Abstract
Introduction
Young people living with HIV (YPLHIV) are at increased risk of developing chronic kidney disease (CKD) which is associated with high mortality and morbidity. Early diagnosis is important to halt progression. We aimed to estimate the prevalence and factors associated with CKD among YPLHIV in Kampala, Uganda, and to compare serum creatinine and cystatin C for early diagnosis of CKD in this population.
Methods
A cross-sectional study with YPLHIV aged 10 to 24 years was conducted in seven HIV clinics. Participants provided a urine and blood sample to measure urinary albumin, proteinuria, serum creatinine and cystatin C levels at baseline and after three months. The estimated glomerular filtration rate (eGFR) was calculated using CKDEPI 2021, Cockroft-Gault and bedside Schwartz equations using creatinine or cystatin C. The albumin creatinine ratio (ACR) and proteinuria were measured. CKD was defined as either eGFR < 60 ml/min/1.73m2 or < 90 ml/min/1.73m2 or ACR above 30 mg/g on two separate occasions. Univariable and multivariable logistic regression were used to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CI) for factors associated with CKD.
Results
A total of 500 participants were enrolled. Most were female (56%; n = 280) and aged 10 to 17 years (66.9%; n = 335). CKD prevalence ranged from 0 to 23% depending on the criteria, equation and biomarker used. Cystatin C-based equations estimated higher prevalence of CKD compared to creatinine-based ones. Prevalence of ACR above 30 mg/g was 10.1% and of proteinuria 29%. Factors independently associated with CKD were age (aOR = 1.42; 95% CI:1.30–1.51) and male sex (aOR = 3.02; 95% CI:1.68–5.43).
Conclusion
CKD prevalence among YPLHIV varied substantially depending on definitions used and the current definition would likely lead to missed cases of CKD among YPLHIV. Estimating equations should be validated against measured GFR in YPLHIV and the optimal definition of CKD in this vulnerable population should be revised to optimise detection and opportunities for reducing disease progression.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03865-8.
Keywords: Prevalence, Chronic kidney disease, Young people, HIV, Africa, HIV comorbidities
Introduction
Prevalence of chronic kidney disease (CKD) is increasing globally [1]. CKD is defined as abnormalities in kidney structure or function present for three or more months [2]. The Global Burden of Disease study estimates CKD prevalence at 9.1% (95% CI 8.5%-9.8%) with geographic variation [3]. Studies in Sub-Saharan Africa (SSA) have found prevalence ranging from 6 to 48% depending on the population, the definitions used, and the measurements taken [4–6].
CKD is associated with high morbidity and mortality as diagnosis is usually delayed, often occurring after kidney failure due to its insidious onset [7]. Kidney failure can only be treated with expensive kidney replacement therapies (KRT) that are not readily available in low and middle-income countries [8]. Early diagnosis before marked loss of kidney function and severe damage is important to minimise risk of progression to kidney failure and cardiovascular events [9–11].
Diagnosis of CKD is based on the level of glomerular filtration rate (GFR) and markers of kidney damage such as protein excretion into the urine shown by proteinuria or albuminuria [12]. GFR can either be measured directly (mGFR) or estimated (eGFR) with a specific biomarker and one of the estimating equations [13]. Most commonly, serum creatinine and cystatin C estimating equations are used to estimate GFR [14]. Serum creatinine is widely available and relatively cheap [15] but has limitations as it is influenced by muscle mass, physical activity and general health status [16] as well as high analytic variability [15]. Cystatin C is not affected by these conditions as it is produced by most nucleated cells and has uniform generation despite individual differences in people and situations [16–18]. However, it is affected by conditions of high inflammation, corticosteroid use and thyroid disease [16, 19]. Cystatin C more accurately estimated measured GFR compared to creatinine in a large cohort study done across Uganda, Malawi and South Africa that recommended the use of Cystatin C in African populations [4].
Young people living with HIV (YPLHIV) are at higher risk of CKD than young people not living with HIV [20]. CKD risk is associated with high HIV viremia (> 4000 copies per ml), severe immunosuppression (CD4 cell count < 200 cells/ml), infection with hepatitis C virus, diabetes mellitus (DM), hypertension, use of drugs that treat opportunistic infections, and toxicity due to anti-retroviral therapy (ART) from tenofovir disoproxil fumarate (TDF) and indinavir [6, 21–24]. Further, YPLHIV in SSA are particularly vulnerable to developing CKD compared to adults living with HIV due to late HIV diagnosis and initiation on ART, poorer adherence to ART complicated by high viremia and low CD4 cell counts [25–27].
Although YPLHIV are at high risk of CKD, little is known about CKD prevalence, the best biomarker to diagnose CKD and factors associated with CKD in this vulnerable group. Therefore, we sought to study this among YPLHIV in Kampala, Uganda.
Methods
Study design and setting
This cross-sectional study was conducted in the HIV clinics of seven urban public health facilities from the 12th of April 2023 to 31st January 2024 in Kampala, Uganda. These offer comprehensive HIV care to children (aged below 18 years) and adults (aged 18 years and above).
Study population and sampling
The study included YPLHIV aged 10–24 years with presumed perinatal HIV infection (defined as being diagnosed with HIV before 10 years of age with self-report of no sexual debut or blood transfusion prior to diagnosis). Pregnant YPLHIV were excluded. Systematic random sampling was used to identify potential participants from all YPLHIV enrolled in the seven HIV clinics from electronic medical records. They were ordered by age at diagnosis and every third person invited to join the study. A sample size of 500 was powered to detect a prevalence of CKD between 16% and 24%.
Study procedures
Eligible participants were invited to the HIV clinic through a phone call where they were screened, consented, and enrolled. A trained study team member conducted an interview with the participant and completed a questionnaire to record demographic information, symptoms, risk factors and the relevant medical history such as prior diagnosis with diabetes mellitus (DM) or hypertension. Anthropometric measurements (mid-upper arm circumference (MUAC), weight and height) were taken. Weight was assessed using a digital weighing scale, height using a stadiometer and blood pressure (BP) using a digital BP machine with a paediatric cuff for younger participants. Body composition monitoring was conducted using bioimpedance impedance spectroscopy (BIS) to measure body fat, muscle mass and visceral fat. Participants provided a spot urine sample (20 mls) as well as 8 ml of venous blood. Urine dipstick was done at the facility to determine proteinuria and other urinary abnormalities. The samples were stored in a cooler box before transfer to the study laboratory on the same day. Participants that had kidney abnormalities (eGFR < 90 ml/min/1.73m2 or ACR > = 30 mg/g) at the initial visit were invited back for another urine and blood test at three months to confirm CKD.
