Abstract
Background
Argonaute (AGO), Dicer-like (DCL), and RNA-dependent RNA polymerase (RDR) are essential components of RNA silencing pathways in plants. These components are crucial for the generation and regulatory functions of small RNAs, especially in plant development and response to environmental stresses. Despite their well-characterized functions in other plant species, there is limited information about these genes and their stress responses in centipedegrass (Eremochloa ophiuroides), a key turfgrass species.
Results
Using genome-wide analysis we identified 20 AGO, 6 DCL, and 10 RDR members in centipedegrass and provided a comprehensive overview of their characteristics. We performed the chromosomal location, gene duplication, syntenic analysis, conserve motif, gene structure, and cis-acting elements analysis. And conducted phylogenetic analyses to clarify the evolutionary relationships among the EoAGO, EoDCL, and EoRDR gene families. Three-dimensional modeling prediction of EoAGO, EoDCL, and EoRDR proteins supported the phylogenetic classification. Furthermore, we examined the expression patterns of these genes in different tissues (spike, stem, leaf, root, and flower) and under different stress conditions (cold, salt, drought, aluminum, and herbicide) using RT-qPCR. The results revealed that most of EoAGO, EoDCL, and EoRDR genes were upregulated in response to multiple abiotic stresses, while some exhibited unique responses, suggesting potential specialized regulatory functions.
Conclusion
In this study, we performed a comprehensive genome‑wide identification, and phylogenetic and expression pattern analyses of the EoAGO, EoDCL and EoRDR gene families. Our analysis provides a foundation for future research on the RNA silence elements of turfgrass, and affords scientific basis and insights for clarifying the expression patterns of EoAGO, EoDCL and EoRDR genes under adversity stress. Further functional validation and molecular breeding of these genes can be carried out for enhancing the stress resistance of centipedegrass.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-11062-y.
Keywords: AGO, DCL, RDR, Gene family, Centipedegrass, Abiotic stresses
Introduction
Small noncoding RNAs in plants, such as microRNAs (miRNAs) and short interfering RNAs(siRNAs), comprise 21–24 nucleotides. These molecules regulate various aspects of plant growth and development, abiotic and biotic stress response, and signal transduction [1–3]. RNA interference (RNAi) is a conserved gene silencing process mediated by small RNAs (sRNAs), which regulates translational inhibition, RNA degradation, and chromatin modifications in eukaryotes [4]. This mechanism is essential in gene silencing and post-transcriptional regulation, with numerous RNAi technologies developed to address biotic and abiotic stress in plants [5, 6]. Previous studies have reported that sRNAs generation and functions are directly associated with the proteins encoded by three key RNAi gene families: the Argonaute (AGO), Dicer-like (DCL), and RNA -dependent RNA polymerases (RDR) [7, 8]. As fundamental elements of the RNAi mechanism, RDRs utilize RNA as a template to synthesize double stranded RNAs (dsRNA), while the RNase III-type DCLs are responsible for cleaving dsRNA into various sRNAs, including siRNAs and miRNAs. Subsequently, these sRNAs can bind to AGO family proteins and integrate into the core of RNA-induced silencing complexes (RISCs) [9–11]. The RISCs, guided by sRNAs, can recognize target genes through complementary base pairing and regulate gene expression at the post-transcriptional level [post-transcriptional gene silencing, (PTGS)] and the transcriptional level [transcriptional gene silencing, (TGS)] [12, 13]. In PTGS, dsRNA is processed by the DCL enzyme into siRNAs, which subsequently associate with AGO protein to form the RISC. The RISC complex subsequently recognizes and binds to complementary sequences on the target mRNA, leading to its degradation [14]. TGS involves modifying chromatin structure to inhibit gene expression, mediated by sRNAs that guide chromatin-modifying enzymes to specific genomic loci. Chromatin modification affects the gene silencing effect mediated by sRNA molecules by modifying the structure and accessibility of chromatin, including histone modification and DNA methylation [15]. miRNAs originate from single-stranded transcripts (pri-miRNAs) generated by RNA polymerase II, while siRNA is primarily processed from fully complementary dsRNA. Based on the mechanism of dsRNA production and the functional distinctions of siRNA, siRNA can be divided into various types, heterochromatic siRNAs (hc-siRNAs), phased secondary siRNAs (phasiRNAs) [16], trans-acting small interfering RNAs (ta-siRNAs), natural antisense siRNAs (nat-siRNAs), long siRNAs (lsiRNAs) [17, 18], long miRNAs (lmiRNAs) [19], and DCL-independent siRNA (sidRNAs) [20]. While miRNAs, ta-siRNAs, and nat-siRNAs mediate PTGS, hc-siRNAs, lmiRNAs, and sidRNAs direct DNA methylation thus inducing TGS [21].
The fundamental elements of the RNA silencing pathway, namely AGO, DCL, and RDR, have been identified in various species. For instance, a total of 20 genes were identified in Arabidopsis [22], 32 genes in rice [23], 28 genes in maize [24], and 26 in sorghum [25]. These studies have demonstrated that AGO, DCL, and RDR genes are essential in plant development, including flower and fruit development, and nutrient organs growth, including roots, stems, and leaves [26]. Additionally, they are essential in plant stress responses and defense against pathogens. Previous studies reported that some AGO, DCL, and RDR genes exhibit upregulated or downregulated expression changes in rice [23, 27], cucumber [28], and maize [29] in response to abiotic stresses, including drought, and high salt and temperatures.
Centipedegrass (Eremochloa ophiuroides) is an essential perennial (C4) warm-season diploid turfgrass species (2n = 2x = 18) belonging to the Eremochloa genus of the Poaceae family [30]. This grass species originates from the Yangtze River region in China; however, it is now widely distributed across southeast Asia, the southern United States, and the northern and eastern regions of Australia [31, 32]. Centipedegrass is one of the most popular turfgrasses because of its beautiful leaf color, excellent adaptability to nutrient-deficient soils, and minimal maintenance requirements, making it a preferred choice for lawn and slope preservation and landscaping purposes [33, 34]. Comparing with other warm-season turfgrasses, centipedegrass exhibits superior drought tolerance, robust disease resistance, and thrives in acidic to slightly alkaline soil conditions [35, 36].
Recently, many plants have been compelled to endure extreme environments including drought, salinity, cold and metal stresses, which seriously endangering their growth and development [28]. RNA silencing family genes expression is dynamically regulated during plant development and stress response, indicating the influential role of RNA silencing regulation in environmental signals response [1–3]. Therefore, performing a comprehensive analysis of the RNA silencing family in centipedegrass is essential. With the availability of a high-quality draft genome, it is possible for us to study on RNA silencing mechanism of centipedegrass. Further study on the regulatory mechanisms of RNAi, it may reveal the essential stress response genes that enhance stress tolerance, disease resistance, and overall quality in grass. In this study, we comprehensively analyzed EoAGO, EoDCL, and EoRDR genes, including the gene structures, conserved domains, duplication events, cis-acting elements and phylogenetic analysis. Furthermore, we examined the expression profiles of these identified genes across various tissues and under diverse abiotic stress conditions. This study provided the basic genomic information and clarified the expression patterns of EoAGO, EoDCL and EoRDR genes under adversity stress. Through further functional validation and molecular breeding of these genes can be carried out for improving the stress resistance of centipedegrass.
Materials and methods
Plant genome sequence acquisition and identification of EoAGO, EoDCL and EoRDR gene family members
The genome sequence of Arabidopsis was obtained from the TAIR database (https://www.arabidopsis.org/), while genome sequences of other plants (sorghum, maize, and rice) were downloaded from the Phytozome database (https://phytozome-next.jgi.doe.gov/). The genome assembly and protein sequences of centipedegrass were obtained from the Figshare database (https://figshare.com/s/8256acffdb73bb050045) [37]. The Hidden Markov Model (HMM) profiles of the AGO, DCL, and RDR structural domains were AGO (PAZ PF02170, PIWI PF02171), DCL (DEAD PF00270, Helicase-C PF00271, Dicer-dimer PF03368, PAZ PF02170, RNaseIII PF00636), and RDR (RdRP PF05183). EoAGO, EoDCL, and EoRDR proteins in centipedegrass were identified using the default settings of HMMER 3.0 software (version 3.0), SMART (https://smart.embl.de/), Pfam(http://pfam-legacy.xfam.org/), and the Conserved Domain Database in NCBI were used to confirm finally resulted EoAGO, EoDCL, and EoRDR members. The resulting genes were named based on their phylogenetic relationship with the members of similar gene families in sorghum, maize, rice, and Arabidopsis. The ExPASy-ProtParam tool was used to determine the physio-chemical properties of the EoAGO, EoDCL, and EoRDR genes [38]. WoLF PSORT was used for subcellular localization predictions [39].
Multiple sequence alignment, phylogenetic analysis and classification of the EoAGO, EoDCL and EoRDR gene families
The EMBL-EBI-Clustal Omega tool with default settings was used to perform multiple sequence alignment of the predicted EoAGO, EoDCL, and EoRDR proteins and was viewed using the Jalview software (version 2.11) (https://www.jalview.org/) [40]. The neighbor-joining method with 1,000 times bootstrap-replicates in MEGA11 software was used to build the phylogenetic tree [41]. EoAGO, EoDCL, and EoRDR genes were assigned to different subgroups based on the classification of orthologous genes from Arabidopsis, sorghum, maize, and rice.
