Abstract
Purpose
Vascular cognitive impairment(VCI) ranks as the second most prevalent type of dementia.Increasing evidence has shown that inflammation and multi-faceted neuro-immune interactions integrate systemic and central inflammatory pathways, thereby inducing vascular tissue injury and contributing to the development of vascular cognitive impairment (VCI).V-type immunoglobulin-like suppressor of T cell activation (VISTA) is an Negative checkpoint regulators(NCR) that is associated with CNS homeostasis, interactions with peripheral immunity and CNS inflammation.The primary objective of this study was to seek the correlation between VISTA and VCI in patients with cardiovascular risk factors.Our secondary objective was to explore the potential of VISTA as a biomarker for VCI.
Patients and Methods
We enrolled individuals with cardiovascular risk factors in this cross-sectional study research and categorized them into two groups: without cognitive impairment (control) and with cognitive impairment (VCI). VISTA expression in peripheral blood mononuclear cells (PBMCs) was analyzed using relative quantitative polymerase chain reaction. VISTA expression was identified in monocyte subsets using flow cytometry. We use Enzyme linked immunosorbent assay to detect inflammatory factors in serum.
Results
In PBMC in patients with VCI, the expression of VSIR was significantly reduced. In contrast to controls, fasting glucose, fibrosis, and the levels of interleukin 6 (IL-6) in VCI patients were noticeably higher, and uric acid levels were significantly lower. Vsir mRNA expression in PBMCs correlated negatively with IL-6 levels, Trail Making Test B scores, and Hachinski scores and positively with Boston Naming Test scores. In intermediate monocytes, flow cytometry showed reduced Vsir expression, which was connected with VCI. The percentage of intermediate monocytes, uric acid, and the VISTA mean fluorescence intensity on intermediate monocytes were shown to be independent factors to VCI by multivariate logistic regression analysis.
Conclusion
Decreased VISTA promotes the occurrence of VCI in patients with cardiovascular risk factors by promoting monocytes toward the proinflammatory intermediate monocyte subset. VISTA may serve as a potential biomarker for distinguishing VCI in individuals with cardiovascular risk factors.
Keywords: neuroinflammation, intermediate monocyte, biomarker, flow cytometry
Introduction
After Alzheimer’s disease (AD), vascular cognitive impairment (VCI) is the second most frequent type of dementia, ranging from mild cognitive impairment to dementia.1 Numerous conditions linked to cerebrovascular risk factors and illnesses are the cause of VCI.2 The main characteristics of vascular dementia (VaD) are cerebral microangiopathy and the breakdown of the blood-brain barrier, which are primarily caused by thickening of the vascular wall, buildup of collagen along blood arteries and capillaries, atrophy of smooth muscle cells, and thinning of the lumen.3 Low-grade inflammatory disorders of the arterial wall are caused by vascular changes, and the innate immune system, particularly the monocyte-derived macrophages, has a significant impact on the onset and progression of the illness.4
Immune checkpoints are essential for preserving the delicate equilibrium between protective immune responses and excessive inflammation, which can cause unnecessary tissue damage and autoimmune diseases.5 VISTA(also known as Vsir,PD-1H)6 is a cell surface inhibitory molecule predominantly expressed in the hematopoietic compartment, particularly in myeloid cells such as monocytes, macrophages, dendritic cells, and neutrophils, as well as in naïve CD4+ and CD8+ T cells, and regulatory Foxp3+ T cells.VISTA attaches the ligand V-set and immunoglobulin domain-containing protein 3 and binds the co-inhibitory receptor P-selectin glycoprotein ligand-1 in acidic environments, acting as both a ligand and a receptor, and this sets it apart from antigens linked to cytotoxic T lymphocytes and programmed cell death 1.7 Recent data indicate that VISTA may play a role in myeloid cell chemotaxis and migration via direct and indirect signaling.8 Because Ccl2 is a chemoattractant for monocytes, VISTA may indirectly control the recruitment of monocytes. In multiple mouse models of inflammation, VISTA knockout (KO) is associated with elevated levels of pro-inflammatory cytokines, which are derived from both T cells and myeloid cells.8–11 Furthermore, it has been demonstrated that VISTA KO can impair peripheral tolerance, contributing to the development of chronic inflammation in multiple organs.11 Additionally, Borggrewe et al have shown that VISTA is predominantly expressed in microglial cells in the brains of humans and mice, with its expression being significantly downregulated in microglial cells within tissues affected by multiple sclerosis (MS).12 Despite the regulatory role of VISTA in immunological responses, the pattern and function of the expression of the protein-coding gene, v-set immunoregulatory receptor (Vsir), in patients with VCI remain unclear.
The objective of this investigation was to examine the degree of Vsir expression in patients’ PBMCs who had cardiovascular risk factors, supplying fresh and potential biomarkers for VCI screening. In addition, we applied flow cytometry to show the VISTA expression in monocyte subsets and used an enzyme-linked immunosorbent assay (ELISA) to measure the serum inflammatory markers in an effort to shed more light on VISTA’s function in the VCI process.