Laboratory methods and testing
In the laboratory, serum creatinine, urinary albumin and cystatin C levels were determined. Those with an albumin creatinine ratio (ACR) > = 30 mg/g or eGFR < 60 ml/min/1.73m2 at baseline were followed-up after three months to confirm the KDIGO guideline-recommended clinical diagnosis of CKD. Cystatin C was measured by particle-enhanced immunoturbidimetric assay on Roche Cobas C311 platform with Tina-quant Cystatin C Gen.2. Creatinine was measured using the enzymatic calorimetric method using an isotope dilution mass spectroscopy (IDMS) traceable standard reference material on the Cobas Integra 400 plus machine with Creatinine Plus Version 2 (CREP2), Roche Diagnostics. The urine albumin was quantified using the immunoturbidimetric assay on the Roche Cobas C311 platform using Tina-quant Albumin Gen2, (Roche Diagnostics). Prior to testing, the machines were calibrated according to manufacturer instructions. Urinalysis by dipstick was done with AYDMED urinalysis Reagent Test Strips (Sungo Europe B.V Amsterdam) to determine presence of urobilinogen, bilirubin, ketones, blood, proteins, nitrites, leucocytes, glucose, specific gravity, pH, and ascorbic acid [28].
Diagnosis of CKD was based on the kidney disease improving global outcomes (KDIGO) guidelines and staging [29], i.e. (1) markers of kidney damage such as an albumin: creatinine ratio > = 30 mg/g, or (2) eGFR < 60 ml/min/1.73m2, with these abnormalities confirmed with a repeat test after three months [30]. Since a normal GFR is between 90 and 120 ml/min/1.73m2, we also considered an eGFR cut-off below 90 ml/min/1.73m2 which is regarded as stage 2 CKD as abnormal in such a young population [31]. The initial baseline measurement of either eGFR below 90 ml/min/1.73m2 or ACR above 30 mg/g was regarded as kidney impairment as CKD status was not yet established at that point.
To explore CKD definition in this cohort that included children and where chronic disease had affected pubertal development and mean body and muscle mass, we primarily used a range of GFR estimating equations and eGFR cut offs that reflected contemporary practice for adults and children and/or sought to adjust for body size. eGFRscr was estimated using the following creatinine-based equations: CKD Epidemiology collaboration (CKDEPI) 2021 [14] which is the current KDIGO recommended equation for GFR estimation in adults, the Bedside Schwartz [32] which is KDIGO recommended for children, and Cockroft-Gault because it takes into consideration stature by including height. eGFRcystc was estimated using the following cystatin C-based equations: Schwartz cystatin C [33] and CKDEPI 2012 [34]. For completeness prevalence was also estimated using other relevant equations such as Full Age Spectrum [35], CKDEPI40 [36] and Pierce U25 [37] among others, and in combination with ACR and proteinuria.
Data management and statistical analysis
Data were collected in REDCap and analysed with STATA statistical software version 18 (STATA Corp USA). Demographic data were collected on age, sex, address, religion, tribe, assets one owned and marital status. Symptoms such as loss of weight, anorexia, nausea, vomiting, facial puffiness in the morning and urinary volume abnormalities were collected. Risk factors such as prior diagnosis with hypertension, diabetes mellitus, tuberculosis, prolonged use of non-steroidal anti-inflammatory drugs, type of anti-retroviral drugs used, duration living with HIV and viral suppression were collected. Viral suppression was considered as an HIV viral load below 1000 copies/ml. Hypertension was classified according to the American Academy of Paediatrics (AAP) guidelines as being above the 95th percentile for age and sex below 13 years and above 130/80mmhg in those above 13 years [38]. Muscle mass was abnormal if below 33.3 for males and 24.3 for females. Social economic status was divided into three using principal component analysis. Demographic data were summarised in percentages or means (standard deviation) and median (interquartile range). We determined the eGFR at the initial visit and at the three month follow up visit. The distribution of eGFRs estimated with different equations was shown in a Kernel density plot. CKD prevalence diagnosed by either creatinine or cystatin C was calculated. Univariable logistic regression was used to estimate odds ratios (OR) of factors associated with CKD for each of the five equations used, respectively. All variables with p < 0.2 in the univariable model, and a-priori identified variables known to be associated with CKD (age, sex, HIV viral suppression, blood pressure) were then included in a multivariable logistic regression model for each of the five equations. We assessed for multicollinearity to ensure the accuracy of the estimates, between age, blood pressure, body mass index, mid upper arm circumference using the variance inflation factor. If the factors were below 5 and the tolerance not close to 0, multicollinearity was not present [39].
Ethical considerations
Ethical approval was received from the Uganda Virus Research Institute (UVRI) Research Ethics Committee (reference number GC/127/946), the Uganda National Council of Science and Technology (HS2578ES) and the London School of Hygiene and Tropical Medicine institutional review board (28797). Information about the study appropriate for adults, semi-literate adults and children was provided in an information booklet that was read to the participants and caregivers. All the participants more than 18 years of age provided a written informed consent. Those below 18 years of age provided assent, and their caregivers provided written informed consent. If a child refused to provide assent even after their caregiver had provided consent, that child was not enrolled into the study. All participants had the option to withdraw at any point during the research. All participants with suspected CKD were referred to a nephrologist for management.
Results
Of 532 YPLHIV invited to participate, 500 were enrolled as 32 declined to participate (Table 1). The majority were female (56.0%; n = 280), children aged 10–17 years (66.9%; n = 335) and living in Kampala (58.9%; n = 295). Females had better nutritional indicators than males - they were less likely to be underweight (26.4% vs. 48.9%; p < 0.001), not stunted (85.6% vs. 76.9%; p = 0.03), and to have normal mid upper circumference (92.9% vs. 87.7%; p = 0.05). No participant had a prior diagnosis of DM or hypertension.
Table 1.