Chromosomal location, gene duplication, and syntenic analysis
Chromosomal location information of EoAGO, EoDCL, and EoRDR genes was obtained from GFF and sequencing files. The MCScanX was used to conduct gene duplication analysis [42, 43] and displayed by Circos plot [44]. The dual synteny plotter software (https://github.com/CJ-Chen/TBtools-II) was used to visualize the syntenic relationships between the EoAGO, EoDCL, and EoRDR genes of centipedegrass and other four plants (Arabidopsis, sorghum, maize, and rice). All these analyses were visualized using TBtools [45].
Conserve motif, gene structure, cis-acting elements analysis and 3-Dimensional structure prediction
The NCBI-CDD was demployed to obtain conserved domains of the EoAGO, EoDCL and EoRDR proteins. The exon–intron organizations of the EoAGO, EoDCL, and EoRDR genes were determined using TBtools. The PlantCare online software (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/), visualized in TBtools, was used to predict the Cis-acting elements in the 2,000 bp upstream region of each gene.
SWISS-MODEL (https://swissmodel.expasy.org/) was used for EoAGO, EoDCL and EoRDR protein 3D structure prediction. The template selection is based on coverage and similarity (identity ≥ 30%). GMQE represents the accuracy and coverage of a model, with a credibility range of 0–1. A higher value indicates better quality.
Plant materials and stress treatments
Seeds of the centipedegrass cultivar ‘Wuling’ (provided by the Sichuan Academy of Grassland Science, Chengdu, China) were sown in square pots filled with quartz sand and cultivated under greenhouse conditions: temperature 23 /19 °C (12 h day/12 h night). After 2 months, a part of the seedlings underwent stress treatments: cold (4 ℃), drought (20% PEG-6000), salt (200 mmol L–1 NaCl), Al (100 mmol L–1 AlCl3), and herbicide (6 mmol·L–1 glufosinate) stresses. Control plants were irrigated with ½ Hoagland nutrient solution. During the treatments, samples were collected at 0, 0.5, 1.5, 3, 6, 12, 24, 48, and 72 h during the treatments. The remaining seedlings cultivated under normal conditions were used to collect flower, stem, spike, root and leaf at during the flowering period of grasses. All collected samples were immediately frozen in liquid nitrogen for RNA extraction. Three samples at each treatment time point and tissue were taken for RNA extraction and RT-qPCR.
RNA isolation, cDNA synthesis and RT-qPCR expression analysis
Total RNA from centipedegrass was isolated with TRIzol reagent (Invitrogen, USA). The ABScript III Gdna Remover and Genious 2X SYBR qPCR Mix (Abclonal, Wuhan) were used for the synthesis of cDNA and RT-qPCR follow the manufacturer's protocol, the CXF Connect™ Real-Time System (Bio-Rad) was used for reactions. Based on the previous studies published by group [46], the most stable gene under different stresses was selected as the reference genes for calculating the gene expression using the 2−ΔΔCt method [47], where the UBC (ubiquitin-conjugating enzyme) gene was used for cold and Al stress, the MD (malate dehydrogenase) gene was used for salt, drought stresses, and different tissues, and the RIP (60S ribosomal protein L2) gene was used for herbicide stress. Based on the location on the phylogenetic tree and the amino acid identity with each other, only seven EoAGOs, three EoDCLs, and four EoRDRs genes were selected for the RT-qPCR expression analysis. Primer software (version 5) was used to design the 14 primer pairs (Supplementary Table S4). RNA-seq data of centipedegrass under cold treatment were obtained from previous studies [35].
Results
Genome-wide identification of AGOs, DCLs and RDRs in centipedegrass
The HMM profiles of conserved domains were used to identify the EoAGO, EoDCL and EoRDR gene families. Analysis revealed 20 EoAGOs genes, 6 EoDCLs genes, and 10 EoRDRs genes in the centipedegrass genome database. The characteristics of the identified RNA silencing genes are summarized in Table 1.
Table 1.
Basic information of AGO, DCL and RDR genes in centipedegrass
| Gene Name | Locus ID | Chromosomal location | ORF length (bp) | Mw (Da) | pI | Instability index | GRAVY | Subcellular localization | |
|---|---|---|---|---|---|---|---|---|---|
| AGO | EoAGO1a | evm.model.ctg497.10 | chr7:3,531,971–3,539,712 | 3321 | 121,829 | 9.55 | 49.29 | -0.528 | Nucleus |
| EoAGO1b | evm.model.ctg498.5 | chr7:3,648,204–3,655,953 | 3321 | 121,841.1 | 9.55 | 49.29 | -0.524 | Nucleus | |
| EoAGO1c | evm.model.ctg780.217 | chr9:55,644,807–55,660,515 | 3318 | 122,401 | 9.53 | 49.87 | -0.521 | Nucleus | |
| EoAGO1d | evm.model.ctg391.46 | chr5:102,512,987–102,520,102 | 2745 | 101,090.7 | 9.13 | 53.74 | -0.438 | Nucleus | |
| EoAGO1e | evm.model.ctg624.33 | chr8:81,742,442–81,750,079 | 3132 | 115,567.5 | 9.47 | 52.77 | -0.509 | Nucleus | |
| EoAGO2 | evm.model.ctg782.50 | chr9:60,264,310–60,269,206 | 3069 | 110,606.8 | 9.40 | 44.48 | -0.432 | Nucleus | |
| EoAGO4a | evm.model.ctg573.10 | chr6:71,861,539–71,866,868 | 2754 | 102,410.1 | 8.99 | 47.48 | -0.373 | Chloroplast | |
| EoAGO4b | evm.model.ctg21.9 | chr1:21,004,110–21,012,014 | 2694 | 100,670.6 | 9.11 | 49.21 | -0.380 | Nucleus | |
| EoAGO4c | evm.model.ctg136.122 | chr2:63,976,662–63,988,327 | 2241 | 84,242.57 | 8.73 | 51.45 | -0.412 | Chloroplast | |
| EoAGO4d | evm.model.ctg21.7 | chr1:20,971,506–20,976,544 | 2637 | 97,980.53 | 9.16 | 44.73 | -0.264 | Chloroplast | |
| EoAGO5a | evm.model.ctg374.195 | chr3:17,707,456–17,747,083 | 3366 | 121,870.4 | 9.62 | 49.92 | -0.426 | Nucleus | |
| EoAGO5b | evm.model.ctg382.112 | chr4:5,126,642–5,134,952 | 3384 | 124,299.7 | 9.36 | 51.19 | -0.406 | Nucleus | |
| EoAGO5c | evm.model.ctg254.4 | chr3:15,520,044–15,531,252 | 3465 | 126,281.1 | 9.46 | 46.94 | -0.381 | Nucleus | |
| EoAGO5d | evm.model.ctg253.270 | chr3:15,614,325–15,622,564 | 2655 | 98,881.25 | 9.04 | 45.89 | -0.317 | Chloroplast | |
| EoAGO6 | evm.model.ctg415.78 | chr5:76,081,544–76,089,848 | 2523 | 94,313.97 | 9.29 | 47.11 | -0.318 | Nucleus | |
| EoAGO7 | evm.model.ctg312.44 | chr4:82,793,205–82,797,989 | 3546 | 132,281.8 | 9.59 | 48.88 | -0.336 | Chloroplast | |
| EoAGO10 | evm.model.ctg402.94 | chr5:93,406,197–93,421,012 | 2418 | 91,252.04 | 9.00 | 41.72 | -0.334 | Chloroplast | |
| EoAGO18a | evm.model.ctg194.36 | chr3:91,247,450–91,252,794 | 3174 | 115,172.3 | 9.35 | 40.54 | -0.497 | Nucleus | |
| EoAGO18b | evm.model.ctg373.62 | chr4:20,391,739–20,397,027 | 2784 | 103,422.4 | 9.04 | 39.57 | -0.402 | Nucleus | |
| EoAGO18c | evm.model.ctg462.4 | chr5:24,108,058–24,113,635 | 2442 | 90,521.62 | 8.66 | 42.57 | -0.351 | Nucleus | |
| DCL | EoDCL1 | evm.model.ctg278.43 | chr4:112,660,845–112,671,572 | 5811 | 216,143.1 | 6.09 | 43.02 | -0.380 | Nucleus |
| EoDCL2a | evm.model.ctg366.102 | chr4:26,660,222–26,676,924 | 3579 | 134,465.1 | 6.22 | 45.93 | -0.116 | Nucleus | |
| EoDCL2b | evm.model.ctg367.10 | chr4:26,521,666–26,538,358 | 3705 | 139,249.9 | 6.49 | 45.42 | -0.113 | Nucleus | |
| EoDCL3a | evm.model.ctg62.68 | chr1:80,081,933–80,090,656 | 4413 | 165,747 | 6.29 | 42.68 | -0.260 | Nucleus | |
| EoDCL3b | evm.model.ctg359.151 | chr4:33,024,764–33,036,537 | 4986 | 186,822.8 | 6.18 | 44.80 | -0.154 | Nucleus | |
| EoDCL4 | evm.model.ctg110.32 | chr2:41,402,507- 41,414,032 | 2475 | 93,886.41 | 5.94 | 43.44 | -0.117 | Nucleus | |
| RDR | EoRDR1a | evm.model.ctg634.45 | chr8:72,843,667–72,849,516 | 3339 | 127,570.1 | 6.84 | 39.82 | -0.285 | Chloroplast |
| EoRDR1b | evm.model.ctg635.6 | chr8:72,681,119–72,686,934 | 3339 | 127,563.1 | 6.86 | 39.69 | -0.281 | Chloroplast | |
| EoRDR2 | evm.model.ctg769.18 | chr9:45,305,488–45,311,415 | 3390 | 125,811.8 | 6.52 | 44.76 | -0.200 | Chloroplast | |
| EoRDR3a | evm.model.ctg722.8 | chr9:7,674,410–7,683,240 | 1872 | 69,692.43 | 5.68 | 47.27 | -0.355 | Chloroplast | |
| EoRDR3b | evm.model.ctg722.9 | chr9:7,683,242–7,698,311 | 1302 | 50,129.07 | 5.19 | 38.95 | -0.407 | Chloroplast | |
| EoRDR4a | evm.model.ctg7.29 | chr1:4,513,777–4,572,529 | 2925 | 108,886.9 | 5.67 | 41.15 | -0.269 | Chloroplast | |
| EoRDR4b | evm.model.ctg7.31 | chr1:4,576,824–4,588,094 | 3474 | 130,552.2 | 8.15 | 46.64 | -0.399 | Chloroplast | |
| EoRDR6a | evm.model.ctg398.176 | chr5:97,820,643–97,823,154 | 1392 | 52,150.5 | 5.53 | 35.00 | -0.326 | Chloroplast | |
| EoRDR6b | evm.model.ctg599.18 | chr7:21,144,141–21,148,060 | 3624 | 135,837 | 8.3 | 44.59 | -0.241 | Chloroplast | |
| EoRDR6c | evm.model.ctg38.11 | chr1:42,871,533–42,876,902 | 3615 | 135,381.9 | 6.85 | 39.15 | -0.260 | Chloroplast |
The genomic length of 20 EoAGOs varied from 2241 to 3546 bp corresponding to EoAGO4c (evm.model.ctg136.122) and EoAGO7 (evm.model.ctg312.44), with their pI values exhibiting basic characteristics (pI value 8.66 ~ 9.59). The six EoDCLs ORF ranged from 2475 to 5811 bp corresponding to EoDCL4 (evm.model.ctg.110.32) and EoDCL1 (evm.model.ctg278.43). All EoDCL proteins exhibited acidic properties (pI value 5.94 ~ 6.49). The ORF length of EoRDRs varies between 1302 and 3624 bp corresponding to EoRDR3b (evm.model.ctg722.9) and EoRDR6b (evm.model.ctg599.18), respectively. Most EoRDRs exhibit acidic properties, while EoRDR4b and EoRDR6b demonstrate higher pI values of 8.15 and 8.3. Regarding subcellular localization predictions, 13 EoAGOs (65%) and 10 EoRDRs (100%) were predicted to be localized in the nucleus, while seven EoAGOs (35%) and six EoDCLs (100%) were predicted to be localized in the chloroplast.
