Abstract
Introduction
Over half of patients who spend >48 hours in the intensive care unit (ICU) are fed via a nasogastric (NG) tube. Current guidance recommends continuous delivery of feed throughout the day and night. Emerging evidence from healthy human studies shows that NG feeding in an intermittent pattern (rather than continuous) promotes phasic hormonal, digestive and metabolic responses that are important for effective nutrition. It is not yet known whether this will translate to the critically ill population. Here, we present the protocol for a proof-of-concept study comparing diurnal intermittent vs continuous feeding on hormonal and metabolic outcomes for patients in the ICU.
Methods and analysis
The study is a single-centre, prospective, randomised, open-label trial comparing intermittent enteral nutrition with the current standard practice of continuous enteral feeding. It aims to recruit participants (n=30) needing enteral nutrition via an NG tube for >24 hours who will be randomised to a diurnal intermittent or a continuous feeding regimen with equivalent nutritional value. The primary outcome is peak plasma insulin/c-peptide within 3 hours of delivering the morning intermittent feed on the second study day, compared with that seen in the continuous feed delivery group at the same time point. Secondary outcomes include feasibility, tolerability, efficacy and metabolic/hormonal profiles.
Ethics and dissemination
We obtained ethical approval from the Wales Research Ethics Committee 3 prior to data collection (reference 23/WA/0297). We will publish the results of this study in an open-access peer-reviewed journal.
Trial registration number
Keywords: Adult intensive & critical care, NUTRITION & DIETETICS, Randomized Controlled Trial, INTENSIVE & CRITICAL CARE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The intermittent feeding intervention in this study has been designed to replicate typical human feeding patterns and to replicate diurnal rhythms, with a 13-hour nightly fasting period between daytime feeding cycles.
Intensive hourly blood sampling around the time of a meal will enable us to create a detailed profile of metabolic hormones for continuous and intermittent feeding regimens.
As intensive blood samples are taken around only one feed, we may fail to detect differences in metabolism present at other time points during a course of enteral feeding.
The eligibility criteria are broad, which should facilitate a sample representative of the intensive care unit patient population.
This is an open-label, single-centre study, which may have introduced bias and limit the generalisability of the results.
Introduction
In the UK, around 200 000 patients are admitted to critical care units annually (icnarc.org), and between 30% and 50% of those patients are malnourished at the time of admission.1 International guidelines emphasise the importance of providing early adequate enteral nutrition (EN) for critically ill patients.2,4 Approximately, half of these critically ill patients will be fed via a gastric tube because they are unable to feed themselves for a prolonged period.5 There is uncertainty about the optimal enteral feeding regimen in these critically ill patients and nutritional targets are frequently missed.6
The current standard of care is continuous delivery of feed, throughout the day and night. This feeding pattern may be unphysiological, both in the sense that it fails to trigger acute mealtime metabolic/hormonal and gastrointestinal responses and that there are none of the usual postprandial periods aligned with circadian rhythms in metabolism.7 Other feed patterns have been described, such as intermittent feeding, where feed is delivered in divided doses over a period of between 20 min to an hour, with breaks of several hours in between.7 The existent recommendation in favour of continuous gastric feeding is based on the results of meta-analyses suggesting intermittent administration increases the risk of adverse gastrointestinal sequelae including diarrhoea, vomiting, constipation, abdominal distension and aspiration.4 8 However, the evidence synthesised in these meta-analyses was deemed to be of low quality, with small numbers of participants.
