Abstract
Objective
Maturity onset diabetes of the young (MODY) occurs due to mutations in genes involved in pancreatic beta cell function and insulin secretion, has heterogeneous clinical and laboratory features, and account for 1-5% of all diabetes cases. The prevalence and distribution of MODY subtypes vary between countries. The aim of this study was to evaluate the clinical and laboratory characteristics, mutation distribution, and phenotype-genotype relationship in a large case series of pediatric Turkish patients genetically diagnosed with MODY.
Methods
MODY cases from 14 different pediatric endocrinology departments were included. Diagnosis, treatment, follow-up data, and results of genetic analysis were evaluated.
Results
A total of 224 patients were included, of whom 101 (45%) were female, and the mean age at diagnosis was 9.4±4.1 years. Gene variant distribution was: 146 (65%) GCK; 43 (19%) HNF1A; 8 (3.6%) HNF4A, 8 (3.6%) KLF11 and 7 (3.1%) HNF1B. The remaining 12 variants were: PDX (n=1), NEUROD1 (n=3), CEL (n=1), INS (n=3), ABCC8 (n=3) and KJNC11 (n=1). Of the cases, 197 (87.9%) were diagnosed with incidental hyperglycemia, 16 with ketosis (7%) and 7 (3%) with diabetic ketoacidosis (DKA), while 30% presented with classical symptoms of diabetes. Two-hundred (89%) had a family history of diabetes. Anti-GAD antibody was detected in 13 cases, anti-islet antibody in eight and anti-insulin antibody in four. Obesity was present in 16. Distribution of therapy was: 158 (71%) diet only; 23 (11%) intensive insulin treatment; 17 (7.6%) sulfonylureas; 10 (4.5%) metformin; and 6 (2.7%) insulin and oral anti-diabetic treatment.
Conclusion
This was the largest genetically diagnosed series from Turkey. The most common gene variants were GCK and HNF1A with much lower proportions for other MODY types. Hyperglycemia was the most common presenting symptom while 11% of patients had diabetes-associated autoantibodies and 7% were obese. The majority of patients received dietary management only.
Keywords: Childhood, MODY, diagnosis
What is already known on this topic?
Maturity onset diabetes of the young (MODY) is the term used to describe a group of inherited, non-autoimmune forms of diabetes mellitus. MODY diagnosis is often made at a young age (under 25 years of age), pancreatic autoantibodies are not present, there is a low insulin requirement, often a family history of autosomal dominant diabetes, no history of obesity and no history of diabetic ketoacidosis (DKA).
What this study adds?
This large-series national study showed that the diagnostic criteria in the prediagnostic process of MODY should be reconsidered because some patients with genetically diagnosed MODY had a history of DKA, pancreatic antibody positivity and no family history of diabetes.
Introduction
Maturity onset diabetes of the young (MODY) is the term used to describe a group of inherited, non-autoimmune forms of diabetes mellitus (DM). MODY was initially thought to be relatively rare but it has become apparent that it is more common than first thought. Despite increasing awareness of MODY, it has been estimated that up to 1-5% of MODY cases remain undiagnosed. Since the clinical spectrum is variable due to the range of genetic defects, there may be diagnostic delays, and some cases may not be diagnosed until adulthood. Furthermore, cases may sometimes be misdiagnosed as type 1 (T1DM) or type 2 DM (T2DM) (1, 2, 3).
Given the natural history of MODY, that there is cost-effective treatment, and the potential impact on more than one family member, it is essential to diagnose these cases correctly. The presence of family history, clinical and physical examination findings incompatible with T1DM or T2DM, and negative diabetes autoantibodies when present, strengthen the diagnosis of MODY (3). In addition, urinary c-peptide/creatinine ratio >0.2 nmol/mmol has high specificity and sensitivity in differentiating MODY from T1DM (4).
Genetic testing is important for definitive diagnosis and identification of other family members with the same variant. Mutation analyses classify the type of MODY and this in turn informs about the expected clinical courses and guides treatment planning. In patients with HNF4A and HNF1A mutations, forms of MODY which are responsive to sulphonylurea therapy, it is important to change treatment regimens from insulin treatment, as low-dose sulphonylurea treatment may provide good glycemic control for years and, in patients with GCK mutations, will prevent unnecessary insulin treatment. Mutation screening is also important for asymptomatic relatives with a 50% risk of inheriting the mutation.
The distribution of MODY types differs between countries. Although HNF1A MODY is reported to be the most common type globally, there are countries where GCK MODY is observed more frequently (5). Therefore, knowing which of the MODY types is more common in one’s own country is important. Determination of the MODY type will provide the most accurate approach for the pediatric endocrinologist to manage MODY patients. Moreover, managing clinicians should understand the phenotype-genotype relationship in MODY.
The aim of this study was to investigate the distribution of MODY types in a pediatric population from Turkey and to investigate the phenotype-genotype relationship.