Chromosomal localization, phylogenetic and syntenic analysis
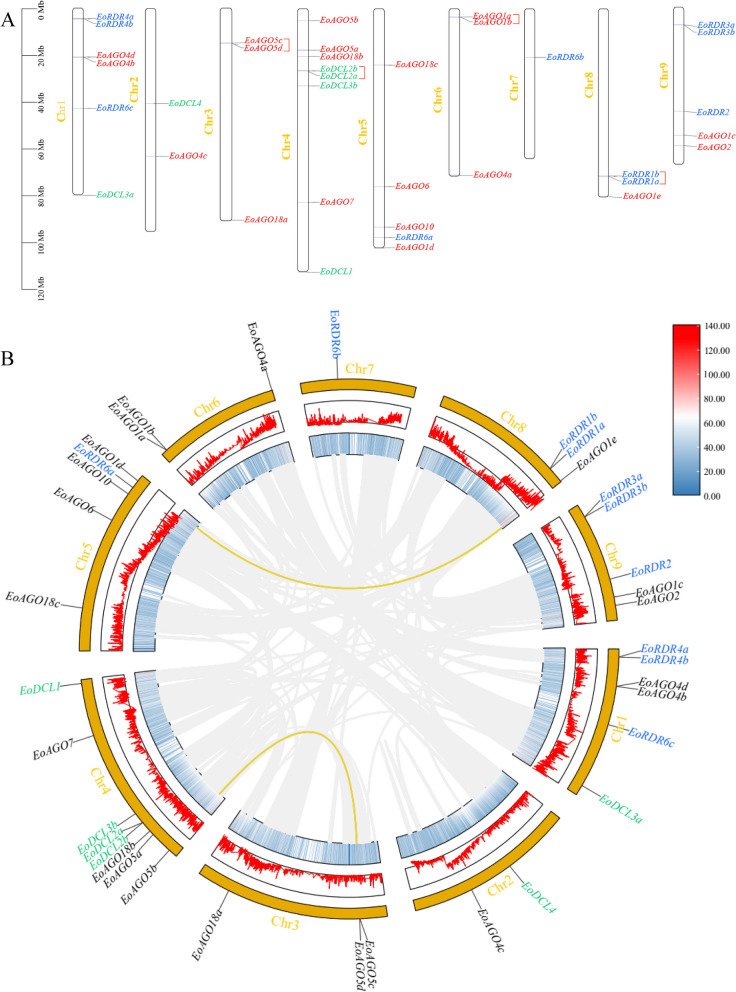
We demonstrated the chromosomal distribution of the EoAGO, EoDCL, and EoRDR genes utilizing the GFF data from the centipedegrass genome (Fig. 1A). The EoAGO, EoDCL, and EoRDR genes exhibited uneven distribution across 9 chromosomes. We identified 20 EoAGO genes on almost all chromosomes except chromosome 7, while six EoDCL genes were solely located on chromosomes 1 2, and 4. Another 10 EoRDR genes were distributed among chromosomes 1, 5, 7, 8, and 9. Chromosome 4 contained the highest number of genes (eight), while chromosome 7 contained the fewest (one). Chromosome 1 contains all three kinds of genes. There were two pairs of AGO tandem duplications genes (EoAGO1a-EoAG1b and EoAGO5c-EoAGO5d) from chromosomes 3 and 6. Besides, DCL and RDR each possess a pair of tandem duplication genes (EoDCL2a-EoDCL2b and EoRDR1a-EoRDR1b) from chromosomes 4 and 8.
Fig. 1.
A Distribution and location of EoAGO, EoDCL, and EoRDR genes on nine chromosomes. AGO: red, DCL: blue, RDR: green. The red-colored brackets indicate the tandem duplication gene pairs. B Schematic representations of the interchromosomal relationships of EoAGO, EoDCL, and EoRDR genes. The gray lines represent all synteny blocks in the centipedegrass genome. The yellow lines indicate the segmental duplication of gene pairs. The different colored box on the right represents the gene density of the heatmap in innermost circle
Furthermore, we also performed a collinearity analysis to identify duplication events in the EoAGO, EoDCL, and EoRDR genes (Fig. 1B). Only two pairs of segmental duplication genes were identified, both comprising EoAGO genes (EoAGO1d-EoAGO1e and EoAGO5b-EoAGO5d). Other EoDCL and EoRDR lack any segmental duplication genes. These findings indicate that the proliferation EoAGO, EoDCL, and EoRDR families may have been influenced by tandem and segmental duplication events, with tandem duplication possibly being the primary driving force.
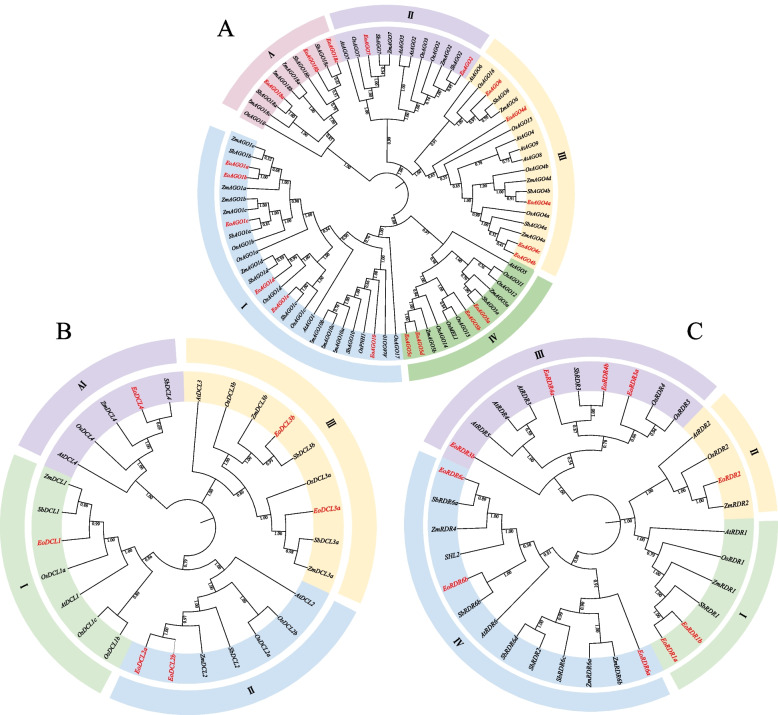
The protein sequences of AGO, DCL, and RDR from four different plant species, including sorghum, maize, rice, and Arabidopsis, were used to create the phylogenetic tree to investigate the evolutionary relationship of the EoAGO, EoDCL, and EoRDR gene families. The AGO, DCL, and RDR gene families were categorized into several clades, based on the homology and classification within species, using Neighbor-joining method (NJ) approach with high bootstrap support (Fig. 2). The 20 EoAGO genes were separated into four different clades (Fig. 2A): Clade I comprised AGO1 and AGO10, clade II comprised AGO2 and AGO7, clade III comprised AGO4 and AGO6, clade IV comprised AGO5, and clade V comprised AGO18. The AGO18 member was absent in Arabidopsis. However, it existed in sorghum, maize, rice, and centipedegrass, indicating the specificity and similar evolution of AGO18 in these grasses. Based on the phylogenetic analysis, all DCL genes were divided into four clades (Fig. 2B): clade I contained EoDCL1, clade II comprised EoDCL2a and EoDCL2b, clade III contained EoDCL3a and EoDCL3b, and clade IV contained EoDCL4. Besides, the 10 EoRDR genes were separated into four clades: clade I contained EoRDR1a and EoRDR1b, clade II contained EoRDR2, clade III contained EoRDR3a, EoRDR3b, EoRDR4a, and EoRDR4b, and clade IV contained EoRDR6a, EoRDR6b, and EoRDR6c (Fig. 2C). The EoAGO, EoDCL and EoRDR genes were named based on the location on the phylogenetic tree and combined with the blast result of amino acid identity with each other.