Several studies of intermittent feeding have been conducted in the intensive care unit (ICU). These have been relatively small, each recruiting fewer than 200 patients and have failed to demonstrate improvements in morbidity or mortality.7 9 10 These intermittent feeding regimens frequently continue to deliver food during the night or do not include a prolonged fasting period which may diminish the potential benefits.10,16 Primary outcomes have focused on measures of gastrointestinal tolerance while largely neglecting potentially important hormonal, metabolic, circadian, sleep or delirium outcomes.7 9 17 Only a few of these studies have published mortality and length of stay data as secondary outcomes, and none have assessed longer-term functional patient-centred outcomes. A recent review of the research agenda for nutrition in the ICU cited the need for phase II trials to study the effects of different feeding patterns on biological markers of metabolism with a view to a phase III randomised controlled trial (RCT) focusing on mortality and physical function as the main outcomes.2
Diurnal feeding describes the alignment of wake/light cycles with feeding and sleep/dark cycles with fasting. There are several reasons why diurnal intermittent feeding might be beneficial for critically ill patients, compared with the usual continuous administration of nasogastric (NG) feed. Of particular interest are the potential metabolic benefits of intermittent diurnal feeding. Evidence from animal studies highlights the importance of circadian rhythms in regulating digestion and metabolism.18,20 Hormonal secretion, regulated by the circadian clock, affects the capacity for glucose, protein and lipid metabolism.7 21 Critically ill patients are particularly susceptible to dysregulation of the circadian rhythm, which is influenced heavily by exogeneous cues (zeitgebers) such as the timing of nutritional intake.22 23
Studies in humans have shown that the patterns of feeding/fasting cycles affect metabolism. Muscle protein synthesis—of great importance in the critically ill population—is stimulated more effectively by ‘pulsed’ ingestion of protein than a continuous supply of amino acids. This is thought to be due to the ‘leucine trigger hypothesis’, where a critical dose of protein is required to maximise anabolism.24,26 In health, with normal oral intake of meals, metabolic hormones such as insulin and ghrelin are released in a pulsatile manner in response to feeding.27 This pattern is maintained with intermittent gastric feeding in healthy adults, but lost with continuous feeding, with important implications for skeletal muscle autophagy and gut motility.28 Intermittent feeding was also shown to increase splanchnic blood flow, which may be beneficial for gastrointestinal tolerance of EN.28 Our deep phenotyping study investigating the metabolic and immune consequences of intermittent versus continuous NG feed delivery in healthy volunteers has highlighted a loss of the typical patterns of circulating glucose, fatty acid, triglycerides and urea, as well as a loss of the normal diurnal variation in insulin and glucagon-like peptide-1, alongside modulation of neutrophil metabolism, when feeding is delivered continuously.29 A study in critically ill patients found intermittent feeds resulted in a lower insulin requirement, with no additional risk of dysglycaemia.30
From a more pragmatic perspective, there are other potential benefits to intermittent feeding as continuous gastric feeding imposes restrictions on patient mobility and has to be interrupted for procedures or investigations. The frequency of these pauses in continuous feeding may explain why intermittent feeding has been shown to help reach targets for enteral calories earlier than continuous feeding.10 12 Additionally, a meta-analysis suggests that although intermittent feeding carries an increased risk of diarrhoea, this was balanced by a reduced incidence of constipation, with no difference in other gastrointestinal outcomes between the groups.31
These plausible beneficial effects of intermittent feeding, together with the proposed benefits of diurnal feeding (alignment of feed with the circadian clock during wake/light cycles), may improve tolerance to and recovery from critical illness. Optimising the delivery of nutrition to critically ill patients has the potential to provide several benefits: improved metabolic function with maintained insulin sensitivity; reduced catabolism and sarcopenia, which would hasten rehabilitation and improve long-term functional status; altered immune response to improve outcomes in sepsis and better entrainment of circadian rhythms with improved sleep/wake cycles, potentially resulting in reduced delirium.10 23 32 These benefits for patients could include a shorter, less complicated, recovery from critical illness and lower mortality. There are potential cost savings in shortened ICU and hospital stay. Crucially, implementation would not involve new drugs or technology and be straightforward and cost effective to implement as well as potentially saving staff time.
The DINE-N study aims to provide evidence to assess whether diurnal intermittent rather than continuous feed is advantageous. The study will focus on hormonal and metabolite profiles in response to intermittent versus continuous gastric feeds. The primary outcome will be peak plasma insulin within 3 hours of an intermittent feed compared with the equivalent time points in continuously fed patients. Insulin was chosen as the primary outcome because it is a pivotal hormone in metabolism, influencing anabolism/catabolism in the fed state, skeletal muscle autophagy, cellular energy supply and glycaemic control.33 To differentiate endogenous insulin secretion from exogenous insulin administration, we will simultaneously measure C-peptide.
Aim & objectives
The aim of this project is to establish whether intermittent feeding with overnight fasting, compared with the current standard of care in critically ill patients, produces an equivalent response in physiological, hormonal and metabolic responses to that seen in healthy volunteers. The research objectives will be to establish the clinical feasibility, tolerability and efficacy of intermittent diurnal feeding in critically ill adults.
Methods and analysis
Trial design
The study will be a prospective, parallel-group, randomised, open-label trial (prospectively registered at ClinicalTrials.gov: NCT06115044). The protocol for this study has been reported according to the Standard Protocol Items: Recommendations for Interventional Trials guidelines (online supplemental appendix 1).34 The setting is the mixed ICU of Southmead Hospital, Bristol, a 996-bed teaching hospital in the southwest of England, and a major trauma centre serving an adult population of approximately 2.3 million. The ICU has 48 beds with approximately 2500 admissions annually. The study is planned to open to recruitment in November 2023 and close to recruitment in May 2024.
Patient and public involvement
A patient and public involvement (PPI) group was involved in the design of the study protocol. They informed key decisions regarding the acceptability of the intervention; blood sampling to answer the research question and emergency waiver consent approach. One PPI representative will be included as part of the trial oversight committee to review the study conduct and outcomes.
Population
The study will recruit a population of critically ill adult patients who are anticipated to require prolonged feeding via a gastric tube (>48 hours). Participants will be recruited within 24 hours of starting EN.
Patients must meet all the inclusion and none of the exclusion criteria as listed below.
Inclusion criteria:
Adults (≥18) on intensive care.