Demographic and clinical characteristics of the study participants by sex
| Male | Female | Total | |
|---|---|---|---|
| N = 220 (44%) | N = 280 (56%) | N = 500 | |
| Age in years (mean, SD) | 16.5 (3.8) | 16.3 (3.5) | 16.4 (3.6) |
| Age category | |||
| Children | 143 (65.0) | 191 (68.2) | 334 (66.8) |
| Adults | 77 (35.0) | 89 (31.8) | 166 (33.2) |
| Address | |||
| Kampala | 123 (55.9) | 171 (61.1) | 294 (58.8) |
| Wakiso | 80 (36.4) | 99 (35.4) | 179 (35.8) |
| Other districts | 17 (7.7) | 10 (3.6) | 27 (5.4) |
| Religion | |||
| Christian | 158 (71.8) | 204 (72.9) | 362 (72.4) |
| Moslem | 62 (28.2) | 73 (26.1) | 135 (27.0) |
| Other | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| Social Economic Status | |||
| Lowest | 78 (35.5) | 100 (35.7) | 178 (35.6) |
| Middle | 61 (27.7) | 94 (33.6) | 155 (31.00 |
| Highest | 81 (36.8) | 86 (30.7) | 167 (33.4) |
| School going | |||
| No | 58 (26.4) | 67 (23.9) | 125 (25.0) |
| Yes | 162 (73.6) | 213 (76.1) | 375 (75.0) |
| Marital Status | |||
| Married | 3 (1.4) | 16 (5.7) | 19 (3.80) |
| Never married | 217 (98.6) | 264 (94.3) | 481 (96.2) |
| Tribe | |||
| Ganda | 149 (67.7) | 174 (62.1) | 323 (64.6) |
| Other tribes | 65 (29.5) | 86 (30.7) | 151 (30.2) |
| Non-Ugandan | 6 (2.7) | 20 (7.1) | 26 (5.2) |
| Weight in Kg mean (SD) | 48.0 (12.4) | 49.4 (12.4) | 48.8 (12.4) |
| Body Mass Index1 | |||
| Normal | 173 (79.0) | 214 (76.4) | 387 (77.6) |
| Underweight (< 18.5 kg/m2) | 38 (17.3) | 24 (8.6) | 62 (12.4) |
| Overweight (> 25 kg/m2) | 8 (3.7) | 42 (15.0) | 50 (10.0) |
| Stunting** | |||
| Not stunted | 123 (76.9) | 184 (85.6) | 307 (81.9) |
| Stunted | 37 (23.1) | 31 (14.4) | 68 (18.3) |
| Mid Upper Arm Circumference 2 | |||
| Normal | 192 (87.7) | 260 (92.9) | 452 (90.6) |
| Malnourished | 27 (12.3) | 20 (7.1) | 47 (9.4) |
| Blood pressure3 | |||
| Normal | 137 (62.8) | 215 (77.1) | 352 (70.8) |
| Elevated | 39 (17.9) | 27 (9.7) | 66 (13.3) |
| Hypertensive | 42 (19.3) | 37 (13.3) | 79 (15.9) |
| On TDF regimen | |||
| No | 61 (27.7) | 66 (23.6) | 127 (25.4) |
| Yes | 159 (72.3) | 214 (76.4) | 373 (74.6) |
| On Dolutegravir | |||
| Yes | 210 (95.5) | 273 (97.5) | 483 (96.6) |
| No | 10 (4.5) | 7 (2.5) | 17 (3.4) |
| Virally suppressed | |||
| Yes | 195 (89.5) | 247 (88.5) | 442 (88.9) |
| No | 23 (10.5) | 32 (11.5) | 55 (11.1) |
| Muscle mass 2 | |||
| Normal muscle mass | 164 (82.0) | 235 (87.7) | 399 (85.3) |
| Abnormal muscle mass | 36 (18.0) | 33 (12.3) | 69 (14.7) |
* Living outside Kampala/Wakiso region. # included those with no religion and those of African traditional religion. **Only those aged less than 19 years. 1 one missing, 2. 32 missing as their measurements were below threshold of the BIS machine.3 three missing a blood pressure reading KG-Kilogram, N-number, SD-standard deviation, TDF Tenofovir disoproxil fumarate
Comparison of serum creatinine and cystatin C
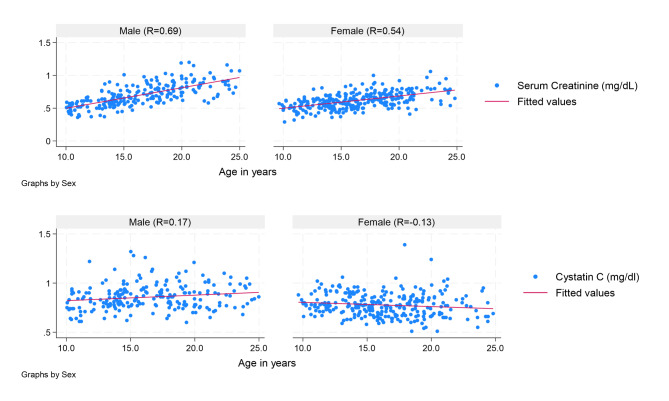
The mean serum creatinine (scr) was 0.63 mg/dl (SD 0.15) with a range of 0.29 to 1.2 mg/dl. The mean scr was significantly different according to sex, age, presence of stunting or viral suppression. The mean cystatin C was 0.81 mg/dl (SD 0.13) with a range of 0.51 to 1.39 mg/dl. The mean cystatin C was higher in males at 0.86 mg/dl versus 0.78 mg/dl in females but with no other differences (Supplemental Table 1). Serum creatinine but not cystatin C was correlated with age and sex (Fig. 1).
Fig. 1.
Relationship between serum creatinine and cystatin C and age for males and females
Sample size was 494
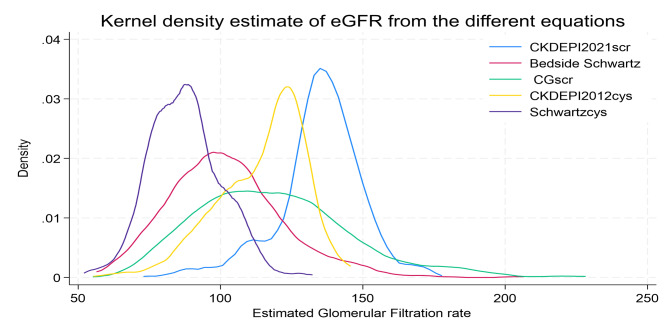
Distribution of the eGFR
CKDEPI consistently gave higher eGFR readings for both creatinine and cystatin C, and the Schwartz cystatin C equation gave the lowest eGFR values (Fig. 2).
Fig. 2.
