | 0.437 |
The data are presented as the mean ± SD. ***p < 0.001
Therapeutic effects of ERGT
Primary outcome
A comparison between the baseline and final assessments revealed that the 5MWS significantly increased in both the RT + CT (p < 0.01) and CT (p < 0.001) groups. Furthermore, the improvement in the 5MWS did not differ between the two groups (Table 2). Remarkably, using the MCID as the criterion, we found that the proportion of patients whose improvement exceeded the MCID for 5MWS (MCID = 0.16 m/s) was significantly greater in the RT + CT group (70.59%) than in the CT group (43.21%, p = 0.040, 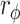 = 0.208; Table 3), suggesting that the ERGT is more likely to induce clinically meaningful improvements in the walking speed than conventional training alone.
= 0.208; Table 3), suggesting that the ERGT is more likely to induce clinically meaningful improvements in the walking speed than conventional training alone.
Table 2.
Clinical outcomes of the RT + CT and CT groups at baseline and at the end of the training program
| Variable | RT + CT group | CT group | Independent t-test | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Difference | Baseline | Final | Difference | P value | Cohen’s d | |
| BI | 59.1 ± 5.9 | 72.6 ± 6.4*** | 13.5 ± 8.1 | 56.3 ± 10.6 | 65.4 ± 11.9*** | 9.1 ± 10.3 | 0.097 | 0.482 |
| BBS | 44.6 ± 8.7 | 52.6 ± 4.4*** | 7.9 ± 6.2 | 44.4 ± 6.0 | 50.8 ± 4.4*** | 6.4 ± 4.1 | 0.330 | 0.300 |
| 5MWS (s) | 12.1 ± 5.9 | 7.2 ± 2.1** | -4.9 ± 4.9 | 11.1 ± 5.8 | 8.0 ± 3.6*** | -3.1 ± 4.1 | 0.123 | 0.390 |
| 6MWT (m) | 252.6 ± 105.8 | 334.1 ± 88.6*** | 81.5 ± 41.4 | 239.2 ± 114.7 | 332.2 ± 119.4*** | 93.0 ± 79.8 | 0.395 | 0.181 |
| FMA-UE | 49.6 ± 16.1 | 57.4 ± 13.3*** | 7.8 ± 7.1 | 46.1 ± 16.7 | 50.6 ± 14.8*** | 4.5 ± 7.8 | 0.114 | 0.440 |
The data are presented as the mean ± SD
**, p < 0.01; ***, p < 0.001 for the baseline vs. final assessment; statistical analysis by the paired samples t test
Table 3.
Comparison between the two groups in terms of the proportions of patients who exceeded the MCID for functional improvement
| Variable | MCID criterion (Anchor-based) | MCID Exceeded (Y/N, %) | χ2 | P value |
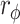
|
|
|---|---|---|---|---|---|---|
| RT + CT (n = 17) | CT (n = 81) | |||||
| Primary outcome | ||||||
| 5MWS | 0.16 m/s | 12/5 (70.59%) | 35/46 (43.21%) | 4.220 | 0.040* | 0.208 |
| Secondary outcomes | ||||||
| BIF |
16.18 (ischemic stroke) 5.02 (hemorrhagic stroke) |
7/10 (41.18%) | 14/67 (17.28%) | 0.047* | 0.220 | |
| FMA-UEF | 9 | 7/10 (41.18%) | 15/66 (18.52%) | 0.056 | 0.206 | |
| BBSF | 12.5 | 4/13 (23.53%) | 8/73 (9.88%) | 0.213 | 0.158 | |
| 6MWT |
44 m (initial gait speed < 0.40 m/s) 71 m (initial gait speed ≥ 0.40 m/s) |
11/6 (64.71%) | 46/35 (56.79%) | 0.362 | 0.548 | 0.061 |
*, p < 0.05; 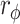 , phi coefficient; Y/N: Yes/No
, phi coefficient; Y/N: Yes/No
F, Fisher’s exact test
Secondary outcomes
Comparisons between the baseline and final assessments revealed improvements in the BI, BBS, 6MWT, and FMA-UE in both the RT + CT and CT groups (both p < 0.05). However, these improvements did not differ between the two groups (all p > 0.05) (Table 2). Using the MCID as the criterion, it was observed that the proportion of patients whose improvement exceeded the MCID for the BI was greater in the RT + CT group (41.18%) than in the CT group (17.28%) (p = 0.047, 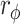 = 0.220, Table 3), suggesting that the ERGT yields a higher proportion of patients in the improvement of activities of daily living as measured by the BI.