Planned for gastric EN (anticipated duration >48 hours).
Exclusion criteria:
>24 hours since starting EN.
Parenteral or jejunal nutrition.
Trophic feed only (eg, lactate >4).
High risk of refeeding syndrome.35
Gastrointestinal surgery or pathology.
Diabetic emergencies.
Pregnancy.
Prone positioning.
Trial intervention
The intervention is an adjustment to the normal pattern of delivery of gastric feed to deliver intermittent feeds (see figure 1). The intervention period of 48 hours will start with the initiation of the overnight fast on day 0 (19:00 hours) and will end on day 2. Feeds will be given at 8:00, 13:00 and 18:00 hours each day via a volumetric pump over a period of 30–60 min. On study day 1, each feed will be 200 mL (600 mL/24 hours). On study day 2, each feed will be one-third of individual daily caloric requirements, established using a weight-based equation set by specialist intensive care dieticians following the local nutrition guideline (online supplemental appendix 2).4 The feed type will be Nutrison Protein Plus (Nutricia, UK).
Figure 1. Feeding and sampling timeline.
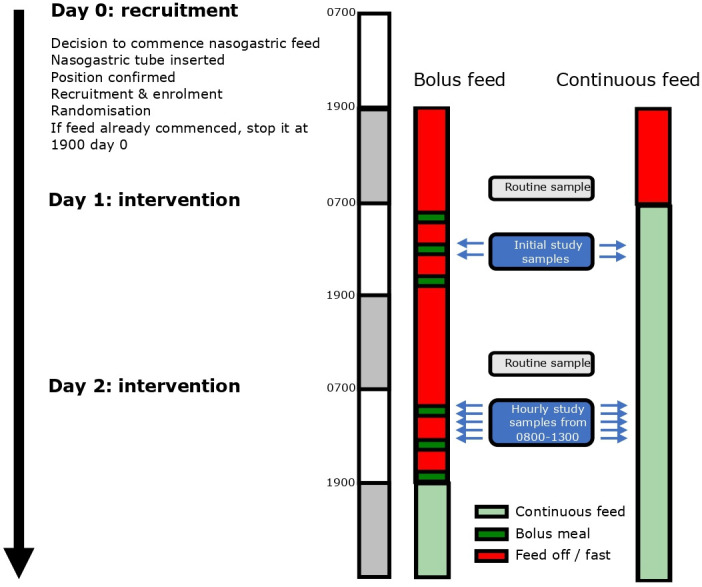
Patients in the diurnal intermittent feeding group will restart the usual local continuous enteral feeding regimen at 12:00 on day 3 to prevent overfeeding. There will be a further 12 hours of monitoring for adverse events potentially attributable to the intervention.
Comparator
The comparator group will have a continuous enteral feeding regimen via volumetric pump using Nutrison Protein Plus. On day of study enrolment (day 0), the continuous feed will stop at 19:00 then restart at 08:00 on study day 1 at 25 mL/hour (600 mL/24 hours), equivalent in calorie delivery and 24-hour volume to the intervention group for study day 1. At 08:00, on study day 2, the rate of continuous feeding will be adjusted to individual daily caloric requirements, established using a weight-based equation set by specialist intensive care dieticians following the local nutrition guideline (online supplemental appendix 2). At the end of the 48-hour intervention period, these patients will continue on the usual local continuous enteral feeding regimen.
Patients in either group who are either hyperkalaemic or fluid-restricted in the absence of renal replacement therapy will have the same calorie target but will receive a reduced volume of feed using Nutrison Concentrated (Nutricia, UK).
Outcomes
Primary outcome: The primary outcome is peak plasma insulin (and c-peptide) within 3 hours of a feed compared with the equivalent time in the continuous feed group. This will be measured for the first feed (8:00) on day 2 of the study (samples taken hourly between 8:00 and 13:00 to allow peak identification).
Secondary outcomes include:
-
Endocrine and metabolic (all blood plasma)
Glucose.
Ketones (beta-hydroxybutyrate).
Urea.
GLP-1.
Non-esterified fatty acids.
Triglyceride.
Glycerol.
-
Feasibility
% target nutrition achieved (per 24-hour period).
Absolute calories delivered.
Protocol compliance.
-
Tolerability
Episodes of vomiting/24-hour period.
Episodes of aspiration of feed.
Delayed gastric emptying (Gastric Residual Volume >250 mL×2 in a 24-hour period).
Ileus.
Diarrhoea (passage of type 6 or 7 stool according to the Bristol Stool Chart or >3 stool/24 hours).
Constipation.
-
Efficacy
ICU and hospital length of stay.
ICU and hospital mortality.
Delta-SOFA (Sequential Organ Failure Assessment) between day 0 and day 2.
The schedule of assessments is provided in online supplemental appendix 3.