Material and Methods
Recruitment
The First Affiliated Hospital Ethics Committee of Harbin Medical University granted approval for this study, which was carried out from April 2023 to January 2024 in accordance with the Declaration of Helsinki’s criteria. An informed consent form was signed by each study participant or their duly authorized representative before to the trial.The clinical trial registration number is NCT06342661.
54 patients with cardiovascular risk factors from Harbin Medical University’s First Affiliated Hospital were retrospectively included. Among the 54 participants were 27 patients with VCI (case group) and 27 individuals of who had normal cognitive function. The baseline characteristics were balanced and comparable between the VCI and the control groups.Patients with cardiovascular risk factors who met the following criteria were included: I) a diagnosis of VCI based on the National Institute of Neurological Disorders and Stroke(NINDS) and the Association Internationale pour la Recherche et I’Enseignement en Neurosciences (AIREN) criteria13 and the Diagnostic and Statistical Manual of Mental Disorders V(DSM)14 and II) an age of 50 years or older. The followings were the exclusion criteria: I)an autoimmune diagnosis, II) serious systemic diseases such as severe bone and joint, liver, kidney, hematopoietic system, chronic obstructive pulmonary and endocrine system diseases, as well as cancer,15 and III) a history of depression or mental disorder.
Baseline Measurements
Data on baseline demographic characteristics, vascular risk factors, and history of stroke (ischemic or hemorrhagic) were collected as previously described.15
Neurocognitive Evaluation
Initially, all patients would undergo testing for Mini-Mental State Examination and Montreal Cognitive Assessment scores for screening of cognitive decline, which are recommended by NINDS-AIREN. The minor results contained the capability in five cognition fields: (1) verbal memory, Hopkins Verbal Learning Test;16 (2) visual and spatial capability, Clock Drawing test;17 (3) administrative capability, Trail Making test (TMT) (B time);18 (4) attention, TMT (A time);18 and (5) language tests, Boston Naming test2 (BNT-2).19 In addition, we used the Instrumental Activities of Daily Living20 to assess socialization or activities of daily living status and the Neuropsychiatric Inventory to assess mental behavioral symptoms.
Neuroimaging
CT and MRI were performed on admission for all patients in the study. Neuroimaging was assessed using the Fazekas scale21 and the Vascular Behavioral and Cognitive Disorders criteria.22
Blood Sampling and PBMCs
Blood was obtained in the morning after overnight fasting using EDTA vacutainers. Peripheral PBMCs were isolated using a Ficoll–Hypaque gradient (TBD, Tianjin, China). Serum and plasma were kept at −80°C until needed. PBMCs were treated with an extraction kit (SEVEN, China) to extract total RNA.Total RNA was measured using NanoDrop ND-2000 (Thermo Fisher Scientific, Inc., Wilmington, DE, USA), and 1 μg of RNA was used for cDNA synthesis using a specific kit (Monad; Wuhan, China).
Relative Quantitative Polymerase Chain Reaction (PCR)
Relative quantitative PCR was performed using a Gene 9600 system (Applied Biosystems, Shanghai, China). Every sample had its vsir gene expression examined, with GAPDH serving as an internal control. For the relative quantitative PCR, the following parameters were followed: primer annealing for 60s at 60°C, denaturation for 135 s at 95°C, and initial denaturation for 3 min at 95°C.The following were the precise primer sequences for Vsir:F:5′-GCGGATGGACAGCAACATT-3′; R:5′-TTGGAGAGTCAGGGACAGGG-3′
GAPDH:F:5′-GGAGCGAGATCCCTCCAAAAT-3′;R:5′-GGCTGTTGTCATACTTCTCATGG-3′.
Cytokine Measurements
We use Enzyme linked immunosorbent assay kit (JL14113-96T, Jianglai Biotechnology, Shanghai, China) to detect the inflammatory factor interleukin-6 (IL-6) in serum.