= 0.220, Table 3), suggesting that the ERGT yields a higher proportion of patients in the improvement of activities of daily living as measured by the BI.
The effect of ERGT over time
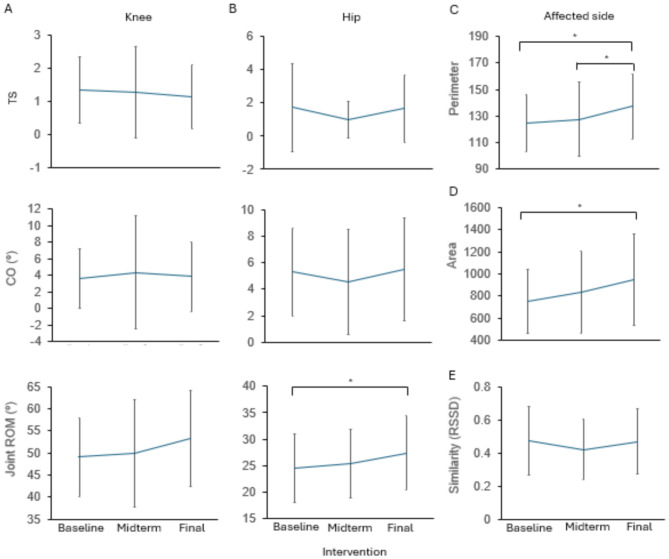
Figure 2 illustrates the kinematic parameters obtained in a sample patient in the RT + CT group at the baseline, midterm, and final assessments. Figure 4 shows the gait parameters for the hip and knee joints, including the TS, CO, area, perimeter, joint ROMs, and RSSD, during walking (the data are shown in Supplementary Table 1). For data collected in free mode, the perimeter and area of the cyclogram and the ROM of the hip on the affected side increased from baseline to the final assessment (final vs. baseline, p = 0.046 for the perimeter, p = 0.014 for the area, and p = 0.011 for the hip ROM) and increased from the midterm to the final assessment (final vs. midterm, p = 0.048 for the perimeter). However, the increases in the symmetry of joint motion, including the TS and CO, and hip-knee cyclogram similarity, including the RSSD, were not significant over time. These findings suggest that the power provided by the UIPER to the knees may facilitate specific characteristics of gait function in patients with stroke.
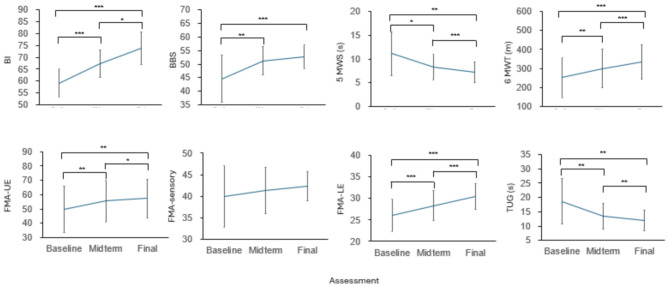
Fig. 4.
Changes in kinematic data in the RT + CT group at the three assessment time points. (A-B) Symmetry-related analysis of the TS and CO and joint ROM in the knee (A) and hip (B) of the affected side. (C) Cyclogram perimeter. (D) Cyclogram area in the affected lower limb for hip-knee coordination during walking. (E) Gait similarity analysis according to the RSSD between the affected and unaffected sides. The data are presented as the mean ± SD. *p < 0.05
We further explored changes in the results of the assessments across the baseline, midterm, and final assessments in the RT + CT group (Fig. 5 and Supplementary Table 2, one-way repeated-measures ANOVA). Except for the FMA-sensory and BBS, the improvements in the BI, FMA-UE, 5MWS, 6MWT, FMA-LE, and TUG were significantly different over time, indicating improvements in functional assessments over the course of training in the RT + CT group.
Fig. 5.