Management of feed delivery
The study will use a standardised regimen from the local nutrition guideline for the assessment and management of gastric residual volumes (online supplemental appendix 2). The timing of gastric residual volume checks differs slightly from the guideline to account for the diurnal intermittent feeding pattern. During the intervention period, residual volumes will be checked at 08:00, 13:00 and 18:00 hours with further checks overnight at 22:00, 02:00 and 06:00 hours. Management of nausea, vomiting, diarrhoea, aspiration, ileus or constipation associated with EN will be at the discretion of the treating intensivist.
If feed is interrupted or delayed (eg, in the case of transfer for imaging or surgery) the feeding regimen will be adjusted:
Intermittent diurnal feed: if there are 2 hours or more until the next scheduled feed the missed feed will be given in entirety followed by the next feed at the scheduled time. If there are less than 2 hours until the next scheduled feed, the missed feed will be given in full, followed by a 1-hour gap and then the next scheduled feed.
Continuous feed: the feed rate will be increased to compensate for the hours missed to achieve the prescribed 24-hour target.
Concomitant medication
There is no restriction on concomitant medication. Enteral feed may interact with the absorption of some medications including certain antiretrovirals and antibiotics, antiepileptics and immunosuppressants. Daily medication review by a pharmacist is routine in the ICU. Alternative routes of administration and therapeutic drug monitoring will be undertaken as advised.
Assessment of compliance
The routinely collected feeding administration record for each patient will be used to assess compliance with the intervention.
Blood sampling
The study primary and several secondary outcomes will be measured from blood samples taken on study day 2 from 08:00 (before the start of the morning feed in the diurnal intermittent group), and at hourly intervals until and including 13:00, just before the start of the next feed in the diurnal intermittent group (see figure 1). Blood samples will be taken from an indwelling catheter (10 mL per sample, arterial line or central venous line) then immediately distributed into an EDTA (ethylenediaminetetraacetic acid) and serum collection tube. All samples will be centrifuged at 3461×g for 10 min for removal of the plasma or serum supernatant then frozen at −80°C pending analysis.
Analysis of samples will use standard techniques and commercially available assay kits for the relevant primary and secondary outcomes.
Baseline data
Baseline demographic and health-related data will be collected as follows:
Sex.
Ethnicity.
Age.
Weight.
Height.
Severity of illness (Acute Physiology and Chronic Health Evaluation II (APACHE II) and Intensive Care National Audit and Research Centre (ICNARC) physiology score).
Organ failure assessment (SOFA score).
Primary reason for admission to ICU.
Time of admission.
Time of gastric tube insertion.
Time enteral feeding started prior to enrolment.
Time of enrolment.
Presence/absence of diabetes (including type if present).
Medication (insulin, oral hypoglycaemic agents, statins).
Assignment to intervention
The study flow diagram is illustrated in figure 2. Participants will be randomly allocated into two groups to receive either diurnal intermittent or continuous feed for the following 48 hours with regular monitoring. Participants will be randomised in a 1:1 ratio to intervention or control group, stratified by sex, using sealed opaque envelopes. The National Cancer Institute Clinical Trial Randomization Tool was used by a member of the ICU team independent from the study to generate the allocation sequence (https://ctrandomization.cancer.gov/). Eligibility will be confirmed, and randomisation performed, by an appropriately trained healthcare professional on the study delegation log. Participants are screened for eligibility 7 days a week, helping to achieve an adequate rate of participant enrolment.
Figure 2. Study flowchart.
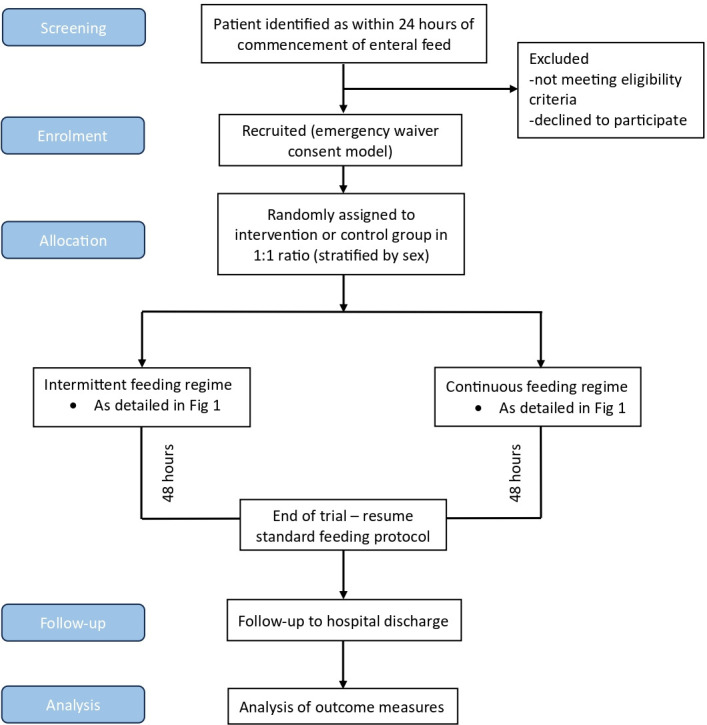
The study is open-label. Trial participants, care providers and the research team will not be masked to group allocation. The study statistician will conduct the analysis masked to group allocation.