Flow Cytometry
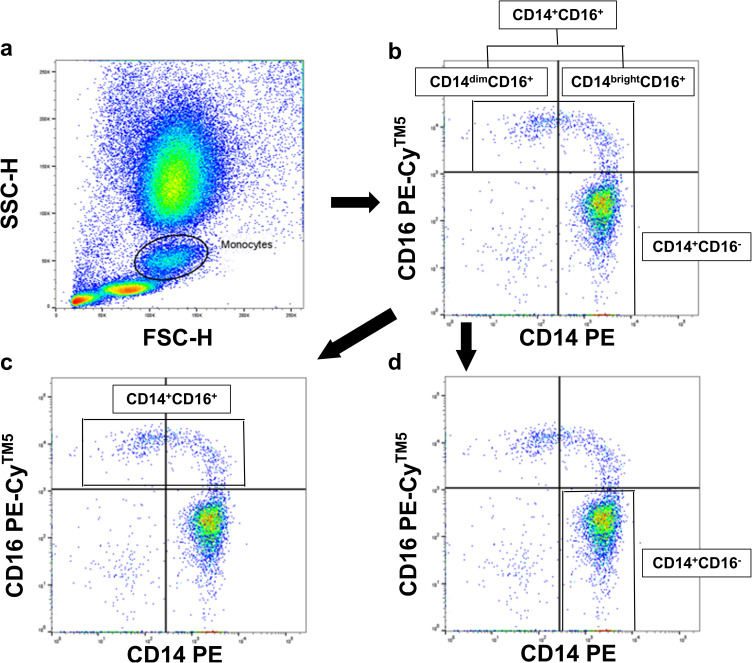
For cytometric analysis, cells were incubated for 15 min on ice in Fc receptor blocking solution (Absin, Bioscience Inc., Shanghai, China) and subsequently stained with Alexa Fluor® 488 anti-VISTA (Clone #730804, Abcam, Hangzhou, China) for 30 min on reverse transcription. The use of CD14 (555398, PE Mouse Anti-Human, BD Biosciences, Franklin Lakes, NJ, USA) and CD16 (555408, PE-CyTM5 Mouse Anti-Human, BD Biosciences, Franklin Lakes, NJ, USA) antibodies remained the same as ever.23–26Correspondingly isotype antibodies (555574,PE mouse IgG2aκisotype, and PECyTM5 mouse IgG1κisotype,BD Biosciences,Franklin Lakes, NJ, USA) served as the negative controls. One hundred microliters of blood were incubated at room temperature in the dark for fifteen minutes. Three milliliters of lysis solution were added for leukocyte fixation and erythrocyte lysis. (eBioscience, San Diego, CA, USA). There was a cytometric analysis carried out with a flow cytometer (FACSAriaTM, BD Biosciences) and the BD FACSDiva Software System (BD Biosciences). In order to assess 3-color fluorescence within the monocyte gate, monocytes were first gated in a forward scatter/sideward scatter (FSC/SSC) dot-plot, as illustrated in Figure 1. The term “CD14+CD16-” refers to monocytes that express CD14 but not CD16.CD14+CD16+ cells are defined as monocytes expressing CD16 with either high (CD14brightCD16+) or low (CD14dimCD16+) CD14expressing.27 Three monocyte subgroups have been recognized in the most recent classification of monocyte heterogeneity: nonclassical (CD14+CD16+), intermediate (CD14++CD16+), and classical (CD14++CD16-).28
Figure 1.
Fluorescence-activated cell scanner analysis.
Notes: (a) Monocytes were gated in a FSC/SSC dot-plot. (b–d) CD14+CD16- cells were defined as monocytes expressing CD14 but not CD16 (lower right quadrant). CD14 +CD16+ cells were defined as monocytes expressing CD16 and either high levels of CD14 (upper right quadrant; CD14brightCD16+) or lower levels of CD14 (upper left quadrant; CD14dimCD16+).
Abbreviation: FSC/SSC, forward scatter/sideward scatter.
Statistical Analysis
The data analysis application is SPSS Windows 26 version (SPSS Inc., Chicago, IL, USA). An independent sample t-test was used to compare continuous variables, which are represented as mean ± standard deviation for variables with a normal distribution. Using the Wilcoxon rank-sum test, continuous variables with non-normal distributions are represented as the median (P25–P75). Utilizing the chi-squared test, categorical data were compared and are shown as n (%). The correlation coefficient (rs) between the VISTA mean fluorescence intensity (MFI) on the intermediate monocyte, the levels of IL-6, and cognitive fields and the expression of Vsir mRNA in PBMCs was determined using Spearman correlation analysis.Contributors to VCI were identified using a multivariable logistic regression model. The multivariable logistic analysis includes variables that showed P<0.05 in the univariate analysis. Since the expression of Vsir mRNA in PBMCs and VISTA MFI in intermediate monocytes were significantly correlated with IL-6, IL-6 was not included. Statistical significance was set at P<0.05, and receiver operating characteristic (ROC) curve analysis of Vsir expression in PBMCs and intermediate monocytes was used for predicting VCI. The sample size was determined using a power analysis. The power estimated to be 90% with a minimum of 15 participants in each group at a two-sided significance level of 0.05, actually achieves 92.384% power with group sample sizes of 15 and 15. For each outlier, I accepted outliers as much as possible unless it was clear that they represented errors or bad data.
Results
Patient Characteristics
54 patients with cardiovascular risk factors from Harbin Medical University’s First Affiliated Hospital were retrospectively included. Among the 54 participants were 27 patients with VCI (case group) and 27 individuals who had normal cognitive function.Table 1 provides a summary and comparison of the participants’ fundamental traits for the two groups.The VCI and control groups did not significantly differ in terms of age, sex, education, diabetes, body mass index(BMI), hypertension, low-density lipoprotein cholesterol, total cholesterol, triglycerides, homocysteine, white blood cell count, neutrophil percentage, serum creatinine, uric acid, history of stroke, smoking, drinking, or Fazekas scale scores (Table 2).The case group exhibited considerably greater levels of IL-6(P<0.001),FIB (P<0.05), and fasting glucose (P<0.05), compared to the control group. Conversely, the case group’s uric acid level (P<0.05) was considerably lower than that of the control group.
Table 1.