Changes in functional outcomes in the RT + CT group at the three assessment time points. The data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
In the present study, we examined the clinical effectiveness of the UIPER for ERGT. The results showed that ERGT induced a robust improvement in the functional assessment and gait parameter results. Specifically, for the primary outcome, the 5MWS, there was significant improvement in the RT + CT group compared to the CT group, indicating that ERGT facilitates gait recovery. Importantly, an improvement in the perimeter and area of the cyclogram across training sessions led to better performance in terms of the kinematic parameters. These findings suggest that the application of ERGT when combined with conventional training can induce a better improvement in walking speed and gait kinematics in the stroke patient as compared with conventional training only.
Almost all functional assessment outcomes significantly improved in both groups; however, patients in the RT + CT group were more likely to reach the MCID for the 5MWS and BI than those in the CT group. Coupled with extensive insurance coverage, comprehensive functional assessments, and prolonged hospital stays, PAC patients generally achieve superior poststroke outcomes. Throughout their stay, PAC patients undergo a high frequency of intensive rehabilitation, including physical therapy, occupational therapy, and speech therapy, which is provided in a multidisciplinary rehabilitation team approach according to the patient’s ability [41]. It is hypothesized that a more robust improvement in walking speed and activities of daily living induced by extra ERGT is based on a combination of robot-assisted and conventional rehabilitation trainings. It is possible that the effectiveness of ERGT is only observed in the early stage after stroke, and its effectiveness in late-stage patients remains unclear.
It has been shown that combining robot-assisted and conventional therapy can improve gait performance [14, 42]. It is important to explore the possible mechanisms underlying this improvement. Previous pilot studies indicated that the ERGT can enhance walking abilities [10, 43]. It is possible that the torque generated by the actuators in the knees of this UIPER is designed to compensate for the movement of the limbs on the affected side,. Furthermore, it has been shown that knee extension is crucial for achieving dynamic balance [44]. The present study indicates that gait outcomes improve along the course of ERGT using UIPER training, a finding that is consistent with the previous findings [10, 43]. Among gait parameters, the ROM of hip and the perimeter and area of the hip-knee cyclogram increased after training, a finding indicating that ERGT using UIPER that supports knee activation may also facilitate hip function and thus enhances gait performance by showing a faster walking speed. However, more studies are still needed for further characterizing the kinesthetic dynamics induced by ERGT using UIPER.
It is noteworthy that the UIPER we applied was originally intended to use for specific functionalities, such as the walking speed. A previous single-arm pilot study using Keeogo targeted at subacute stroke patients with stroke onset time less than 6 months and reported that walking speed, walking distance, FMA-LE, and gait parameters improved after 20 training sessions within 4 weeks [43]. These results are consistent with our findings although the dosage of robot-assisted training in the present study is less than that in the previous study in terms of frequency, session duration, and total sessions. Although the improvements on walking speed and distance are also observed in the robot-assisted training using treadmill-based Lokomat with body-weight support for subacute stroke patients in a study for stroke within 3 weeks, a setup that is analogous to the present study [45]. It is important to notice that Keeogo is intended to apply minimal or no body-weight supports and provide less mechanical assistance. It is because patients in this motor recovery stage have better balance and hip initiation, but could still have more pronounced impairment in knee flexion. This intended use is thus more restrictive than other partial-weight-bearing robots such as Lokomat, in which hip flexion or good trunk control are not prerequisites for their application. Clinicians need to be aware of the optimal types of patients for each rehabilitation robot so that the patient’s improvement can be optimized.
Notably, the RT + CT group had a longer duration poststroke onset than did the CT group, with a difference of approximately 14 days, although these two groups did not differ in other demographic data or functional measurements. This difference can be attributed to the fact that a proportion of patients did not meet the inclusion criteria when they were admitted to the rehabilitation ward. These patients became eligible for ERGT after approximately 14 days of conventional rehabilitation. The additional intervention sessions in the CT group may have influenced the outcomes. For this issue, we selected data from the CT group (n = 10) to control the homogeneity between groups. Although the similar results were found (supplemental Results and Supplementary Tables 3, 4, and 5), a prospective randomized controlled study is still needed to confirm these findings.