Consent
Informed consent will be sought from all participants. Many participants will lack capacity because of illness or the required interventions. Owing to the time-critical nature of enteral feeding, and as prolonged feeding prior to intervention may bias the study, it is not practicable to wait until capacity returns. Seeking personal consultee opinion in an emergency may cause additional distress for relatives. As such, an emergency waiver of consent model will be used, with informed consent sought once patients regain capacity (online supplemental appendix 4). Participants (or their personal or professional consultee) are free to withdraw at any time from the study without giving reasons and without prejudicing any further treatment. This approach was deemed appropriate in a PPI consultation. See online supplemental appendix 5 for the participant information sheet and online supplemental appendix 6 for the participant consent sheet approved by the Research Ethics Committee.
The biological samples will be used only for the predefined schedule of assessments in this study and then discarded. The data generated from samples can be used for ancillary studies.
Participant study exit criteria
The trial intervention will be terminated if any of the following events occur (within 48 hours of study entry):
Death.
Withdrawal of life-sustaining treatment.
Early discharge from the ICU.
Decision by attending clinician that the delivery of feed should be stopped or adjusted on safety grounds.
Development of any of the conditions listed in the exclusion criteria (ie, need for prone positioning or trophic feeding).
Post-trial care
After the trial intervention period, participants will be managed and monitored following the local ICU unit guidelines in general and specifically for nutrition (online supplemental appendix 2).
Participant withdrawal
Participants, or their personal or professional consultees, may withdraw from the study at any time. No reason needs to be given and usual medical care will not be affected. Only data essential for study monitoring and oversight will be retained.
End of trial
The trial will end on completion of the follow-up period for the final participant or if required by the sponsor, research ethics committee or the trial oversight committee.
Data collection, management and analysis
Data will be collected on a paper case report form (CRF) according to the schedule assessments outlined in online supplemental appendix 3. All missing data must be explained on the CRF. Paper copies of the CRF will be kept in a secure location (locked cabinet). An online REDCap database will be used to store and assimilate clinical and assay data. All documents will be stored securely and only accessible by trial staff and authorised personnel. Data will be collected and retained in accordance with the relevant data protection legislation. Access to the data will be granted to authorised representatives from the sponsor, host institution and the regulatory authorities to permit trial-related monitoring, audits and inspections.
Retention in the study is promoted by 7-day research nurse support and education of clinical teams. Follow-up for ICU and hospital outcomes uses routinely collected national clinical audit data. No distinction is made in data collection between participants who deviate or discontinue from intervention protocols.
Statistical analysis
In a study of healthy participants, the intermittently fed group had a mean (SD) peak plasma insulin concentration at 2 hours after the intermittent feed of 373±204 pmol/L compared with the continuous feed group 58±41 pmol/L.29 This effect size of 2.14 would require 6 subjects per group to have a 90% power (p=0.05). We expect a smaller effect size in the critically ill population as the volume of intermittent feeds is less than in the healthy participant studies and they are likely to have less physiological capacity to mount a response. In addition, a smaller response in critically ill patients would be clinically relevant and there is likely to be more variability in the data given the heterogeneity in the patient sample and the potential for violation of the assumption of equal variances. We have, therefore, adjusted the sample size calculation to be able to detect a smaller effect of 1.26 whereby the study would have 80% power with 11 patients per group, and 90% power with 15 patients per group. The sample size has been adjusted to 30 to detect this more conservative effect size estimate while still retaining at least 80% power in the case of a 25% drop-out rate.
All data will be analysed using an intention-to-treat analysis set. Analysis will be undertaken by the study statistician, masked to group allocation. A full review of the data (data veracity, verification and validity) will be undertaken prior to inferential analysis. If the amount of missing data on an outcome is between 20% and 40%, we will carry out a sensitivity analysis under ‘missing not At random’ scenarios. If the amount of missing data on an outcome is ≥40%, these data will be reported descriptively.
Two-sided statistical tests will be used throughout and a p<0.05 will be taken as statistically significant. The primary outcome is peak plasma insulin within 3 hours of the second-day morning intermittent feed compared with the equivalent time point in continuously fed patients. A robust comparison of means on the primary outcome will be undertaken using the most appropriate form of the two-sample t-test (independent samples, Welch test or bootstrap equivalent if there is a severe violation of underpinning assumptions). Effect size will be reported using 95% CIs. The same analyses at the 3 hours of intermittent feed compared with the equivalent time point in continuously fed patients will be undertaken for hormonal and metabolic indicators (C-peptide, fatty acid, glycerol, triglyceride, GLP-1, glucose, ketones and urea). Area under the curve of hormonal and metabolic measures tested hourly on study day 2 will be compared between groups using the same methodology. Haematology, biochemistry and acid-base balance measures derived from routine daily blood sampling will be compared between randomised arms for each day using the above methodology, assessed for changes over time using the paired samples t-test, and with change scores assessed between randomised arms. There are no planned interim analyses.