Comparisons of Clinical Characteristics Between VCI and Control Groups
| VCI Group (n=27) | Control Group (n=27) | χ2/t/Z | P | |
|---|---|---|---|---|
| Age, years | 69.93±8.467 | 66.59±7.996 | 1.487 | 0.143 |
| Sex, male | 18 (66.7%) | 23 (85.2%) | 2.533 | 0.111 |
| Cardiovascular risk factors | ||||
| Hypertension | 24 (88.9%) | 20 (74.1%) | 1.964 | 0.161 |
| Diabetes mellitus | 14 (51.9%) | 7 (25.9%) | 3.818 | 0.051 |
| Body mass index, kg/m2 | 24.2 (22.49, 25.34) | 24.2 (22.03, 25.95) | −0.087 | 0.931 |
| Smoking | 13 (48.1%) | 15 (55.6%) | 0.297 | 0.586 |
| History of CVD | 18 (66.7%) | 18 (66.7%) | 0.000 | 1.000 |
| Fazekas scale | 5 (4, 5) | 3 (2, 6) | −1.917 | 0.055 |
| Drinking | 12 (44.4%) | 13 (48.1%) | 0.074 | 0.785 |
| Education, years | 8 (5, 11) | 9 (7, 12) | −1.934 | 0.053 |
| MMSE | 19 (13, 20) | 26 (25, 28) | −5.728 | 0.000 |
| MoCA | 14 (8, 16) | 25 (25, 26) | −6.326 | 0.000 |
| Verbal Memory | ||||
| HVLT | 21 (18, 23) | 25 (23, 28) | −4.244 | 0.000 |
| Visual-spatial ability | ||||
| CDT | 2 (1, 3) | 4 (3, 4) | −4.409 | 0.000 |
| Executive function | ||||
| TMT-B | 180 (100, 268) | 150 (80, 225) | −1.213 | 0.225 |
| Attention | ||||
| TMT-A | 115 (74, 150) | 70 (60, 110) | −3.105 | 0.002 |
| Language tests | ||||
| BNT-2 | 21 (14, 24) | 23 (20, 28) | −2.082 | 0.037 |
| IDAL | 13 (11, 27) | 8 (8, 13) | −3.134 | 0.002 |
| NPI | 4 (0, 12) | 0 (0, 3) | −2.858 | 0.004 |
| Hachinski | 11 (8, 14) | 7 (5, 12) | −2.635 | 0.008 |
Notes: Data are mean value ± SD, n (%) or median [interquartile range].
Abbreviations: CVD, cerebrovascular disease; MMSE, Mini-Mental Status Examination; MoCA, Montreal Cognitive Assessment; BNT, Boston Naming Test; CDT, Clock Drawing test; TMT, Trail Making Test; IDAL, Instrumental Activities of Daily Living; NPI, Neuropsychiatric Inventory.
Table 2.
Comparisons of Laboratory Measurements Between VCI and Control Groups
| VCI Group (n=27) | Control Group (n=27) | χ2/t/Z | P | |
|---|---|---|---|---|
| Total cholesterol, mmol/l | 4.06 (3.57, 5.36) | 4.46 (3.8, 5.62) | −1.210 | 0.226 |
| LDL-C,mmol/l | 2.24 (1.96, 3.43) | 2.49 (2.21, 3.4) | −0.863 | 0.388 |
| Triglyceride,mmol/l | 1.66 (1.11, 2.1) | 1.54 (1.02, 1.91) | −0.730 | 0.466 |
| Fasting glucose,mmol/l | 5.85 (4.69, 6.89) | 5 (4.51, 5.45) | −2.042 | 0.041 |
| Homocysteine,mmol/l | 16.15 (12.05, 21.59) | 15.5 (11.08, 17.51) | −0.801 | 0.423 |
| FIB,g/l | 3.43 (2.69, 4.43) | 2.64 (2.35, 3.43) | −2.337 | 0.019 |
| White blood cells,*10–9/l | 7.39 (5.71, 8.7) | 7.18 (5.61, 8.75) | −0.303 | 0.762 |
| Neutrophils percentage,% | 68.72±10.299 | 68.90±9.604 | −0.066 | 0.948 |
| Serum creatinine,umol/l | 82.2 (74.1, 102.8) | 85.85 (74.65, 94.1) | −0.427 | 0.669 |
| Uric acid,umol/l | 335.6 (274.4, 365.8) | 378.1 (327.9, 481.5) | −2.473 | 0.013 |
| IL-6 | 8.10 (5.98, 14.65) | 4.42 (2.47, 5.03) | −5.129 | 0.000 |
| Vsir mRNA in the PBMCs | 0.09 (0.07, 0.11) | 0.65 (0.14, 5.91) | −5.303 | 0.000 |
| VISTA MFI on intermediate monocytes | 1126.5 (884.25, 1658.75) | 2884 (2150, 3957.5) | −4.180 | 0.000 |
Notes: Data are mean value ± SD, n (%) or median [interquartile range].
Abbreviations: LDL-C, low-density lipoprotein cholesterol; IL-6, interleukin-6; MFI, mean fluorescence intensity.