Kernel density plot showing the distribution of the eGFR according to different estimating equations and biomarkers
Scr serum creatinine, CG Cockroft Gault, cys cystatin C
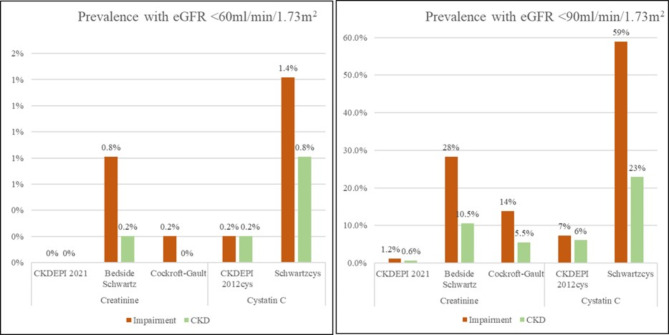
Prevalence of CKD and kidney impairment using eGFR
CKD and kidney impairment prevalence varied according to the eGFR cut-off, and the biomarker used. Using an eGFR < 60 ml/min/1.73m2 cut-off, the highest prevalence was with the Schwartz cystatin C equation which showed a 1.4%; 95% CI: 0.5–2.9% prevalence of kidney impairment and a 0.8%; 95% CI: 0.2–2.1% prevalence of CKD at the 3-month follow-up. The lowest prevalence was with the CKDEPI 2021 equation with a 0%; 95% CI 0-0.07% prevalence of both kidney impairment and CKD.
Similarly, using eGFR < 90 ml/min/1.73m2 cut-off, the highest prevalence was with the Schwartz cystatin C equation that showed a 58.9% (95% CI: 54.4–63.3%) prevalence of kidney impairment and a 23% (95% CI 19.2–26.8%) prevalence of CKD. The lowest with CKDEPI with a kidney impairment prevalence of 1.2% (95% CI 0.5–2.6%) and a 0.6%; 95% CI: 0.01–1.7% prevalence of CKD (Fig. 3). Prevalence using other eGFR equations ranged from 0 to 27.5% (Supplementary Table 2).
Fig. 3.
Prevalence of CKD according to the different estimating equations and biomarkers
Sample size was 494
Prevalence of CKD according to eGFR and ACR
All participants were staged according to combined baseline eGFR using cystatin C and ACR to assess risk of progression [29]. Overall, 436 (88.3%) participants had low risk of progression 1, 53 (10.7%) had intermediate risk of progression 2 and 5 (1.0%) were at high risk of progression 3 with no one in the highest risk category 4. (Table 2).
Table 2.
All participants’ CKD status staged according to estimated GFR from cystatin C and albumin creatinine ratio at baseline
| eGFR and ACR categories | ACR categories in mg/g | ||||
|---|---|---|---|---|---|
| < 30 Normal to mildly increased |
30–300 Moderately increased |
> 300 Severely increased |
Total numbers | ||
| eGFR in ml/min/1.73m2 | Stage | A1 | A2 | A3 | |
| >=90 Normal and high | G1 | 178 (36.0%)1 | 24 (4.9%)2 | 1 (0.2%)3 | 203 (41.1%) |
| 60–89 Mild reduction | G2 | 258 (52.2%)1 | 24 (4.9%)2 | 2 (0.2%)3 | 284 (57.5%) |
| 45–59 Mild to moderate reduction | G3a | 5 (1.0%)2 | 2 (0.4%)3 | 0 (0%)4 | 7 (1.4%) |
| 441 (89.3%) | 50 (10.1%) | 3 (0.6%) | 494* (100%) | ||
eGFR estimated glomerular filtration rate, ACR Albumin creatinine ratio, *6 participants were missing serum creatinine and cystatin C results. 1 low risk of progression, 2 intermediate risk of progression, 3 high risk of progression, 4 highest risk of progression
Prevalence of CKD according to markers of kidney damage
Urinalysis showed that 143 (29%) participants had proteinuria on dipstick. Prevalence of proteinuria was similar for those with eGFR > = 90 ml/min/1.73m2 and < 90 ml/min/1.73m2 (24.6% vs. 31.7% p = 0.17). At baseline, 10.1% [50] and at follow up, 3.8% [19] participants had an ACR > = 30 mg/g.
Factors associated with CKD
Factors associated with CKD varied with the equation and biomarker used for those with an eGFR < 90 ml/min/1.73m2 (Table 3) but was largely associated with male sex (with the exception of CKDEPI2021), viral non-suppression (by the cystatin C based equations), increasing age (by the CKDEPI and Bedside Schwartz equations), and being overweight (with the exception of the Cockroft-Gault equation). CKD was also associated with proteinuria (by the CKDEPI 2012 equation) and being on a TDF-based regimen (by the Bedside Schwartz equation). There was no evidence that CKD was associated with high blood pressure, muscle mass, and ACR.
Table 3.