Fig. 2.
Phylogenetic tree for AGO, DCL and RDR gene families from centipedegrass, Arabidopsis, rice, maize and sorghum. Gene families were separated into several clades, highlighted with colors. EoAGO, EoDCL and EoRDR genes were colored in red, A: AGO, B: DCL, and C: RDR
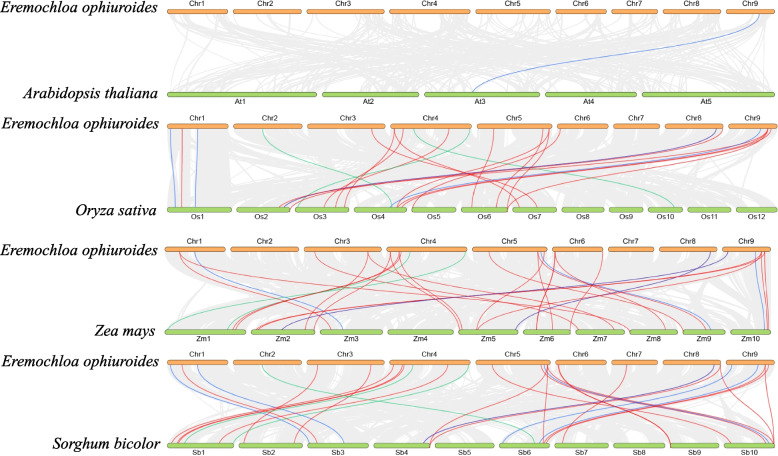
Synteny, which occurs within and between species, is another avenue for the rapid evolution of gene families. A comparative syntenic analysis was conducted using four representative species to further explore the evolutionary clues of the EoAGO, EoDCL, and EoRDR genes, three monocotyledonous plants (rice, maize, and sorghum), and one dicotyledonous plant (Arabidopsis) (Fig. 3). The findings revealed 29 syntenic pairs between centipedegrass and maize, followed by sorghum (28) and rice (23), while one syntenic pair was identified between centipedegrass and Arabidopsis. More syntenic pairs between centipedegrass and monocotyledons than dicotyledons suggests close evolutionary relationships between centipedegrass and other monocotyledons.
Fig. 3.
Synteny analysis of AGO, DCL and RDR genes between centipedegrass and four representative plant species. Gray lines indicate the collinear blocks, whereas the colored lines indicate the syntenic AGO (red); DCL (green) and RDR (blue) gene pairs
Conserved domains, multiple sequence alignment, gene structure, cis-elements analysis in the promoter regions and 3-Dimensional structure prediction
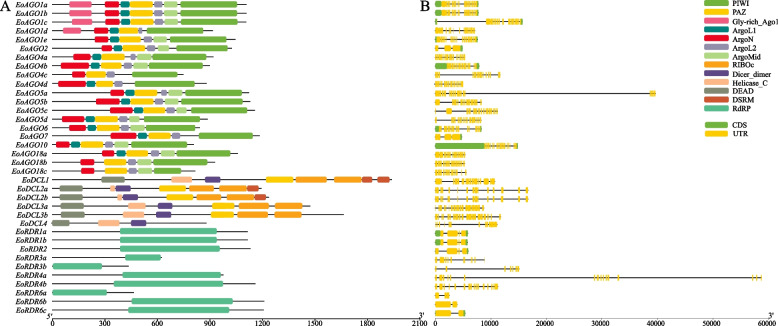
The conserved domains of the EoAGO, EoDCL and EoRDR proteins were identified demonstrating the structural domain conservatism and diversity among the proteins (Fig. 4A, Table S1). All EoAGO proteins possess four domains: ArgoN, PAZ, ArgoL2, and PIWI. Additional conserved domains, including ArgoL1, Gly-rich_Ago1 and ArgoMid, were identified in most EoAGOs. The additional Gly-rich domain in AGO1 of Arabidopsis, sorghum, and maize, was observed in EoAGO1 (EoAGO1a, EoAGO1b, EoAGO1c, and EoAGO1d). Furthermore, the multiple sequence alignment, indicates that the PIWI domain exhibits a structure similar to RNaseH, and is essential in cleaving target mRNA. This function is facilitated by a conserved metal-chelating motif known as Asp–Asp–His/Asp (DDH/D) (Fig. S1) [48, 49]. The sequence alignment results of the PIWI domains revealed that there were 11 EoAGO proteins exhibited the conserved DDH/H tetrad identical to those in AtAGO1 (Fig. S1). The DDD/H motif identified in EoAGO2 protein, is identical to AtAGO2 and AtAGO3 proteins. However, DDH/H motif is not essential and may be replaced into DDH/P and DDW/P, as observed in Arabidopsis, rice, maize and sorghum. The DDH/P motif was identified in EoAGO4a, EoAGO4b and EoAGO4d proteins, similar to the AtAGO6 and AtAGO8 proteins. The DDW/P motif was exclusively observed in EoAGO6 protein. Furthermore, the other four (EoAGO4c, EoAGO18a, EoAGO18b, and EoAGO18c) exhibited different deficiencies in PIWI domain catalytic residue(s) illustrated in Table S2.
Fig. 4.
Analysis of conserved domains and gene structure of EoAGO, EoDCL and EoRDR genes. A Conserved domain composition analysis. B Gene structure analysis
The EoDCL proteins exhibited all the common domains found in the Dicer family, including DEAD, Helicase_C, Dicer_dimer, PAZ, two tandem RIBOc (RNaseIIIa and RNaseIIIb), and dsRM. However, EoDCL3a and EoDCL3b lacked the DSRM domain, and EoDCL4 lacked the PAZ, RIBIc, and DSRM domains (Fig. 4A). Besides, the EoDCL1 contained two DSRM domains, which were observed in DCL1 in Arabidopsis, maize, and sorghum. The sequence alignment demonstrated that all the EoDCL proteins, except EoDCL4, possess the RNase III catalytic sites within the two tandem RIBOc domains at the glutamate (E), aspartate (D), aspartate (D), glutamate (E) (EDDE) position (Fig. S1), a characteristic of plant DCL proteins [50, 51].
A conserved RdRP domain has been identified in all EoRDR proteins similar to the corresponding proteins in other plants (Fig. 4A). The sequence alignment of the EoRDR proteins identified the conserved DxDGD catalytic motif within the RdRP conserved domain, except for EoRDR3a, which lacks a portion of the DxDGD catalytic motif (Fig. S1) [52, 53].
Analyzing different exon–intron structures of the EoAGO, EoDCL, and EoRDR genes help us facilitates comprehension of their genetic variations and potential evolutionary processes (Fig. 4B). The coding sequences of the complete EoAGO, EoDCL, and EoRDR families were interrupted by introns. Most of the EoAGO genes exhibited an intron number ranging from 17 to 24, consistent across various in different groups. However, the EoAGO2 and EoAGO7 genes belong to clade II, possessing two and three introns, respectively. Regarding EoDCLs, the introns number ranges from 14 to 25. Most EoRDRs contained 1–3 introns, while clade III (EoRDR3 and EoRDR4) contained 6–17 introns. The intron number of EoAGO, EoDCL, and EoRDR genes exhibited significant variation between clades but remained relatively conserved among members in the same clade, similar to that of other plants.
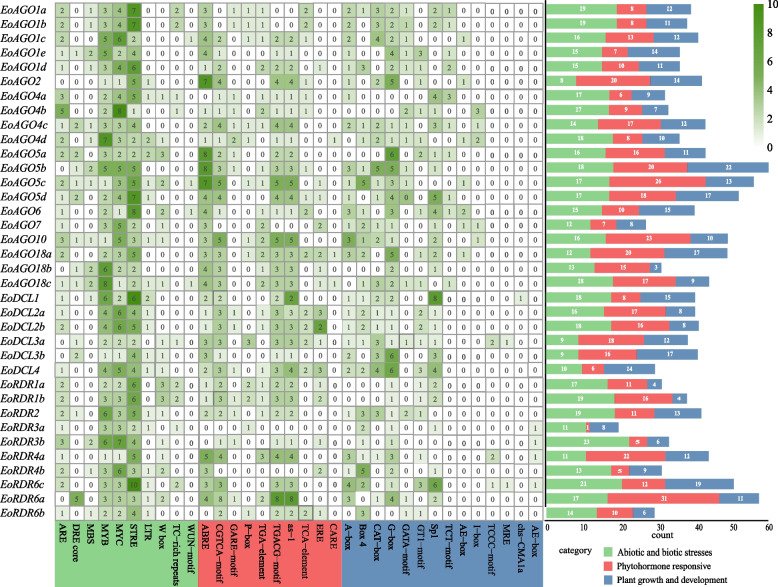
We analyzed 34 cis-element elements across all members of the EoAGO, EoDCL, and EoRDR genes families, categorizing them into three groups: abiotic and biotic stress, phytohormone responsiveness, plant growth and development (Fig. 5). Among the genes, EoAGO5b exhibited the highest number of cis-acting elements, while EoRDR3a exhibited the fewest. Light-responsive, abscisic acid-responsiveness elements, and MeJA-responsiveness elements were the most prevalent in the EoAGO, EoDCL, and EoRDR families (Fig S2, Table S3). Other cis-acting elements associated with meristem expression, auxin responsiveness, defense and stress responsiveness, low-temperature responsiveness, and wound responsiveness were identified. The findings indicate that EoAGOs, EoDCLs, and EoRDRs are essential in regulating processes, including photoperiodic control of flowering, hormone signaling pathways (MeJA, salicylic acid, and abscisic acid), and stress responses.