VISTA Expression in PBMCs in the VCI and Control Groups
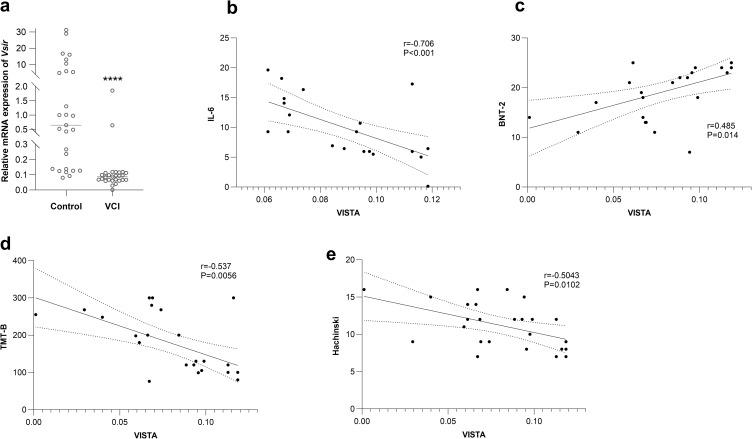
Compared to control PBMCs, patients with VCI showed a declining trend in the mean expression value of Vsir (Figure 2a). In the peripheral blood monolayer cells (PBMCs) of patients with VCI, the expression of Vsir mRNA was strongly inversely linked with the levels of IL-6 (r=−0.706, P<0.001) (Figure 2b), TMT-B (r=−0.537, P=0.0056) (Figure 2c), and Hachinski (r=−0.5043, P=0.0102) (Figure 2d). In the PBMCs of individuals with VCI, there was a strong positive correlation between BNT-2 (r=0.485, P=0.014) and the expression of Vsir mRNA (Figure 2e).
Figure 2.
The expression of Vsir mRNA in the PBMCs of the Control and VCI groups and the correlation between it and IL-6, cognition domains.
Notes: (a) Vsir expression is downregulated in the PBMCs of VCI patients compared to the control.**** indicates p<0.0001.(b, d and e) The level of IL-6, TMT-B, and Hachiski have a significant negative correlation with the expression of Vsir mRNA in the PBMCs of VCI patients. (c) BNT-2 has a significant positive correlation with the expression of Vsir mRNA in the PBMCs of VCI patients.
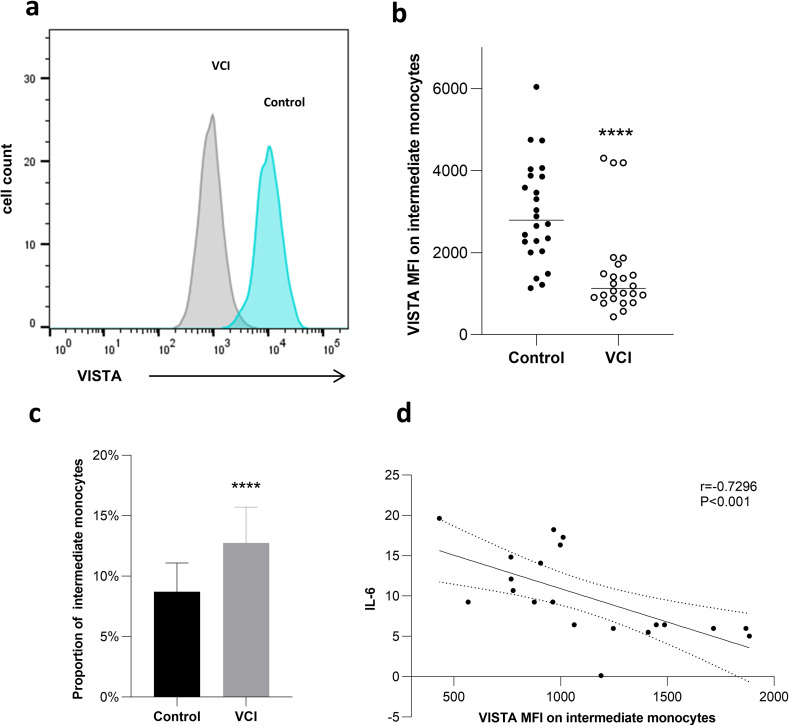
VISTA Expression in Intermediate Monocytes
When comparing the intermediate monocytes of patients with VCI to controls, flow cytometry analysis showed that Vsir expression was downregulated (Figure 3a and b). Figure 3c shows that the proportion of intermediate monocytes was considerably greater in patients with VCI ([12.76 ± 2.947]%) compared to the controls (vs [8.71 ± 2.389]%, P<0.0001). In the intermediate monocytes of VCI patients, there was a strong negative correlation between the amount of IL-6 (r=−0.7296, P<0.001) and VISTA MFI (Figure 3d).
Figure 3.
Vista expression in intermediate monocytes.
Notes: A representative histogram (a) and cumulative data (b) of VISTA expression on CD14brightCD16+ gated cells from control and VCI groups. MFI, mean fluorescence intensity. ****indicates p<0.0001. (c) Comparison of proportion of intermediate monocytes between 2 patient groups.**** indicates p<0.0001. (d) The level of IL-6 has a significant negative correlation with the VISTA MFI on intermediate monocytes of VCI patients.