Methods
The study included pediatric patients with genetically diagnosed MODY from 14 pediatric endocrinology and diabetes departments in Turkey. Ethical approval was obtained from Ankara University Faculty of Medicine (decision number: 12-112-20, date: 13.02.2020). The National Pediatric Endocrinology and Diabetes Association (ÇEDD) supported the study. An electronic case registration form was created in the ÇEDD-NET data system of the association, and centers were asked to record their data using this system. The data entry took one year. Retrospective medical history (at least three generations of family history), clinical characteristics (age at diagnosis, year of diagnosis, duration of diabetes, presence of classical diabetes symptoms), laboratory results (blood glucose, insulin, c-peptide, glycated hemoglobin (HbA1c) levels, lipid profile, presence of acidosis at diagnosis, presence of autoantibodies at diagnosis, and reserve insulin values) and genetic data were transferred from electronic case record forms to Microsoft Excel (Microsoft Inc., Redmond, USA) and Statistical Package for the Social Sciences (SPSS), version 20.0 (IBM Inc., Armonk, NY, USA) files for statistical evaluation.
Informed consent was obtained from the parents/guardians of the patient from all centers.
Patients with a positive family history in which at least two consecutive generations were affected, early-onset hyperglycemia, negative diabetes autoantibodies, and no classical features of T1DM or T2DM (not insulin-dependent or not requiring insulin treatment even after three years of insulin treatment, or having insulin reserve with serum c-peptide level >0.60 ng/mL) were sought from the clinics. Genetic analysis was performed with a presumptive prediagnosis of MODY. Patients had already had genetic analyses and they were genetically diagnosed.
Body mass index (BMI) values of the cases were evaluated according to the Neyzi et al. (6) data. adjusted for age and sex, and cases with BMI >95th percentile were considered obese. Ketonemia was defined as a blood ketone level of 1 mg/dL, and diabetic ketoacidosis (DKA) was defined as a blood glucose level above 200 mg/dL with blood pH <7.3 or HCO3 <15 mmol/L. Autoantibody status and reserve insulin values were recorded at diagnosis.
Mutation Interpretation
Genetic analyses of the cases were performed by next-generation sequencing and screening for 14 currently known mutations. Each patient was included in the study with the genetic diagnosis of MODY being made in the unit where they were followed.
MODY DNA sequence analysis panel was performed on each patient. All results were assessed by genetic specialist according to ACGM classification. Results, which were pathogenic or likely pathogenic were accepted as positive (7).
Statistical Analysis
Descriptive statistics were calculated for all patients taking part in the study using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA). Data were expressed as the mean±standard deviation (SD), median (minimum; maximum). The Kolmogorov-Smirnov test evaluated the normality of variables. Descriptive analyses are presented using means and SD for normally distributed variables. A p value <0.05 was assumed to indicate a statistically significant result in all analyses.
Results
General Features
A total of 224 cases were included in the study. Of these 101 (45%) were female, and the mean age at diagnosis was 9.4±4.1 years and ranged widely from 0.2 to 17 years. On MODY genotyping, 146 (65%) cases were diagnosed as GCK, 43 (19%) cases were diagnosed as HNF1A, 8 (3.6%) cases were diagnosed as HNF4A and a further eight with KLF11 while 7 (3.125%) patients had an HNF1B variant. In the remaining twelve cases there were three (1.3%) each with NEUROD1, INS and ABCC8 mutations while the last three had PDX (n=1), CEL (n=1) and KJNC11 (n=1) mutations, respectively (Figure 1).
Figure 1.
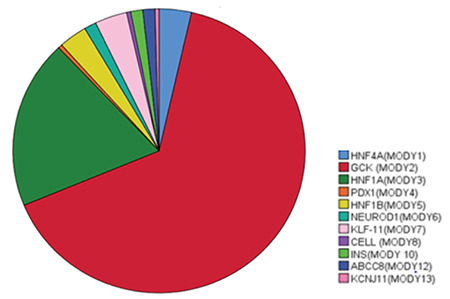
Distribution of gene variants found in the 224 MODY cases from a Turkish pediatric population
Most of the cases (n=197, 87.9%) were diagnosed with incidental hyperglycemia, while 16 (7%) had ketosis and 7 (3%) had DKA; 30% had diabetes symptoms. There were 200 cases (89%) with a family history of DM; 93 (41%) had diabetes in two generations and 84 (37%) had diabetes in three generations. Relatives of 162 patients with diabetes were under the age of 40 years. In 24 patients, no family history was found. A proportion of the patients with genetically diagnosed MODY presented with T1DM-associated autoantibodies, including 13 (5.8%) with anti-GAD, 8 (3.6%) with anti-islet and 4 (1.8%) with anti-insulin antibodies. These cases were in HNF4A, GCK, HNF1A, KLF11, KJNC11 MODY (type 1, 2, 3, 7, 12 MODY) types. Sixteen of the cases were obese. Clinical and laboratory characteristics of MODY types are given in Table 1.