Fig. 5.
The cis-acting elements distribution of types and numbers analysis of EoAGO, EoDCL and EoRDR gene promoters. The green gradient legend indicates the number of cis-acting elements
The three-dimensional structures of the EoAGO, EoDCL, and EoRDR proteins were predicted using the SWISS-MODEL [54] (refer to Fig S3 and Table S5). Each target exhibited greater than 30% identity with the template, a threshold indicative of successful modeling, as noted in the advancements of homology protein structure modeling. The GMQE values, ranging from 0.54 to 0.92, signified the high-quality nature of all the models. The majority of the models employed for predicting the 3D structures of EoAGO, EoDCL, and EoRDR were derived from rice, maize, and sorghum species, highlighting the close genetic affinity between centipedegrass and these species. This proximity suggests the possibility of functional parallels among the genes.
Expression analysis of EoAGO, EoDCL, and EoRDR genes in different tissues
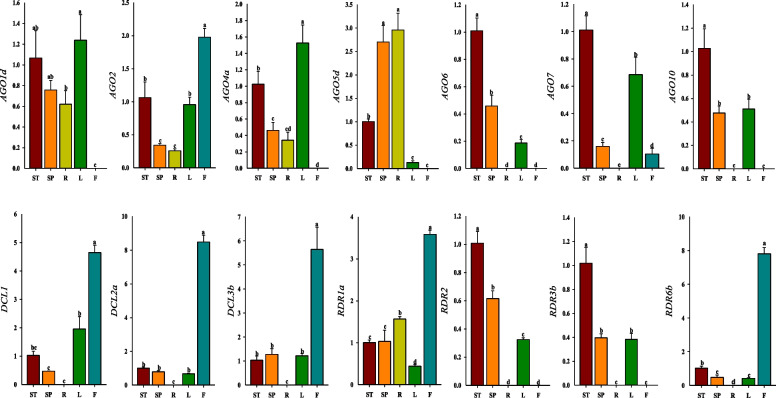
We selected seven EoAGO, three EoDCL, and four EoRDR genes from different phylogenetic tree clades to analyze their expression using RT-qPCR in other tissues, including stem, spike, root, leaf, and flower. All selected genes were expressed, however, EoAGO2, EoDCL3b, and EoRDR1a were expressed in all tissues (Fig. 5). Furthermore, all selected genes were expressed in stem, spike, and leaf with EoAGO1d, EoAGO5d, and EoDCL1 demonstrating the highest expression level, respectively. In addition, numerous genes exhibited low expression in root and flower, while EoAGO1d and EoDCL2a demonstrated the highest expression levels, respectively. Among the EoAGO genes, five genes (EoAGO1d/4/5d/6/10) exhibited no expression in the flower, while EoAGO2/7 exhibited higher expression levels in other tissues. Most EoAGO genes exhibited lower spike and root expression levels than in stem and leaf. However, EoAGO5d exhibited higher expression in spike and root than in stem and leaf. Of the three EoDCL genes (EoDCL1/2a/3b), exhibited significantly higher flower expression levels. However, they did not express themselves in the root. Among EoRDR genes, EoRDR1a was expressed in all five tissues, and EoRDR1a and EoRDR6b exhibited high expression levels in the flowers especially for EoRDR6b. Another two genes (EoRDR2/3b) exhibited higher expression in the stem than in the spike and leaf, however, they exhibited no detectable expression in root and flower tissue.
Expression analysis of EoAGO, EoDCL, and EoRDR genes under five abiotic stresses
We analyzed the expression pattern of seven EoAGO, three EoDCL, and four EoRDR genes under various treatments, including cold, salt, PEG, Al, and herbicide stresses. The results revealed that all EoAGO, EoDCL, and EoRDR genes are differentially expressed under five abiotic stresses (Fig. 7).
Fig. 7.
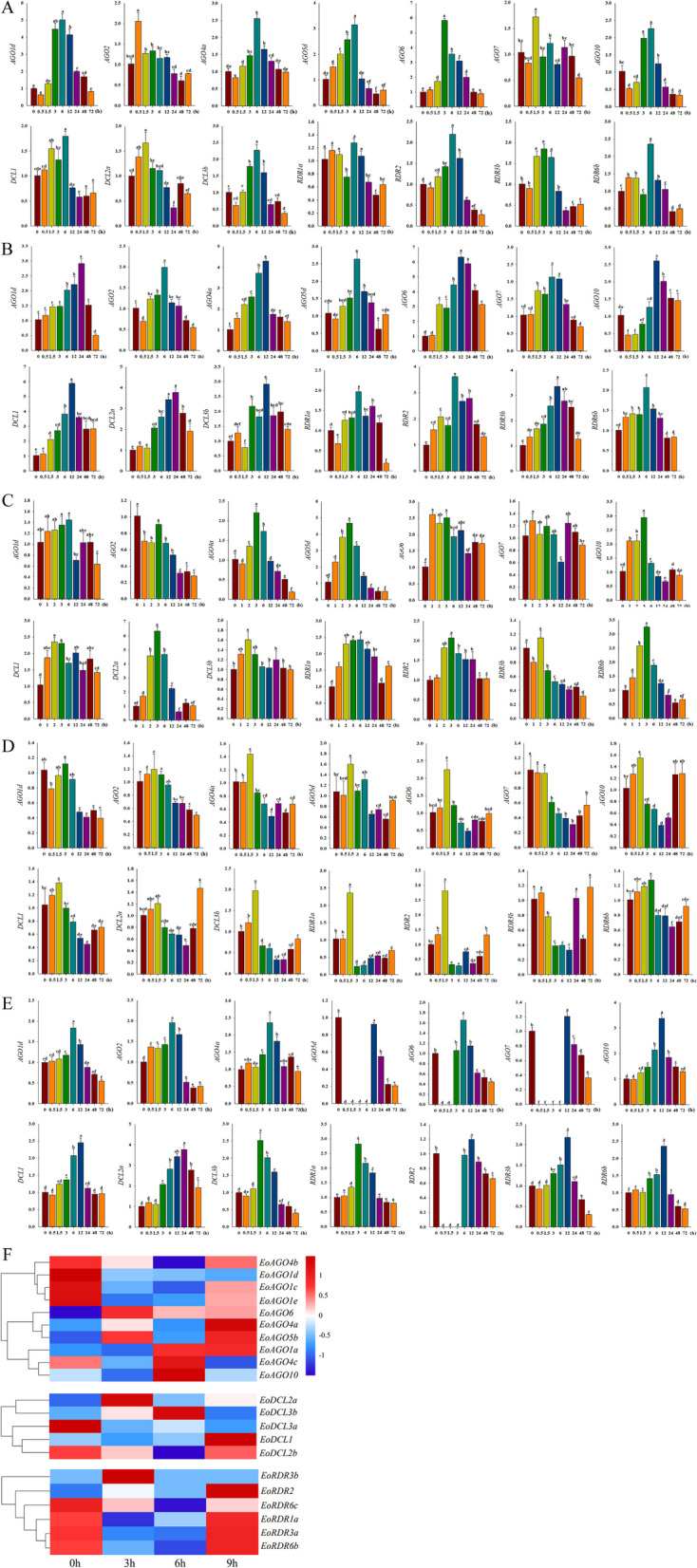
Gene expression of EoAGO, EoDCL and EoRDR genes under A cold, B salt, C drought, D Al and E herbicide stress conditions analyzed using RT-qPCR assay after treatment for 0, 0.5, 1.5, 3, 6, 12, 24, 48, 72 h. Small letter(s) indicated significant differences (p < 0.05, Duncan) between different time stages. F EoAGO, EoDCL and EoRDR genes expression under cold stress based on RNA-seq data
Under cold stress, five EoAGO, one EoDCL, and three EoRDR genes exhibited significant upregulation at 3 h (EoAGO6, EoRDR3b) or 6 h (EoAGO1d/4/5d/6/10, EoDCL3b, EoRDR2/6b). Subsequently, the expression levels declined (p < 0.05) (Fig. 7A). Moreover, we employed existing RNA-seq datasets to examine the transcript levels of EoAGO, EoDCL, and EoRDR genes in response to cold treatments. We identified ten EoAGO, five EoDCL, and six EoRDR genes using the protein blast of NCBI (identify > 90%). The findings indicated that a subset of EoAGO, EoDCL, and EoRDR genes exhibited differential expression patterns in response to cold treatments (Fig. 7F), which was partially consistent with the RT-qPCR findings, including EoAGO1a/4a/6/10, EoDCL1/3b, and EoRDR2/3b/6b genes.
All selected genes exhibited varying degrees of upregulation at 6, 12, and 24 h under salt treatment, followed by a subsequent decrease in expression levels (p < 0.05), and EoAGO4a, EoAGO6, EoDCL1, EoRDR2, and EoRDR3b exhibited a significant upregulation (Fig. 7B). Regarding PEG treatments, the EoAGO4A/5d/10, EoDCL2a, and EoRDR6b significantly expressed at 3 h (p < 0.05), and followed by rapid decline (Fig. 7C). Additionally, other genes expression decreased with prolonged exposure time, especially for EoAGO2 and EoRDR3b after PEG treatment for 3 h. For Al treatment, EoAGO1d/2/4a/5d/7/10, EoDCL1/2a/3b, and EoRDR1/2/3 were downregulated after treatment for 1.5 h (p < 0.05). However, most of them were upregulated again after treatment for 12 h (Fig. 7D). Regarding herbicide stress, almost all selected genes were induced at 12 h (p < 0.05), and EoAGO10 and EoDCL2a were significantly expressed. Moreover, most of these genes were decreased at 24 h (p < 0.05) by herbicide treatment (Fig. 7E).