The present study has several limitations. First, the UIPER provides mechanical support that may influence a patient’s gait function, even in free mode without power support. Consequently, the kinematic data obtained from the sensors of the robot may not accurately reflect the patient’s gait without using the device. The relationship between functional improvements and kinematic patterns offers valuable insights into the mechanisms underlying the functional improvements induced by ERGT. Second, the UIPER was used to guide the patient’s gait to fit a certain pattern; thus, a personalized gait training protocol is necessary, as each patient might need a specific training protocol. This approach inevitably induces variability in the treatment protocol. Third, the dosage in both of the RT + CT and CT groups were not equal, ERGT in the RT + CT group was an extra intervention that might yield a bias in outcome measurements when compared with the CT group. Therefore, in the future, a study design with equal training dosage between the two arms is necessary. Finally, the sample size in this study might not be adequate for drawing definitive conclusions regarding the treatment effect of ERGT. Future randomized studies with larger sample sizes and follow-up assessments are needed to provide more robust evidence.
Conclusions
ERGT using UIPER facilitates the recovery of walking speed in patients after stroke, and its effectiveness can also be observed in the improvement of gait parameters as measured by the sensors of the robot. However, to establish the efficacy of this approach, randomized controlled studies with larger sample sizes are still necessary. Additionally, long-term follow-up assessments are warranted to evaluate the sustainability of the observed improvements and further refine the rehabilitation protocols.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The present project was supported by the Ministry of Science and Technology, Taiwan, and Chang Gung Medical Foundation. These funding agencies did not involve in the design of the study, data collection, data analysis, and drafting of the manuscript. Besides, we also thanks for assistants of Hui-Xuan Qiu and Cheng-En Chien for assisting this study.
Abbreviations
- UIPER
User-initiated powered exoskeletal robot
- ERGT
Exoskeletal robot-assisted gait training
- RT
Robot-assisted therapy
- CT
Conventional therapy
- MCID
Minimum clinically important different
- 5MWS
5-meter walking test
- PAC
Post-acute care
- mRS
Modified Rankin score
- AROM
Active range of motion
- MAS
Modified Ashworth scale
- NRS
Numerical rating scale
- MMSE
Mini-mental state examination
- RSSD
The square root of the sum of squared deviations
- TS
Trend symmetry
- CO
Cyclogram orientation
- BBS
Berg balance test
- BI
Barthel index
- 6MWT
6-minute walking testing
- FMA
Fugl-Meyer assessment
- UE
Upper extremity
- LE
Lower extremity
- TUG
Time up and go test
- ANOVA
Analysis of variance
Author contributions
YCP, JJH, and SCC designed the present study. LCL, CHC, and SCC conducted the experiments and collected the data. JJH, LCL, CHC, YHC, and YCP analyzed the data. JJH, SCC, and YCP interpreted the data. JJH and YCP prepared and revised the manuscript. All authors have reviewed and approved the final manuscript.
Funding
1. Ministry of Science and Technology, Taiwan (NMRPG3M0442, NSTC112-2218-E-182 A-001).
2. Chang Gung Medical Foundation (BMRPB67).
3. Chang Gung Medical Foundation (CMRPG3M1651-2).
Data availability
Data are made available in supplementary materials.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 202200822B0) on 11th July 2022 and all participants in the present study provided informed consent.