Table 1. Demographic characteristics of pediatric genetically confirmed MODY cases in a Turkish cohort (n=224).
|
HNF4A n=8 |
GCK n=146 |
HNF1A n=43 |
PDX1 n=1 |
HNF1B n=7 |
NEUROD1 n=3 |
KLF-11 n=8 |
CEL n=1 |
INS n=3 |
ABCC8 n=3 |
KJNC11 n=1 |
Mean |
|
|
Age at diagnosis (years) |
10.3±4.6 |
8.8±3.9 |
10.6±4.3 |
3.9 |
12.4±3 |
9±7.8 |
11.1±2 |
6 |
9.1±2.3 |
10.5±1.8 |
8.5 |
9.4±4.1 (range 0.15-17) |
|
Sex F/M |
3/5 |
63/83 |
22/21 |
0/1 |
3/4 |
1/2 |
4/4 |
1/0 |
1/2 |
2/1 |
1 |
101/123 (45%/55%) |
|
Symptom (+) (-) |
6 2 |
23 123 |
5 18 |
1 0 |
5 2 |
0 3 |
7 1 |
0 1 |
0 3 |
1 2 |
1 0 |
30% (49/224) |
|
Presentation (n) IH DK DKA |
5 1 2 |
145 0 0 |
31 9 2 |
0 0 1 |
5 1 2 |
2 0 1 |
3 3 0 |
1 0 0 |
3 1 0 |
3 1 0 |
1 0 0 |
197 (87%) 16 (7%) 8 (3.5%) |
|
Negative family history (n) |
2 |
17 |
4 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
24/224 (11%) |
F/M: female/male, IH: incidental hyperglycemia, DK: diabetic ketosis, DKA: diabetic ketoacidosis, MODY: maturity onset diabetes of the young
While 158 (71%) of the patients were followed up with diet alone, 23 (11%) received intensive insulin therapy, 17 (7.6%) received sulfonylurea, 10 (4.5%) received metformin, and 6 (2.7%) received insulin and oral antidiabetic therapy (Table 2a, 2b). Seven patients presented with DKA or developed DKA during follow-up and these patients’ genotypes were: HNF4A (n=2, 0.89%), HNF1A (n=2, 0.89%), HNF1B (n=1, 0.44%), NEUROD (n=1, 0.44%), and ABCC8 (n=1, 0.44%). The clinical and laboratory characteristics of the patients who presented with DKA are shown in Table 3. An INS gene mutation was found in one patient who developed DKA during follow-up.
Table 2a. Treatment.
|
PDX1 n=1 |
HNF1B n=7 |
NEUROD1 n=3 |
KLF-11 n=8 |
CEL n=1 |
INS n=3 |
ABCC8 n=3 |
KJNC11 n=1 |
|
|
Literature |
D OAD (metformin, DPP4) Insulin |
Insulin |
D OAD Insulin |
OAD Insulin |
OAD Insulin |
D OAD Insulin |
OAD |
Diet OAD Insulin |
|
Treatment |
D: 1 |
D: 1 intensive insuline: 4 Basal ins+OAD: 1 |
D: 2 Intensive ins: 1 |
D: 1 M: 1 Intensive ins: 4 Basal ins+OAD: 2 |
D: 1 |
OAD: 1 Intensive ins: 1 |
OAD: 2 |
OAD: 1 |
OAD: oral anti-diabetic, M: metformin, DPP4: dipeptidyl peptidase-4 inhibitors, D: diet, ins: insuline
Table 2b. Treatment.
|
Treatment |
HNF4A n=8 |
GCK n=146 |
HNF1A n=43 |
|
Lecture |
Diet SU GLP-1 RA Insulin |
Diet |
SU Meglitinide, GLP-1 RA, SGLT-2 Insulin |
|
In our cases |
D: 4 SU: 2 Intensive ins: 2 |
D: 135 SU: 3 OAD (M): 3 Insulin: 2 Intensive ins: 3 |
D: 13 OAD (S): 11 OAD (M): 4 ins: 7 Basal ins+OAD: 2 |
OAD: oral anti-diabetic, M: metformin, D: diet, SU: sulphonylureas, GLP-1 RA: glucagon-like peptide 1 receptor agonist, SGLT-2: sodium-glucose transport protein 2 inhibitors, ins: insuline
Table 3. Characteristics of patients presenting with acidosis or developing acidosis during follow-up.
|
HNF4A-MODY n=2 |
HNF1A-MODY n=2 |
HNF1B-MODY n=1 |
NEUROD1-MODY n=1 |
INS-MODY n=1 |
|||
|
Sex |
M |
M |
M |
F |
M |
F |
F |
|
Age of diagnosis, years |
8.2 |
10 |
12 |
15 |
15 |
18 |
16.6 |
|
GAD |
Negative |
Negative |
Negative |
Negative |
Negative |
Negative |
Negative |
|
ICA |
No data |
Negative |
Negative |
Positive |
Negative |
Negative |
Negative |
|
Anti insulin |
Negative |
Negative |
Negative |
Negative |
Negative |
Negative |
Negative |
|
Family history |
No |
No |
Yes (grandfather) |
Yes (brother, grandmother, grandfather) |
Yes (father) |
Yes (father uncle, aunt) |
Yes Father |
|
Glucose at Dx (mg/dL) |
674 |
548 |
443 |
210 |
600 |
281 |
269 |
|
C-peptide (ng/mL) |
0.60 |
0.53 |
0.1 |
0.1 |
0.1 |
0.1 |
3.47 |
|
HbA1c (%) |
11.78 |
10.6 |
13.2 |
9 |
8 |
7.5 |
11.2 |
|
Treatment |
Int insulin the same treatment continued |
Int insulin after diet |
Int insulin the same treatment continued |
Int insulin the same treatment continued |
Int insulin the same treatment continued |
Int insulin the same treatment continued |
Int insulin the same treatment continued |
F: female, M: male, HbA1c: glycated hemoglobin, int: initially, MODY: maturity onset diabetes of the young
The characteristics of patients receiving intensive insulin treatment are shown in Table 4. Insulin treatment was needed in 35 (15%) of the patients at diagnosis or follow-up. Of these patients, 13 (5%) had HNF1A and 6 (2%) had HNF1B MODY.