Factors associated with having CKD (eGFR < 90 ml/min/1.73m2) among study participants according to the different estimating equations and biomarkers
| eGFR < 90 ml/min/1.73m2 from Cystatin C based Equations | eGFR < 90 ml/min/1.73m2from Serum Creatinine Based Equations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CKD EPI 2012 (n = 36/494) | Schwartz Cystatin (n = 291/494) | CKDEPI 2021 (n = 6/494) | Bedside Schwartz (n = 140/494) | Cockroft Gault (n = 68/493) | ||||||
| Unadjusted OR | Adjusted OR* | Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | |
| Age in years | 1.14 (1.03–1.25) | 1.13 (1.01–1.27) | 0.98 (0.94–1.04) | 0.99 (0.94–1.04) | 1.50 (1.13–1.98) | 1.45 (1.01–2.14) | 1.40 (1.30–1.51) | 1.42 (1.28–1.58) | 0.99 (0.94–1.04) | 1.08 (0.96–1.22) |
| Age categorized | ||||||||||
| > 18 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| < 18 | 0.47 (0.33–0.92) | 1.06 (0.73–1.55) | 0.09 (0.01–0.83) | 0.17 (0.11–0.26) | 0.89 (0.52–1.52) | 0.63 (0.23–1.77) | ||||
| Sex | ||||||||||
| Females | 1 | 1 | 1 | 1 | 1 | |||||
| Males | 2.42 (1.2–4.92) | 2.84 (1.27–6.31) | 3.21 (2.18–4.71) | 3.13 (2.12–4.65) | 0.64 (0.12–3.52) | 0.75 (0.11–4.92) | 2.22 (1.49–3.32) | 3.02 (1.68–5.43) | 3.21 (2.18–4.71) | 0.32 (0.16–0.60) |
| Blood pressure | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Elevated | 0.59 (0.17–1.98) | 0.41 (0.86–1.90) | 1.69 (0.95–2.98) | 1.42 (0.79–2.59) | 0.07 (0.00-0.63) | 0.35 (.-.) | 1.19 (0.66–2.17) | 1.02 (0.45–2.31) | 0.41 (0.21–0.78) | 0.26 (0.08–0.80) |
| Hypertensive | 0.98 (0.39–2.45) | 0.69 (0.26–1.89) | 1.25 (0.76–2.06) | 1.14 (0.67–1.94) | 0.45 (0.04–4.04) | 1.61 (0.24,10.63) | 2.42 (1.46–4.02) | 1.69 (0.89–3.29) | 0.73 (0.35–1.50) | 0.58 (0.18–1.86) |
| Viral suppression | ||||||||||
| Suppressed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Non suppressed | 3.11 (1.38–7.04) | 3.27 (1.29–8.32) | 2.09 (1.11–3.97) | 2.29 (1.18–4.44) | 1.01 (0.00-7.12) | 0.92 (.,.) | 0.42 (0.19–0.91) | 0.34 (0.13–0.89) | 0.63 (0.24–1.64) | 0.61 (0.21–1.74) |
| CD4 T cell count at baseline | ||||||||||
| > 500 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| 200–500 | 2.3 (0.98–5.38) | 1.41 (0.55–3.59) | 1.54 (0.96–2.48) | 1.52 (0.03–29.58) | 1.86 (1.03–3.04) | 0.66 (0.35–1.25) | 1.08 (0.56–2.10) | |||
| < 200 | 3.84 (1.5–9.38) | 2.59 (0.97–6.92) | 1.76 (0.96–3.22) | 5.34 (0.38–75.78) | 1.89 (1.04–3.46) | 0.65 (0.29–1.44) | 1.22 (0.56–2.68) | |||
| Social Economic Status | ||||||||||
| Least | 1 | 1 | 1 | 1 | 1 | |||||
| Middle | 1.03 (0.42–2.49) | 0.96 (0.62–1.49) | 2.28 (0.81–2.21) | 1.34 (0.62–1.49) | 1.32 (0.69–2.51) | 1.04 (0.56–1.92) | ||||
| Highest | 1.47 (0.66–3.31) | 1.15 (0.74–1.78) | 1.85 (1.15–2.99) | 1.15 (0.75–1.78) | 1.40 (0.75–2.64) | 0.81 (0.43–1.52) | ||||
| Body Mass Index | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Underweight | 1.69 (0.82–3.43) | 1.36 (0.92–2.01) | 0.29 (0.00-2.30) | 0.31 (0.18–0.51) | 0.74 (0.32–1.68) | 4.03 (2.29–7.09) | 7.23 (3.33–15.7) | |||
| Overweight | 1.27 (0.35–4.56) | 1.24 (0.63–2.43) | 3.11 (0.27–22.51) | 1.81 (0.94–3.51) | 1.98 (0.68–5.74) | 0.19 (0.00-1.12) | ||||
| Weight in Kg | 1.02 (0.99–1.04) | 1.01 (0.99–1.02) | - | 1.06 (1.04–1.09) | 0.99 (0.96–1.04) | |||||
| Stunting | ||||||||||
| Not stunted | 1 | 1 | 1 | 1 | 1 | |||||
| Stunted | 1.42 (0.50–4.02) | 0.88 (0.52–1.51) | - | 0.67 (0.31–1.43) | 1.81 (0.87–3.73) | |||||
| Mid Upper Arm Circumference | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| Malnourished | 1.64 (0.60–4.44) | 0.74 (0.40–1.36) | - | 0.43 (0.18–0.98) | 0.82 (0.29–2.39) | 4.05 (2.07–7.93) | 1.81 (0.79–4.13) | |||
| Muscle mass | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| Abnormal | 1.10 (0.41–2.98) | 1.28 (0.76–2.18) | 3.89 (0.64–23.66) | 1.44 (0.84–2.48) | 0.96 (0.45–2.05) | |||||
| Proteinuria | ||||||||||
| Negative | 1 | 1 | 1 | 1 | 1 | |||||
| Positive | 3.40 (1.7–6.78) | 3.71 (1.75–7.88) | 1.44 (0.96–2.15) | 1.22 (0.11–8.69) | 1.49 (0.98–2.27) | 1.12 (0.64–1.95) | ||||
| Albumin creatinine ratio | ||||||||||
| < 30 | 1 | 1 | ||||||||
| >=30 | 1.74 (0.69–4.4) | 0.76 (0.43–1.34) | 1.67 (0.19–14.6) | 0.89 (0.47–1.71) | ||||||
| On Tenofovir based regimen | ||||||||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 1.45 (0.62–3.41) | 0.97 (0.64–1.46) | 1.72 (0.19–14.88) | 3.22 (1.85–5.61) | 1.87 (0.94–3.73) | 0.63 (0.36–1.08) | ||||
| Duration on ART in years | ||||||||||
| < 5 | 1 | 1 | 1 | 1 | 1 | |||||
| 6 to 10 | 0.49 (0.17–1.43) | 0.79 (0.46–1.39) | 0.75 (-,-) | 0.92 (0.49–1.71) | 0.78 (0.37–1.61) | |||||
| > 10 | 1.11 (0.43–2.89) | 1.12 (0.64–1.98) | 1.27 (-,-) | 1.31 (0.71–2.42) | 0.62 (0.11–0.39) | |||||
OR Odds ratio ART Anti-retroviral therapy. N Number, eGFR estimated glomerular filtration rate* Adjusted for age, sex, blood pressure, viral suppression proteinuria, baseline CD4 T cell count
Results were similar when using CKD defined by eGFR < 60 ml/min/1.73m2 (Supplementary Table 3).
Discussion
This is the first study to compare the prevalence and factors associated with CKD diagnosed by creatinine and cystatin C among YPLHIV in Uganda according to standard guidelines. We found highly variable prevalence depending on the definition, the estimating equation and the biomarker used. This was compounded by the commonly used GFR estimating equations being recommended for adults (CKDEPI 2021) or children (Schwartz) only, despite the highly variable physical and sexual maturity within this important age group where long-term disease management is critical. Using cystatin C eGFR measures consistently gave a substantially higher prevalence of CKD: using the Schwartz cystatin equation approximately 60% of YPLHIV had eGFR < 90mls/min/1.73m2. While dipstick proteinuria is anticipated in this population largely treated with anti-retroviral drugs, 10% of participants had substantially elevated levels of albuminuria. However, when participants with baseline abnormalities were remeasured at three months according to the gold-standard definition, overall prevalence of CKD was much lower. This might have been due to transient derangements in kidney functions caused by dehydration, physical stress, fever or acute kidney diseases that had resolved by the three-month follow-up visit [40, 41].