Kaplan-Meier analyses coupled with the log-rank test will be used to compare the length-of-stay outcomes between randomised arms. The percentage achieving target nutrition (per 24-hour period) and protocol compliance will be reported by randomised arm with 95% CI for between-group differences.
Monitoring
The study will be monitored in accordance with local hospital guidelines according to a plan agreed by the sponsor. All trial-related documents will be made available on request for monitoring and audit by the research sponsor, research ethics committee and for inspection by the Medicines and Healthcare products Regulatory Authority or other licensed bodies. A formal data monitoring committee was not considered to be required given the limited scope of the study. The trial management group consisting of the study investigators and sponsor’s representative meets quarterly to review study’s progress. The oversight committee of at least two independent clinicians and one PPI representative will review the reports and recommendations of the trial management group. Any suspected adverse events will be reported to the study sponsor.
Ethics and dissemination
The study has received Health Research Authority (HRA) approval from Wales—Cardiff Research Ethics Committee 3 (reference 23/WA/0297). Any amendments to the current protocol will be communicated to the research ethics committee via the research sponsor.
The trial will be reported in accordance with the Consolidated Standards of Reporting Trials guidelines (www.consort-statement.org). The main report will be written by the trial management group with authorship determined according to the internationally agreed criteria for authorship (www.icmje.org).
The study will be presented at scientific and clinical meetings and uploaded to a preprint server prior to publication in an open access peer-reviewed journal. Participants will be asked if they wish to have a lay summary of the findings.
The coapplicants, collaborators and sponsor will have access to the final trial dataset. Applications for access to the final trial dataset will be considered by the chief investigator and sponsor after publication of trial results. Participants consented to use of anonymised trial data to support future research in the participant consent form (see online supplemental appendix 6).
Discussion
Despite the widely recognised importance of adequate nutrition for a large proportion of the critically ill population, there is a shortage of evidence comparing outcomes between different feeding strategies and specifically between continuous and diurnal intermittent regimens. The existing trials are small (typically less than 100 participants in each arm) and implement a variety of intermittent feeding regimens, which have been difficult to synthesise in meta-analyses. Many studies fail to delineate adequate fasting periods, particularly overnight, which limits the ability to contrast with continuous feeding regimens (as gradual stomach emptying likely smooths out the delivery of nutrients to the gut). Recent reviews highlight the paucity of studies looking at metabolic and hormonal outcomes from intermittent diurnal feeding.31 36 We detected differences in metabolic and immune profiles in healthy volunteers, but it is not yet known whether these findings translate to the ICU population, whose physiology may be altered by critical illness. This study will start to address this knowledge gap in the literature by focusing on the metabolic and hormonal effects of continuous versus diurnal intermittent gastric feeding.
The potential for intermittent feeding to increase the risk of adverse gastrointestinal outcomes continues to be a source of controversy. There is a suggestion of a trend towards increased incidence of constipation in continuous feeding groups and increased incidence of diarrhoea in intermittent feeding groups. This study will contribute towards clarifying the side effect profile for continuous and intermittent feeding regimens. Even if there are differences this may indicate the need to individualise the choice of different feeding patterns depending on patient factors.37
A strength of the study includes the involvement of dieticians, specialists in nutrition and metabolism, and PPI in the research design and delivery. The eligibility criteria are broad, which should help to generate a representative sample of the ICU patient population. The diurnal intermittent feeding intervention in this study has been carefully considered, and involves sufficient breaks between feeds, with an overnight fast, to allow us to capture feeding/fasting cycles in metabolites. Hourly blood sampling around the time of a meal represents an innovative approach that allows detailed profiling of metabolic hormones and will increase our ability to detect a difference between continuous and diurnal intermittent feeding regimens.
We anticipate several limitations to this study. An open-label design has been chosen, which may bias the interpretation of the results. Only a single centre will be studied, limiting the generalisability and external validity of the study. Despite intensive blood sampling, there is a chance that, by only sampling on day 2 of the trial period, we may fail to detect differences in metabolism that are present at different time points. If we do detect a difference in metabolic outcomes between the groups, it may be difficult to tease out which differences can be attributed to the diurnal aspect or intermittent nature of the intervention. In addition, there may be caloric intake independent of the enteral feed (eg, drugs diluted in dextrose solution or propofol solubilised in a lipid emulsion). Although this should theoretically be balanced between groups by randomisation, we have not taken specific account of non-nutritional calories in this protocol. Finally, it is understandably not feasible to undertake any more invasive tissue/mechanistic measurements in this exploratory study.