Table 4. Characteristics of patients on insulin.
|
HNF4A |
GCK |
HNF1A |
HNF1B |
NEUROD1 |
KLF-11 |
INS |
ABCC8 |
Total |
|
|
Number of patients |
2 |
5 |
13 |
6 |
1 |
6 |
1 |
1 |
35 |
|
Features at presentation |
K: 1 DKA: 1 DM symptom at diagnose: 2 |
K: 0 DKA: 0 Diabet symptom: 2 |
K: 5 DKA: 2 Diabet symptom: 5 |
K: 1 DKA: 2 Diabet symptom: 6 |
K: 1 DKA: 1 Diabet symptom: 1 |
K: 3 DKA: 0 Diabet symptom: 6 |
K: 0 DKA: 0 Diabet symptom: 0 |
K: 0 DKA: 0 Diabet symptom: 0 |
|
|
HbA1c % |
11.7, 12.9 |
5.8, 6, 6.4, 7.2, 7.4 |
6, 7, 7.4, 8, 8.4, 8.8, 9, 11.30 12.5, 13.6, 14.6, 20 |
6, 7.5, 7.7, 8, 9.5, 17.5 |
7.4 |
6.8, 9.8, 10, 11.9 14.7, 15.6 |
11.2 |
5.8 |
K: ketosis, DKA: diabetic ketoacidosis, F: female, M: male, HbA1c: glycated hemoglobin, DM: diabetes mellitus
Genotype-phenotype Characteristics and Treatment According to MODY Types
GCK MODY
GCK mutation was found in 146 (65%) of 224 patients. The mean±SD age was 8.8±3.9 years. Although all of the GCK MODYs were diagnosed with incidental hyperglycemia, 23 of them had typical symptoms of T1DM. In 50% of the cases, there was a family history of diabetes in at least two generations. At the time of diagnosis, the mean blood glucose level was 125±21 mg/dL, and c-peptide was 1.4±1.3 mg/dL. HbA1c ranged from 5.4-10.2% with a mean of 6.3±0.3%. Anti-GAD (n=6), anti-insulin (n=1) and islet cell antibodies (n=5) were positive in some. Comorbidities included arachnoid cyst, short stature, asthma, cystic fibrosis, precocious puberty, connective tissue diseases, dyslipidaemia and undescended testis. While 135 patients were followed up with diet, six received oral anti-diabetic therapy, and five received intensive insulin treatment.
HNF1A MODY
Nearly a fifth (19.2%, n=43) of the patients had this mutation. The mean±SD age at diagnosis was 10.6±4.3 years. Typical symptoms of T1DM were present in five patients, and 31 patients presented with incidental hyperglycemia. Thirty-five patients had a positive family history. At diagnosis, mean blood glucose was 209±162 mg/dL, and c-peptide was 1.5±0.96 ng/mL. Mean HbA1c was 8.8±3.1%. Ten patients had autoantibody positivity. Comorbidities included exudative retinopathy (n=2), asthma (n=1), growth hormone deficiency (n=1), celiac disease (n=1), pelviectasis (n=1), hearing loss (n=2), and sensory neuropathy (n=1). Thirteen of the patients were on diet therapy, 15 were on oral anti-diabetic therapy and nine were on insulin therapy.
HNF4A MODY
This MODY subtype constituted 3.5% (n=8) of the cases. The mean age at diagnosis was 10.3±4.6 years. There was one case with a birth weight of over 4000 g. Five cases had incidental hyperglycemia, and six cases had typical symptoms of diabetes. Acidosis was present in two cases, and autoantibody positivity was observed in one case. At the time of diagnosis, mean blood glucose was 306±216 mg/dL, and c-peptide was 1.9±1.2 ng/mL. while HbA1c range was 4.7-11.7%. One patient was obese at diagnosis. The only comorbidity was spinocerebellar syndrome (n=3). While four of the patients were being managed with the diet, two were being treated with oral anti-diabetic therapy and two were being treated with insulin.
HNF1B MODY
This mutation was detected in 3.1% (n=7) of the cases. The mean age at diagnosis was 12.4±3 years. Five patients had incidental hyperglycemia, and five patients had symptoms of diabetes. Two patients presented with DKA. Six patients had a family history. At the time of diagnosis, blood glucose was 308±177 mg/dL, and c-peptide was 1.18±1.0 ng/mL. HbA1c range was 5.4-14.5%. No autoantibody positivity was found in any of the patients. Additional anomalies were chronic renal failure and elevated transaminases in one, focal segmental glomerulosclerosis in polycystic kidney in two, and renal cyst in one. Four of the patients were receiving insulin, one was on diet only and one was receiving oral anti-diabetic therapy and basal insulin.