Discussion
RNA silencing is a regulatory mechanism for gene expression regulation and a defense strategy common among eukaryotes. Moreover, it is essential in plant stress physiology. Plants can process diverse sRNAs through different RDR-DCL-AGO gene interactions, thereby indirectly regulating multiple biological pathways [16, 55]. This study conducts a comprehensive genome-wide analysis of EoAGO, EoDCL, and EoRDR genes in centipedegrass. The study investigated the correlation between these genes and their equivalents in other grass species, and their expression patterns in response to various abiotic stress factors.
Duplication events, evolution relationships and expression characteristics among AGO family in centipedegrass
AGO is an essential component of RISC and regulates plant leaf polarity differentiation. With the complete sequencing of plant genomes, the AGO gene family has been identified in many plants, including rice (19 members) [23], maize (18 members) [24], sorghum (14 members) [25], Arabidopsis (10 members) [22], and so on. In this study, 20 AGO genes were found from the centipedegrass genome, indicating the diversification and duplication events of AGO genes have occurred during evolution [56]. Previous studies reported that clade I, II and V all belong to AGO1/5/10/18 group [57, 58]. Arabidopsis contains AGO1 and one AGO10, while centipedegrass has five AGO1 homologs, similar to the four homologs in rice and sorghum, and five in maize, which suggest redundant and specialized functions [59]. In the AGO1 gene in centipedegrass, we identified one pair of tandem duplication genes (EoAGO1a-EoAGO1b) and one pair of segmental duplication genes (EoAGO1d-EoAGO1e), indicating the significant role of duplication events in AGO1 gene expansion. The AGO5 family was expanded in Poaceae plants [60], with five homologs in rice, and three each in maize and sorghum, indicating a conserved role in the 21-nt phasiRNA pathway, which is essential for developmental processes and male reproductive ability [61]. Tandem duplication genes (EoAGO5c-EoAGO5d) and segmental duplication genes (EoAGO5b-EoAGO5d) were identified among the EoAGO5 genes. The AGO18 family, which is grass-specific, is essential in plant reproduction and viral defense. For instance, the AGO18 gene in rice could induced by viral infection, while EoAGO18a/18b/18c was not detected in centipedegrass [27, 62]. In the AGO2/7 group in clade III, it was observed that AGO2 probably diverged from AGO7 from their common ancestor of seed plants, as evidenced by the different catalytic triad sequences (DDH in AGO7 and DDD in AGO2) (Fig. S1, Table S2). This change from H to D in the catalytic triad may indicate functional divergence. AGO7 is highly conserved and known to bind miR390, which initiates the generation of TAS3-derived trans-acting siRNAs (tasiRNAs) that regulate auxin response factors (ARF) in flowering plants [63, 64]. Clade IV belongs to the AGO4/6/8/9 group. EoAGO4a/4b/4c/4d and EoAGO6 were contained in this clade, without AGO7 and AGO9 in centipedegrasss, similar in rice and sorghum. EoAGO4 gene exhibits more complex evolutionary patterns, while EoAGO6 is highly conserved, presumably due to involvement in different sRNA pathways. The AGO4/6/8/9 group should be classified into AGO4 and AGO6 clades, representing two distinguishable clades in plants, consistent with findings in centipedegrass and other Poaceae species [62]. Among the seven EoAGO genes for RT-qPCR, only EoAGO2 and EoAGO7, clade III member, were expressed in flower, suggesting their involvement in the flower development pathway in centipedegrass. In addition, most EoAGO genes exhibited higher expression levels in the stem and leaf, while demonstrating lower expression in the root (Fig. 6). EoAGO5d exhibited high expression in the spike, indicating active involvement in reproductive tissues. In Arabidopsis, AGO5 was reported to show high expression during all stages of flower and seed formation [63]. In response to cold stress, five out of seven EoAGO genes (EoAGO1d/4a/5d/6/10) were upregulated at 6 h and subsequently declined (Fig. 1a), mirroring the expression pattern of ZmAGO1a/1b/4/5a/5/10b in maize [29]. The overall expression patterns were largely in line with the RNA-seq data analysis (Fig. 7F). Under salt treatment, all EoAGO genes responded at 6 h or 24 h, exhibiting varying gene expression patterns. As for PEG treatment, EoAGO4a/5d/10 were significant upregulated, suggesting their essential roles in gene regulation during drought stress. Moreover, all seven EoAGO genes displayed varying response levels under Al and herbicide treatments, although further research is needed to confirm these results.
Fig. 6.
The expression patterns of 14 EoAGO, EoDCL and EoRDR genes in the stem (ST), spike (SP), root (R), leaf (L), and flower(F) by RT-qPCR assay. Lowercase letter(s) indicate significant differences (p < 0.05, Duncan) among the tissues
DCL family in centipedegrass demonstrated distinct characteristics and specific expression in flower
DCL is an essential element in sRNA biogenesis, originally discovered in animals [65]. A previous study suggest that the plant DCL protein emerged after the plant-animal split but before the monocot-dicot divergence around 150 million years ago [66]. In Arabidopsis, four DCL proteins, named DCL1 to DCL4, have specialized functions in the sRNAs biogenesis [67]. For instance, DCL1 is mainly involved in the biogenesis of miRNAs [68], the function loss of OsDCL1 affects miRNA accumulation and causes developmental defects in rice [69], DCL2 is associated with viral dsRNA processes, DCL3 is necessary for color modification and long-distance RNAi signaling [70, 71], and DCL4 regulates plant development by transacting on the biogenesis of siRNAs (ta-siRNAs) and reproductive phasiRNAs [72]. In the evolution of monocot plants, whole-genome duplications (WGD) greatly affect the growth of the DCL gene family [73]. Studies have demonstrated that grass ancestors had one WGD and two chromosome fusion events [74, 75]. In rice, the DCL gene experienced functional differentiation after the ρ-WGD event, helping with virus defense and gene regulation [74]. In addition, the DCL gene may evolve new functions through subfunctionalization and neofunctionalization [76]. It was reported in rice [77] and maize [78] that DCL3b (also called DCL5) emerged and evolved in monocots and is involved in producing reproductive phasiRNAs in anthers. In this study, we identified six EoDCL genes distributed across four clades, each containing at least one EoDCL member (Fig. 2B). Clade II contains two DCL2 genes, similar to the OsDCL2 genes. EoDCL2a and EoDCL2a were considered tandem duplication genes because of their proximity on chromosome 4 and 96% amino acid sequence identity (Fig. 1A), suggesting that gene duplication events contributed to DCL2 gene expansion. Clade III own two DCL3 members, EoDCL3a and EoDCL3b, which exhibit low similarity, consistent with the observations in sorghum, rice, and maize but contrasting with those in Arabidopsis and soybean [25]. This indicates that the emergence of the DCL3b paralog in monocots occurred after the split of monocots and dicots [79]. The function of DCL3a and DCL3b in rice and maize is diversified; OsDCL3a and ZmDCL3a are involved in hc-siRNAs biogenesis, however, OsDCL3a and ZmDCL3b (ZmDCL5) are engaged in 24-nt phasiRNAs biogenesis [77, 78]. Therefore, EoDCL3b may be designated as EoDCL5 within a new clade. However, the functional divergence and expression patterns between EoDCL3a and EoDCL3b require further investigation. Although plants evolved four different DCL groups, their functions overlap and can compensate for each other without one [80]. Expression patterns indicate that three selected EoDCL genes exhibit consistent expression across all tissues, with high expression levels in flowers (Fig. 6). In Arabidopsis, DCL genes are essential in inducing flowering, as dcl1/dcl3 mutants exhibited delayed flowering [81]. Consequently, EoDCL genes may be crucial in flowering and related miRNA and siRNA biogenesis. Regarding abiotic stresses, EoDCL1/2a/3b exhibited significant upregulation under cold, salt, and herbicide treatments, while all the expressions were downregulated under Al treatment (Fig. 7). The changes in EoDCL1 and EoDCL3b expression levels under cold treatment were consistent with RNA-seq dataset results findings (Fig. 7F). EoDCL2a exhibited significant upregulation under drought treatment, similar to ZmDCL2/3b in maize [24]. These results revealed that the three EoDCL1/2a/3b genes could be regulated by various abiotic stresses.
RDR family in centipedegrass own a conserved domain and demonstrated potential function in reproductive growth
RDR is essential for siRNA biogenesis as it facilitates the conversion of single-stranded RNA into double-stranded RNA through the RdRP conserved domain. According to the phylogenetic tree analysis, RDRs can be classified into four groups in centipedegrass (Fig. 2 C). Clade I contains tandem duplication genes (EoRDR1a-EoRDR1b) in centipedegrass, while other species have one member (Fig. 2). It was found that RDR1/2 were sister genes to RDR6, mainly involved in virus defense response [82]. Besides, RDR2 has been associated with DNA methylation and repressive chromatin modifications in several plant species including Arabidopsis and maize [29, 83, 84]. We identified three RDR6 orthologues in centipedegrass, similar to those in maize, indicating a conserved role of RDR6 in plants with the production of highly conserved 21-nt tasiRNAs pathway [85]. Furthermore, RDR6 participates reproductive phasiRNAs generation, with EoRDR6b exhibiting significantly high expression in flowers, indicating a potential role in reproductive growth in centipedegrass (Fig. 6). RDR3 considered ancestral to other RDR types [27]. Four EoRDRs members (EoRDR3a/3b/4a/4b) were identified in clade III. However the specific function of RDR3 remains unknown. Under various abiotic stresses, all four EoRDRs exhibited different responses (Fig. 7). The EoRDR2, EoRDR3b and EoRDR6b exhibited low expression levels in various tissues, but they were significantly upregulated under drought stress, indicating a potential function in response to specific environmental stresses.