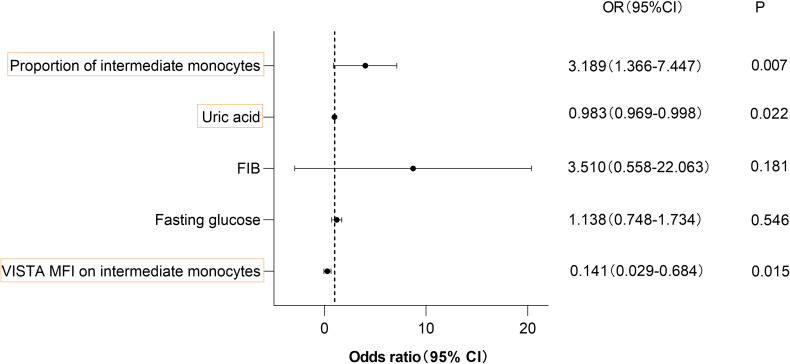
VISTA as a Predictive Marker of VCI
A multivariable logistic regression model was employed to identify the factors that contributed to VCI, as seen in Figure 4; The multivariable logistic analysis included the factors (fasting glucose, uric acid, FIB, and the proportion of intermediate monocytes) that had P<0.05 in the univariate analysis. Since the expression of Vsir mRNA in PBMCs and VISTA MFI in intermediate monocytes were significantly correlated with IL-6, IL-6 was omitted. VISTA MFI on intermediate monocytes (OR: 0.141; 95% CI: 0.029–0.684, P<0.05), uric acid (OR: 0.983; 95% CI: 0.969–0.998, P<0.05), and the proportion of intermediate monocytes (odds ratio [OR]: 3.189; 95% confidence interval [CI]: 1.366–7.447, P<0.05) were independent contributors to VCI, according to multivariate logistic regression analysis (Figure 4).
Figure 4.
A multivariable logistic regression model was used to determine contributors to VCI.
Notes: VISTA MFI on intermediate monocytes values are in the thousands.
Abbreviations: OR, odds ratio; CI, confidence interval.
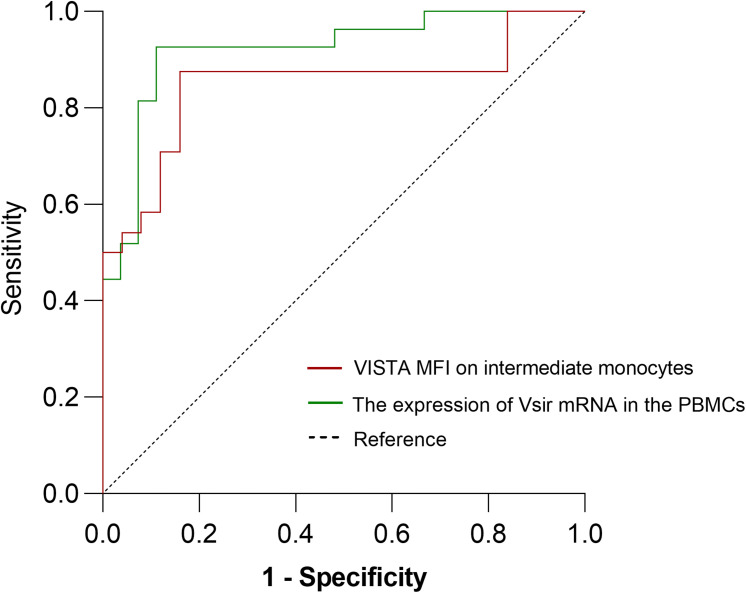
Figure 5 displays the ROC curves for Vsir mRNA expression in PBMCs and VISTA MFI in intermediate monocytes. The expression of Vsir mRNA in PBMCs demonstrated a predictive value for VCI, with an area under the ROC curve (AUC) of 0.8483. Similarly, the VISTA mean fluorescence intensity (MFI) in intermediate monocytes showed an AUC of 0.9204. A threshold degree of 1943.5 on intermediate monocytes exhibited the highest combined sensitivity (84%) and specificity (87.5%) for the detection of VCI in terms of VISTA MFI; Vsir mRNA expression in PBMCs at a threshold level of 0.12 provided 88.9% sensitivity and 92.6% specificity for identifying VCI.
Figure 5.
ROC curve analysis of Vista MFI on intermediate monocytes and the expression of Vsir mRNA in the PBMCs for predicting VCI.
Notes: The areas under the ROC curve were 0.8483 for VISTA MFI on intermediate monocytes and 0.9204 for the expression of Vsir mRNA in the PBMCs.
Abbreviations: VCI, vascular cognitive impairment; ROC, receiver-operating characteristic.
Discussion
There are no approved treatments for VaD.2 In comparison to controls, this investigation revealed a declining trend in Vsir expression in the PBMCs and intermediate monocytes of patients with VCI. In addition, We discovered that reduced Vsir expression in VCI patients’ peripheral blood mononuclear cells was linked to lower BNT-2 and greater TMT-B, Hachinski, and IL-6 levels. Patients with VCI showed a shift toward the intermediate monocyte subgroup according to flow cytometry analysis, indicating reprogramming of myelopoiesis at the bone marrow level.