KLF MODY
KLF MODY was present in 3.5% (n=8) of the patients. The mean age at diagnosis was 11.2±2.0 years. Three patients presented with incidental hyperglycemia, seven had symptoms of diabetes and three patients with diabetic ketosis. Family history was present in all cases. At diagnosis, blood glucose was 278±175 mg/dL, and c-peptide was 2.2±2.0 ng/mL. The range of HbA1c was 5.6-10.2%. Only one patient had autoantibodies (anti-GAD). One patient was on a diet, one on oral anti-diabetic therapy, two on oral anti-diabetic therapy and basal insulin, and four on intensive insulin treatment.
NEUROD1 MODY
NEUROD1 MODY was found in 1.3% (n=3) of the cases. Their age was 1, 12 and 15 years old and one patient presented with DKA. No autoantibody positivity was detected. Additional anomalies included epilepsy in one patient and mental motor retardation (MMR) in one. At diagnosis, blood glucose was 164±100 mg/dL and c-peptide was 0.8±0.7 mg/dL. HbA1c was in the range of 5.5-8%. Family history was not found in any patient. Two patients were on a diet, and one received intensive insulin treatment.
INS MODY
This MODY subtype was found in 1.3% (n=3) cases. Their ages were 7.5, 7.8 and 11 years old. Two patients had no symptoms of diabetes, and one patient presented with DKA. All three patients had a family history. Acidosis developed in one patient during follow-up. No autoantibody positivity was detected. At the time of diagnosis, blood glucose was 200±64 mg/dL, and c-peptide was 8.5±4.3 mg/dL. HbA1c ranged from 6.2 to 14%. MMR was associated with one patient. One patient was on a diet, and two were receiving intensive insulin.
ABCC8 MODY
This subtype of MODY was found in 1.3% (n=3) of the cases. Their age was 8.5, 11 and 12 years and diabetes symptoms were absent in two. Family history was present in all patients, and one patient was positive for autoantibodies. At diagnosis, blood glucose was 140±26 mg/dL, and c-peptide was 2.4±0.58 mg/dL. Two of the patients were taking oral anti-diabetic therapy and the other was managed with diet.
PDX MODY
A 3.9-year-old patient presented with DKA. Family history was positive. No autoantibodies were detected. Admission HbA1c was 5.2%. Blood glucose was 135 mg/dL, and c-peptide was 3.4 mg/dL at the time of diagnosis. He was being managed with diet only.
CEL MODY
CEL variant associated MODY was detected in one patient, 6 years old, who presented with incidental hyperglycemia. There was no family history. Insulin was not started during the follow-up. Autoantibodies were negative and HbA1c was 5.6%. At diagnosis, blood glucose was 137 mg/dL, and c-peptide was 0.7 mg/dL. He was managed with diet only.
KJNC11 MODY
This form of MODY was detected in one case who was 8.5 years old. Symptoms of diabetes and autoantibody positivity were present at presentation. There was a family history of diabetes. HbA1c was 7.8%, and insulin was not started during follow-up. At the time of diagnosis, blood glucose was 191 mg/dL, and c-peptide was 2.1 mg/dL. The patient was followed up with oral anti-diabetic therapy.
Of the 224 patients evaluated, eight patients presented with acidosis. These cases were limited to the MODY types 1, 3, 5, and 6 and their detailed characteristics are shown in Table 3. DKA developed in one patient with INS mutation during follow-up. Table 5 shows the characteristics of the patients with positive autoantibodies.
Table 5. Cases with autoantibody positivity.
|
HNF4A n=8 |
GCK n=146 |
HNF1A n=43 |
PDX1 n=1 |
HNF1B n=7 |
NEUROD1 n=3 |
KLF-11 n=8 |
CELL n=1 |
INS n=3 |
ABCC8 n=3 |
KJNC11 n=1 |
Total |
|
|
Anti-GAD Anti-insulin Anti-islet |
Anti-GAD: 1 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 6 Anti-insulin: 1 Anti-islet: 5 |
Anti-GAD: 4 Anti-insulin: 3 Anti-islet: 3 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 1 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 1 Anti-insulin: 0 Anti-islet: 0 |
Anti-GAD: 0 Anti-insulin: 0 Anti-islet: 0 |
13 4 8 |
|
1 |
12 |
10 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
25 |
Discussion
In this study, which is the largest genetically diagnosed series from Turkey, the most common form of MODY was GCK-MODY (65%) followed by HNF1A-MODY (19%), while the other types were much rarer. Although the majority of cases were diagnosed because of incidental hyperglycemia, there were also cases with DKA and ketosis without a family history. Obesity was rarely associated with MODY, while comorbidities were seen in some MODY types.