Conclusion
We identified and characterized 20 EoAGO, 6 EoDCL and 10 EoRDR genes in the centipedegrass genome, comprehensively analyzing their critical roles in RNA silencing mechanisms. A total of two tandem and two segmental duplication pairs were identified in the AGO family, contributing to the gene expansion. EoDCL1/2a/3b and EoRDR6b exhibited high flowers expression levels, suggesting a significant role in flowering. Furthermore, some of the EoAGO, EoDCL and EoRDR genes could be regulated by various stresses such as cold, drought, salt, Al, and herbicide, indicating that these genes play an essential role in the stress resistance of centipedegrass. Combining RNA-seq data analysis with RT-qPCR findings, reavealed that EoAGO4a/6/10, EoDCL2a/3b and RDR3b exhibited significant responses under cold stress. Future research will focus on the functional verification of these cold responsive genes, and through genetic transformation and breeding programs to cultivate new varieties of centipedegrass with higher stress resistance, laying the foundation for creating new germplasm.
Supplementary Information
Additional File 1: Fig S1. Sequences alinement of AGO, DCL and RDR genes. Fig S2. Promoter classification and proportion analysis of EoAGO, EoDCL and EoRDR genes. Fig S3. Three-dimensional (3D) protein structures of EoAGO, EoDCL and EoRDR proteins. Table S1. The alignment details and features of conserved domains of EoAGO, EoDCL and EoRDR genes. Table S2. AGO proteins with missing catalytic residue(s) in PIWI domains. Table S3. Promoter analysis of EoAGOs, EoDCLs and EoRDRs. Table S4. Primers used in the RT-qPCR analysis. Table S5. The detailed information of the predicted three-dimensional (3D) proteins.
Authors’ contributions
SY L and JM Z experimental conception and design. SY L, X L, X M, and JM Z identification and bioinformatic analysis. XY W, TQ L, and W M material cultivation and treatments. SY L, CS X, DD K, and Y L genes expression by RT-qPCR. SY L, X L original manuscript preparation. JM Z and X L experiments supervision and manuscript revision. JM Z and X L funding acquisition. All authors have read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32071885), the Sichuan Science and Technology Program (2024YFHZ0300) and the Sichuan Forestry and Grassland Science and Technology Innovation Team Funding (CXTD2024005).
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/s/8256acffdb73bb050045.
Declarations
Ethics approval and consent to participate
All the experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, were carried out in accordance with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Siyu Liu and Xiong Lei contributed equally to this work .
References
- 1.Chen X. Small RNAs and their roles in plant development. Ann Rev Cell Dev. 2009;25:21–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu S, Ying S, Vincent LC. Stress-responsive microRNAs in Populus. Plant J. 2008;55(1):131–51. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Wang JW. The Crosstalk between MicroRNAs and Gibberellin Signaling in Plants. Plant Cell Physiol. 2020;61(11):1880–90. [DOI] [PubMed] [Google Scholar]
- 4.Finnegan EJ, Matzke MA. The small RNA world. J Cell Sci. 2003;116(23):4689–93. [DOI] [PubMed] [Google Scholar]
- 5.Gualtieri C, Leonetti P, Macovei A. Plant miRNA cross-kingdom transfer targeting parasitic and mutualistic organisms as a tool to advance modern agriculture. Front Plant Sci. 2020;11:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordoloi KS, Agarwala N. MicroRNAs in plant insect interaction and insect pest control. Plant Gene. 2021;26.
- 7.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301(5631):336–8. [DOI] [PubMed] [Google Scholar]
- 8.Voinnet O. Origin, Biogenesis, and activity of plant microRNAs. Cell. 2009;136(4):669–87. [DOI] [PubMed] [Google Scholar]
- 9.Valencia MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515–24. [DOI] [PubMed] [Google Scholar]
- 10.Pratt AJ, MacRae IJ. The RNA-induced silencing complex: A versatile gene-silencing machine. J Biol Chem. 2009;284(27):17897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fei Q, Xia R, Meyers BC. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell. 2013;25(7):2400–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26(5):611–23. [DOI] [PubMed] [Google Scholar]
- 13.Vaucheret H. Plant argonautes. Trends Plant Sci. 2008;13(7):350–8. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Li Y, Ding SW. Small RNA-based antimicrobial immunity. Nat Rev Immunol. 2019;19(1):31–44. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JH, Guo HS. RNA silencing: from discovery and elucidation to application and perspectives. J Integr Plant Biol. 2022;64(2):476–98. [DOI] [PubMed] [Google Scholar]
- 16.Borges F, Martienssen RA. The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol. 2015;6(12):727–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matzke MA, Mosher RA. RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat Rev Genet. 2014;15(6):394–408. [DOI] [PubMed] [Google Scholar]
- 18.Creasey KM, Zhai J, Borges F, Van E, Regulski M, Meyers BC, Martienssen RA. miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature. 2014;508(7496):411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, Qi Y. DNA methylation mediated by a microRNA pathway. Mol Cell. 2010;38(3):465–75. [DOI] [PubMed] [Google Scholar]
- 20.Ye R, Chen Z, Lian B, Rowley MJ, Xia N, Chai J, Li Y, He XJ, Wierzbicki AT, Qi Y. A Dicer-Independent Route for Biogenesis of siRNAs that Direct DNA Methylation in Arabidopsis. Mol Cell. 2016;61(2):222–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Qi Y. RNAi in Plants: An Argonaute-Centered View. Plant Cell. 2016;28(2):272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez F. Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci. 2006;11(9):460–8. [DOI] [PubMed] [Google Scholar]
- 23.Kapoor M, Arora R, Lama T, Nijhawan A, Khurana JP, Tyagi AK, Kapoor S. Genome-wide identification, organization and phylogenetic analysis of Dicer-like, Argonaute and RNA-dependent RNA Polymerase gene families and their expression analysis during reproductive development and stress in rice. BMC Genomics. 2008;9(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Y, Cheng Y, Cheng X, Jiang H, Zhu S, Cheng B. Identification and characterization of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families in maize. Plant Cell Rep. 2011;30:1347–63. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Lu T, Dou YC, Yu B, Zhang C. Identification of RNA silencing components in soybean and sorghum. BMC Bioinformatics. 2014;15(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabbione A, Daurelio L, Vegetti A, Talón M, Tadeo F, Dotto M. Genome-wide analysis of AGO, DCL and RDR gene families reveals RNA-directed DNA methylation is involved in fruit abscission in Citrus sinensis. BMC Plant Biol. 2019;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Yang Z, Wang Y, Zheng L, Ye R, Ji Y, Zhao S, Ji S, Liu R, Xu L, Zheng H, Zhou Y, Zhang X, Cao X, Xie L, Wu Z, Qi Y, Li Y. Viral-inducible Argonaute18 confers broad-spectrum virus resistance in rice by sequestering a host microRNA. Elife. 2015;4. [DOI] [PMC free article] [PubMed]
- 28.Gan D, Zhan M, Yang F, Zhang Q, Hu K, Xu W, Lu Q, Zhang L, Liang D. Expression analysis of argonaute, Dicer-like, and RNA-dependent RNA polymerase genes in cucumber (Cucumis sativus L.) in response to abiotic stress. J Genet. 2017;96:235–49. [DOI] [PubMed] [Google Scholar]
- 29.Zhai L, Teng F, Zheng K, Xiao J, Deng W, Sun W. Expression analysis of Argonaute genes in maize (Zea mays L.) in response to abiotic stress. Hereditas. 2019;156:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai XY, Fu JY, Li X, Peng L, Yang L, Liang Y, Jiang M, Ma J, Sun L, Guo B, Yu X. Low-molecular-weight organic acid-mediated tolerance and Pb accumulation in centipedegrass under Pb stress. Ecotoxicol Environ Saf. 2022;241:113755. [DOI] [PubMed] [Google Scholar]
- 31.Hook JE, Hanna WW, Maw BW. Quality and growth response of centipedegrass to extended drought. Agron J. 1992;84(4):606–12. [Google Scholar]
- 32.Li J, Guo H, Zong J, Chen J, Li D, Liu J. Genetic diversity in centipedegrass [Eremochloa ophiuroides (Munro) Hack.]. Hortic Res. 2020;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islam MA, Hirata M. Centipedegrass (Eremochloa ophiuroides (Munro) Hack.): growth behavior and multipurpose usages. Grassl Sci. 2005;1(3):183–90. [Google Scholar]
- 34.Johnson BJ, Carrow RN. Influence of soil pH and fertility programs on centipedegrass. Agron J. 1992;84(1):21–6. [Google Scholar]
- 35.Liu Y, Xiong Y, Zhao J, Bai S, Li D, Chen L, Feng J, Li Y, Ma X, Zhang J. Molecular Mechanism of Cold Tolerance of Centipedegrass Based on the Transcriptome. Int J Mol Sci. 2023;24(2):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katuwal KB, Xiao B, Jespersen D. Root physiological and biochemical responses of seashore paspalum and centipedegrass exposed to iso-osmotic salt and drought stresses. Crop Sci. 2020;60(2):1077–89. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Zi H, Wang R, Liu J, Wang H, Chen R, Li L, Guo H, Chen J, Li J, Zong J. A high-quality chromosome-scale assembly of the centipedegrass [Eremochloa ophiuroides (Munro) Hack.] genome provides insights into chromosomal structural evolution and prostrate growth habit. Horticulture Research. 2021;8(2):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasteiger E, Hoogland C, Gattiker A, Duvaud SE, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools in the ExPASy server. Totowa, NJ. Humana Press. 2005:571–607.