The highest prevalence (59%) using an eGFR cutoff < 90 ml/min/1.73m2 and the Schwartz cystatin C equation, at baseline which fell to 23% at three months follow-up, was very high. This is similar to a study done in 96 Nigerian YPLHIV aged 15 to 29 years which found 53.3% prevalence [42] and a Tanzanian study among 240 YPLHIV aged less than 14 years that showed a prevalence of 28% [43]. When kidney function was determined by eGFR below 60 ml/min/1.73m2 on two separate occasions at least three months apart, the prevalence of CKD was 0.8%. This is lower than in a study done in Zambia among children living with HIV aged 1 to 18 years that found a prevalence of 3.8% after 3 months [44]. However, the children in this Zambian study were younger than the ones in our study.
Using a eGFR cut off of less than 60 ml/min/1.73m2 excludes a large proportion of YPLHIV who are already showing signs of impaired kidney function such as an ACR > = 30 mg/g, proteinuria and hypertension, and who would benefit from early intervention to halt progression of their kidney disease [45]. Using this KDIGO recommended GFR cut off that was based on adult kidney function and markers can lead to underdiagnosis of CKD in children. Pottel et al. have shown that clinical manifestations of decreased kidney function in young people start at GFR less than 75 ml/min/1.73m2; they recommend that the CKD definition should be revised to reflect this [46]. This could be even higher for those with chronic conditions such as HIV. The recommendations should consider them as well.
KDIGO recommends that screening and surveillance for CKD be tailored to the specific high risk group [47]. Our study suggests that using sequential estimation of eGFR over three months excludes YPLHIV at risk of CKD, and might be misleading to the public health response whose goal is to halt progression and to predict those who are in danger of kidney failure or development of cardiovascular complications [47]. KDIGO further recommends that screening frequency should be based on the risk profile of the individual and potential to progress [48]. YPLHIV have the potential to progress due to the continued insult to the kidney, one abnormal eGFR measurement that shows reduced kidney function should be sufficient for them to be followed up regularly and managed.
Estimating GFR in our population was challenging as the different estimating equations and biomarkers gave very different results. This was worse as one transitioned from equations meant for those below 18 years to those equations meant for adults above 18 years. The difference in the eGFR was wide even in the same individual. It is difficult to determine the true estimate for CKD among YPLHIV using these estimating equations yet knowing the true estimate is important to plan the public health response for CKD [7]. Clinicians who seek to diagnose CKD and plan management may get confused about the true CKD status of an individual. Misdiagnosis and classification of YPLHIV removes the opportunity to intervene early to halt progression to kidney failure [49]. The KDIGO recommended CKDEPI equation was developed from Caucasians with a mean age of 47 years and might not be appropriate for younger adults [50]. It is not surprising that each of the estimating equations gave a different prevalence since each estimating equation reflects the characteristics of the population/dataset that was used to develop it [51]. There is an urgent need to develop estimating equations for Africans living in Africa.
The use of GFR alone doesn’t predict progression or mortality risk and other markers of kidney damage such as albuminuria or proteinuria are used [26, 52]. This has also been shown in a meta-analysis with over 27 million adult participants that showed lower levels of GFR and high levels of ACR were associated with more adverse outcomes such as kidney failure, cardiovascular events and hospitalisations [53]. When ACR was used, the prevalence was 10.1%. This is lower than that reported among a Tanzanian cohort of YPLHIV aged 1 to 14 years which found a prevalence of 20.1% [43]. However, the ACR was determined at a single time point and included younger children. Proteinuria prevalence was 29% which was high in such a young population. Proteinuria is an early marker of HIV associated nephropathy [54] and if persistent, is predictive of CKD status in children [55]. However, we measured proteinuria only at baseline and yet two positive out of three readings are used to diagnose persistent proteinuria [47].
Cystatin C emerged as a better biomarker than serum creatinine as eGFR calculated from Cystatin C was above CKD stage 1 more consistently for all those that had an increased ACR, proteinuria or hypertension which are markers of abnormal kidney function [12]. In this study, 12.4% of the participants had a BMI < 18.5 kg/m2, the use of serum creatinine to estimate GFR in this group would lead to underdiagnosis of CKD given the dependence of creatinine on nutritional status or muscle mass. Cystatin C was recommended by a recent study in three countries (Uganda, Malawi, and South Africa) as the better biomarker in Africans [4]. Cystatin C is not as widely available in Uganda and is more expensive than serum creatinine making it harder to access [8]. Cystatin C should be recommended for the diagnosis of CKD in YPLHIV as well.
The associated factors varied by equation and biomarker used. We found that age, sex, and HIV viral non-suppression were associated with CKD for most equations and that proteinuria, CD4 cell count, blood pressure, and being on a TDF regimen were not associated for all of them. Age was not associated with CKD for the cystatin C-based equations. A study among perinatally infected YPLHIV in South Africa with a mean age of 12.0 years found sex, but not age or blood pressure were associated with CKD [56]. Males were also found to have more CKD than females in a study in Zimbabwe [57]. TDF use was also not associated with CKD status in a cohort of American children with CKD [58].
One of the strengths of this study is that we estimated the eGFR and ACR at two different time points more than three months apart as recommended by KDIGO and were able to ascertain those that actually had CKD according to the standard definition of CKD. However, most of the GFR estimating equations and normal serum creatinine have not been validated in YPLHIV in resource-limited settings especially in Africa and this makes it that much harder to determine the abnormal values in YPLHIV [4, 59]. This could explain the low correlation between eGFR and the markers of kidney damage found in this study. We determined both markers of kidney damage (albuminuria and proteinuria) and function and could tell YPLHIV that were at risk of CKD progression. The biggest limitation is that we did not measure the GFR using either ioxehol or the nuclear tracers 99mTc-diethylenetriaminepentaacetic acid (DTPA) or 51Cr-EDTA [10] and so we are unable to tell how accurate the eGFR was. This study was carried out in an urban setting and the prevalence might be different in the rural parts of Uganda.