This study will unveil detailed information on the effects of diurnal intermittent versus continuous feeding on metabolic hormone profiles. We aim to use the study findings to inform the design of a multicentre RCT powered to demonstrate the efficacy of different NG feeding regimens on length of stay, mortality and important long-term patient-centred outcomes such as physical function. This preliminary study may highlight the benefits of diurnal intermittent feeding regimen from a practical standpoint, for example, in terms of patient mobility and acceptability to patients and staff, so consideration will be given to a definitive mixed methods study design to investigate these factors. Any benefits of the diurnal intermittent regimen may also be translated to resource-poor settings where the limited availability of pumps may prevent continuous administration.
Trial status
Active, not recruiting.
supplementary material
Footnotes
Funding: This study is funded by the Southmead Hospital Charity Research Fund. AEP and MA are supported by MRC funding for studies of metabolism related to ICU care (MR/W029138/1). North Bristol NHS Trust is the sponsor of the trial and contact details are given below. Neither funder nor sponsor have a role in study design, delivery, analysis, interpretation or decision to submit for publication.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-086540).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Clodagh Emer Beattie, Email: clodaghbea01@hotmail.co.uk.
Matt Thomas, Email: matt.thomas@nbt.nhs.uk.
Borislava Borislavova, Email: borislava.borislavova@nbt.nhs.uk.
Harry A Smith, Email: harryasmith95@gmail.com.
Michael Ambler, Email: mike.ambler@bristol.ac.uk.
Paul White, Email: paul.white@uwe.ac.uk.
Kati Hayes, Email: kati.hayes@nbt.nhs.uk.
Danielle Milne, Email: danielle.milne@nbt.nhs.uk.
Aravind V Ramesh, Email: ar17163@bristol.ac.uk.
Javier T Gonzalez, Email: jg833@bath.ac.uk.
James A Betts, Email: jb335@bath.ac.uk.
Anthony E Pickering, Email: tony.pickering@bristol.ac.uk.
References
- 1.Lew CCH, Yandell R, Fraser RJL, et al. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review. J Parenter Enteral Nutr . 2017;41:744–58. doi: 10.1177/0148607115625638. [DOI] [PubMed] [Google Scholar]
- 2.Arabi YM, Casaer MP, Chapman M, et al. The intensive care medicine research agenda in nutrition and metabolism. Intensive Care Med. 2017;43:1239–56. doi: 10.1007/s00134-017-4711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compher C, Bingham AL, McCall M, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J Parenter Enteral Nutr. 2022;46:12–41. doi: 10.1002/jpen.2419. [DOI] [PubMed] [Google Scholar]
- 4.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Binnekade JM, Tepaske R, Bruynzeel P, et al. Daily enteral feeding practice on the ICU: attainment of goals and interfering factors. Crit Care. 2005;9:R218–25. doi: 10.1186/cc3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendavid I, Singer P, Theilla M, et al. NutritionDay ICU: A 7 year worldwide prevalence study of nutrition practice in intensive care. Clin Nutr. 2017;36:1122–9. doi: 10.1016/j.clnu.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Bear DE, Hart N, Puthucheary Z. Continuous or intermittent feeding: pros and cons. Curr Opin Crit Care. 2018;24:256–61. doi: 10.1097/MCC.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Sun R, Wu A, et al. Prognostic Value of Prolonged Feeding Intolerance in Predicting All‐Cause Mortality in Critically Ill Patients: A Multicenter, Prospective, Observational Study. J Parenter Enteral Nutr . 2020;44:855–65. doi: 10.1002/jpen.1693. [DOI] [PubMed] [Google Scholar]
- 9.Van Dyck L, Casaer MP. Intermittent or continuous feeding: any difference during the first week? Curr Opin Crit Care. 2019;25:356–62. doi: 10.1097/MCC.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 10.McNelly AS, Bear DE, Connolly BA, et al. Effect of Intermittent or Continuous Feed on Muscle Wasting in Critical Illness: A Phase 2 Clinical Trial. Chest. 2020;158:183–94. doi: 10.1016/j.chest.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Kadamani I, Itani M, Zahran E, et al. Incidence of aspiration and gastrointestinal complications in critically ill patients using continuous versus bolus infusion of enteral nutrition: a pseudo-randomised controlled trial. Aust Crit Care. 2014;27:188–93. doi: 10.1016/j.aucc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 12.MacLeod JBA, Lefton J, Houghton D, et al. Prospective randomized control trial of intermittent versus continuous gastric feeds for critically ill trauma patients. J Trauma. 2007;63:57–61. doi: 10.1097/01.ta.0000249294.58703.11. [DOI] [PubMed] [Google Scholar]