Consent for publication
All participants in the present study received instruction about the experimental procedures and content and the experiments were conducted after participants had completed the consent forms.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wevers L, van de Port I, Vermue M, Mead G, Kwakkel G. Effects of task-oriented circuit class training on walking competency after stroke: a systematic review. Stroke. 2009;40(7):2450–9. [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, Katz RC, Lamberty K, Reker D. Management of adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36(9):e100–143. [DOI] [PubMed] [Google Scholar]
- 3.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et al. Guidelines for adult Stroke Rehabilitation and Recovery: a Guideline for Healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–169. [DOI] [PubMed] [Google Scholar]
- 4.Abdullahi A, Truijen S, Umar NA, Useh U, Egwuonwu VA, Van Criekinge T, Saeys W. Effects of Lower Limb Constraint Induced Movement Therapy in people with stroke: a systematic review and Meta-analysis. Front Neurol. 2021;12:638904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyaert C, Vasa R, Frykberg GE. Gait post-stroke: pathophysiology and rehabilitation strategies. Neurophysiol Clin. 2015;45(4–5):335–55. [DOI] [PubMed] [Google Scholar]
- 6.Van Criekinge T, Vermeulen J, Wagemans K, Schroder J, Embrechts E, Truijen S, Hallemans A, Saeys W. Lower limb muscle synergies during walking after stroke: a systematic review. Disabil Rehabil. 2020;42(20):2836–45. [DOI] [PubMed] [Google Scholar]
- 7.Lauziere S, Betschart M, Aissaoui R, Nadeau S. Understanding spatial and temporal gait asymmetries in individuals post stroke. Int J Phys Med Rehabil. 2014;2(3):201. [Google Scholar]
- 8.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: characteristics. Gait Posture. 1996;4(2):136–48. [Google Scholar]
- 9.Balaban B, Tok F. Gait disturbances in patients with stroke. Pm&r. 2014;6(7):635–42. [DOI] [PubMed] [Google Scholar]
- 10.McLeod JC, Ward SJ, Hicks AL. Evaluation of the Keeogo Dermoskeleton. Disabil Rehabil Assist Technol. 2019;14(5):503–12. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T, Sankai Y, Takata S, Kobayashi Y, Ando Y, Nakagawa M, Saito T, Saito K, Ishida C, Tamaoka A, et al. Cybernic treatment with wearable cyborg hybrid assistive limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: a multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001). Orphanet J Rare Dis. 2021;16(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer E, Opheim A, Jorgensen V. Implementing the exoskeleton Ekso GT(TM) for gait rehabilitation in a stroke unit - feasibility, functional benefits and patient experiences. Disabil Rehabil Assist Technol. 2022;17(4):473–9. [DOI] [PubMed] [Google Scholar]
- 13.de Miguel-Fernandez J, Lobo-Prat J, Prinsen E, Font-Llagunes JM, Marchal-Crespo L. Control strategies used in lower limb exoskeletons for gait rehabilitation after brain injury: a systematic review and analysis of clinical effectiveness. J Neuroeng Rehabil. 2023;20(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Reviews 2020(10). [DOI] [PMC free article] [PubMed]
- 15.Louie DR, Eng JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. J Neuroeng Rehabil. 2016;13(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil. 2018;15(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGibbon CA, Sexton A, Jayaraman A, Deems-Dluhy S, Gryfe P, Novak A, Dutta T, Fabara E, Adans-Dester C, Bonato P. Evaluation of the Keeogo exoskeleton for assisting ambulatory activities in people with multiple sclerosis: an open-label, randomized, cross-over trial. J Neuroeng Rehabil. 2018;15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth EJ, Merbitz C, Mroczek K, Dugan SA, Suh WW. HEMIPLEGIC GAIT: relationships between walking speed and other temporal parameters: 1. Am J Phys Med Rehabil. 1997;76(2):128–33. [DOI] [PubMed] [Google Scholar]
- 19.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–6. [DOI] [PubMed] [Google Scholar]
- 20.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Changes in gait symmetry and velocity after stroke: a cross-sectional study from weeks to years after stroke. Neurorehabilit Neural Repair. 2010;24(9):783–90. [DOI] [PubMed] [Google Scholar]
- 21.Krasovsky T, Levin MF. Toward a better understanding of coordination in healthy and poststroke gait. Neurorehabilit Neural Repair. 2010;24(3):213–24. [DOI] [PubMed] [Google Scholar]
- 22.Goswami A. A new gait parameterization technique by means of cyclogram moments: application to human slope walking. Gait Posture. 1998;8(1):15–36. [DOI] [PubMed] [Google Scholar]