MODY is reported with different frequencies depending on the ethnicity, selected patient population, number of genes analyzed and technical characteristics. The distribution of subgroups may also differ according to the method, environment, and technique. In the present study, which presents a representation of several centers from across Turkey, MODY2 (GCK-MODY) was the most common diagnosis, as reported previously (7, 8). While GCK mutations were found incidentally, as expected, diabetes symptoms were observed in patients with HNF1A and HNF4A variants. Treatment was changed in 37 (16%) of our patients after genetic diagnosis or during follow-up (5, 7, 8, 9, 10, 11).
In the largest series from Germany/Austria, MODY1 was found in 44, MODY2 in 609, MODY3 in 230, and MODY5 in 35 of 1047 MODY cases from 76,836 children under 20 years of age with diabetes (12). In the UK, MODY prevalence and subtype studies have been performed extensively and it has been reported that HNF1A-MODY is the most common type (52%) with GCK-MODY the second most common. While MODY due to HNF1A mutation is most common in European countries (13), GCK-MODY is the most common type in Japan, although there are few studies from Asian and Far Eastern countries (14).
Studies from Italy have shown that the selected patient population is important in determining the frequency of the MODY subtype. In the MODY subgroup in which patients with symptomatic hyperglycemia were evaluated, HNF1A- and HNF1B-MODY were most prevalent, while in the group with incidental hyperglycemia, GCK-MODY was most common. This is also a factor in the difference in frequency of MODY subtypes in adult and pediatric studies. For an accurate ranking, both age groups should be evaluated together (15).
Family history has been used as an essential criterion in MODY probability calculation models. This probability calculation reduces the probability of MODY in the absence of family history, but case reports and the present series show that there may be some exceptional cases. In a large series reported from Japan, 18% of the cases with genetically proven MODY did not have a family history (14). Furthermore, novel mutation was demonstrated in 7.3% of patients with the four most common MODY subtypes reported from Slovakia (16). In our series, the de novo mutation rate was 11%.
The use of anti-GAD positivity as a criterion for exclusion of MODY is a matter of debate. In a large series, anti-GAD was found to be positive in only 5 of 508 MODY cases, and this rate was similar to the normal population rate. However, in a Czech study, anti-GAD positivity was found in 7 of 28 MODY cases, and IAC2 positivity was found in one of 28 MODY cases. In these patients, the course of diabetes was worse, and HbA1c values were higher. Autoantibody negativity has been reported with appropriate therapeutic control of diabetes. Schober et al. (17) found a positive autoantibody rate of 17% in a German-Austrian series (18, 19). In the present study, autoantibodies were positive in 25 patients. This may be due to a condition termed “double diabetes”. Polygenic diabetes has been reported to affect 3-4% of MODY cases, and the coexistence of T2DM and MODY is more common than T1DM and MODY (20). In the present series, only three patients with GCK mutation had increased HbA1c values and insulin requirement during follow-up. In a Polish study, increased HbA1c values were also reported in patients with GCK mutation. This was explained by T1DM or T2DM accompanying monogenic diabetes. Thus, monogenic DM does not preclude the existence of other types. In 8 of 285 cases with GCK mutation, HbA1c values above 7.5% were reported. Steele et al. (21) also found HbA1c values between 8.2-9.5% in 6 of 235 cases with GCK mutation.
Although obesity is used to differentiate cases with T2DM from MODY, obesity associated with rare types of MODY, such as those caused by variants in IPF1/PDX1 (MODY 4), NEUROD1 (MODY 6), BLK (MODY 11) and ABCC8 (MODY 12), and even common types (HNF1A- and HNF4A-MODY) have been reported in recent years. Therefore, obesity in MODY may be more common than previously thought, probably due to the general increase in global obesity prevalence (14). In the present series, obesity was found in 16 (7%) cases at diagnosis. In a Japanese study, the rate of overweight was 8.2%, and insulin resistance was 22% (21).
While MODY due to variants in HNF4A, HNF1A and HNF1B may present with DKA, INS, NEUROD1 and PDX, which are rare types, may also present with DKA (20). Consistent with the literature, our patients also had these mutations and presented with acidosis due to insulin deficiency. It seems that using acidosis as an exclusion criterion for MODY may exclude some cases.
The criteria of three generations affected with diabetes, autoantibodies being negative, and insulin reserve present do not always hold true. In the present series, autoantibodies were positive in 25 patients, seven patients presented with DKA, and no family history was found in 24 patients. Approximately 50% of positive MODY cases do not fulfill the classical MODY diagnostic criteria; some patients with genetically confirmed MODY may also have pancreatic antibodies, and some de novo mutations may have occurred (21).
Over time and with more extensive research, knowledge of the natural history of MODY has increased. The ability to partially or completely change the treatment of people diagnosed with MODY from insuline to oral hypoglycaemic agents has saved patients from injections and unnecessary insulin therapy. However, while GCK mutations are followed up only with diet, PDX, INS, BLK and APPL1 variant-associated MODY cases require early insulin, and, HNF4A, HNF1A and PAX4 defects may also require oral antidiabetic or insulin. MODY due to ABCC8 and KCNJ11 mutations respond well to oral antidiabetics. In HNF1B, NEUROD1, KLF11 and CEL mutations, the response to oral antidiabetics is variable and insulin requirement develops over time (22). In our series, treatment changes were made in 37 patients during follow-up. While 71% of the patients were followed up only by adjusting their diet, 10% were receiving intensive insulin treatment, and 7% were receiving sulfonylurea.