Myeloid cells of the immune cell compartment, such as neutrophils, monocytes, macrophages, and dendritic cells, are the main sources of VISTA expression.29 Our study provides the first evidence of a declining trend in Vsir expression in PBMCs and in intermediate monocytes in patients with VCI, suggesting an important role for VISTA, not only in multiple sclerosis,30 as was previously reported, but also in the pathophysiology of VCI. According to recent research, monocytes from individuals with atherosclerosis who have already developed or who have risk factors for the disease exhibit a trained immunity phenotype, which is the name for monocytes that have reprogrammed themselves to adopt a long-term proinflammatory phenotype.31 Additionally, inflammation plays a significant role in the development of neurodegenerative illnesses such Parkinson’s disease, AD, and VCI.3,32,33 Although they make up 2–5% of monocytes, intermediate monocytes are more prevalent in patients with inflammatory diseases, autoimmune disorders, sepsis, and ischemic heart disease.27 According to flow cytometry analysis, patients with VCI have a considerably higher frequency of intermediate monocytes.
The trained immunity theory was recently investigated in brain microglia. In this investigation, following a repeated peripheral immunological assault with lipopolysaccharides, the production of cytokines in tissue-resident microglia was evaluated. We concentrated on how the innate immune system contributes to the development of VCI for two reasons. First, circulating inflammatory markers such as IL-6, which stimulate monocytes and macrophages, have recently been linked to VCI.3 Second, there may be similar pathophysiological pathways between VCI and atherosclerosis owing to the sharing of risk factors (diabetes mellitus, smoking, and hypertension). Monocyte-derived macrophages have a significant part in the pathophysiology of atherosclerosis.4
The case group in this study had low levels of uric acid and high levels of proinflammatory factors (IL-6), suggesting that oxidative stress and inflammation may be features of VCI.34 High fibrinogen levels were linked to a higher risk of AD and VaD, according to another study, which is consistent with our conclusions.35 White matter hyperintensity progression was observed to be significantly correlated with the percentage of CD14++CD16+ intermediate monocytes in a prior investigation involving 51 older participants.36 Similar to previously reported findings on intermediate monocytes, the percentage of intermediate monocytes was discovered to be a biomarker of VCI risk.Unexpectedly, we identified a potential role for Vsir expression in PBMCs and in intermediate monocytes as a biomarker for predicting VCI risk.
Conclusion
While our study yields promising findings, it is important to note that the modest sample size places certain limitations on the strength and generalizability of our results. The sample size may not fully capture the variability present in the broader population, which could affect the external validity of our conclusions.Therefore, further prospective research on the inflammatory properties of immune cells in larger patient cohorts should be conducted to gain a deeper comprehension of the role and importance of PD-1H in VCI pathogenicity. One such research project would be to isolate and examine intermediate monocytes that exhibit reduced Vsir expression.
In conclusion, our findings suggest that in people with cardiovascular risk factors, VISTA may be associated with the inflammation that occurs when VCI develops. By altering the inflammatory function of intermediate monocytes, decreased VISTA and an increased proportion of IL-6 and intermediate monocytes may have an impact on the development of VCI. Furthermore, When identifying VCI in patients with cardiovascular risk factors, VISTA may be a helpful biomarker.
Acknowledgments
An unauthorized version of the Chinese MMSE was used by the study team without permission, however this has now been rectified with PAR. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com).
Funding Statement
This work was supported in part by Natural Science Foundation of Heilongjiang Province of China (LH2023H025).
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Disclosure
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Frances A, Sandra O, Lucy U. Vascular cognitive impairment, a cardiovascular complication. World J Psychiat. 2016;6(2):199–207. doi: 10.5498/wjp.v6.i2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8 [DOI] [PubMed] [Google Scholar]
- 3.Wang -X-X, Zhang B, Xia R, Jia Q-Y. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020;24(18):9601–9614. doi: 10.26355/eurrev_202009_23048 [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. doi: 10.1038/nri3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Rubinstein R, Lines JL, et al. Vista, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(4):1537–1541. doi: 10.4049/jimmunol.1100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Wu G, Manick B, et al. VSIG-3 as a ligand of Vista inhibits human T-cell function. Immunology. 2019;156(1):74–85. doi: 10.1111/imm.13001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H, Li X, Hu L, et al. A crucial role of the PD-1H coinhibitory receptor in suppressing experimental asthma. Cell Mol Immunol. 2018;15(9):838–845. doi: 10.1038/cmi.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceeraz S, Sergent PA, Plummer SF, et al. Vista Deficiency Accelerates the Development of Fatal Murine Lupus Nephritis. Arthritis Rheumatol. 