- 39.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007;32(2):585–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Procter JB, Carstairs G, Soares B, Mourão K, Ofoegbu TC, Barton D, Lui L, Menard A, Sherstnev N, Roldan-Martinez D, Duce S, David M, Martin A, Barton GJ. Alignment of Biological Sequences with Jalview. US: Springer; 2021. p. 203–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Tang H, Debarry J, Tan X, Li J, Wang X, Lee T, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holub EB. The arms race is ancient history in Arabidopsis, the wildflower. Nat Rev Genet. 2001;2(7):516. [DOI] [PubMed] [Google Scholar]
- 44.Chen C, Wu Y, Xia R. A painless way to customize Circos plot: From data preparation to visualization using TBtools. Imeta. 2022;1(3). [DOI] [PMC free article] [PubMed]
- 45.Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, Xia R. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol Plant. 2023;16(11):1733–42. [DOI] [PubMed] [Google Scholar]
- 46.Wang XY, Shu X, Su XL, Xiong YL, Xiong Y, Chen ML, Tong Q, Ma X, Zhang JB, Zhao JM. Selection of Suitable Reference Genes for RT-qPCR Gene Expression Analysis in Centipedegrass under Different Abiotic Stress. Genes. 2023;14(10):1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 48.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12(4):340–9. [DOI] [PubMed] [Google Scholar]
- 49.Carbonell A, Fahlgren N, Garcia-Ruiz H, Gilbert KB, Montgomery TA, Nguyen T, Cuperus JT, Carrington JC. Functional analysis of three Arabidopsis ARGONAUTES using slicerdefective mutant. Plant Cell. 2012;24(9):3613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Axtell MJ, Fedoroff NV. The helicase and RNaseIIIa domains of Arabidopsis DICER-LIKE1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 2012;159(2):748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579(26):5822–9. [DOI] [PubMed] [Google Scholar]
- 52.Zong J, Yao X, Yin J, Zhang D, Ma H. Evolution of the RNA-dependent RNA polymerase (RdRP) genes: duplications and possible losses before and after the divergence of major eukaryotic groups. Gene. 2009;447(1):29–39. [DOI] [PubMed] [Google Scholar]
- 53.Cao JY, Xu YP, Li W, Li SS, Rahman H, Cai XZ. Genome-wide identification of DICER-LIKE, ARGONAUTE, and RNA-DEPENDENT RNA POLYMERASE gene families in Brassica species and functional analyses of their Arabidopsis homologs in resistance to Sclerotinia sclerotiorum. Front Plant Sci. 2016;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018;46:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bologna NG, Voinnet O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol. 2015;65:473–503. [DOI] [PubMed] [Google Scholar]
- 56.Singh RK, Gase K, Baldwin IT, Pandey SP. Molecular evolution and diversification of the Argonaute family of proteins in plants. BMC Plant Biol. 2015;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Xia R, Meyers BC, Walbot V. Evolution, functions, and mysteries of plant ARGONAUTE proteins. Curr Opin Plant Biol. 2015;27:84–90. [DOI] [PubMed] [Google Scholar]
- 58.Rogers K, Chen X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell. 2013;25(7):2383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y. Rice MicroRNA effect or complexes and targets. Plant Cell. 2009;21(11):3421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, Li W, Guo M, Liu S, Liu L, Yu Y, Mo B, Chen X, Gao L. Origin, evolution and diversification of plant ARGONAUTE proteins. Plant J. 2022;109(5):1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Huang Y, Pan L, Zhao Y, Huang W, Jin W. Male sterile 28 encodes an ARGONAUTE family protein essential for male fertility in maize. Chromosome Res. 2021;29:189–201. [DOI] [PubMed] [Google Scholar]
- 62.Bélanger S, Zhan J, Meyers BC. Phylogenetic analyses of seven protein families refine the evolution of small RNA pathways in green plants. Plant Physiol. 2023;192(2):1183–203. [DOI] [PMC free article] [PubMed]
- 63.Havecker ER, Wallbridge LM, Hardcastle TJ, Bush MS, Kelly KA, Dunn RM, Schwach F, Doonan JH, Baulcombe DC. The Arabidopsis RNA-directed DNA methylation argonautes functionally diverge based on their expression and interaction with target loci. Plant Cell. 2010;22(2):321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou C, Han L, Fu C, Wen J, Cheng X, Nakashima J, Ma J, Tang Y, Tan Y, Tadege M, Mysore KS, Xia G, Wang ZY. The trans-acting short interfering RNA3 pathway and no apical meristem antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell. 2013;25(12):4845–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margis R, Fusaro AF, Smith NA, Curtin SJ, Watson JM, Finnegan EJ. Waterhouse PM. The evolution and diversification of Dicers in plants. FEBS Letters. 2006;580(10):2442–50. [DOI] [PubMed] [Google Scholar]
- 66.Henderson IR, Zhang X, Lu C, Johnson L, Meyers BC, Green PJ, Jacobsen SE. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38(6):721–5. [DOI] [PubMed] [Google Scholar]
- 67.Voinnet O. Use, tolerance and avoidance of amplified RNA silencing by plants. Trends Plant Sci. 2008;13(7):317–28. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa Y, Hiraguri A, Moriyama H, Fukuhara T. The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Mol Biol. 2007;63(6):777–85. [DOI] [PubMed] [Google Scholar]
- 69.Liu B, Li P, Li X, Liu C, Cao S, Chu C, Cao X. Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol. 2005;139(1):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2(5): e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Zhang BS, Wu HW, Liu CL, Guo HS, Zhao JH. The RNA-binding domain of DCL3 is required for long-distance RNAi signaling. aBIOTECH. 2023;5(1):114. [DOI] [PMC free article] [PubMed]
- 72.Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320(5880):1185–90. [DOI] [PubMed] [Google Scholar]
- 73.Patel P, Mathioni SM, Hammond R, Harkess AE, Kakrana A, Arikit S, Dusia A, Meyers BC. Reproductive phasiRNA loci and DICER-LIKE5, but not microRNA loci, diversified in monocotyledonous plants. Plant Physiol. 2021;185(4):1764–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salse J, Bolot S, Throude M, Jouffe V, Piegu B, Quraishi UM, Calcagno T, Cooke R, Delseny M, Feuillet C. Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell. 2008;20(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murat F, Xu JH, Tannier E, Abrouk M, Guilhot N, Pont C, Messing J, Salse J. Ancestral grass karyotype reconstruction unravels new mechanisms of genome shuffling as a source of plant evolution. Genome Res. 2010;20(11):1545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen S, Liu W, Naganuma M, Tomari Y, Iwakawa HO. Functional specialization of monocot DCL3 and DCL5 proteins through the evolution of the PAZ domain. Nucleic Acids Res. 2022;50(8):4669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song X, Li P, Zhai J, Zhou M, Ma L, Liu B, Jeong D, Nakano M, Cao S, Liu C. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J. 2012;69(3):462–74. [DOI] [PubMed] [Google Scholar]
- 78.Teng C, Zhang H, Hammond R, Huang K, Meyers BC, Walbot V. Dicer-like 5 deficiency confers temperature-sensitive male sterility in maize. Nat Commun. 2020;11(1):2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shoemaker RC, Polzin K, Labate J, Specht J, Brummer EC, Olson T, Young N, Concibido V, Wilcox J, Tamulonis JP, Kochert G, Boerma HR. Genome duplication in soybean (Glycine subgenus soja). Genetics. 1996;144(1):329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16(21):2733–42. [DOI] [PubMed] [Google Scholar]
- 81.Schmitz RJ, Hong L, Fitzpatrick KE, Amasino RM. DICER-LIKE 1 and DICER-LIKE 3 redundantly act to promote flowering via repression of FLOWERING LOCUS C in Arabidopsis thaliana. Genetics. 2007;176(2):1359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin L, Mo N, Zhang Y, Muhammad T, Zhao G, Zhang Y, Liang Y. CaRDR1, an RNA-dependent RNA polymerase plays a positive role in pepper resistance against TMV. Front Plant Sci. 2017;8:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hunter LJR, Brockington SF, Murphy AM, Pate AE, Gruden K, Macfarlane SA, Palukaitis P, Carr JP. RNA-dependent RNA polymerase 1 in potato (Solanum tuberosum) and its relationship to other plant RNA-dependent RNA polymerases. Sci Rep. 2016;6(1):23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xia R, Xu J, Meyers BC. The Emergence, Evolution, and Diversification of the miR390-TAS3-ARF Pathway in Land Plants. Plant Cell. 2017;29(6):1232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Fig S1. Sequences alinement of AGO, DCL and RDR genes. Fig S2. Promoter classification and proportion analysis of EoAGO, EoDCL and EoRDR genes. Fig S3. Three-dimensional (3D) protein structures of EoAGO, EoDCL and EoRDR proteins. Table S1. The alignment details and features of conserved domains of EoAGO, EoDCL and EoRDR genes. Table S2. AGO proteins with missing catalytic residue(s) in PIWI domains. Table S3. Promoter analysis of EoAGOs, EoDCLs and EoRDRs. Table S4. Primers used in the RT-qPCR analysis. Table S5. The detailed information of the predicted three-dimensional (3D) proteins.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/s/8256acffdb73bb050045.