Strengths of the present study include the high number of cases compared to early studies from Turkey, the detailed information about clinical characteristics of the identified cases and that this was a nationally supported study.
Study Limitations
However, the limitations include the fact that not all centers participated, the MODY panels used varied from center to center and the interpretation of the MODY reports were performed by several geneticists.
Conclusion
This large-series national study showed that the diagnostic criteria in the prediagnostic process of MODY should be reconsidered in the presence of DKA, the presence of antibody positivity and the absence of family history in patients with genetically diagnosed MODY in the Turkish population. Therefore, it does not seems appropriate to use obesity and/or the absence of DKA as absolute criteria for suggesting that a patient does not have MODY, and this will need to be considered in the development of updated MODY screening guidelines.
Ethics
Ethics Committee Approval: Ethical approval was obtained from Ankara University Faculty of Medicine (decision number: 12-112-20, date: 13.02.2020).
Informed Consent: Informed consent was obtained from the parents/guardians of the patient from all centers.
Acknowledgments
The authors thank the children and parents for participating in the study. The authors thank to Jeremy Jones for editing.
Footnotes
Authorship Contributions
Concept: Elif Özsu, Zehra Aycan, Merih Berberoğlu, Zeynep Şıklar, Design: Elif Özsu, Zehra Aycan, Zeynep Şıklar, Data Collection or Processing: Elif Özsu, Semra Çetinkaya, Semih Bolu, Nihal Hatipoğlu, Şenay Savaş Erdeve, Olcay Evliyaoğlu, Firdevs Baş, Atilla Çayır, İsmail Dündar, Emine Demet Akbaş, Seyid Ahmet Uçaktürk, Merih Berberoğlu, Zeynep Şıklar, Şervan Özalkak, Nursel Muratoğlu Şahin, Melikşah Keskin, Ülkü Gül Şiraz, Hande Turan, Ayşe Pınar Öztürk, Eda Mengen, Elif Sağsak, Fatma Dursun, Nesibe Akyürek, Sevinç Odabaşı Güneş, Zehra Aycan, Analysis or Interpretation: Elif Özsu, Zehra Aycan, Merih Berberoğlu, Zeynep Şıklar, Literature Search: Elif Özsu, Writing: Elif Özsu, Zehra Aycan, Zeynep Şıklar.
Conflict of interest: None declared.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Tattersall RB. Mild familial diabetes with dominant inheritance. Q J Med. 1974;43(170):339–357. [PubMed] [Google Scholar]
- 2.Shields B, Colclough K. Towards a systematic nationwide screening strategy for MODY. Diabetologia. 2017;60(4):609–612. doi: 10.1007/s00125-017-4213-7. [DOI] [PubMed] [Google Scholar]
- 3.Irgens HU, Molnes J, Johansson BB, Ringdal M, Skrivarhaug T, Undlien DE, Søvik O, Joner G, Molven A, Njølstad PR. Prevalence of monogenic diabetes in the population-based Norwegian Childhood Diabetes Registry. Diabetologia. 2013;56(7):1512–1519. doi: 10.1007/s00125-013-2916-y. [DOI] [PubMed] [Google Scholar]
- 4.Ozsu E, Cizmecioglu FM, Yesiltepe Mutlu G, Yuksel AB, Calıskan M, Yesilyurt A, Hatun S. Maturity Onset Diabetes of the Young due to Glucokinase, HNF1-A, HNF1-B, and HNF4-A Mutations in a Cohort of Turkish Children Diagnosed as Type 1 Diabetes Mellitus. Horm Res Paediatr. 2018;90:257–265. doi: 10.1159/000494431. [DOI] [PubMed] [Google Scholar]
- 5.Gökşen D, Yeşilkaya E, Özen S, Kor Y, Eren E, Korkmaz Ö, Berberoğlu M, Karagüzel G, Er E, Abacı A, Evliyaoğlu O, Akbaş ED, Ünal E, Bolu S, Nalbantoğlu Ö, Anık A, Tayfun M, Büyükinan M, Abalı S, Can Yılmaz G, Kor D, Söbü E, Şıklar Z, Polat R, Darcan Ş. Molecular Diagnosis of Monogenic Diabetes and Their Clinical/Laboratory Features in Turkish Children. J Clin Res Pediatr Endocrinol. 2021;13:433–438. doi: 10.4274/jcrpe.galenos.2021.2021.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neyzi O, Bundak R, Gökçay G, Günöz H, Furman A, Darendeliler F, Baş F. Reference Values for Weight, Height, Head Circumference, and Body Mass Index in Turkish Children. J Clin Res Pediatr Endocrinol. 2015;7:280–293. doi: 10.4274/jcrpe.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Özdemir TR, Kırbıyık Ö, Dündar BN, Abacı A, Kaya ÖÖ, Çatlı G, Özyılmaz B, Acar S, Koç A, Güvenç MS, Kutbay YB, Erdoğan KM. Targeted next generation sequencing in patients with maturity-onset diabetes of the young (MODY). J Pediatr Endocrinol Metab. 2018;31:1295–1304. doi: 10.1515/jpem-2018-0184. [DOI] [PubMed] [Google Scholar]