Conclusion
CKD prevalence among YPLHIV in Uganda was much lower using serum creatinine-based estimates compared to cystatin C based estimates. Prevalence of kidney impairment was much higher at baseline compared to three months later. Cystatin C diagnosed more people with both kidney impairment and CKD but is more expensive in Uganda. The substantial prevalence of albuminuria and reduced eGFR suggest that HIV programs should prioritize screening for CKD among YPLHIV. The definition of CKD and best biomarker to use in YPLHIV should be further investigated to optimise detection of those with early abnormalities of kidney function. Estimating equations should be validated against measured GFR in young people to define how best to estimate GFR across older children and young adults in Africa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the health workers and peers at the participating facilities where this data was collected. The HIV program at the participating health facilities is supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through CDC. We acknowledge Evelyn Natuha, Stella Mirembe, Malcolm Lwanga and Aidah Nanvuma for supporting the research study.
Abbreviations
- ACR
Albumin Creatinine Ratio
- AIDS
Acquired Immune Deficiency Syndrome
- ART
Anti-Retroviral Therapy
- BIS
Bioimpedance Spectroscopy
- BMI
Body Mass Index
- CALHIV
Children and Adolescents living with HIV
- CD4
Cluster of differentiation 4
- CKD
Chronic Kidney Disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- DM
Diabetes Mellitus
- FAS
Full Age Spectrum
- GFR
Glomerular Filtration Rate
- HB
Haemoglobin
- HIV
Human Immunodeficiency Virus
- HIVAN
HIV associated Nephropathy
- HW
Health Worker
- IQR
Interquartile Range
- KDIGO
Kidney Disease Improving Global Outcomes
- KRT
Kidney replacement therapy
- NCD’s
Non-Communicable Diseases
- PLHIV
People Living with HIV AIDS
- SSA
Sub Saharan Africa
- USA
United States of America
- WHO
World Health Organization
- YPLHIV
Young People Living with HIV
Author contributions
EN, LT, RK, CDC, DN, BC, YCM, HW contributed to the conceptualization and design of the study, data collection, analysis, and interpretation. EN, LT, RK drafted the manuscript. CDC, BC, RK, DN, YCM edited the draft manuscript. HW was responsible for the overall supervision of this work. All authors reviewed and approved the manuscript before submission for publication.
Funding
Support for research was provided by Fogarty International Centre, National Institutes of Health (grant #2D43TW009771-06) HIV and co-infections in Uganda. HAW is funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (Grant 1: MR/R010161/1). EN, Doctoral Research Fellow, NIHR131273 is funded by the NIHR for this research project. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
Data availability
The data supporting the findings of this study are openly available in repository https://datacompass.lshtm.ac.uk/.
Declarations
Human ethics and consent to participate declarations
Ethical approval was received from the Uganda Virus Research Institute (UVRI) Research Ethics Committee (reference number GC/127/946), the Uganda National Council of Science and Technology (HS2578ES) and the London School of Hygiene and Tropical Medicine institutional review board (28797). Information about the study appropriate for adults, semi-literate adults and children was provided in an information booklet that was read to the participants and caregivers. All the participants more than 18 years of age provided written informed consent. Those below 18 years of age provided assent, and their caregivers provided written informed consent. If a child refused to provide assent even after their caregiver had provided consent, that child was not enrolled into the study. All participants had the option to withdraw at any point during the research. All participants with suspected CKD were referred to a nephrologist for management.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Supplements. 2022;12(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Disease. 2002;39:S1–266. [PubMed] [Google Scholar]
- 3.Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabian J, Kalyesubula R, Mkandawire J, Hansen CH, Nitsch D, Musenge E, et al. Measurement of kidney function in Malawi, South Africa, and Uganda: a multicentre cohort study. Lancet Global Health. 2022;10(8):e1159–69. [DOI] [PubMed] [Google Scholar]
- 5.Fabian J, George JA, Etheredge HR, van Deventer M, Kalyesubula R, Wade AN, et al. Methods and reporting of kidney function: a systematic review of studies from Sub-saharan Africa. Clin Kidney J. 2019;12(6):778–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naicker S, Fabian J. Risk factors for the development of chronic kidney disease with HIV/AIDS. Clin Nephrol. 2010;74(Suppl 1):S51–6. [DOI] [PubMed] [Google Scholar]
- 7.Stanifer JW, Muiru A, Jafar TH, Patel UD. Chronic kidney disease in low-and middle-income countries. Nephrol Dialysis Transplantation. 2016;31(6):868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyesubula R, Makanga G, Gyagenda JO, Atuhe D, Kansiime G, Kiggundu D et al. Nephrology in Uganda. Nephrol Worldw. 2021:75–83.
- 9.George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB, Kengne AP. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health. 2017;2(2):e000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR Estimation. Clin J Am Soc Nephrol. 2020;15(8):1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New Creatinine- and cystatin C–Based equations to Estimate GFR without Race. N Engl J Med. 2021;385(19):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani K, Rosner MH, Ostermann M, Creatinine. From physiology to clinical application. Eur J Intern Med. 2020;72:9–14. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(1):58–66. [DOI] [PubMed] [Google Scholar]
- 18.Mauss S, Berger F, Kuschak D, Henke J, Hegener P, Wolf E, et al. Cystatin C as a marker of renal function is affected by HIV replication leading to an underestimation of kidney function in HIV patients. London, England: SAGE Publications Sage UK; 2008. [PubMed] [Google Scholar]
- 19.Mian AN, Schwartz GJ. Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis. 2017;24(6):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frigati LJ, Ameyan W, Cotton MF, Gregson CL, Hoare J, Jao J, et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc Health. 2020;4(9):688–98. [DOI] [PubMed] [Google Scholar]
- 21.Jotwani V, Atta MG, Estrella MM. Kidney disease in HIV: moving beyond HIV-Associated Nephropathy. J Am Soc Nephrol. 2017;28(11):3142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalyesubula R, Wearne N, Semitala FC, Bowa K. HIV-associated renal and genitourinary comorbidities in Africa. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S68–78. [DOI] [PubMed] [Google Scholar]