- 13.Nasiri M, Farsi Z, Ahangari M, et al. Comparison of Intermittent and Bolus Enteral Feeding Methods on Enteral Feeding Intolerance of Patients with Sepsis: A Triple-blind Controlled Trial in Intensive Care Units. Middle East J Dig Dis. 2017;9:218–27. doi: 10.15171/mejdd.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoney DH, Parker D, Jr, Formea CM, et al. Tolerability of bolus versus continuous gastric feeding in brain-injured patients. Neurol Res. 2002;24:613–20. doi: 10.1179/016164102101200456. [DOI] [PubMed] [Google Scholar]
- 15.Serpa LF, Kimura M, Faintuch J, et al. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:9–14. doi: 10.1590/s0041-87812003000100003. [DOI] [PubMed] [Google Scholar]
- 16.Steevens EC, Lipscomb AF, Poole GV, et al. Comparison of continuous vs intermittent nasogastric enteral feeding in trauma patients: perceptions and practice. Nutr Clin Pract. 2002;17:118–22. doi: 10.1177/0115426502017002118. [DOI] [PubMed] [Google Scholar]
- 17.Qu J, Xu X, Xu C, et al. The effect of intermittent versus continuous enteral feeding for critically ill patients: a meta-analysis of randomized controlled trials. Front Nutr. 2023;10:1214774. doi: 10.3389/fnut.2023.1214774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutagouga Boudjadja M, Culotta I, De Paula GC, et al. Hypothalamic AgRP neurons exert top-down control on systemic TNF-α release during endotoxemia. Curr Biol. 2022;32:4699–706. doi: 10.1016/j.cub.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Ganeshan K, Nikkanen J, Man K, et al. Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell. 2019;177:399–413. doi: 10.1016/j.cell.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston JD. Physiological responses to food intake throughout the day. Nutr Res Rev. 2014;27:107–18. doi: 10.1017/S0954422414000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metab Clin Exp. 2018;84:11–27. doi: 10.1016/j.metabol.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skene DJ, Skornyakov E, Chowdhury NR, et al. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc Natl Acad Sci U S A. 2018;115:7825–30. doi: 10.1073/pnas.1801183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouw IWK, Heilbronn LK, van Zanten ARH. Intermittent feeding and circadian rhythm in critical illness. Curr Opin Crit Care. 2022;28:381–8. doi: 10.1097/MCC.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherton PJ, Etheridge T, Watt PW, et al. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–8. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 25.Davis TA, Fiorotto ML, Suryawan A. Bolus vs. continuous feeding to optimize anabolism in neonates. Curr Opin Clin Nutr Metab Care. 2015;18:102–8. doi: 10.1097/MCO.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaromskyte G, Prokopidis K, Ioannidis T, et al. Evaluating the Leucine Trigger Hypothesis to Explain the Post-prandial Regulation of Muscle Protein Synthesis in Young and Older Adults: A Systematic Review. Front Nutr. 2021;8:685165. doi: 10.3389/fnut.2021.685165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury AH, Murray K, Hoad CL, et al. Effects of Bolus and Continuous Nasogastric Feeding on Gastric Emptying, Small Bowel Water Content, Superior Mesenteric Artery Blood Flow, and Plasma Hormone Concentrations in Healthy Adults: A Randomized Crossover Study. Ann Surg. 2016;263:450–7. doi: 10.1097/SLA.0000000000001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HA, Davis M, Watkins JD, et al. Effect Of Nutrient Delivery Pattern On 24-h Rhythms In Blood Biochemistry, Neutrophilic Autophagy And The Skeletal Muscle Transcriptome. Med Sci Sports Exerc. 2023;55:780–1. doi: 10.1249/01.mss.0000987184.05947.3a. [DOI] [Google Scholar]
- 30.Sjulin TJ, Strilka RJ, Huprikar NA, et al. Intermittent gastric feeds lower insulin requirements without worsening dysglycemia: A pilot randomized crossover trial. Int J Crit Illn Inj Sci. 2020;10:200–5. doi: 10.4103/IJCIIS.IJCIIS_112_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heffernan AJ, Talekar C, Henain M, et al. Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis. Crit Care. 2022;26:325. doi: 10.1186/s13054-022-04140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez JT, Dirks ML, Holwerda AM, et al. Intermittent versus continuous enteral nutrition attenuates increases in insulin and leptin during short-term bed rest. Eur J Appl Physiol. 2020;120:2083–94. doi: 10.1007/s00421-020-04431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 34.Chan A-W, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence . NICE; 2006. Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. (Nice Clinical Guideline CG32)https://www.nice.org.uk/guidance/cg32 Available. [PubMed] [Google Scholar]
- 36.Ma Y, Cheng J, Liu L, et al. Intermittent versus continuous enteral nutrition on feeding intolerance in critically ill adults: A meta-analysis of randomized controlled trials. Int J Nurs Stud. 2021;113:103783. doi: 10.1016/j.ijnurstu.2020.103783. [DOI] [PubMed] [Google Scholar]
- 37.Wischmeyer PE, Bear DE, Berger MM, et al. Personalized nutrition therapy in critical care: 10 expert recommendations. Crit Care. 2023;27:261. doi: 10.1186/s13054-023-04539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