- 9.Haliloglu B, Hysenaj G, Atay Z, Guran T, Abalı S, Turan S, Bereket A, Ellard S. GCK gene mutations are a common cause of childhood-onset MODY (maturity-onset diabetes of the young) in Turkey. Clin Endocrinol (Oxf) 2016;85(3):393–399. doi: 10.1111/cen.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ağladıoğlu SY, Aycan Z, Çetinkaya S, Baş VN, Önder A, Peltek Kendirci HN, Doğan H, Ceylaner S. Maturity onset diabetes of youth (MODY) in Turkish children: sequence analysis of 11 causative genes by next generation sequencing. J Pediatr Endocrinol Metab. 2016;29:487–496. doi: 10.1515/jpem-2015-0039. [DOI] [PubMed] [Google Scholar]
- 11.Karaoglan M, Nacarkahya G. Clinical and laboratory clues of maturity-onset diabetes of the young and determination of association with molecular diagnosis. J Diabetes. 2021;13:154–163. doi: 10.1111/1753-0407.13097. [DOI] [PubMed] [Google Scholar]
- 12.Warncke K, Kummer S, Raile K, Grulich-Henn J, Woelfle J, Steichen E, Prinz N, Holl RW. Frequency and Characteristics of MODY 1 (HNF4A Mutation) and MODY 5 (HNF1B Mutation): Analysis From the DPV Database. J Clin Endocrinol Metab. 2019;104(3):845–855. doi: 10.1210/jc.2018-01696. [DOI] [PubMed] [Google Scholar]
- 13.Nkonge KM, Nkonge DK, Nkonge TN. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin Diabetes Endocrinol. 2020;6(1):20. doi: 10.1186/s40842-020-00112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorifuji T, Higuchi S, Kawakita R, Hosokawa Y, Aoyama T, Murakami A, Kawae Y, Hatake K, Nagasaka H, Tamagawa N. Genetic basis of early-onset, maturity-onset diabetes of the young-like diabetes in Japan and features of patients without mutations in the major MODY genes: Dominance of maternal inheritance. Pediatr Diabetes. 2018;19(7):1164–1172. doi: 10.1111/pedi.12714. [DOI] [PubMed] [Google Scholar]
- 15.Lorini R, Klersy C, d'Annunzio G, Massa O, Minuto N, Iafusco D, Bellannè-Chantelot C, Frongia AP, Toni S, Meschi F, Cerutti F, Barbetti F, Italian Society of Pediatric Endocrinology and Diabetology (ISPED) Study Group. Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care. 2009;32(10):1864–1866. doi: 10.2337/dc08-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanik J, Dusatkova P, Cinek O, Valentinova L, Huckova M, Skopkova M, Dusatkova L, Stanikova D, Pura M, Klimes I, Lebl J, Gasperikova D, Pruhova S. De novo mutations of GCK, HNF1A and HNF4A may be more frequent in MODY than previously assumed. Diabetologia. 2014;57:480–484. doi: 10.1007/s00125-013-3119-2. [DOI] [PubMed] [Google Scholar]
- 17.Schober E, Holl RW, Grabert M, Thon A, Rami B, Kapellen T, Seewi O, Reinehr T. Phenotypical aspects of maturity-onset diabetes of the young (MODY diabetes) in comparison with Type 2 diabetes mellitus (T2DM) in children and adolescents: experience from a large multicentre database. Diabet Med. 2009;26(11):466–473. doi: 10.1007/s00431-005-1709-9. [DOI] [PubMed] [Google Scholar]
- 18.McDonald TJ, Colclough K, Brown R, Shields B, Shepherd M, Bingley P, Williams A, Hattersley AT, Ellard S. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med. 2011;28:1028–1033. doi: 10.1111/j.1464-5491.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 19.Urbanová J, Rypáčková B, Procházková Z, Kučera P, Cerná M, Anděl M, Heneberg P. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med. 2014;31:466–471. doi: 10.1111/dme.12314. [DOI] [PubMed] [Google Scholar]
- 20.Fendler W, Małachowska B, Baranowska-Jazwiecka A, Borowiec M, Wyka K, Malecki MT, Jarosz-Chobot P, Mysliwiec M, Mlynarski W. Population-based estimates for double diabetes amongst people with glucokinase monogenic diabetes, GCK-MODY. Diabet Med. 2014;31:881–883. doi: 10.1111/dme.12449. [DOI] [PubMed] [Google Scholar]
- 21.Steele AM, Wensley KJ, Ellard S, Murphy R, Shepherd M, Colclough K, Hattersley AT, Shields BM. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS One. 2013;8:e65326. doi: 10.1371/journal.pone.0065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aarthy R, Aston-Mourney K, Mikocka-Walus A, Radha V, Amutha A, Anjana RM, Unnikrishnan R, Mohan V. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY) - A review. J Diabetes Complications. 2021;35:107640. doi: 10.1016/j.jdiacomp.2020.107640. [DOI] [PubMed] [Google Scholar]