- 23.Lai CL, Tsai MM, Luo JY, Liao WC, Hsu PS, Chen HY. Post-acute care for stroke - a retrospective cohort study in Taiwan. Patient Prefer Adherence. 2017;11:1309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picelli A, Bacciga M, Melotti C, E LAM, Verzini E, Ferrari F, Pontillo A, Corradi J, Tamburin S, Saltuari L, et al. Combined effects of robot–assisted gait training and botulinum toxin type A on spastic equinus foot in patients with chronic stroke: a pilot, single blind, randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(6):759–66. [PubMed] [Google Scholar]
- 25.Longworth JA, Chlosta S, Foucher KC. Inter-joint coordination of kinematics and kinetics before and after total hip arthroplasty compared to asymptomatic subjects. J Biomech. 2018;72:180–6. [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Lee H, Cho J-s, Kim I, Lee J, Jang SH. Effects of knee osteoarthritis severity on inter-joint coordination and gait variability as measured by hip-knee cyclograms. Sci Rep. 2021;11(1):1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HS, Ryu H, Lee S-U, Cho J-s, You S, Park JH, Jang S-H. Analysis of gait characteristics using hip-knee cyclograms in patients with hemiplegic stroke. Sensors. 2021;21(22):7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82(7):707–15. [PubMed] [Google Scholar]
- 29.Pilkar R, Ramanujam A, Chervin K, Forrest GF, Nolan KJ. Cyclogram-based joint symmetry assessment after utilization of a foot drop stimulator during post-stroke hemiplegic gait. J Biomech Eng 2018, 140(12). [DOI] [PubMed]
- 30.Crenshaw SJ, Richards JG. A method for analyzing joint symmetry and normalcy, with an application to analyzing gait. Gait Posture. 2006;24(4):515–21. [DOI] [PubMed] [Google Scholar]
- 31.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, Duncan PW. Locomotor experience Applied Post Stroke Investigative T: meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacelon CS. The Barthel Index and other indices of functional ability. Rehabilitation Nurs. 1986;11(4):9–11. [DOI] [PubMed] [Google Scholar]
- 33.Chang YC, Lin HF, Chen YF, Chen HY, Shiu YT, Shi HY. Minimal clinically important difference (MCID) in the functional status measures in patients with stroke: inverse probability treatment weighting. J Clin Med 2023, 12(18). [DOI] [PMC free article] [PubMed]
- 34.Berg K, Wood-Dauphinee S, Williams J. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27(1):27–36. [PubMed] [Google Scholar]
- 35.Song M-J, Lee J-H, Shin W-S. Minimal clinically important difference of Berg Balance Scale scores in people with acute stroke. Phys Therapy Rehabilitation Sci. 2018;7(3):102–8. [Google Scholar]
- 36.Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–41. [DOI] [PubMed] [Google Scholar]
- 37.Fulk GD, He Y. Minimal clinically important difference of the 6-Minute walk test in people with stroke. J Neurol Phys Ther. 2018;42(4):235–40. [DOI] [PubMed] [Google Scholar]
- 38.Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil. 2011;18(Suppl 1):599–610. [DOI] [PubMed] [Google Scholar]
- 39.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73(7):447–54. [DOI] [PubMed] [Google Scholar]
- 40.Chan PP, Tou JIS, Mimi MT, Ng SS. Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch Phys Med Rehabil. 2017;98(11):2213–20. [DOI] [PubMed] [Google Scholar]
- 41.Chien SH, Sung PY, Liao WL, Tsai SW. A functional recovery profile for patients with stroke following post-acute rehabilitation care in Taiwan. J Formos Med Assoc. 2020;119(1 Pt 2):254–9. [DOI] [PubMed] [Google Scholar]
- 42.Moucheboeuf G, Griffier R, Gasq D, Glize B, Bouyer L, Dehail P, Cassoudesalle H. Effects of robotic gait training after stroke: a meta-analysis. Annals Phys Rehabilitation Med. 2020;63(6):518–34. [DOI] [PubMed] [Google Scholar]
- 43.Lin T-Y, Tang SF, Chen H-C, Ho C-S. Exoskeleton-assisted gait training for Subacute Stroke patients: Feasibility Study and preliminary results. Arch Phys Med Rehabil. 2023;104(3):e28–9. [Google Scholar]
- 44.Carter ND, Khan KM, Mallinson A, Janssen PA, Heinonen A, Petit MA, McKay HA. Knee extension strength is a significant determinant of static and dynamic balance as well as quality of life in older community-dwelling women with osteoporosis. Gerontology. 2002;48(6):360–8. [DOI] [PubMed] [Google Scholar]
- 45.Talaty M, Esquenazi A. Feasibility and outcomes of supplemental gait training by robotic and conventional means in acute stroke rehabilitation. J Neuroeng Rehabil. 2023;20(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are made available in supplementary materials.