2017;69(4):814–825. doi: 10.1002/art.40020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceeraz S, Eszterhas SK, Sergent PA, et al. Vista deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther. 2017;19(1):270. doi: 10.1186/s13075-017-1474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Xu W, Yuan Y, et al. Immune-checkpoint protein Vista critically regulates the IL-23/IL-17 inflammatory axis. Sci Rep. 2017;7(1):1485. doi: 10.1038/s41598-017-01411-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borggrewe M, Grit C, Den Dunnen WFA, et al. Vista expression by microglia decreases during inflammation and is differentially regulated in CNS diseases. Glia. 2018;66(12):2645–2658. doi: 10.1002/glia.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250 [DOI] [PubMed] [Google Scholar]
- 14.Roehr B. American Psychiatric Association explains DSM-5. BMJ. 2013;346:f3591. doi: 10.1136/bmj.f3591 [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Zhang J, Liang Y, et al. Network topology and machine learning analyses reveal microstructural white matter changes underlying Chinese medicine Dengzhan Shengmai treatment on patients with vascular cognitive impairment. Pharmacol Res. 2020;156:104773. doi: 10.1016/j.phrs.2020.104773 [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Tian J, Wei M, Miao Y, Wang Y. The utility of the Hopkins Verbal Learning Test (Chinese version) for screening dementia and mild cognitive impairment in a Chinese population. BMC Neurol. 2012;12(1):136. doi: 10.1186/1471-2377-12-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiai S, Sugishita M, Ichikawa T, Gono S, Watabiki S. Clock-drawing test and unilateral spatial neglect. Neurology. 1993;43(1):106–110. doi: 10.1212/wnl.43.1_part_1.106 [DOI] [PubMed] [Google Scholar]
- 18.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol. 1972;28(2):167–169. doi: [DOI] [PubMed] [Google Scholar]
- 19.Deng YM, Ding ZY, Liu YF, Wu JW, Wang YJ, Zhao XQ. A Case-Control Study: infectious Burden Increased the Occurrence of Vascular Cognitive Impairment No Dementia. CNS Neurosci Ther. 2016;22(12):1012–1014. doi: 10.1111/cns.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedrosa H, De Sa A, Guerreiro M, et al. Functional evaluation distinguishes MCI patients from healthy elderly people--The ADCS/MCI/ADL scale. J Nutr Health Aging. 2010;14(8):703–709. doi: 10.1007/s12603-010-0102-1 [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 22.Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–138. doi: 10.1016/j.jacc.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 24.Tsujioka H, Imanishi T, Ikejima H, et al. Post-reperfusion enhancement of CD14(+)CD16(-) monocytes and microvascular obstruction in ST-segment elevation acute myocardial infarction. Circ J. 2010;74(6):1175–1182. doi: 10.1253/circj.cj-09-1045 [DOI] [PubMed] [Google Scholar]
- 25.Kuroi A, Imanishi T, Suzuki H, et al. Clinical characteristics of patients with Kawasaki disease and levels of peripheral endothelial progenitor cells and blood monocyte subpopulations. Circ J. 2010;74(12):2720–2725. doi: 10.1253/circj.cj-10-0317 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Imanishi T, Ikejima H, Tsujioka H, Ozaki Y. Association between circulating monocyte subsets and in-stent restenosis after coronary stent implantation in patients with ST-elevation myocardial infarction. Circulation J. 2010;74(12):2585–2591. doi: 10.1253/circj.cj-10-0544 [DOI] [PubMed] [Google Scholar]
- 27.Ozaki Y, Imanishi T, Taruya A, et al. Circulating CD14+CD16+ Monocyte Subsets as Biomarkers of the Severity of Coronary Artery Disease in Patients With Stable Angina Pectoris. Circ J. 2012;76(10):2412–2418. doi: 10.1253/circj.CJ-12-0412 [DOI] [PubMed] [Google Scholar]
- 28.NCBI, Nomenclature of monocytes and dendritic cells in blood - PubMed. Accessed February 2, 2024. https://pubmed.ncbi.nlm.nih.gov/20628149/.
- 29.Shibru B, Fey K, Fricke S, et al. Detection of Immune Checkpoint Receptors - A Current Challenge in Clinical Flow Cytometry. Front Immunol. 2021;12:694055. doi: 10.3389/fimmu.2021.694055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derakhshani A, Asadzadeh Z, Baradaran B, et al. The expression pattern of Vista in the PBMCs of relapsing-remitting multiple sclerosis patients: a single-cell RNA sequencing-based study. Biomed Pharmacother. 2022;148:112725. doi: 10.1016/j.biopha.2022.112725 [DOI] [PubMed] [Google Scholar]
- 31.Wong KL, Yeap WH, Tai JJY, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53(1–3):41–57. doi: 10.1007/s12026-012-8297-3 [DOI] [PubMed] [Google Scholar]
- 32.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- 33.P M, Ir A, M G, B L, C A. Inflammation in Parkinson’s Disease: mechanisms and Therapeutic Implications. Cells. 2020;9(7):1687. doi: 10.3390/cells9071687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19(4):1331–1336. doi: 10.3233/JAD-2010-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoki S, Tanaka T, Ihara M. Diagnostic and prognostic blood biomarkers in vascular dementia: from the viewpoint of ischemic stroke. Neurochem Int. 2021;146:105015. doi: 10.1016/j.neuint.2021.105015 [DOI] [PubMed] [Google Scholar]
- 36.Noz MP, Ter Telgte A, Wiegertjes K, et al. Trained Immunity Characteristics Are Associated With Progressive Cerebral Small Vessel Disease. Stroke. 2018;49(12):2910–2917. doi: 10.1161/STROKEAHA.118.023192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.