- 23.Purswani MU, Chernoff MC, Mitchell CD, Seage GR, Zilleruelo G, Abitbol C, et al. Chronic kidney disease associated with perinatal HIV infection in children and adolescents. Pediatr Nephrol. 2012;27(6):981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhimma R, Purswani MU, Kala U. Kidney disease in children and adolescents with perinatal HIV-1 infection. J Int AIDS Soc. 2013;16(1):18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Innes S, Patel K. Non-communicable diseases in adolescents with perinatally-acquired HIV-1 infection in high-and low-income settings. Curr Opin HIV AIDS. 2018;13(3):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunupuradah T, Phupitakphol T, Sophonphan J, Prasitsuebsai W, Anugulruengkitt S, Jantarabenjakul W, et al. Prevalence of persistent renal dysfunction in perinatally HIV-infected Thai adolescents. Pediatr Infect Dis J. 2018;37(1):66–70. [DOI] [PubMed] [Google Scholar]
- 27.Bernheimer JM, Patten G, Makeleni T, Mantangana N, Dumile N, Goemaere E, et al. Paediatric HIV treatment failure: a silent epidemic. J Int AIDS Soc. 2015;18:20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Queremel Milani DA, Jialal I, Urinalysis. StatPearls Publishing, Treasure Island (FL); 2020 2020. [PubMed]
- 29.Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco AL, De Jong PE, et al. Kidney disease: improving global outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Supplements. 2013;3(1):1–150. [Google Scholar]
- 30.Levey AS, Eckardt K-U, Dorman NM, Christiansen SL, Cheung M, Jadoul M et al. Nomenclature for kidney function and disease: executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Kidney Diseases. 2020;6(5):309 – 17. [DOI] [PMC free article] [PubMed]
- 31.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrology: JASN. 2009;20(3):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dialysis Transplantation. 2016;31(5):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Björk J, Nyman U, Larsson A, Delanaye P, Pottel H. Estimation of the glomerular filtration rate in children and young adults by means of the CKD-EPI equation with age-adjusted creatinine values. Kidney Int. 2021;99(4):940–7. [DOI] [PubMed] [Google Scholar]
- 37.Pierce CB, Muñoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ. Age-and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 2021;99(4):948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn JT, Falkner BE. New Clinical Practice Guideline for the management of high blood pressure in children and adolescents. Hypertens (Dallas Tex: 1979). 2017;70(4):683–6. [DOI] [PubMed]
- 39.Thompson CG, Kim RS, Aloe AM, Becker BJ. Extracting the variance inflation factor and other multicollinearity diagnostics from typical regression results. Basic Appl Soc Psychol. 2017;39(2):81–90. [Google Scholar]
- 40.Bongers C, Alsady M, Nijenhuis T, Tulp ADM, Eijsvogels TMH, Deen PMT, et al. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol Rep. 2018;6(11):e13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC et al. Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney international. 2021;100(3):516 – 26. [DOI] [PubMed]
- 42.Okafor UH, Unuigbe EI, Chukwuonye E. Prevalence and clinical and laboratory characteristics of kidney disease in anti-retroviral-naive human immunodeficiency virus-infected patients in South-South Nigeria. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation. Saudi Arabia. 2016;27(1):129–34. [DOI] [PubMed] [Google Scholar]
- 43.Fredrick F, Francis JM, Ruggajo PJ, Maro EE. Renal abnormalities among HIV infected children at Muhimbili National Hospital (MNH)-Dar es Salaam, Tanzania. BMC Nephrol. 2016;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimba KM. Prevalence and factors associated with renal dysfunction in HIV positive paediatric patients on highly active antiretroviral therapy at the paediatric centre of excellence of the university teaching hospital, in Lusaka, Zambia. Pediatr Nephrol. 2016;31(10):1778. [Google Scholar]
- 45.Uhlig K, Johnson CA, Levey AS. New guidelines spell out approach to chronic kidney disease: early diagnosis of chronic kidney disease widens the window of opportunity to put effective treatments to work. Recent National Kidney Foundation clinical practice guidelines provide a simple definition, a practical staging system, and a stage-appropriate action plan. Patient Care. 2003;37(7):38–45. [Google Scholar]
- 46.Pottel H, Hoste L, Delanaye P. Abnormal glomerular filtration rate in children, adolescents and young adults starts below 75 mL/min/1.73 m 2. Pediatr Nephrol. 2015;30:821–8. [DOI] [PubMed] [Google Scholar]
- 47.Levey A, Atkins R, Coresh J, Cohen E, Collins A, Eckardt K-U, et al. Chronic kidney disease as a global public health problem: approaches and initiatives–a position statement from kidney disease improving global outcomes. Kidney Int. 2007;72(3):247–59. [DOI] [PubMed] [Google Scholar]
- 48.Shlipak MG, Tummalapalli SL, Boulware LE, Grams ME, Ix JH, Jha V, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34–47. [DOI] [PubMed]
- 49.Bhimma R. HIV-Related kidney diseases. In: Bobat R, editor. HIV infection in children and adolescents. Cham: Springer International Publishing; 2020. pp. 143–52. [Google Scholar]
- 50.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller WG. Perspective on new equations for estimating glomerular filtration rate. Clin Chem. 2021;67(6):820–2. [DOI] [PubMed] [Google Scholar]
- 52.Bökenkamp A. Proteinuria—take a closer look! Pediatr Nephrol. 2020;35(4):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Consortium WGftCP. Estimated glomerular filtration rate, Albuminuria, and adverse outcomes: an individual-participant data Meta-Analysis. JAMA. 2023;330(13):1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palau L, Menez S, Rodriguez-Sanchez J, Novick T, Delsante M, McMahon BA, et al. HIV-associated nephropathy: links, risks and management. HIV AIDS (Auckl). 2018;10:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beng H, Rakhmanina N, Moudgil A, Tuchman S, Ahn S-Y, Griffith C, et al. HIV-associated CKDs in children and adolescents. Kidney Int Rep. 2020;5(12):2292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frigati L, Mahtab S, Nourse P, Ray P, Perrazzo S, Machemedze T, et al. Prevalence of risk factors for chronic kidney disease in South African youth with perinatally acquired HIV. Pediatric nephrology (Berlin. Germany). 2018;34(2):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byers BW, Drak D, Shamu T, Chimbetete C, Dahwa R, Gracey DM. Assessing renal impairment in treatment-naïve adolescents living with HIV commencing antiretroviral therapy in Zimbabwe. AIDS. 2023;37(5):789–94. [DOI] [PubMed] [Google Scholar]
- 58.Purswani M, Patel K, Kopp JB, Seage GR 3rd, Chernoff MC, Hazra R, et al. Tenofovir treatment duration predicts proteinuria in a multiethnic United States Cohort of children and adolescents with perinatal HIV-1 infection. Pediatr Infect Dis J. 2013;32(5):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanepoel CR, Atta MG, D’Agati VD, Estrella MM, Fogo AB, Naicker S, et al. Kidney disease in the setting of HIV infection: conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies Conference. Kidney Int. 2018;93(3):545–59. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available in repository https://datacompass.lshtm.ac.uk/.